Abstract
Azorhizobium caulinodans is able to fix nitrogen in the free-living state and in symbiosis with the tropical legume Sesbania rostrata. The bacteria accumulate poly-β-hydroxybutyrate (PHB) under both conditions. The structural gene for PHB synthase, phbC, was inactivated by insertion of an interposon. The mutant strains obtained were devoid of PHB, impaired in their growth properties, totally devoid of nitrogenase activity ex planta (Nif−), and affected in nucleotide pools and induced Fix− nodules devoid of bacteria. The Nif− phenotype was the consequence of the lack of nifA transcription. Nitrogenase activity was partially restored to a phbC mutant by constitutive expression of the nifA gene. However, this constitutive nifA expression had no effect on the nucleotide content or on growth of the phbC mutant. It is suggested that PHB is required for maintaining the reducing power of the cell and therefore the bacterial growth. These observations also suggest a new control of nifA expression to adapt nitrogen fixation to the availability of carbon and reducing equivalents.
Azorhizobium caulinodans, isolated from stem nodules of the tropical legume Sesbania rostrata, is able to grow in the free-living state at the expense of dinitrogen (11, 12). Expression of the nitrogen fixation genes (nif genes) and the fixABCX operon depends on the transcriptional activator NifA, as in other members of the class Proteobacteria (2, 36). Expression of nifA, in turn, is controlled by both the oxygen and the ammonia status of the cell, via the FixLJ, FixK, and NtrBC/NtrYX proteins and the HF-I-like protein, NrfA (18, 19, 22, 35). A. caulinodans can adapt its energy metabolism to a wide range of oxygen concentrations (5, 15, 21, 25, 29) and accumulate reserves of poly-β-hydroxybutyrate (PHB) both in the free-living state and during symbiosis (43).
PHB and other polyhydroxyalkanoate granules are frequently found in bacteria. These polymers serve as carbon and energy storage compounds and also as a sink for reducing equivalents (1). The most common biosynthetic pathway for PHB, first described for Alcaligenes eutrophus (44), starts with the formation of acetoacetyl coenzyme A (acetoacetyl-CoA) by condensation of two acetyl-CoA molecules catalyzed by a β-ketothiolase. An NADPH-dependent acetoacetyl-CoA reductase catalyzes the reduction of acetoacetyl-CoA to β-hydroxybutyryl–CoA. Finally, PHB synthase, encoded by the phbC gene, polymerizes the β-hydroxybutyryl residues to a polyester molecule. The PHB molecule can be depolymerized and converted back to acetyl-CoA via a PHB depolymerase and an NAD-dependent β-hydroxybutyrate dehydrogenase. The regulation of PHB synthesis and degradation is controlled at the level of β-ketothiolase and β-hydroxybutyrate dehydrogenase enzyme activities (1, 41), allowing rapid modulation of the level of reductive power in the cell. This fine-tuning of the reducing power may prevent inhibition of several enzymes of the tricarboxylic acid (TCA) cycle (13).
Whether PHB is a source of carbon and energy during nitrogen fixation and in symbiosis is unclear. Rhizobia such as Rhizobium tropici, Rhizobium etli, Bradyrhizobium japonicum, and A. caulinodans accumulate PHB in the free-living state (43, 46) and in mature nodules (16, 23, 34), whereas Sinorhizobium meliloti accumulates PHB in the free-living state and during the early stages of nodule development only (17). A phbC mutant strain of R. etli displays higher nitrogenase activity in symbiosis than does the wild type (9). In contrast, Bergersen et al. (4) reported that stored PHB in isolated B. japonicum bacteroids can support nitrogenase activity during darkness and thereby prolong the period of nitrogen fixation beyond that of a bacteroid suspension containing less PHB.
Here, we investigated the involvement of PHB accumulation in the energy metabolism in free-living A. caulinodans bacteria, and its role in sustaining nitrogen fixation, by studying the phenotype of phbC mutants.
MATERIALS AND METHODS
Bacterial strains, plasmids, growth conditions, and nitrogenase and β-galactosidase assays.
The bacterial strains and plasmids are listed in Table 1 or schematized in Fig. 1. All media used for A. caulinodans are based on the same mineral salt solution previously described (12). Nitrogen-free minimal medium used routinely for nitrogenase assays was LSO, which contains lactate and succinate (12). LSN medium is LSO containing 20 mM ammonium sulfate as a nitrogen source. The carbon source concentrations used for growth in nitrogen-containing medium were 56 mM lactate (LN), 18 mM succinate (SN), and 24 mM pyruvate (PN). YLS is LSN medium containing 0.1% yeast extract. Nitrogenase assays of bacteria in the free-living state were performed as reported previously (10, 12). In selected experiments, the oxygen tension in the gas phase was adjusted to 1, 3, 6, or 10%. β-Galactosidase assays of bacteria under the free-living state were performed as described previously (32). Escherichia coli strains were grown in Luria-Bertani medium, and R. etli strains were grown in PY rich medium (6). Batch cultures of R. etli were grown in minimal medium complemented with 10 mM pyruvic acid as previously described (8). Antibiotics were used at the following concentrations: for A. caulinodans, 200 μg of carbenicillin per ml and 100 μg of kanamycin per ml; for R. etli, 30 μg of kanamycin per ml; for E. coli, 100 μg of ampicillin per ml, 25 μg of kanamycin per ml, and 30 μg of gentamicin per ml; for all, 10 μg of tetracycline per ml and 50 μg of spectinomycin per ml.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Description | Reference |
|---|---|---|
| Strains | ||
| ORS571 | A. caulinodans wild-type strain | 11 |
| 5795 | ORS571 ethyl methanesulfonate mutant; Nif− Fix− | 10 |
| 57601 | ORS571 fixK mutant; Nif− Fix− | 22 |
| 57592 | ORS571 fixL mutant; Nif− Fix− | 19 |
| 57593 | ORS571 fixJ mutant; Nif− Fix− | 19 |
| CA430 | ORS571 phbC::Ω-Spcr | This work |
| CA439 | ORS571 phbC::Ω-Spcr | This work |
| SAM100 | R. etli phaC::Ω-Kmr | 9 |
| Plasmids | ||
| pΔ10.59 | R. etli phaC gene | This laboratory |
| pRS1022 | ORS571 nifA gene expressed constitutively from Kmr promoter | 18 |
| pLRSA2 | ORS571 nifA gene expressed from its own promoter | 36 |
| pRS2002 | Translational nifH-lacZ fusion | 19 |
| pRS2010 | Transcriptional nifA-lacZ fusion | 18 |
| pRS2028 | Transcriptional nifAΔ-lacZ fusion | 20 |
| pRS2004 | Translational fixK-lacZ fusion | 22 |
| pRS3012 | Transcriptional fixN-lacZ fusion | 29 |
FIG. 1.
