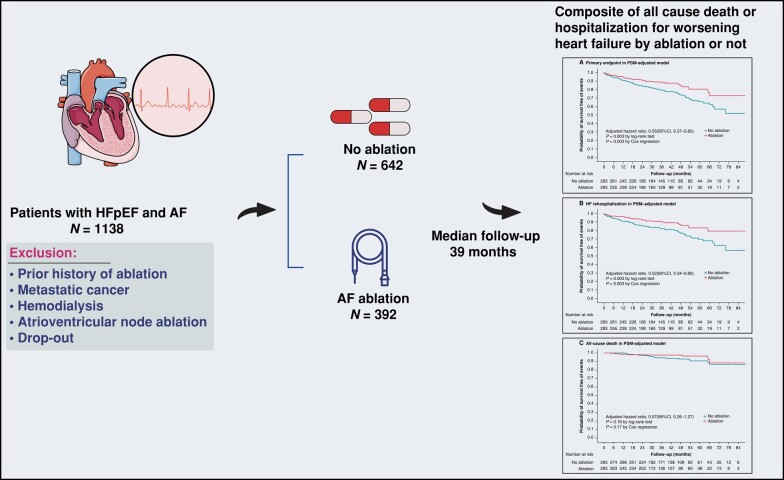
Abstract
Aims
Patients with heart failure with preserved ejection fraction (HFpEF) and atrial fibrillation (AF) have worse clinical outcomes than those with sinus rhythm (SR). We aim to investigate whether maintaining SR in patients with HFpEF through a strategy such as AF ablation would improve outcomes.
Methods and results
This is a cohort study that analysed 1034 patients (median age 69 [63–76] years, 46.2% [478/1034] female) with HFpEF and AF. Of these, 392 patients who underwent first-time AF ablation were assigned to the ablation group, and the remaining 642 patients, who received only medical therapy, were assigned to the no ablation group. The primary endpoint was a composite of all-cause death or rehospitalization for worsening heart failure. After a median follow-up of 39 months, the cumulative incidence of the primary endpoint was significantly lower in the ablation group compared to the no ablation group (adjusted hazard ratio [HR], 0.55 [95% CI, 0.37–0.82], P = 0.003) in the propensity score-matched model. Secondary endpoint analysis showed that the benefit of AF ablation was mainly driven by a reduction in rehospitalization for worsening heart failure (adjusted HR, 0.52 [95% CI, 0.34–0.80], P = 0.003). Patients in the ablation group showed a 33% relative decrease in atrial tachycardia/AF recurrence compared to the no ablation group (adjusted HR, 0.67 [95% CI, 0.54–0.84], P < 0.001).
Conclusion
Among patients with HFpEF and AF, the strategy of AF ablation to maintain SR was associated with a lower risk of the composite outcome of all-cause death or rehospitalization for worsening heart failure.
Keywords: Heart failure with preserved ejection fraction, Atrial fibrillation, Ablation, Death, Rehospitalization
Graphical Abstract
Graphical Abstract.
AF, atrial fibrillation; HFpEF, heart failure with preserved ejection fraction.
What’s new?
The strategy of atrial fibrillation (AF) ablation significantly reduced the primary composite endpoint of all-cause mortality or rehospitalization for worsening heart failure in patients with heart failure with preserved ejection fraction (HFpEF) and AF.
The clinical benefit of ablation was primarily attributed to a notable decrease in rehospitalization for worsening heart failure in patients with HFpEF and AF.
Heart failure with preserved ejection fraction patients in the ablation group showed a 33% relative decrease in atrial tachycardia/AF recurrence compared to the no ablation group.
The treatment effects of AF ablation were more significant in HFpEF patients with New York Heart Association class I to II symptoms or hypertension.
The clinical benefit of AF ablation in HFpEF patients was only observed in patients receiving catheter ablation but not for surgical ablation.
Introduction
Heart failure with preserved ejection fraction (HFpEF) is a major epidemic with a poor prognosis.1 Screening for and treating underlying causes and comorbidities has been recommended to manage HFpEF patients.2–4 Atrial fibrillation (AF) often coexists with HFpEF due to shared risk factors and complex pathophysiological interactions.5 Epidemiological studies show that AF occurs in two-thirds of HFpEF patients throughout the disease, and when coexists with HFpEF, AF leads to more significant physical limitations, symptom burden, and poorer prognosis than those in sinus rhythm (SR).6–10 Scilicet, AF is an independent risk factor, rather than a marker, in patients comorbid with AF and HFpEF.11 The optimal therapy strategy for AF in HFpEF patients has recently become a topic of interest.
Catheter ablation is a well-established therapy option for AF. The latest guidelines for AF recommend it to improve survival and decrease heart failure (HF) hospitalization in selected AF patients with heart failure with reduced ejection fraction (HFrEF).12,13 Several studies comparing catheter ablation in AF patients with HFrEF and HFpEF have shown equivalent efficacy and safety in maintaining SR.14–18 Single-arm studies of catheter ablation in patients with HFpEF have suggested potential improvements in left ventricular function and haemodynamic parameters for patients who maintain SR.19–22 However, it is not yet clear whether catheter ablation is superior to medical therapy alone in terms of clinical outcomes in these populations. No randomized controlled trials have compared ablation vs. drug therapy in HFpEF, and post hoc analyses have produced inconsistent results.23,24 The HF subgroup of the CABANA trial showed a 43% relative reduction in all-cause mortality with AF ablation compared to medical therapy within a median follow-up of 48.5 months, but the enrolled patients were HFrEF and HFpEF.23 The pre-specified subset of HFpEF in the recent RAFT-AF trial showed no significant difference for the primary endpoint of all-cause mortality and HF events.24 Real-world data from the Swedish Heart Failure Registry (SwedeHF) showed that catheter ablation was associated with a lower risk of both first and recurrent HF hospitalization in HFpEF patients within a median follow-up of 2.6 years, but HFpEF was less representive in the registry.25 The other real-word data from the Keio Interhospital Cardiovascular Studies–Atrial Fibrillation registry (KiCS-AF) showed that catheter ablation was significantly associated with a clinically meaningful improvements in the Atrial Fibrillation Effect on QualiTy-of-life Overall Summary score in HF patients with left ventricular ejection fraction (LVEF) ≥ 50%.26 Regrettably, this study had insufficient power for the analysis of hard endpoint in the HFpEF subgroup due to the limited cardiovascular events.26 Other small retrospective studies have also reported similar results.27,28 However, these findings are not yet conclusive.
The present study, based on a large and dedicated cohort of HFpEF patients, aims to investigate whether the strategy of AF ablation to maintain SR, compared to no ablation, is associated with improved all-cause death and rehospitalization for worsening HF in patients with HFpEF and AF.
Methods
Study design and participants
Consecutive patients with HFpEF and AF referred to Zhongshan Hospital between January 2015 and December 2019 were retrospectively enrolled in the cohort. The criteria used for diagnosing patients with HFpEF were as follows: (1) a history of hospitalization for HF with symptoms classified as New York Heart Association (NYHA) class II, III, or IV; (2) LVEF ≥ 50%; (3) at least one of the following cardiac structural abnormalities identified by echocardiography: left ventricular hypertrophy, left atrial enlargement, or diastolic dysfunction; and (4) elevated levels of N-terminal pro-brain natriuretic peptide (NT-proBNP), with a threshold of ≥400 pg/mL for patients with SR at admission and ≥600 pg/mL for patients with AF at admission.29 The attending physicians confirmed the diagnosis of AF based on the patient’s prior history of AF episodes and/or the 12-lead electrocardiogram (ECG) performed at the time of admission. Patients who sought treatment for HFpEF or AF were both included in this study. However, regardless of the reason for the patient’s visit, in accordance with the standard treatment procedure in our centre, all patients admitted to the hospital first received symptomatic treatment as needed. The cardiologist then further evaluated the comprehensive treatment strategy for HFpEF and AF, including the evaluation of ablation therapy. In general, the decision to offer ablation or medical therapy alone to a patient was made jointly by the physicians, the patients, and the patient’s family. Patients who have indications and consent to undergo AF ablation were offered AF ablation; while the remaining patients, including those who have indications but are concerned about potential benefits, risks and cost, were treated with medical therapy alone (rhythm control and/or rate control drug). Different therapy strategies were performed during the index hospitalization.
