Abstract
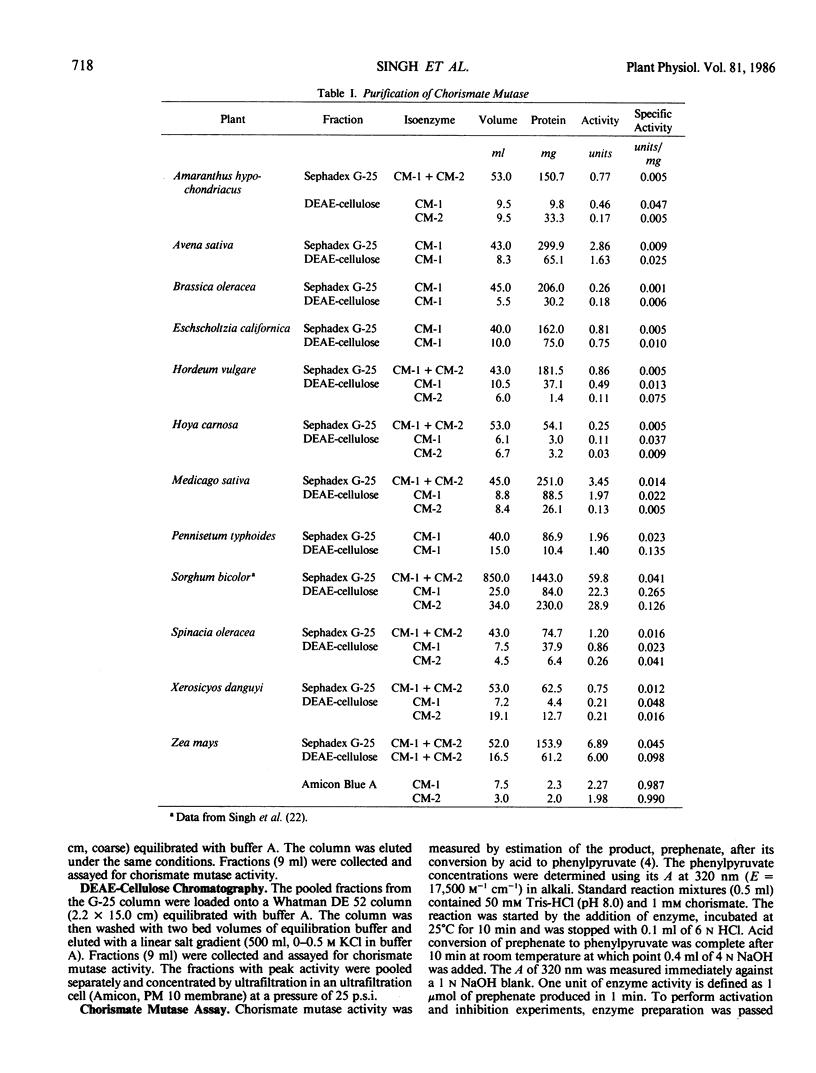
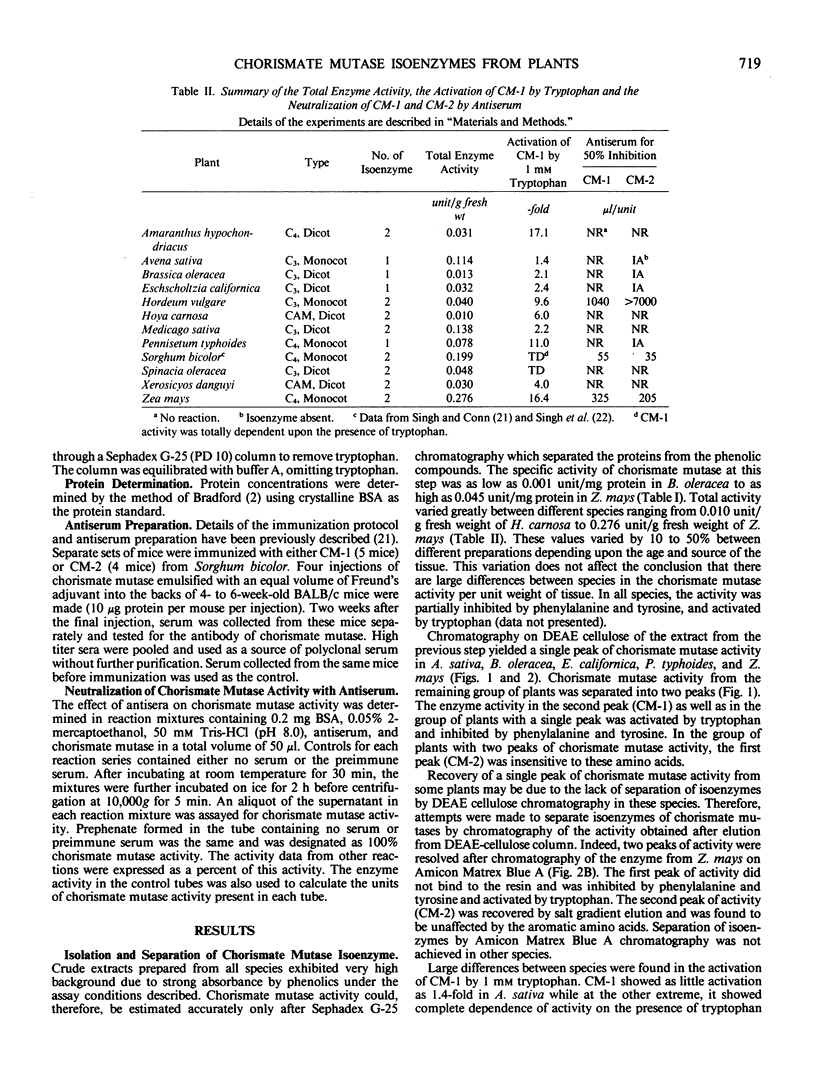
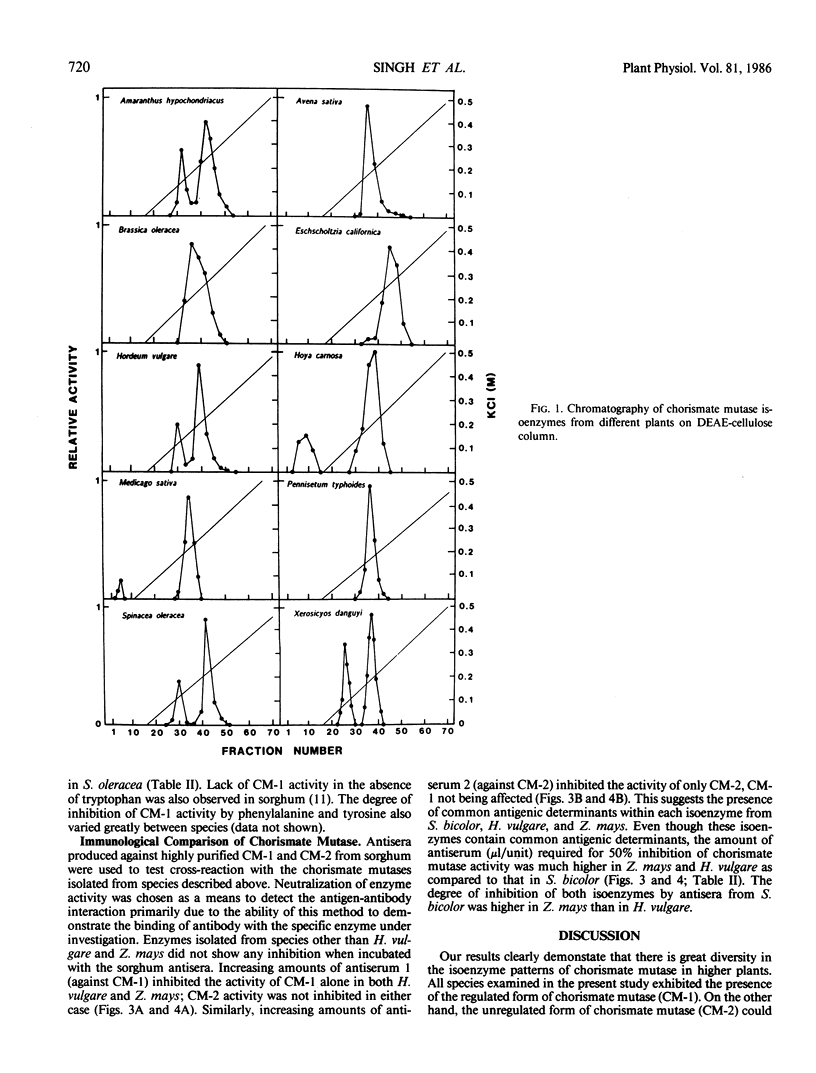
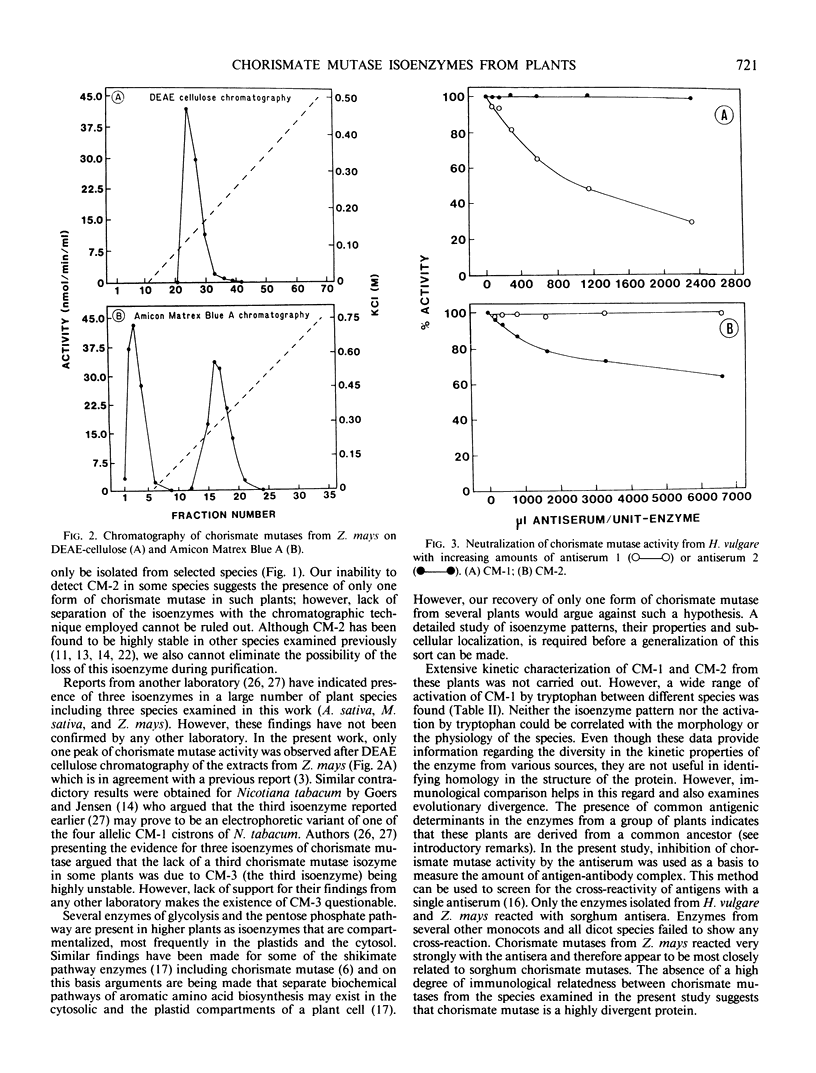
The isoenzyme pattern of chorismate mutase (EC 5.4.99.5) was examined by diethylaminoethyl-cellulose chromatography in a wide variety of plants. All plants contained a regulated form of chorismate mutase (CM-1), and most contained an additional, unregulated form (CM-2). The regulatory properties of CM-1 differed significantly between plants. Antisera prepared against CM-1 and CM-2 from Sorghum bicolor were used to test immunological cross reaction of chorismate mutases from other plants. There was a high degree of similarity between chorismate mutase isoenzymes from Sorghum bicolor and Zea mays and some with Hordeum vulgare, but all other species studied were antigenically distinct from sorghum. No homology between the structure of CM-1 and CM-2 was detected within any species.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Crawford I. P. Gene rearrangements in the evolution of the tryptophan synthetic pathway. Bacteriol Rev. 1975 Jun;39(2):87–120. doi: 10.1128/br.39.2.87-120.