Genetic and physical map of the A. caulinodans phbC region. The positions of phbC and nac genes are indicated by arrows. Restriction site abbreviations: B, BamHI; P, PstI; R, EcoRI; S, SacI; Sc, ScaI; X, XhoI. Ω-Spr represents the Ω-streptomycin/spectinomycin resistance interposon. Ω-gus-Spcr represents the glucuronidase-streptomycin/spectinomycin resistance interposon. The horizontal bar indicates the DNA fragment whose nucleotide sequence was determined.
Recombinant DNA techniques and construction of plasmids and mutant strains.
Plasmid isolation, DNA ligation, restriction mapping, exonuclease III treatment, transformation, genomic DNA extraction, Southern blotting, and hybridization were performed by standard methods, as described previously (3). An E. coli S17-1 colony containing pRS592, a plasmid of an A. caulinodans Sau3A genomic DNA bank inserted into pLA29-17 (18), was selected by complementation of the Nif− phenotype of the mutant strain 5795 (10). The 12-kb XhoI fragment from pRS592, containing phbC, was transferred into pVK100 (26) to yield pRS5930 (Fig. 1) and into the single SalI site of pJQ200mp18 (39) to yield pSP1600. Plasmid pRSΩ6 (Fig. 1) is a derivative of pRS5930 obtained after partial digestion with EcoRI and ligation with a 4-kb EcoRI fragment carrying a Ω-Gus-Smr Spcr (glucuronidase-spectinomycin resistant) interposon from pWM5 (31). The location of the interposon at the correct EcoRI site in the phbC gene was verified by restriction analysis. Similarly, after partial digestion of pSP1600 with EcoRI, the 2-kb EcoRI Ω-Spcr interposon from pHP45Ω-Spcr (38) was inserted into the phbC gene, to yield pSP1601. The 13-kb XhoI/PstI fragment of pSP1601, containing the interposon, was then recovered as a PstI fragment (with the PstI site of the vector polylinker) and ligated into the PstI site of pSUP202 (42) to yield pSP1605 (Fig. 1). Plasmid pSP1611 is a derivative of pSP1605 in which the tetracycline resistance gene is interrupted with the kanamycin resistance gene from pHP45Ω-Kmr (14) (Fig. 1). To construct phbC mutant strains, spectinomycin-resistant, kanamycin-sensitive strains were isolated from two independent experiments after introducing pSP1611 into A. caulinodans wild type by conjugation and subsequent recombination of the interposon in the genome. Mutants resulting from double recombination of the Ω-Spr interposon at the correct location in the phbC gene were verified by Southern hybridization of their genomic DNA with DNA fragments of pSP1600 and the interposon as probes. Plasmids pRS2010 and pRS2028 are suicide vectors carrying transcriptional nifA-lacZ fusions that were used for recombination of the fusions in the host genome. Plasmid pRS2028 carries the same lacZ-Km cartridge from pKOK5 (27) at position +268 as in pRS2010 (18) and a deletion of the nifA coding sequence from the nucleotides +103 to +211 (positions are in reference to the transcription start site). The details of the construction of pRS2028 are reported elsewhere (20).
Deletion mapping of the phbC region.
pTZ18P2 is a derivative of pTZ18R (Pharmacia), containing two PacI restriction sites introduced by ligation of appropriate oligonucleotide duplexes on either side of the polylinker. pRK415P1 is a derivative of pRK415 (24) into which a single PacI restriction site has been introduced. To obtain sequential deletions of the pRS5930 12-kb XhoI fragment which restore nitrogen fixation to the mutant strain 5795, the fragment was first cloned into the SalI site of pTZ18P2 in the two possible orientations. The resulting plasmids were digested with KpnI and XbaI, treated with exonuclease III (Pharmacia), and religated. The deleted fragments were then recovered as PacI fragments and ligated into pRK415P1. One of these deletions enabled us to isolate a 2.5-kb EcoRI fragment (one EcoRI site from the plasmid and the other from the fragment) which, once inserted into pRK415 (pRS5951 and pRS5952 [Fig. 1]), still restored nitrogen fixation of strain 5795 in the free-living state. This 2.5-kb EcoRI fragment was transferred into pBKS+ (Pharmacia), yielding pRS5961 and pRS5962.