Patients with a history of left ventricular systolic dysfunction as evidenced by any prior measurement of LVEF < 50% on echocardiogram, including those with AF-mediated cardiomyopathy (LVEF < 50%) were not included in this study. The other major exclusion criteria included patients with (1) a prior history of left atrium radiofrequency ablation, cryoablation, or surgical ablation for AF; (2) a prior history of ablation for supraventricular tachycardia or ventricular tachycardia; (3) metastatic cancer; and (4) a requirement for haemodialysis due to terminal renal failure. The investigation conforms with the principles outlined in the Declaration of Helsinki. Prior to the commencement of the study, the ethics committee at Zhongshan Hospital, Fudan University provided approval and written informed consent was obtained from all patients who participated.
Ablation procedure
Patients who underwent catheter ablation or surgical ablation procedures (thoracoscopy-assisted minimally invasive radiofrequency ablation of AF) were included in the cohort. Before ablation, transoesophageal echocardiography was performed on all patients to evaluate whether there was a left atrial thrombus present. If a thrombus was detected, the patient was treated with anticoagulant therapy, and the ablation was postponed until the thrombus was dissolved as confirmed by repeated transoesophageal echocardiography. The operators in the centre were experienced, and the ablation procedures were performed in a relatively stable manner. The cornerstone of AF ablation was on circumferential electrical isolation of the bilateral pulmonary veins (PVI), while additional procedures such as linear ablation, complex fractionated atrial electrogram ablation, superior vena cava isolation, and ablation for other intraoperative atrial arrhythmias were performed based on the discretion of the operator. Intravenous injection of amiodarone and external direct current cardioversion may also be administered to restore SR. The most common cause of recurrence is the recovery of pulmonary vein conduction and the recovery of pulmonary vein conduction was more common at 30 and 60 min after the completion of PVI, but rarely occurred at 90 min.30 Our centre usually observed for at least 30 min after the actual ablation. If the SR is maintained continuously, the ablation is considered successful. As for surgical ablation, bilateral pulmonary vein isolation with a bipolar radiofrequency clamp and left atrial appendage resection was performed for all patients, and additional procedures and atrial ablation lines for the left atrium ablation, including, a cut-off of the Marshall ligament, creation of a left atrial roof and bottom connecting lesion, and establishment of a linear lesion connecting the roof-line to the root of the aorta (the junction of the left coronary and non-coronary cusps), were carried out at the operator’s discretion as described previously.31,32 Furthermore, if deemed indicated by the surgeon, the ablation of the right atrium was also performed with four specific lesions created on the superior vena cava, the inferior vena cava, the appendix of the right atrium, and the tricuspid valve annulus.
If there were no bleeding events or other contraindications, anticoagulants were administered beginning on the night following ablation and were continued for a minimum of three months. Thereafter, the continuation of anticoagulants was based on the CHA2DS2-VASc scores. Antiarrhythmic drugs (AADs) were continued for three months post-ablation. In-hospital adverse events related to the ablation procedures, including cardiac tamponade, an acute HF attack, access-site complications (such as groin haematoma, access site bleeding, groin arteriovenous fistula, and groin pseudoaneurysm), and severe respiratory depression caused by narcotic drugs, were treated appropriately. Repeated ablation was recommended for patients with AF recurrence unless there was a contraindication or unwillingness.
Medical therapy of atrial fibrillation and management of heart failure
In patients without ablation, medications for AF were prescribed in accordance with the published clinical practice guidelines at the time, and there were no restrictions on rate control or rhythm control strategies.33,34 The management and therapies of HF are in accordance with the latest Chinese HF guideline recommendations at that time (Chinese HF guideline 2014 and Chinese HF guideline 2018) and mainly include the use of diuretic therapy for patients with fluid storage and the treatment of underlying diseases/complications,33,34 which was generally consistent with the contemporaneous European HF guideline recommendation.35,36 Use of pharmacological treatments at baseline was defined as prior drug therapy documented at admission, in-hospital therapy, and prescriptions at discharge of the index hospitalization. The optimal ventricular rate control target is recommended to be <80 bpm at rest and <110 bpm at moderate exercise according to the Chinese HF guideline 2014; while it was updated to control of ventricular rate at 60–100 bpm and not more than 110 bpm in patients with HF and AF to reduce the symptoms during exercise and resting according to the Chinese HF guideline 2018.33,34
Outcome and follow-up
The primary endpoint was a composite of all-cause death or rehospitalization for worsening HF. The secondary endpoints were comprised of all-cause rehospitalization, rehospitalization for cardiovascular disease, rehospitalization for worsening HF, all-cause death, cardiovascular disease-related death, and incident stroke events. Heart failure hospitalization was defined as an admission to a health care facility for >24 h for worsening of HF, which was deemed as requiring intravenous medication for HF (including diuretics, vasodilators, or inotropic agents) or a substantial increase in oral diuretic therapy for HF (i.e. an increase of furosemide ≥ 40 mg or equivalent, or the addition of a thiazide to a loop diuretic).24,37 Cardiovascular death was defined as death due to HF, sudden cardiac death, myocardial infarction, stroke, or perioperative complications. The recurrence of atrial tachycardia/AF (AT/AF) was also recorded, which was defined as any documented case of AT, atrial flutter, or AF lasting ≥30 s on a 12-lead ECG or Holter monitoring following the ablation procedure. There was a three-month blanking period post-ablation for AT/AF recurrence. In the primary analysis of the hard endpoint, all cardiovascular events after discharge were included and the potential influence of the blanking period on the assessment of outcomes was evaluated as a sensitivity analysis.
All patients were recommended to have scheduled outpatient visits at 1, 3, 6, and 12 months after discharge, and then every 12 months. A cardiovascular specialist performs physical examinations, laboratory tests, and examinations including ECGs and echocardiography during the scheduled visit. A professionally trained follow-up researcher who had access to the admission number and time of the index hospitalization but was blinded to the ablation status inquired about all the follow-up medical records of participating patients. If the patients did not finish the clinic in time after enrolment, they were followed up by telephone according to the registered telephone. Subjects were censored at the last follow-up visit. Time zero was defined as the admission time of the index hospitalization, and the follow-up duration (in months) was measured from time zero to the first episode of events or the last visit. Drop-out was defined as patients who did not return to the hospital outpatient clinic after discharge and could not be contacted by telephone interviews. The other independent follow-up investigator who was also blinded to the ablation status checked and confirmed all endpoint events.
Statistical analysis
For continuous variables, means with standard deviations were reported for normally distributed data, while medians with interquartile ranges were reported for non-normally distributed data. Comparisons between groups were performed using t-tests or Mann–Whitney U tests, depending on the distribution of the data. Categorical variables were presented as counts with percentages and compared using χ2 tests or Fisher’s exact tests when appropriate.
Differences in pre-treatment variables were balanced in three ways. First, imbalanced baseline variables were adjusted in a multivariable-adjusted model. Secondly, propensity score matching (PSM) was adopted to balance differences in pre-treatment variables.38 The propensity score model was generated by fitting a logistic regression with potential covariates that could affect the attending physician’s decision to perform treatment, including demographic characteristics [age, sex, body mass index (BMI)], vital signs (systolic blood pressure, diastolic blood pressure, rest heart rate), AF type, CHA2DS2-VASc score, functional classification of NYHA, pre-existing comorbidities, laboratory markers, echocardiography parameters, and drugs. The primary analysis was performed using complete-case analysis. The PS-matched cohort was formed through a 1:1 pairing of individuals using a nearest-neighbour matching method with a calliper of 0.20. Covariate balance was evaluated using P-values (<0.05) and standardized mean differences (>0.10). Further, an inverse probability of treatment weighting (IPTW) analysis was conducted by weighting patients who received AF ablation with 1/PS and those who did not receive AF ablation with 1/(1 − PS). Patients with PS below 0.1 in the ablation group and PS over 0.9 in the non-ablation group were truncated to overcome the possible influence of large weights.39 The weighted sample mimicked the potential population in a randomized controlled trial drawn from the same target population, which would include all cohort patients.