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M. B., Boyer C. D. Immunological characterization of maize starch branching enzymes. Plant Physiol. 1983 Jul;72(3):813–816. doi: 10.1104/pp.72.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist D. G., Kosuge T. Regulation of aromatic amino acid biosynthesis in higher plants. Properties of an aromatic amino acid-insensitive chorismate mutase (CM-2) from mung bean. Arch Biochem Biophys. 1975 Nov;171(1):36–42. doi: 10.1016/0003-9861(75)90004-1. [DOI] [PubMed] [Google Scholar]
- Gilchrist D. G., Kosuge T. Regulation of aromatic amino acid biosynthesis in higher plants. Properties of an aromatic amino acid-sensitive chorismate mutase (CM-1) from mung bean. Arch Biochem Biophys. 1974 Sep;164(1):95–105. doi: 10.1016/0003-9861(74)90011-3. [DOI] [PubMed] [Google Scholar]
- Gilchrist D. G., Woodin T. S., Johnson M. L., Kosuge T. Regulation of aromatic amino Acid biosynthesis in higher plants: I. Evidence for a regulatory form of chorismate mutase in etiolated mung bean seedlings. Plant Physiol. 1972 Jan;49(1):52–57. doi: 10.1104/pp.49.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J. C., Kerwick R. G. An immunological investigation of the structure and function of ribulose 1,5-bisphosphate carboxylase. Eur J Biochem. 1974 May 15;44(2):481–489. doi: 10.1111/j.1432-1033.1974.tb03506.x. [DOI] [PubMed] [Google Scholar]
- Murphy T. M., Mills S. E. Immunochemical and enzymatic comparisons of the tryptophan synthase alpha subunits from five species of Enterobacteriaceae. J Bacteriol. 1969 Mar;97(3):1310–1320. doi: 10.1128/jb.97.3.1310-1320.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha V., Crawford I. P., Mills S. E. Comparative immunological and enzymatic study of the tryptophan synthetase beta 2 subunit in the Enterobacteriaceae. J Bacteriol. 1972 Jul;111(1):163–168. doi: 10.1128/jb.111.1.163-168.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B. K., Conn E. E. Chorismate mutase isoenzymes from Sorghum bicolor: immunological characterization. Arch Biochem Biophys. 1986 May 1;246(2):617–621. doi: 10.1016/0003-9861(86)90317-6. [DOI] [PubMed] [Google Scholar]