DNA sequencing.
The nucleotide sequences of DNA inserts in pRS5961, pRS5962, and pRS5992 (Fig. 1) were determined with the Taquence kit (U.S. Biochemical). Data were compiled with the Genetics Computer Group program. The Blast and FASTA programs of the National Center for Biotechnology Information server were used for similarity searches.
Other methods and nodulation tests.
PHB was assayed by the spectrophotometric method of Law and Slepecky (28). Spectral absorption was monitored between 200 and 300 nm to ensure that a single and symmetrical peak appeared at 235 nm. Free nucleotide content was assayed as described by Bravo and Mora (8). Immunodetection with antibodies against NifA was performed as previously described (33). Inoculation of S. rostrata and determination of the nitrogenase activity associated with roots were performed as previously described (10). Each series included six plants inoculated with the same strain to assay nitrogenase activity 4 weeks postinoculation. Noninoculated controls were included in each experiment, and no nodules were observed on control plants.
Nucleotide sequence accession number.
The phbC gene EMBL accession no. is AJ006237.
RESULTS
PHB accumulation in A. caulinodans ORS571 wild-type bacteria.
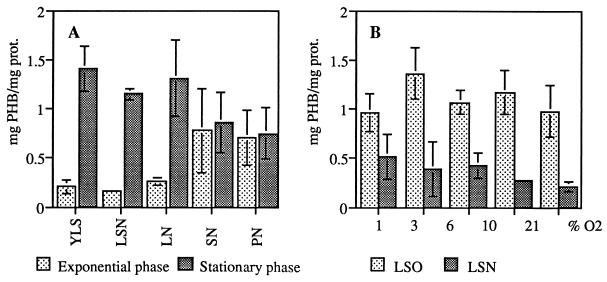
PHB accumulation was assayed in ORS571 cultures grown in minimal medium supplemented with various carbon sources and in rich medium. PHB was detected in all aerated ORS571 cultures (Fig. 2A). During the exponential phase, the PHB content was less than 0.4 mg of PHB/mg of protein when the medium contained lactate as carbon source (YLS, LSN, and LN [Fig. 2A]), whereas it increased during the stationary phase (ca. 1.4 mg of PHB/mg of protein). In contrast, there was little difference in the PHB content between the exponential and stationary phases, during growth in medium with succinate or pyruvate as sole carbon source (SN and PN [Fig. 2A]). An important increase in the PHB content was observed when the cells were transferred from exponential phase in YLS medium (0.2 mg of PHB/mg of protein [Fig. 2A]) to nitrogen-free medium after 3 h of incubation (Fig. 2B). The PHB content was not dependent on oxygen tension in the gas phase, while the addition of ammonia prevented the accumulation of PHB (Fig. 2B).
FIG. 2.
PHB accumulation in the wild-type strain, expressed in milligrams of PHB per milligram of protein (prot.). (A) Aerated cultures with various carbon sources in the presence of ammonia: YLS, rich medium; LSN, lactate and succinate; LN, lactate; SN, succinate; PN, pyruvate. (B) PHB accumulation after 4 h of incubation under various oxygen tensions in the presence (LSN) or the absence (LSO) of ammonia.
PHB accumulation in fixK and nifA mutant strains of A. caulinodans in LSO medium was similar to that in ORS571; thus, it does not appear to be dependent on nitrogen fixation (data not shown).
Identification of the PHB synthase structural gene.
Strain 5795, isolated after ethyl methanesulfonate mutagenesis, was previously described as a regulatory Nif− mutant strain that did not synthesize several polypeptides induced under nitrogen deficiency (10). A plasmid, pRS5930 (Fig. 1), containing a 12-kb XhoI fragment that restored growth of strain 5795 on nitrogen-free medium was first obtained. Different deletions of the fragment were isolated to determine the smallest complementing region. This enabled us to subclone a 2.5-kb EcoRI fragment (pRS5951 and pRS5952 [Fig. 1]) which was sufficient to restore growth and nitrogenase activity to strain 5795. The nucleotide sequence of the 2.5-kb DNA fragment in plasmids pRS5961 and pRS5962 was found to contain two open reading frames (ORFs). The first, of 950 nucleotides (nt), encodes a polypeptide similar to transcriptional regulators of the LysR family, the highest similarity score being with the nac gene product of Klebsiella aerogenes (7). The other ORF, in the opposite orientation, extends beyond the sequenced fragment and corresponds to the 1,300 nt of the 3′ end of a gene whose deduced translation product had significant similarities to phaC/phbC gene products of other bacteria. The nucleotide sequence of the upstream region (pRS5992 [Fig. 1]) led to the identification of a complete ORF of 1,800 nt encoding a 600-amino-acid polypeptide (66.5 kDa). This ORF is preceded by an AGGA motif 6 nt upstream from the putative ATG start codon. The highest similarity scores were obtained with the predicted products of the phbC genes of S. meliloti (47) (57% identity) and R. etli (9) (56% identity). pRS5930 but not pRSΩ6 (which carries an interposon in the ORF) was found to restore wild-type growth on pyruvate as carbon source and to result in 50% of the maximal PHB content for the phbC mutant strain of R. etli SAM100 (9). Thus, the ORF encodes a functional equivalent of the PHB synthase and is hereafter referred to as the phbC gene.