The absolute difference in incidence rate between groups was expressed as incidence rate differences (IRDs). The median duration of follow-up was estimated by using the reverse Kaplan–Meier method. Cumulative event-free survival was estimated by means of the Kaplan–Meier method, and differences in time-to-event distributions were compared by using the log-rank test. Adjusted hazard ratios (HRs) with 95% confidence intervals [CIs] were calculated by means of a Cox proportional hazards model, and the proportional hazards assumptions were assessed by using the Schoenfeld residuals test. Independent variables were analysed in a further stratified model as a stratification factor if they did not meet the equivalence requirement. An adjusted sub-distribution hazard model (Fine and Gray model), accounting for death as a competing risk factor, was used to estimate the incidence rate of AT/AF recurrence while controlling for imbalanced variables.40
Pre-specified subgroup analyses were also conducted to explore potential differences in the risk of the primary outcome among treatment groups based on factors such as age, sex, BMI, LVEF, functional classification of NYHA, CHA2DS2-VASc score, chronic kidney disease, NT-proBNP, AF type, hypertension, and diabetes mellitus. Interactions between AF ablation status and these pre-specified grouping factors were examined by adding an interaction term (subgroup variable × treatment variable) to the Cox model. The heterogeneity (interaction P-values) across subgroup strata was assessed using likelihood ratio tests. The possible relationship between the operation strategy and outcome was also explored by separating the AF ablation group into two subsets based on catheter or surgical ablation.
The robustness of the results about primary and secondary endpoints was also explored in several sensitivity analyses. First, cardiovascular hospitalization events were confined to those after the three-month blanking period. Secondly, patients undergoing the atrioventricular node ablation were also included in the cohort. Thirdly, secondary endpoints were analysed using the sub-distribution hazard model (Fine and Gray model), with all-cause death treated as a competing risk factor. Fourthly, missing variables were imputed using the multivariate imputation method with chained equations.41 Other sensitivity analyses applied for adjustment models, including different variables, calliper, and adjustment of possibly still imbalanced variables (standardized mean differences > 0.05) in the PS-matched cohort and trimming individuals with the most extreme 5% PS values to avoid bias from extreme weights in the IPTW model. The E-value, a novel sensitivity analysis algorithm, was also calculated to assess how robust the association between ablation and the primary outcome is to residual and unmeasured confounding.42
Statistical significance was defined as a two-tailed alpha level of <0.05. Since no adjustments for multiple comparisons were conducted, the results for secondary endpoints, subgroups, and sensitivity analyses should be regarded as exploratory and susceptible to type 1 error. R software version 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria) was used for all statistical analyses.
Results
Patient characteristics and demographics
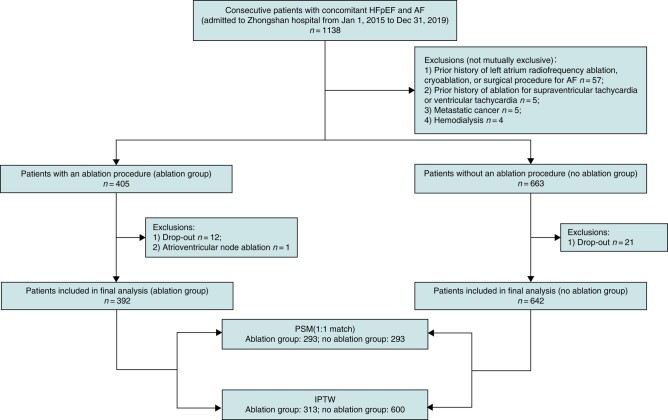
The process of patient enrolment is illustrated in Figure 1. A total of 1138 patients comorbid with HFpEF and AF were identified in our centre between 2015 and 2019. After the exclusion of 70 patients according to pre-specified exclusion criteria, 33 patients who dropped out after the index hospitalization (3.0% of patients [12/405] in the ablation group; 3.2% of patients [21/663] in the non-ablation group) and one patient undergoing the atrioventricular node ablation, a total of 1034 patients were included in subsequent analyses, with 392 in the ablation group and 642 in the no ablation group (Figure 1). Table 1 shows the demographics and baseline characteristics of the patients in both groups. Among the entire study population, the median age was 69 [63–76] years, and 46.2% (478/1034) were female. The median BMI was 24.5 [22.2–27.0] kg/m2. About 55.6% were treated with diuretics. Treatment of underlying diseases/complications of HF included the use of angiotensin-converting enzyme inhibitor/angiotensin II receptor blockade/angiotensin receptor neprilysin inhibitor in 57.2% patients, the use of aldosterone antagonist in 40.3% patients, the use of β-blocker in 67.5% patients, and the use of digoxin in 21.5% patients. Patients in the no ablation group were older and had higher CHA2DS2-VASc scores. While the BMI, diastolic blood pressure and resting heart rate at admission were slightly higher in the ablation group. Comorbidities including stroke, chronic obstructive pulmonary disease, and chronic kidney disease were more prevalent in the no ablation group. Laboratory tests showed the no ablation group had higher levels of serum creatinine, NT-proBNP, and left atrial diameter. As for the drug therapy, aldosterone antagonist, diuretic and digoxin were more likely to be prescribed for patients in no ablation group and the usage of oral anticoagulant was slightly higher in the ablation group. After rigorous PSM procedure, above baseline characteristics between the two groups were well balanced (Table 1; see Supplementary material online, Figures S1–S3).
Figure 1.
The diagram illustrates the flow of patient inclusion in this cohort. The weighted sample size is presented for the IPTW model. AF, atrial fibrillation; HFpEF, heart failure with preserved ejection fraction; IPTW, inverse probability of treatment weighting; PSM, propensity score matching.
Table 1.
Baseline characteristics of patients undergoing ablation or not before and after propensity score matching
| Variable | All patients | Before matching | After 1:1 matching | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No ablation | Ablation | P-value | SMD | No ablation | Ablation | P-value | SMD | ||
| No. patients | 1034 | 642 | 392 | 293 | 293 | ||||
| Clinical characteristics | |||||||||
| Age, years | 69 [63, 76] | 70 [64, 79] | 67 [62, 72] | <0.001 | 0.46 | 67 [62, 73] | 68 [63, 73] | 0.53 | 0.03 |
| Female sex, % | 478 (46.2) | 301 (46.9) | 177 (45.2) | 0.63 | 0.04 | 134 (45.7) | 145 (49.5) | 0.41 | 0.08 |
| BMI, kg/m2 | 24.5 [22.2, 27.0] | 24.1 [21.8, 26.5] | 25.0 [22.9, 27.7] | <0.001 | 0.32 | 24.7 [22.3, 27.4] | 24.7 [22.6, 27.4] | 0.74 | 0.02 |
| Months since AF diagnosis | 10 [2, 46] | 12 [1, 60] | 9 [3, 36] | 0.78 | 0.37 | 9 [1, 48] | 10 [4, 36] | 0.39 | 0.28 |
| Persistent AF, % | 644 (62.3) | 390 (60.7) | 254 (64.8) | 0.22 | 0.08 | 181 (61.8) | 186 (63.5) | 0.73 | 0.04 |
| CHA2DS2-VASc score | 3 [2, 4] | 4 [3, 5] | 3 [2, 4] | <0.001 | 0.40 | 3 [2, 4] | 3 [2, 4] | 0.80 | 0.01 |
| NYHA class I–II, % | 813 (78.6) | 499 (77.7) | 314 (80.1) | 0.41 | 0.06 | 230 (78.5) | 232 (79.2) | 0.92 | 0.02 |
| Systolic BP, mmHg | 128 [118, 140] | 128 [120, 141] | 128 [117, 140] | 0.43 | 0.08 | 125 [120, 140] | 128 [117, 140] | 0.61 | 0.04 |
| Diastolic BP, mmHg | 80 [70, 87] | 77 [70, 84] | 80 [73, 90] | <0.001 | 0.39 | 80 [71, 86] | 80 [72, 89] | 0.23 | 0.10 |
| Heart rate, bpm | 78 [66, 86] | 76 [62, 84] | 80 [70, 88] | <0.001 | 0.31 | 80 [70, 88] | 78 [70, 88] | 0.77 | 0.04 |
| Coexisting conditions | |||||||||
| Hypertension, % | 612 (59.2) | 371 (57.8) | 241 (61.5) | 0.27 | 0.08 | 176 (60.1) | 179 (61.1) | 0.87 | 0.02 |
| Diabetes, % | 181 (17.5) | 121 (18.8) | 60 (15.3) | 0.17 | 0.09 | 51 (17.4) | 50 (17.1) | 1.00 | 0.01 |