- Singh B. K., Connelly J. A., Conn E. E. Chorismate mutase isoenzymes from Sorghum bicolor: purification and properties. Arch Biochem Biophys. 1985 Dec;243(2):374–384. doi: 10.1016/0003-9861(85)90514-4. [DOI] [PubMed] [Google Scholar]
- Singh B. K., Preiss J. Starch Branching Enzymes from Maize : Immunological Characterization using Polyclonal and Monoclonal Antibodies. Plant Physiol. 1985 Sep;79(1):34–40. doi: 10.1104/pp.79.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T. J., Ibrahimi I. M., Wilson A. C. Evolutionary substitutions and the antigenic structure of globular proteins. Nature. 1978 Jul 6;274(5666):92–94. doi: 10.1038/274092a0. [DOI] [PubMed] [Google Scholar]
- Woodin T. S., Nishioka L. Evidence for three isozymes of chorismate mutase in alfalfa. Biochim Biophys Acta. 1973 May 5;309(1):211–223. doi: 10.1016/0005-2744(73)90333-1. [DOI] [PubMed] [Google Scholar]
- Woodin T. S., Nishioka L., Hsu A. Comparison of chorismate mutase isozyme patterns in selected plants. Plant Physiol. 1978 Jun;61(6):949–952. doi: 10.1104/pp.61.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]