Growth characteristics of the phbC mutant strains and PHB accumulation.
phbC mutant strains were constructed (see Materials and Methods) and designated CA430 and CA439. The growth rates of CA430 and CA439 were found to be much lower than that of the wild type in that the mutant strains formed only tiny colonies on rich and minimal solid media after 3 days. Moreover the doubling times of strain CA430 and the wild type were 162 and 132 min, respectively, in rich liquid medium (YLS) and 384 and 192 min, respectively, in LSN medium under 1% oxygen.
The PHB content of strains CA430 and CA439 grown in lactate-containing medium was below 0.14 mg of PHB/mg of protein. Plasmid pRS5930, but not pRSΩ6, restored PHB accumulation to strain CA430 (e.g., 1.67 mg of PHB/mg of protein in LSO medium under 3% O2 in the gas phase). Because the PHB content of strain 5795, grown in lactate, is similarly affected, this phenotype is consistent with a mutation in the phbC gene.
Nitrogenase activity in the free-living state and symbiotic properties of the phbC mutant.
Like strain 5795, strains CA430 and CA439 were unable to grow on nitrogen-free solid medium. Under optimal nitrogen fixation conditions (LSO medium under 3% O2–97% Ar), the phbC mutant CA430 was completely devoid of nitrogenase activity (Fig. 3A). Similarly, no activity was detected at 6 and 1% oxygen in the gas phase (data not shown). Incubation at 3% oxygen tension under nitrogen instead of argon did not result in nitrogenase activity (data not shown). Nitrogenase activity was restored to 80% of the wild-type level (59.5 ± 2.3 versus 70.2 ± 6.2 nmol of C2H4/min/mg of protein) by the introduction of pRS5930, but not of pRSΩ6 (Fig. 3A).
FIG. 3.
(A) Kinetics of nitrogenase activity under 3% oxygen, in A. caulinodans ORS571 (wild type), CA430 (phbC mutant), CA430pRS5930, and CA430pRSΩ6. (B) Kinetics of nitrogenase activity under 3% oxygen, in A. caulinodans ORS571 (wild type), CA430 (phbC mutant), and CA430pRS1022 (phbC mutant with constitutively expressed nifA). prot., protein.
The nodulation properties and nitrogenase activity of ORS571 wild-type and phbC mutant strains were examined 4 weeks after inoculation of S. rostrata plantlets. Plants inoculated with the wild type harbored large nodules which displayed a specific nitrogenase activity of 0.20 ± 0.10 nmol of C2H4/min/mg of nodule (mean of three assays with six plants). Plants inoculated with strain CA430 displayed a Fix− phenotype, and the specific nitrogenase activity was 0.02 ± 0.02 nmol of C2H4/min/mg of nodule (mean of three assays with six plants). The mutant strain CA430 induced essentially numerous small nodules totally devoid of nitrogenase activity which did not contain bacteria. However, we have observed the presence of a few large nitrogen-fixing nodules in addition to the small ones on some plants, which may result from phbC revertant strains. Plants inoculated with the mutant strain containing pRS5930 developed large nodules with specific nitrogenase activities similar to those induced by the wild type (0.14 ± 0.04 nmol of C2H4/min/mg of nodule).
Nucleotide content of ORS571 wild-type and phbC mutant strains.
The absence of nitrogenase activity in strains CA430 and CA439 was surprising since phbC mutants of other rhizobia have been reported to be able to fix nitrogen in the free-living state and during symbiosis (9, 37). We postulated that the absence of PHB causes an imbalance in carbon metabolism, impairing the TCA cycle and, consequently, the supply of energy to nitrogenase. Therefore, the free nucleotide contents of the wild type and mutant CA430 were quantified under conditions of derepression of nitrogenase activity (LSO medium under 3% O2–97% Ar). The NADH pool was found to be higher in the mutant than in the wild type, reflecting a lower NAD/NADH ratio in the mutant. The AMP level was much higher in the mutant than in the wild type, indicating decreased ATP availability (Table 2). The same results were observed for the R. etli phaC mutant strain (9). Furthermore, wild-type nucleotide contents were restored to the A. caulinodans phbC mutant strain by introducing pΔ10.59 (Table 2), which contains the R. etli phaC gene (Table 1). This indicates that, as in R. etli, PHB is a sink for reductive power in A. caulinodans.
TABLE 2.
Nucleotide contents of A. caulinodans wild-type and phbC mutant strains in nitrogen-free medium under an oxygen tension of 3%
| Nucleotide(s) | Nucleotide content (nmol/mg of protein) for strain:
|
|||
|---|---|---|---|---|
| ORS571 | CA430 | CA430pRS1022 | CA430pΔ10.59 | |
| NAD+ | 1.241 ± 0.150 | 1.626 ± 0.103 | 1.680 ± 0.196 | 1.189 ± 0.110 |
| NADH | 0.426 ± 0.039 | 2.287 ± 0.149 | 2.538 ± 0.315 | 0.284 ± 0.047 |
| NAD+/NADH | 2.946 ± 0.588 | 0.714 ± 0.090 | 0.671 ± 0.127 | 3.822 ± 0.337 |
| AMP | 0.016 ± 0.008 | 0.553 ± 0.057 | 0.550 ± 0.071 | 0.022 ± 0.017 |
| ADP | 0.535 ± 0.050 | 0.188 ± 0.025 | 0.228 ± 0.056 | 0.426 ± 0.109 |
| ATP | 0.291 ± 0.026 | 0.013 ± 0.007 | 0.017 ± 0.015 | 0.236 ± 0.114 |
Analysis of the regulation of nif gene expression in the phbC mutant strain CA430.