| Stroke, % | 156 (15.1) | 109 (17.0) | 47 (12.0) | 0.04 | 0.14 | 39 (13.3) | 39 (13.3) | 1.00 | <0.001 |
| CAD, % | 139 (13.4) | 91 (14.2) | 48 (12.2) | 0.43 | 0.06 | 47 (16.0) | 40 (13.7) | 0.49 | 0.07 |
| HCM, % | 68 (6.6) | 40 (6.2) | 28 (7.1) | 0.66 | 0.04 | 18 (6.1) | 25 (8.5) | 0.34 | 0.09 |
| COPD, % | 16 (1.5) | 14 (2.2) | 2 (0.5) | 0.04 | 0.15 | 6 (2.0) | 2 (0.7) | 0.29 | 0.12 |
| SAHS, % | 5 (0.5) | 3 (0.5) | 2 (0.5) | 1.00 | 0.006 | 1 (0.3) | 1 (0.3) | 1.00 | <0.001 |
| CKD, %a | 187 (19.0) | 135 (22.1) | 52 (14.0) | 0.002 | 0.21 | 46 (15.7) | 46 (15.7) | 1.00 | <0.001 |
| Laboratory examination | |||||||||
| SCr, μmol/L | 82 [71, 96] | 83 [71, 97] | 81 [71, 92] | 0.049 | 0.21 | 80 [70, 94] | 81 [70, 93] | 0.94 | 0.01 |
| NT-proBNP, pg/mL | 1439 [920, 2177] | 1566 [1000, 2312] | 1179 [843, 1847] | <0.001 | 0.23 | 1493 [934, 2185] | 1319 [874, 1975] | 0.07 | 0.04 |
| Echocardiography | |||||||||
| LVEF, % | 63 [59, 66] | 63 [59, 66] | 63 [59, 66] | 0.89 | 0.002 | 63 [59, 66] | 63 [59, 66] | 0.73 | 0.02 |
| LAD, mm | 46 [43, 51] | 47 [44, 53] | 45 [42, 49] | <0.001 | 0.57 | 45 [43, 48] | 45 [42, 49] | 0.89 | 0.03 |
| LVEDD, mm | 48 [44, 51] | 48 [44, 52] | 48 [45, 51] | 0.47 | 0.10 | 48 [44, 52] | 47 [45, 51] | 0.60 | 0.07 |
| LVESD, mm | 31 [29, 34] | 31 [28, 34] | 31 [29, 33] | 0.55 | 0.08 | 31 [28, 34] | 31 [28, 33] | 0.62 | 0.06 |
| Medicationsb | |||||||||
| ACE inhibitor, ARB or ARNI, % | 591 (57.2) | 382 (59.5) | 209 (53.3) | 0.06 | 0.13 | 161 (54.9) | 155 (52.9) | 0.68 | 0.04 |
| Aldosterone antagonist, % | 417 (40.3) | 281 (43.8) | 136 (34.7) | 0.01 | 0.19 | 107 (36.5) | 110 (37.5) | 0.86 | 0.02 |
| β-blocker, % | 698 (67.5) | 422 (65.7) | 276 (70.4) | 0.14 | 0.10 | 208 (71.0) | 210 (71.7) | 0.93 | 0.02 |
| Diuretic, % | 575 (55.6) | 389 (60.6) | 186 (47.4) | <0.001 | 0.27 | 154 (52.6) | 151 (51.5) | 0.87 | 0.02 |
| Digoxin, % | 222 (21.5) | 151 (23.5) | 71 (18.1) | 0.048 | 0.13 | 55 (18.8) | 54 (18.4) | 1.00 | 0.01 |
| Oral anticoagulant, % | 1006 (97.3) | 614 (95.6) | 392 (100.0) | <0.001 | 0.30 | 293 (100.0) | 293 (100.0) | NA | <0.001 |
| Antiarrhythmic drugs(I/III/IV), % | 218 (21.1) | 123 (19.2) | 95 (24.2) | 0.06 | 0.12 | 76 (25.9) | 74 (25.3) | 0.93 | 0.02 |
Data were expressed as median [Q1 and Q3], or n (%) unless otherwise specified.
ACE, angiotensin-converting enzyme; AF, atrial fibrillation; ARB, angiotensin II receptor blockade; ARNI, angiotensin receptor neprilysin inhibitor; BMI, body mass index; BP, blood pressure; bpm, beats per minute; CAD, coronary artery disease; CHA2DS2-VASc, congestive heart failure, hypertension, age ≥ 75years, diabetes, stroke/transient ischaemic attack, vascular disease, age 65 to 74years, sex category; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; HCM, hypertrophic cardiomyopathy; HFpEF, heart failure with preserved ejection fraction; LAD, left atrial diameter; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic diameter; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association; SAHS, sleep apnoea hypopnoea syndrome; SCr, serum creatinine; SMD, standardized mean difference; Stroke, haemorrhagic stroke, ischaemic stroke, and transient ischaemic attack.
aCKD was defined as estimated glomerular filtration rate < 60 mL/min/1.73 m2 using the Modification of Diet in Renal Disease formula.
bPrior history of drug therapy documented at admission, in-hospital therapy, and prescriptions at discharge of the index hospitalization.
Ablation procedure and complications
Details of the ablation procedure and complications are presented in Supplementary material online, Table S3. Of the 392 patients in the ablation group, 339 underwent catheter ablation and 53 underwent surgical ablation. PVI was successfully performed in all patients, and ancillary procedures were performed in 264 (77.9%) of patients who underwent catheter ablation, compared to 32 (60.4%) of patients who underwent surgical ablation. Bradycardia occurred in seven patients, and four of them required pacemaker implantation. Five patients experienced an acute episode of heart failure after the procedure, and all were treated according to guidelines. Four patients required emergency pericardiocentesis due to echocardiography-evidenced pericardial effusion. Nine patients experienced access-site complications, and two patients with pseudoaneurysm received surgical repair. A total of 41 patients underwent 42 repeat ablation procedures in the ablation group, including one patient who underwent two repeat ablation procedures at the 14th month and 42nd respectively, and the remaining 40 patients underwent one repeat operation procedure within the time frame of 3 months to 49 months after the index procedure (see Supplementary material online, Table S4). Other patients with AT/AF recurrence were prescribed with medication by their physicians.
Primary endpoint
Over a median follow-up period of 39 months, 201 patients with HFpEF and AF reached the primary endpoint in the overall cohort. The Kaplan–Meier curve showed that a significantly higher proportion of patients in the ablation group were free from the primary endpoint (43 out of 392 patients [11.0%] in the ablation group, 158 out of 642 patients [24.6%] in the no ablation group, P < 0.001 by log-rank). Even after adjusting for imbalanced variables, the difference remained statistically significant (adjusted HR, 0.62 [95% CI, 0.43–0.89], P = 0.01; IRD, −0.39 [95% CI, −0.54 to −0.25]) (Table 2). The E-value for the primary endpoint was 2.14, with an upper limit of the CI at 1.38 (see Supplementary material online, Table S6), indicating that a relatively strong unmeasured confounder associated with the treatment strategy and outcome would be needed to explain away the result.
Table 2.
Incidence rate difference and hazard ratio for primary and secondary endpoints under different analysis model
| Outcome | Unmatched | PSM (1:1) | IPTW | ||||||
|---|---|---|---|---|---|---|---|---|---|
| IRD (100 person-month; 95% CI)a | Adjusted HR (95% CI)b | P-value | IRD (100 person-month; 95% CI)a | Adjusted HR (95% CI)c | P-value | IRD (100 person-month; 95% CI)a | Adjusted HR (95% CI)d | P-value | |
| Primary endpointe | −0.39(−0.54 to −0.25) | 0.62(0.43–0.89) | 0.01 | −0.32(−0.52 to −0.11) | 0.55(0.37–0.82) | 0.003 | −0.31(−0.46 to −0.15) | 0.66(0.44–0.98) | 0.04 |
| Second endpoints | |||||||||
| All-cause rehospitalization | −0.34(−0.63 to −0.04) | 0.92(0.72–1.16) | 0.47 | −0.20(−0.58 to 0.19) | 0.86(0.67–1.10) | 0.23 | −0.34(−0.64 to −0.04) | 0.94(0.75–1.18) | 0.58 |
| Cardiovascular rehospitalization | −0.16(−0.41 to 0.10) | 1.02(0.78–1.33) | 0.91 | −0.11(−0.45 to 0.22) | 0.88(0.67–1.16) | 0.38 | −0.19(−0.46 to 0.07) | 1.00(0.78–1.28) | 0.99 |
| Heart failure rehospitalization | −0.31(−0.45 to −0.18) | 0.61(0.41–0.92) | 0.02 | −0.29(−0.48 to −0.10) | 0.52(0.34–0.80) | 0.003 | −0.27(−0.40 to −0.13) | 0.62(0.41–0.95) | 0.03 |
| All-cause death | −0.15(−0.22 to −0.08) | 0.53(0.25–1.14) | 0.11 | −0.07(−0.17 to 0.02) | 0.57(0.26–1.27) | 0.17 | −0.09(−0.17 to −0.02) | 0.61(0.26–1.38) | 0.23 |
| Cardiovascular death | −0.09(−0.14 to −0.04) | 0.47(0.15–1.44) | 0.19 | −0.04(−0.10 to 0.03) | 0.54(0.17–1.77) | 0.31 | −0.05(−0.10 to 0.01) | 0.63(0.18–2.22) | 0.47 |
| Stroke | −0.04(−0.10 to 0.02) | 0.60(0.26–1.40) | 0.24 | −0.03(−0.11 to 0.05) | 0.69(0.28–1.66) | 0.40 | −0.05(−0.11 to 0) | 0.58(0.26–1.32) | 0.20 |
CI, confidence interval; HR, hazard ratio; IPTW, inverse probability of treatment weighting; IRD, incidence rate differences; PSM, propensity score matching.