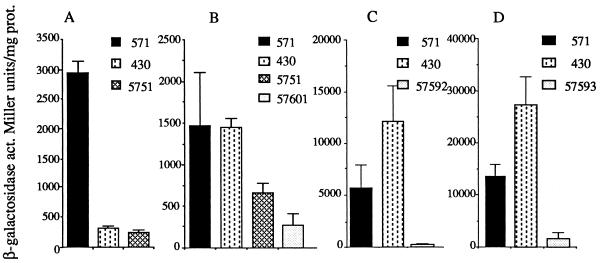
A nifH-lacZ fusion carried by pRS2002 was not expressed in strain CA430 (data not shown), indicating that the lack of nitrogenase activity was due not to the inactivation of nitrogenase but rather to the absence of its synthesis. A chromosomal nifA-lacZ fusion was not expressed in CA430 (Fig. 4A), and this was correlated with the absence of NifA polypeptide synthesis, tested with antibodies against NifA (data not shown). In A. caulinodans, nifA expression is controlled by FixK, whose synthesis of which is controlled by FixLJ in response to microaerobic conditions (19, 22). In order to know if the phbC mutation affected expression or activity of one of these regulators, expression of fixK and fixN (a gene under the control of fixK) was examined. The fixK-lacZ fusion and the fixN-lacZ fusion were more strongly expressed in the phbC mutant than in the wild type but were not expressed in a fixL mutant (57592) and a fixJ mutant (57593), respectively (Fig. 4C and D). Thus, the defective nifA expression in the phbC mutant was not due to the inhibition of FixL, FixJ, or FixK synthesis or activities.
FIG. 4.
Relative expression of nif and fix-lacZ fusions in the wild-type and mutant strains. (A) Chromosome-borne nifA-lacZ; (B) chromosome-borne nifAΔ-lacZ; (C) plasmid-borne fixK-lacZ (pRS2004); (D) plasmid-borne fixN-lacZ (pRS3012). β-Galactosidase activity (act.), expressed in Miller units per milligram of protein (prot.), was assayed in nitrogen-fixing conditions, in wild-type (571), phbC mutant (430), nrfA mutant (5751), fixK mutant (57601), fixL mutant (57592), and fixJ mutant (57593) strains. Data are the means of three independent determinations.
It has recently been shown that a DNA region at the beginning of the nifA coding sequence is involved in the regulation of nifA expression in response to ammonia. This region is linked to the location of NrfA control (18, 20). The expression of a chromosomal transcriptional nifA-lacZ fusion, carrying a deletion of this region (nifAΔ-lacZ), is abolished in a fixK mutant background and was found to partially escape NrfA control (20) (Fig. 4B). In contrast to the nifA-lacZ fusion (Fig. 4A), the nifAΔ-lacZ fusion is fully expressed in the phbC mutant (Fig. 4B), suggesting that this region is also involved in the control by phbC.
When nifA was expressed from a constitutive promoter (pRS1022) in CA430, 30% of the wild-type nitrogenase activity in the free-living state was restored to the mutant (Fig. 3B). This was not observed when nifA was expressed from its own promoter (pLRSA2 [data not shown]). However, pRS1022 did not restore PHB accumulation (data not shown), the nucleotide content (Table 2), or growth on nitrogen-free solid medium or on ammonia-containing medium.
DISCUSSION
Here, we report the characterization and nucleotide sequence of the PHB synthase gene phbC of A. caulinodans. The translation product of A. caulinodans phbC is similar to 18 of the phaC/phbC translation products available in the database, and as expected, the highest similarity was with the alpha subgroup of the Proteobacteria. The phbC gene of A. caulinodans is most probably monocistronic, as in R. etli (9) and S. meliloti (47).
The cloning of an A. caulinodans genomic DNA region, complementing the nitrogen fixation defect of strain 5795, led to the isolation of a DNA fragment containing a complete nac-like gene and the distal two-thirds of phbC. However, a DNA fragment containing the complete nac-like gene and not phbC did not complement the Nif− phenotype of strain 5795. Furthermore, the inactivation of the nac-like gene did not lead to a Nif− phenotype (data not shown). As it was subsequently found that strain 5795 did not accumulate PHB, it was assumed to instead carry a mutation in the phbC gene. Why the Nif− phenotype of strain 5795 was restored by a fragment that contains only the distal two-thirds of phbC remains unclear.
Accumulation of PHB in bacteria in the presence of an excess of the carbon source differs markedly from one species to another and depends on environmental conditions. The main factor that triggers PHB accumulation in members of the family Azotobacteriaceae is oxygen limitation (1). In other bacteria, for example, Azospirillum brasilense, the C/N ratio determines the synthesis of the polymer (45). PHB breakdown in A. caulinodans is inhibited in oxygen-limiting conditions and stimulated by an excess of ammonia (43). We observed an increase in the PHB content during the stationary phase when lactate was the carbon source. In the exponential phase of growth, the synthesis of the polymer was rapid and inversely correlated with nitrogen availability at all oxygen tensions tested. Thus, PHB synthesis in A. caulinodans appears to be controlled mainly by physiological nitrogen.