aIRD indicates an absolute difference in the incidence rate between the two groups.
bHRs estimated using a Cox proportional hazard model and adjusted for potential confounders (unbalanced baseline variables), including age, body mass index, CHA2DS2-VASc score, stroke, chronic kidney disease, chronic obstructive pulmonary disease, N-terminal pro-brain natriuretic peptide, serum creatinine, left atrial diameter, diastolic blood pressure, heart rate, aldosterone antagonist, diuretic, digoxin, and oral anticoagulant in all HFpEF patients for the primary endpoint and second endpoints.
cHRs estimated using a Cox proportional hazard model in patients after matching.
dHRs estimated using a Cox proportional hazard model in all patients with assigning each patient a weight on the basis of the propensity score.
eThe primary endpoint is a composite of all-cause death or rehospitalization for worsening heart failure.
To account for the possibility of an unbalanced distribution of risk factors that could influence the protective association, we conducted PSM and IPTW analyses. In the PS-matched cohort, we successfully matched 586 individuals at a 1:1 ratio with similar baseline characteristics. The protective association of AF ablation was still observed in the PS-matched cohort, with significantly greater freedom from the primary endpoint in the ablation group (adjusted HR, 0.55 [95% CI, 0.37–0.82], P = 0.003) compared to the no ablation group, and an estimated IRD of −0.32 (95% CI, −0.52 to −0.11) (Table 2; Figure 2). The IPTW analysis using the PS as weight also confirmed the protective role of AF ablation, with an adjusted HR of 0.66 (95% CI, 0.44–0.98, P = 0.04) and an IRD of −0.31 (95% CI, −0.46 to −0.15) (Table 2).
Figure 2.
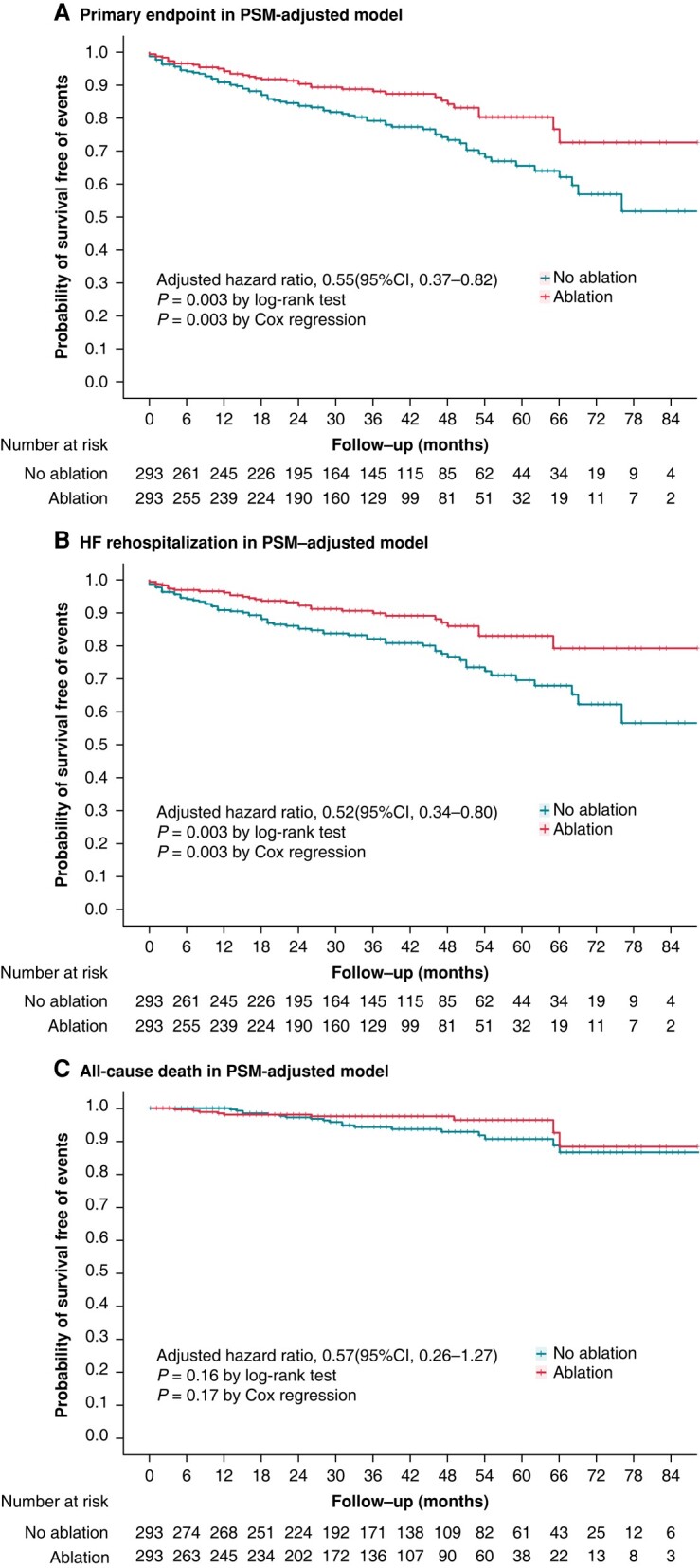
Kaplan–Meier curves stratified by ablation or not for the probability of freedom from (A) primary endpoint (all-cause death or rehospitalization for worsening heart failure), (B) rehospitalization for worsening heart failure, and (C) all-cause death in the PSM-adjusted model. All P-values for the Schoenfeld residuals test were >0.05, indicating that the proportional hazards assumption was not violated for the analyses of all endpoints. CI, confidence interval; HF, heart failure; PSM, propensity score matching.
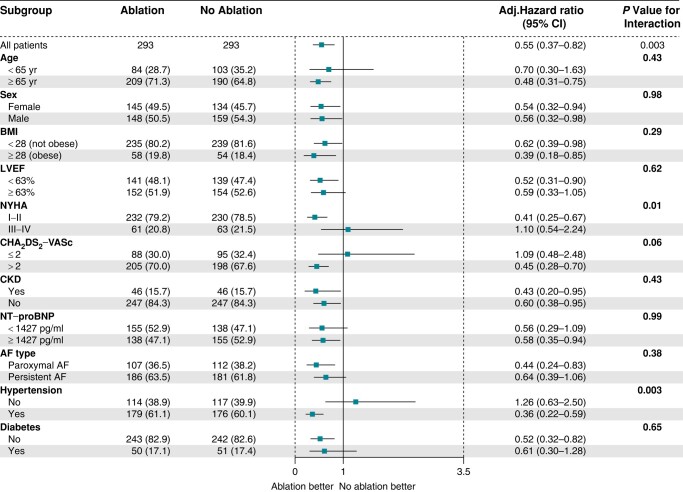
Figure 3 and Supplementary material online, Figure S7 show the primary endpoint in pre-specified subgroups. Significant interactions were found between the primary endpoint and NYHA (P = 0.02 for interaction in the unmatched model; P = 0.01 for interaction in PSM-adjusted model; P = 0.11 for interaction in IPTW-adjusted model) and hypertension (P = 0.01 for interaction in the unmatched model; P = 0.003 for interaction in PSM-adjusted model; P = 0.002 for interaction in IPTW-adjusted model). These results suggest that patients with NYHA I–II or hypertension are more likely to benefit from AF ablation.
Figure 3.
Forest plot of pre-specified subgroup analyses of the primary endpoint in the propensity score matching-adjusted model. The grouping boundary point for LVEF and NT-proBNP is the median. The boundary point of ≥28 kg/m2 for BMI was the definition of obesity for Chinese adults. AF, atrial fibrillation; BMI, body mass index; CHA2DS2-VASc, congestive heart failure, hypertension, age ≥ 75years, diabetes, stroke/transient ischaemic attack, vascular disease, age 65 to 74years, sex category; CI, confidence interval; CKD, chronic kidney disease; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association.
We also performed subset analyses for patients receiving catheter or surgical ablation. Demographics and baseline characteristics of the patients were shown in Supplementary material online, Tables S1 and S2, respectively. Findings from the multivariable-adjusted model in the overall cohort (adjusted HR, 0.63 [95% CI, 0.43–0.92], P = 0.02), the PS-matched model (adjusted HR, 0.58 [95% CI, 0.38–0.88], P = 0.01), and the IPTW model (adjusted HR, 0.65 [95% CI, 0.43–0.96], P = 0.03) all supported that receiving catheter ablation was associated with a lower probability of the primary endpoint compared to the no ablation group (see Supplementary material online, Table S5). However, the superiority of surgical ablation in the primary endpoint compared to no ablation was not observed in all the multivariable-adjusted models in the overall cohort (adjusted HR, 0.46 [95% CI, 0.17–1.29], P = 0.14), the PS-matched model (adjusted HR, 0.50 [95% CI, 0.13–1.94], P = 0.32), or the IPTW model (adjusted HR, 0.67 [95% CI, 0.23–1.94], P = 0.46) (see Supplementary material online, Table S5).
Secondary endpoints
Secondary endpoints were analysed in the overall, PS-matched, and IPTW model, and almost similar results were obtained (Table 2; Figure 2). In the PS-matched cohort, there was no significant difference in all-cause rehospitalization between the ablation group (37.9%; 111 out of 293 patients) and the no ablation group (46.4%; 136 out of 293 patients) (adjusted HR, 0.86 [95% CI, 0.67–1.10], P = 0.23), with an estimated IRD of −0.20 (95% CI, −0.58 to 0.19). Similarly, no significant difference was found in rehospitalization for cardiovascular disease between the ablation group (32.4%; 95 out of 293 patients) and the no ablation group (39.2%; 115 out of 293 patients) (adjusted HR, 0.88 [95% CI, 0.67–1.16], P = 0.38), with an estimated IRD of −0.11 (95% CI, −0.45 to 0.22). However, the incidence of rehospitalization for worsening HF was significantly lower in the ablation group (10.6%; 31 out of 293 patients) compared to the no ablation group (21.5%; 63 out of 293 patients) (adjusted HR, 0.52 [95% CI, 0.34–0.80], P = 0.003), with an estimated IRD of −0.29 (95% CI, −0.48 to −0.10). There was no significant difference in all-cause death between the ablation group (3.1%; 9 out of 293 patients) and the no ablation group (6.5%; 19 out of 293 patients) (adjusted HR, 0.57 [95% CI, 0.26–1.27], P = 0.17), with an estimated IRD of −0.07 (95% CI, −0.17 to 0.02). The incidence of cardiovascular disease-related death was also not significantly different between the ablation group (1.4%; 4 out of 293 patients) and the medical therapy group (3.1%; 9 out of 293 patients) (adjusted HR, 0.54 [95% CI, 0.17–1.77], P = 0.31), with an estimated IRD of −0.04 (95% CI, −0.10 to 0.03). The incidence of stroke events was also not significantly different between the ablation group (2.7%; 8 out of 293 patients) and the medical therapy group (4.4%; 13 out of 293 patients) (adjusted HR, 0.69 [95% CI, 0.28–1.66], P = 0.40), with an estimated IRD of −0.03 (95% CI, −0.11 to 0.05).
Sensitivity analyses
After the inclusion of one patient who underwent rate control by atrioventricular node ablation, the results were generally consistent with the primary analysis (PSM-adjusted model: adjusted HR, 0.49 [95% CI, 0.32–0.75], P = 0.001 for primary endpoint; adjusted HR, 0.52 [95% CI, 0.33–0.81], P = 0.003 for HF rehospitalization). Analyses confining cardiovascular hospitalizations after the three-month blanking period also provided similar results (PSM-adjusted model: adjusted HR, 0.53 [95% CI, 0.35–0.81], P = 0.003 for primary endpoint; adjusted HR, 0.49 [95% CI, 0.31–0.78], P = 0.003 for heart failure rehospitalization). The robustness of the results was also evaluated in other sensitivity analyses, including the imputation of missing baseline variables, the Fine and Gray model for second endpoints, different variables and calliper for the PSM model, and trimming extreme PS values for the IPTW model. All of those analyses confirmed the main findings for the primary endpoint and secondary endpoints (see Supplementary material online, Table S8).
Atrial tachycardia/atrial fibrillation recurrence
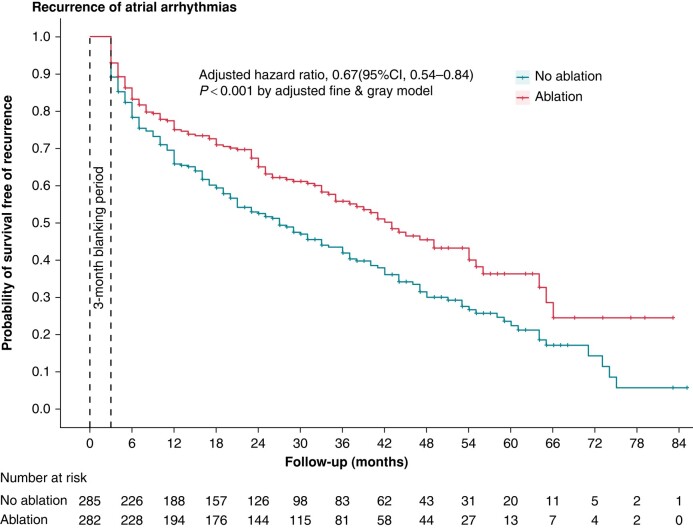
During the follow-up period, 43.5% of patients in the ablation group and 62.0% of patients in the no ablation group had AT/AF recurrence. The adjusted HR for AT/AF recurrence in the multivariable-adjusted Fine and Gray model showed that patients who received AF ablation had a 34% relative decrease in AT/AF recurrence compared to those who did not receive ablation (adjusted HR, 0.66 [95% CI, 0.54–0.81], P < 0.001) (see Supplementary material online, Figure S8; Supplementary material online, Table S9). Analyses from the PSM-adjusted model and IPTW-adjusted model yield similar results (adjusted HR, 0.67 [95% CI, 0.54–0.84], P < 0.001 in PSM-adjusted model; adjusted HR, 0.65 [95% CI, 0.54–0.78], P < 0.001 in IPTW-adjusted model) (Figure 4).
Figure 4.
Kaplan–Meier curve stratified by ablation or not for the probability of freedom from atrial tachyarrhythmia/atrial fibrillation recurrence after a three-month blanking period post-ablation in the propensity score matching-adjusted model. Recurrence analysis only included participants with available data. CI, confidence interval.
Additionally, all patients were further categorized based on whether they experienced AT/AF recurrence during the follow-up. Those who maintained SR, either through ablation or no ablation, had a lower incidence of the primary endpoint event compared to those who experienced AT/AF recurrence (9.1% vs. 28.5%, P < 0.001 by χ2 test).
Discussion
The main findings of this study showed that the strategy of AF ablation in patients with HFpEF and AF significantly reduced the primary composite endpoint of all-cause death or rehospitalization for worsening HF. The clinical benefit was primarily attributed to a notable decrease in rehospitalization for worsening HF. Patients with NYHA class I to II symptoms or hypertension were more likely to benefit from AF ablation. These findings support the idea that AF ablation may be a superior aetiological treatment strategy to alleviate the disease burden in patients with HFpEF.