Inactivation of the phbC gene by insertion of an interposon led to a decrease of growth of the strain. Analysis of CA430 showed that PHB accumulation is required for optimal growth in all conditions tested. Interestingly, its growth rate in a medium containing lactate was low (in particular, at 1% oxygen tension). However, the amount of PHB in wild-type cells under these conditions is low during the exponential phase. This suggests that the absence of PHB synthesis, and hence the absence of PHB turnover, is deleterious for the bacteria, even in conditions where there is no substantial accumulation of the polymer. In addition, the content of reduced nucleotide NADH, under conditions of nitrogen fixation, was higher in the mutant strain than in the wild type. This reflects a decrease in TCA cycle efficiency, which could explain the poor growth of the phbC mutant strain. A phbC mutant strain of R. etli also displays an increased amount of reduced NADH cofactors and a reduced ability to oxidize various substrates (9). In the Azotobacteriaceae, PHB has been suggested to be a redox regulator (1, 41). Thus, PHB metabolism appears essential for maintaining a pool of NADH preventing inhibition of TCA cycle enzymes. This model of TCA cycle regulation proposed for R. etli and Azotobacter beijerinckii is likely to apply to A. caulinodans.
The Nif− phenotype of the phbC mutant of A. caulinodans in the free-living state was due to an impaired nifA expression, whereas the Fix− phenotype results mainly from the lack of bacteroids. When nifA was transcribed from a constitutive promoter, neither the PHB accumulation nor the nucleotide content was restored to the mutant strain. This explains why the nitrogenase activity can only be partially restored in the free-living state, while the growth is strongly impaired in the presence or absence of ammonia.
The nodulation capability of the mutant appeared unaffected, but most of the nodules induced by the CA430 strain were small and did not contain bacteroids. The Fix+ nodules observed among the Fix− nodules on roots of plants inoculated with the mutant strain may result from revertants. The Fix− phenotype of CA430 contrasts with the Fix+ phenotype in symbiosis of R. etli and S. meliloti (9, 37, 47) phbC mutants. This shows that PHB turnover in A. caulinodans is essential for growth both in the free-living state and during symbiosis. In addition, PHB turnover is required for nitrogen fixation, as its absence inhibits nifA transcription.
The inhibition of NifA synthesis was one of the most intriguing properties of the phbC mutant of A. caulinodans. It was suggested that the alteration in the redox state and/or in the pool of ATP observed in the phbC mutant is responsible for the alteration of the activity of one of the regulatory proteins involved in nifA transcription. However, inactivation of NtrBC and NtrYX can be ruled out since nifA transcription in ntr mutants is only partly affected (35). FixK, the other regulatory protein that controls nifA expression, is active, as shown by the expression of a fixN-lacZ fusion in CA430. Interestingly, the nifA deletion, which allows escape from NrfA control, also allowed escape from phbC mutation. This suggests a mode of control of gene expression independent of the promoter region as reported in the case of NrfA. It is unknown whether the phbC mutation acts on NrfA or on another unidentified factor. NrfA, in addition to a role in nifA translation, may be involved in nifA transcription via an effect on the chromosome structure (20). In A. caulinodans, DNA supercoiling of nifA promoter, mediated by DNA gyrase, is necessary for nifA transcription (40). In vitro and in vivo studies of DNA gyrase of E. coli show that its activity depends on the ATP/ADP ratio (30, 48). Thus, the low ATP/ADP ratio in the phbC mutant of A. caulinodans may inhibit the DNA gyrase, thereby specifically affecting the DNA supercoiling of the nifA promoter, and consequently inhibit nifA transcription. To date, the control of nifA expression by NrfA and in response to PHB metabolism is unique to A. caulinodans, whereas other rhizobia impaired in PHB synthesis are Nif+.
In conclusion, the PHB turnover reflects the potential of the cell to adapt anabolism under nitrogen fixation conditions to the availability in carbon and reducing equivalents. Besides a well-established metabolic role of PHB as a redox regulator to maintain optimal cell growth, we propose another mechanism of control through the expression of key regulatory genes that could explain the severe growth defect of the A. caulinodans phbC mutant strain.
ACKNOWLEDGMENTS
N.M.-R. is a recipient of a predoctoral fellowship from the Ministère de l’Enseignement Supérieur et de la Recherche. This work was supported by a grant from Consejo Nacional de Ciencia y Tecnologia, Mexico, CONACyt no. 9111-0954, and partially by DGPA-UNAM.
K. Mandon and N. Michel-Reydellet contributed equally to the work.
We thank Sandra Contreras for technical assistance in the chromatographic determinations.