Patients with HFrEF and AF had approximately three times higher risk for a composite of all-cause death, HF hospitalization, and stroke or systemic embolism after AF ablation compared with patients with HFpEF.18 Since previous studies have shown the superiority of catheter ablation over pharmacological rhythm or rate control in reducing all-cause mortality in HFrEF,37,43 whether clinical benefit of AF ablation compared to medical therapy alone could also apply to patients with HFpEF of whom had significantly lower event rate after AF ablation warrants further study. Subgroup analysis from KiCS-AF and SwedeHF provides evidences that catheter ablation was significantly associated with clinically meaningful improvements in quality of life and lower risk of both first and recurrent HF hospitalization compared to drug therapy alone among HFpEF patients with AF.25,26 However, it should be noted that both those registered studies were conducted across a broader range of patients with concomitant AF and HF and HFpEF patients was therefore less representative by dozens of patients.25,26 Limited sample sizes could lead to inaccurate evaluation, especially for hard endpoints. By contrast, the major strength of the present study was investigated in a large and dedicated cohort of HFpEF patients with AF. The high volume of AF ablation operations and acceptance of referrals from all over the country in our centre ensures that enough patients can be included to support this study. In addition, considering the significant technological advances in surgical ablation procedures, we also included patients who underwent thoracoscopy-assisted minimally invasive radiofrequency ablation of AF that had been less frequently covered in previous studies. More importantly, the cohort in our study was more representative of the current clinical practice of AF ablation and optimized drug therapy in HFpEF patients, since patients in the KiCS-AF registry were included from 2012 to 2017 and SwedeHF was included from 2005 to 2019.25,26
Options of therapy in atrial fibrillation patients with heart failure with preserved ejection fraction in real-world settings
In line with previous real-world data, we found notable differences in baseline characteristics between patients who underwent AF ablation and those who did not.25,26 It suggests that it is a common feature of clinical practice in different regions that relatively healthier HFpEF patients are more likely to undergo AF ablation in real-world settings. This reflects the caution of physicians and patients towards ablation therapy for HFpEF patients in the absence of definite guidelines recommendations. Electrophysiologists often judge the expected success rate and risk of AF ablation by the combinations of age, AF type, tolerance to AF symptoms, history of AADs usage, left atrial diameter, comorbidities, history of HF, and so on. In AF patients comorbid with HF, the severity of HF as evaluated by signs and/or symptoms, functional classification of NYHA and NT-proBNP are the additional key factors affecting the physician’s treatment decision. Patients with poorly controlled or more advanced HF are less tolerant to AF ablation and have a higher risk of post-operative adverse events such as acute episodes of HF.44–46 Therefore, the physicians may be more confident about the tolerability of the procedure and the effectiveness in well-controlled and earlier HFpEF patients. From the patient’s perspective, the degree of distress of symptoms, acceptance of adherence to medications, risk, the expected success rate of AF ablation, and cost are important factors affecting whether to accept AF ablation.
Theoretically, all of these factors affecting treatment decisions should be merged into the adjustment model. In practice, under the standardized operating procedure, most of the clinical indicators that affect doctors’ treatment decisions are relatively objective, so they could be easily included in the model. However, those factors affecting patients’ decisions are commonly subjective and cannot be inserted in a mathematical model, which also lacks previous experiences to deal with. So the balanced cohort that we get through the matching method actually represents a group of patients who are good candidates for AF ablation or drug therapy based on the criteria that doctors judge in clinical practice. It could also be proven by the comparable age, sex distributions, and other variables of the matched cohort in our study to those in previously published trials.23 Admittedly, there may be still residual defects that the final treatment grouping was influenced by unmeasured confounding factors, namely patients’ willingness to treat. Previous studies have also suggested that patients with a more aggressive choice of treatment may have a higher life expectancy, which can be influenced by better adherence to subsequent management of disease.47,48 While this is common to real-world studies, given possible bias, we assessed the impact of those potential unmeasured confounders by introducing an E-value as a sensitivity analysis. The E-value of the primary endpoint was over 2, which means that the HR of 0.55 for the primary outcome in the PSM model could only be explained by an unmeasured confounder that is associated with both receipt of AF ablation and risk of the primary outcome by a risk ratio of more than 2, an intensity that is difficult to achieve in clinical variables (see Supplementary material online, Table S7). That is, unmeasured confounders, including patients’ subjective willingness to receive treatment, are less likely to confound the results of this study. Therefore, in the context that blindness of treatment strategies cannot be reached for patients in ablation-related trials, our real-world study also has certain suggestive significance that the clinical advantage of ablation, as demonstrated in current research, may be predominantly applicable to those healthier HFpEF patients.25
Clinical benefit of atrial fibrillation ablation in heart failure with preserved ejection fraction patients
The rate of primary endpoint of all-cause death and rehospitalization of worsening HF for all patients in the ablation group during follow-up was consistent with previous studies with similar follow-up times.18 Major finding of our study was the confirmation of the association between AF ablation and a lower risk of HF hospitalization rather than all-cause death in HF patients.25 The exploratory analyses also provides further hints of new findings that the clinical benefit was limited to patients undergoing catheter ablation rather surgical ablation. The reasons why benefit from surgical ablation was not observed in our study were unclear. While the relatively high recurrence rate may partly explain, it needs to be verified by studies with larger sample sizes receiving surgical ablation. The finding that the benefit of AF ablation was driving by rehospitalization for worsening HF rather than all-cause death may be explained by the stage of HF in selected patients. As demonstrated in the post hoc analysis of TOPCAT Americas Trial, AF could accelerate a downhill course for ‘AF–HFpEF’ subjects with a selective and novel impact on symptomatic HF status, viz, HF worsening in earlier HF and pump failure mortality in advanced HF.11 In this study, the proportion of patients with NYHA I–II and moderated elevated level of NT-proBNP in the matched cohort suggested an early symptomatic HFpEF population, which was also proven by the relatively moderate all-cause mortality. Thus, our finding that the ability of ablation to reduce HF rehospitalization relative to drug therapy in those patients serves as a mutual confirmation of the results in TOPCAT Americas Trial and adding new evidences in this area.
The selection of heart failure with preserved ejection fraction patients for atrial fibrillation ablation
Another unreported finding previously is that we assessed AF ablation in different NYHA subgroups of HFpEF patients for the first time, and the results suggested that only NYHA class I to II patients could benefit from the ablation. Proper patient selection is a critical issue in the clinical practice of AF ablation in HFpEF patients. The subgroup analysis indicated that not all patients with HFpEF and AF will benefit from AF ablation, a similar finding to the CASTLE-AF trial, which showed that HFrEF patients with NYHA class III symptoms did not benefit from catheter ablation.37 From a pathophysiological perspective, the vicious cycle of HFpEF and AF exacerbates AF burden, left atrial/ventricular haemodynamics, and left atrial/ventricular remodelling. In advanced stages of HF, the worsening of left atrial fibrosis makes it less likely for patients to experience long-term restoration of SR and for cardiac remodelling to be reversed.49 It has been shown that rhythm control therapy initiated within one year of diagnosing AF may provide additive prognostic benefits.50 Similarly, the results indicate that the timing of AF ablation relative to the progression of HF could also affect outcomes. Such results have not been reported and will provide more evidence for the treatment choice of HFpEF patients with AF and population screening in future trials.
Interestingly, our study demonstrated that AF ablation was beneficial for HFpEF patients with hypertension. Hypertension is known to cause pathological left ventricular hypertrophy and diastolic dysfunction.51 In HFpEF patients with comorbid hypertension, severe impairment of left ventricular diastolic function can significantly increase dependence on left ventricular filling from the left atrium. Thus, the restoration of atrial booster pump function and increased ventricular filling after successful ablation could markedly improve HF symptoms in these HFpEF patients with hypertension.
Possible underlying mechanisms
The 3-year recurrence rate of atrial arrhythmias in our cohort was generally consistent with the previous reports.18 An exploratory analysis was conducted to group patients based on whether or not there was a recurrence of AT/AF during the follow-up period. Patients who maintained SR, regardless of whether they underwent ablation or not, had a lower risk of the primary composite endpoint compared to those with recurrent atrial arrhythmias. In combination with previous studies that restoration of SR by PVI in HFpEF patients with concomitant AF induces reverse remodelling, improvement of symptoms, resolution of HFpEF, and subsequently decrease of hospitalizations,20 the relatively lower recurrence rate by AF ablation compared to medical therapy may be an important reason for the endpoint benefit.
The mechanisms by which SR maintenance improves outcomes are a combination of several immediate haemodynamic changes caused by arrhythmias being reversed and the reversal of continuous AF-induced structural changes. Successful cardioversion can restore the atrial booster pump function, enhancing ventricular filling, with the atrial contribution to ventricular filling increasing from 30% to 47% one month after SR restoration.5 Moreover, studies have reported the reversal of structural remodelling of the left atrium and left ventricle three months or more after successful ablation in AF patients with preserved LVEF.16,52,53 These structural changes can contribute to significant improvements in both systolic and diastolic indices of the left ventricle in patients who maintain SR after AF ablation, ultimately leading to the recovery of HFpEF.19,20,53–55 As evidenced by the STALL HFpEF and AF trial, HFpEF patients who maintain SR after AF ablation show significant improvements in peak exercise pulmonary capillary wedge pressure, and almost 45% of patients no longer meet criteria for HFpEF 12 months post-ablation.21 Chieng et al.56 further confirmed above findings in the STALL HFpEF and AF trial by designing a comparative study and found that the improvement of invasive exercise haemodynamic parameters at 6 months was observed in the AF ablation group but not in the optimal medical therapy group in patients with concomitant AF and HFpEF. In addition, 50% of patients following ablation no longer met exercise right heart catheterization-based criteria for HFpEF vs. 7% in the medical arm (P = 0.02). This finding, taken together, provides crucial haemodynamic support for the results of the primary endpoint of our study.