REFERENCES
- 1.Anderson A J, Dawes E A. Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Rev. 1990;54:450–472. doi: 10.1128/mr.54.4.450-472.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arigoni F, Kaminski P A, Celli J, Elmerich C. Transcriptional analysis of the fixABCXORF1 region of Azorhizobium caulinodans suggests post-transcriptional processing of the fixABCXORF1 mRNA. Mol Gen Genet. 1992;235:422–431. doi: 10.1007/BF00279389. [DOI] [PubMed] [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates/Wiley-Interscience; 1987. [Google Scholar]
- 4.Bergersen F J, Peoples M B, Turner G L. A role for PHB in bacteroids of soybean root nodules. Proc R Soc Lond B. 1991;245:59–64. [Google Scholar]
- 5.Bergersen F J, Turner G L, Bogusz D, Appleby C J. Fixation of N2 by bacteroids from stem nodules of Sesbania rostrata. J Gen Microbiol. 1988;134:1807–1810. [Google Scholar]
- 6.Beringer J E. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974;84:188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- 7.Best E A, Bender R A. Cloning of the Klebsiella aerogenes nac gene, which encodes a factor required for nitrogen regulation of the histidine utilization (hut) operons in Salmonella typhimurium. J Bacteriol. 1990;172:7043–7048. doi: 10.1128/jb.172.12.7043-7048.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bravo A, Mora J. Ammonium assimilation in Rhizobium phaseoli by the glutamine synthetase-glutamate synthase pathway. J Bacteriol. 1988;170:980–984. doi: 10.1128/jb.170.2.980-984.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cevallos M A, Encarnación S, Leija A, Mora Y, Mora J. Genetic and physiological characterization of a Rhizobium etli mutant strain unable to synthesize poly-β-hydroxybutyrate. J Bacteriol. 1996;178:1646–1654. doi: 10.1128/jb.178.6.1646-1654.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denèfle P, Kush A, Norel F, Paquelin A, Elmerich C. Biochemical and genetic analysis of the nifHDKE region of Rhizobium ORS571. Mol Gen Genet. 1987;207:280–287. [Google Scholar]
- 11.Dreyfus B L, Elmerich C, Dommergues Y R. Free-living Rhizobium strain able to grow on N2 as the sole nitrogen source. Appl Environ Microbiol. 1983;45:711–713. doi: 10.1128/aem.45.2.711-713.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elmerich C, Dreyfus B L, Reysset G, Aubert J P. Genetic analysis of nitrogen fixation in a tropical fast-growing Rhizobium. EMBO J. 1982;1:499–503. doi: 10.1002/j.1460-2075.1982.tb01197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Encarnación S, Dunn M, Willms K, Mora J. Fermentative and aerobic metabolism in Rhizobium etli. J Bacteriol. 1995;177:3058–3066. doi: 10.1128/jb.177.11.3058-3066.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fellay R, Frey J, Krisch H. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of gram-negative bacteria. Gene. 1987;52:147–154. doi: 10.1016/0378-1119(87)90041-2. [DOI] [PubMed] [Google Scholar]
- 15.Gebhardt C, Turner G L, Gibson A H, Dreyfus B L, Bergersen F J. Nitrogen-fixing growth in continuous culture of a strain of Rhizobium sp. isolated from stem nodules on Sesbania rostrata. J Gen Microbiol. 1984;130:843–848. [Google Scholar]
- 16.Goodchild D J. The ultrastructure of root nodules in relation to nitrogen fixation. Int Rev Cytol Suppl. 1977;6:235–288. [Google Scholar]
- 17.Hirsch A M, Bang M, Ausubel F M. Ultrastructure analysis of ineffective alfalfa nodules formed by nif::Tn5 mutants of Rhizobium meliloti. J Bacteriol. 1983;155:367–380. doi: 10.1128/jb.155.1.367-380.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaminski P A, Desnoues N, Elmerich C. The expression of nifA in Azorhizobium caulinodans requires a gene product homologous to Escherichia coli HF-I, an RNA-binding protein involved in the replication of phage Q beta RNA. Proc Natl Acad Sci USA. 1994;91:4663–4667. doi: 10.1073/pnas.91.11.4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaminski P A, Elmerich C. Involvement of fixLJ in the regulation of nitrogen fixation in Azorhizobium caulinodans. Mol Microbiol. 1991;5:665–673. doi: 10.1111/j.1365-2958.1991.tb00738.x. [DOI] [PubMed] [Google Scholar]
- 20.Kaminski P A, Elmerich C. The control of Azorhizobium caulinodans nifA expression by oxygen, ammonia and by the HF-I like protein, NrfA. Mol Microbiol. 1998;28:603–613. doi: 10.1046/j.1365-2958.1998.00823.x. [DOI] [PubMed] [Google Scholar]
- 21.Kaminski P A, Kitts C L, Zimmerman Z, Ludwig R A. Azorhizobium caulinodans uses both cytochrome bd (quinol) and cytochrome cbb3 (cytochrome c) terminal oxidases for symbiotic N2 fixation. J Bacteriol. 1996;178:5989–5994. doi: 10.1128/jb.178.20.5989-5994.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaminski P A, Mandon K, Arigoni F, Desnoues N, Elmerich C. Regulation of nitrogen fixation in Azorhizobium caulinodans: identification of a fixK-like gene, a positive regulator of nifA. Mol Microbiol. 1991;5:1983–1991. doi: 10.1111/j.1365-2958.1991.tb00820.x. [DOI] [PubMed] [Google Scholar]
- 23.Karr D B, Waters J K, Suzuki F, Emerich D W. Enzymes of the poly-β-hydroxybutyrate and citric acid cycles of Rhizobium japonicum bacteroids. Plant Physiol. 1984;75:1158–1162. doi: 10.1104/pp.75.4.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keen N T, Tamaki S, Kobayashi D, Trolinger D. Improved broad-host-range plasmids for DNA cloning in Gram-negative bacteria. Gene. 1988;70:191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- 25.Kitts C L, Ludwig R. Azorhizobium caulinodans respires with at least four terminal oxidases. J Bacteriol. 1994;176:886–895. doi: 10.1128/jb.176.3.886-895.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knauf V C, Nester E W. Wide host range cloning vectors: a cosmid clone bank of an Agrobacterium Ti plasmid. Plasmid. 1982;8:45–54. doi: 10.1016/0147-619x(82)90040-3. [DOI] [PubMed] [Google Scholar]
- 27.Kokotek W, Lotz W. Construction of a lacZ-kanamycin-resistance cassette, useful for site directed mutagenesis and as a promoter probe. Gene. 1989;84:467–471. doi: 10.1016/0378-1119(89)90522-2. [DOI] [PubMed] [Google Scholar]
- 28.Law J H, Slepecky R A. Assay of poly-β-hydroxybutyric acid. J Bacteriol. 1961;82:33–36. doi: 10.1128/jb.82.1.33-36.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mandon K, Kaminski P A, Elmerich C. Functional analysis of the fixNOQP region of Azorhizobium caulinodans. J Bacteriol. 1994;176:2560–2568. doi: 10.1128/jb.176.9.2560-2568.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maxwell A, Gellert M. The dependence of the ATPase activity of DNA gyrase. J Biol Chem. 1984;259:14472–14480. [PubMed] [Google Scholar]
- 31.Metcalf W W, Wanner B L. Construction of new β-glucuronidase cassettes for making transcriptional fusions and their use with new methods for allele replacement. Gene. 1993;129:17–25. doi: 10.1016/0378-1119(93)90691-u. [DOI] [PubMed] [Google Scholar]
- 32.Michel-Reydellet N, Desnoues N, Elmerich C, Kaminski P A. Characterization of Azorhizobium caulinodans glnB and glnA genes: involvement of the PII protein in symbiotic nitrogen fixation. J Bacteriol. 1997;179:3580–3587. doi: 10.1128/jb.179.11.3580-3587.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morett E, Fischer H M, Hennecke H. Influence of oxygen on DNA binding, positive control, and stability of the Bradyrhizobium japonicum NifA regulatory protein. J Bacteriol. 1991;173:3478–3487. doi: 10.1128/jb.173.11.3478-3487.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.N’doye I, Debilly S F, Vasse J, Dreyfus B, Truchet G. Root nodulation of Sesbania rostrata. J Bacteriol. 1994;176:1060–1068. doi: 10.1128/jb.176.4.1060-1068.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pawlowski K, Klosse U, de Bruijn F J. Characterization of a novel Azorhizobium caulinodans ORS571 two-component regulatory system, ntrYX, involved in nitrogen fixation and metabolism. Mol Gen Genet. 1991;231:124–138. doi: 10.1007/BF00293830. [DOI] [PubMed] [Google Scholar]
- 36.Pawlowski K, Ratet P, Schell J, De Bruijn F. Cloning and characterization of nifA and ntrC genes of the stem nodulating bacterium ORS571, the nitrogen fixing symbiont of Sesbania rostrata: regulation of nitrogen fixation (nif) genes in the free-living versus symbiotic state. Mol Gen Genet. 1987;206:207–219. [Google Scholar]
- 37.Povolo S, Tombolini R, Morea A, Anderson A J, Casella S, Nuti M P. Isolation and characterization of mutants of Rhizobium meliloti unable to synthesize poly-β-hydroxybutyrate. Can J Microbiol. 1994;40:823–829. [Google Scholar]
- 38.Prentki P, Krisch H M. In vitro insertional mutagenesis with selectable DNA fragments. Gene. 1984;29:303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- 39.Quandt J, Hynes M F. Versatile suicide vectors which allow direct selection for gene replacement in gram-negative bacteria. Gene. 1993;127:15–21. doi: 10.1016/0378-1119(93)90611-6. [DOI] [PubMed] [Google Scholar]
- 40.Ratet P, Pawlowski K, Schell J, de Bruijn F J. The Azorhizobium caulinodans nitrogen fixation gene nifA is controlled by the cellular nitrogen and oxygen status. Mol Microbiol. 1989;3:825–838. doi: 10.1111/j.1365-2958.1989.tb00231.x. [DOI] [PubMed] [Google Scholar]
- 41.Senior P J, Dawes E A. The regulation of poly-β-hydroxybutyrate metabolism in Azotobacter beijerinckii. J Biochem. 1973;134:225–238. doi: 10.1042/bj1340225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 43.Stam H, De Vries W, Stouthamer A H, van Verseveld H W. Utilization of poly-β-hydroxybutyrate in free-living cultures of Rhizobium ORS571. FEMS Microbiol Lett. 1986;35:215–220. [Google Scholar]
- 44.Steinbuchel A, Schlegel H G. Physiology and molecular genetics of poly(β-hydroxy-alkanoic-acid) synthesis in Alcaligenes eutrophus. Mol Microbiol. 1991;5:535–542. doi: 10.1111/j.1365-2958.1991.tb00725.x. [DOI] [PubMed] [Google Scholar]
- 45.Tal S, Okon Y. Production of the reserve material poly-β-hydroxybutyrate and its function in Azospirillum brasilense. Can J Microbiol. 1985;31:608–613. [Google Scholar]
- 46.Tombolini R, Nuti M P. Poly(β-hydroxyalkanoate) biosynthesis and accumulation by different Rhizobium species. FEMS Microbiol Lett. 1989;60:299–304. [Google Scholar]
- 47.Tombolini R, Povolo S, Buson A, Squartini A, Nuti M P. Poly-β-hydroxybutyrate (PHB) biosynthetic genes in Rhizobium meliloti 41. Microbiology. 1995;141:2553–2559. doi: 10.1099/13500872-141-10-2553. [DOI] [PubMed] [Google Scholar]
- 48.van Workum M, van Dooren J M, Oldenburg N, Molenaar D, Jensen P R, Snoep J L, Westerhokk H V. DNA supercoiling depends on the phosphorylation potential in Escherichia coli. Mol Microbiol. 1996;20:351–360. doi: 10.1111/j.1365-2958.1996.tb02622.x. [DOI] [PubMed] [Google Scholar]