Limitations
Several limitations should be acknowledged in this study. First, the diagnosis of HFpEF in this study relied on a comprehensive clinical assessment that took into account various factors such as signs and/or symptoms of HF, preserved LVEF, elevated filling pressure as determined by echocardiography, and elevated levels of natriuretic peptides. There is a risk of including atypical HFpEF cohorts without a direct invasive haemodynamics test. Secondly, the data for the analysis of AF recurrence are primarily based on symptom-driven visits rather than continuous heart rhythm monitoring, so analyses related to AF recurrence should be interpreted with caution. Thirdly, the decision to undergo ablation was not randomly assigned, and known and residually unmeasured confounding factors may influence the results. In particular, the patient’s willingness to treat was challenging to insert into a mathematical model. To address this, we used various covariate-adjustment models and sensitivity analyses in several scenarios to assess the robustness of our findings. E-value was also introduced to evaluate the unmeasured confounders, which provides some reassurance that there is no substantial unmeasured confounder that can entirely eliminate the association between catheter ablation and the primary outcome observed in this study. However, it must be admitted that, although we have adopted various methods, the inherent limitations of observational studies make it difficult to entirely rule out the possibility that selection bias may have interfered with the results. Fourthly, the other issue that must be highlighted is that assessing the outcome mainly relies on the clinician’s judgment; hence, although the design in the verification of all endpoint events by two independent, professionally trained follow-up researchers initially unaware of the treatment status, the nature of the non-blinded and the subject to variation may lead to a potential ascertainment bias. Finally, this was a single-centre study. In this cohort of HFpEF patients with AF, the proportion of patients undergoing ablation was higher than those reported in other national registries.25,26 This may be related to the fact that our centre is one of the largest EP centres that treats patients seeking AF ablation from all over the country and increasing confidence in the ablation procedures of both patients and physicians. Electrophysiologists are experienced and of course, this may to some extent affect the generalization of the results.
Conclusion
Based on a large-scale cohort, we found that AF ablation significantly reduced rehospitalization for worsening HF and AF recurrence compared to no ablation in patients with HFpEF. This real-world study provides important supplementary and expanded evidence on the clinical benefits of ablation when HFpEF and AF coexist. Although AF ablation may be effective in alleviating the disease burden in all HF patients, further confirmation is needed through multicentre randomized studies.
Supplementary Material
Acknowledgements
We acknowledge all patients who participated in this study.
Contributor Information
Zhonglei Xie, Department of Cardiology, Zhongshan Hospital, Fudan University, Shanghai Institute of Cardiovascular Diseases, National Clinical Research Center for Interventional Medicine, Fenglin Road 180, Xuhui District, 200032 Shanghai, China.
Baozhen Qi, Department of Cardiology, Zhongshan Hospital, Fudan University, Shanghai Institute of Cardiovascular Diseases, National Clinical Research Center for Interventional Medicine, Fenglin Road 180, Xuhui District, 200032 Shanghai, China.
Zimu Wang, Department of Cardiology, Zhongshan Hospital, Fudan University, Shanghai Institute of Cardiovascular Diseases, National Clinical Research Center for Interventional Medicine, Fenglin Road 180, Xuhui District, 200032 Shanghai, China.
Fuhai Li, Department of Cardiology, The Affiliated Hospital of Qingdao University, Qingdao, China.
Chaofeng Chen, Department of Cardiology, Zhongshan Hospital, Fudan University, Shanghai Institute of Cardiovascular Diseases, National Clinical Research Center for Interventional Medicine, Fenglin Road 180, Xuhui District, 200032 Shanghai, China.
Chaofu Li, Department of Cardiology, Zhongshan Hospital, Fudan University, Shanghai Institute of Cardiovascular Diseases, National Clinical Research Center for Interventional Medicine, Fenglin Road 180, Xuhui District, 200032 Shanghai, China.
Shuai Yuan, Department of Cardiology, Zhongshan Hospital, Fudan University, Shanghai Institute of Cardiovascular Diseases, National Clinical Research Center for Interventional Medicine, Fenglin Road 180, Xuhui District, 200032 Shanghai, China.
Shun Yao, Department of Cardiology, Zhongshan Hospital, Fudan University, Shanghai Institute of Cardiovascular Diseases, National Clinical Research Center for Interventional Medicine, Fenglin Road 180, Xuhui District, 200032 Shanghai, China.
Jingmin Zhou, Department of Cardiology, Zhongshan Hospital, Fudan University, Shanghai Institute of Cardiovascular Diseases, National Clinical Research Center for Interventional Medicine, Fenglin Road 180, Xuhui District, 200032 Shanghai, China.
Junbo Ge, Department of Cardiology, Zhongshan Hospital, Fudan University, Shanghai Institute of Cardiovascular Diseases, National Clinical Research Center for Interventional Medicine, Fenglin Road 180, Xuhui District, 200032 Shanghai, China; Institutes of Biomedical Sciences, Fudan University, Yixueyuan Road 138, Xuhui District, 200032 Shanghai, China.
Supplementary material
Supplementary material is available at Europace online.
Funding
This work was supported by the National Key Research and Development Program of China (grant number 2018YFE0103000) to J.Z.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2017;14:591–602. [DOI] [PubMed] [Google Scholar]
- 2. Ge J. Coding proposal on phenotyping heart failure with preserved ejection fraction: a practical tool for facilitating etiology-oriented therapy. Cardiol J 2020;27:97–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–726. [DOI] [PubMed] [Google Scholar]
- 4. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022;145:e876–94. [DOI] [PubMed] [Google Scholar]
- 5. Kotecha D, Lam CS, Van Veldhuisen DJ, Van Gelder IC, Voors AA, Rienstra M. Heart failure with preserved ejection fraction and atrial fibrillation: vicious twins. J Am Coll Cardiol 2016;68:2217–28. [DOI] [PubMed] [Google Scholar]
- 6. Zakeri R, Chamberlain AM, Roger VL, Redfield MM. Temporal relationship and prognostic significance of atrial fibrillation in heart failure patients with preserved ejection fraction: a community-based study. Circulation 2013;128:1085–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zakeri R, Borlaug BA, McNulty SE, Mohammed SF, Lewis GD, Semigran MJ et al. Impact of atrial fibrillation on exercise capacity in heart failure with preserved ejection fraction: a RELAX trial ancillary study. Circ Heart Fail 2014;7:123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaye DM, Silvestry FE, Gustafsson F, Cleland JG, van Veldhuisen DJ, Ponikowski P et al. Impact of atrial fibrillation on rest and exercise haemodynamics in heart failure with mid-range and preserved ejection fraction. Eur J Heart Fail 2017;19:1690–7. [DOI] [PubMed] [Google Scholar]
- 9. Lam CS, Rienstra M, Tay WT, Liu LC, Hummel YM, van der Meer P et al. Atrial fibrillation in heart failure with preserved ejection fraction: association with exercise capacity, left ventricular filling pressures, natriuretic peptides, and left atrial volume. JACC Heart Fail 2017;5:92–8. [DOI] [PubMed] [Google Scholar]
- 10. Cikes M, Claggett B, Shah AM, Desai AS, Lewis EF, Shah SJ et al. Atrial fibrillation in heart failure with preserved ejection fraction: the TOPCAT trial. JACC Heart Fail 2018;6:689–97. [DOI] [PubMed] [Google Scholar]
- 11. Saksena S, Slee A, Natale A, Lakkireddy DR, Shah D, Di Biase L et al. Atrial fibrillation can adversely impact heart failure with preserved ejection fraction by its association with heart failure progression and mortality: a post-hoc propensity score-matched analysis of the TOPCAT Americas trial. Europace 2023;25:euad095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019;140:e125–51. [DOI] [PubMed] [Google Scholar]
- 13. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 14. Black-Maier E, Ren X, Steinberg BA, Green CL, Barnett AS, Rosa NS et al. Catheter ablation of atrial fibrillation in patients with heart failure and preserved ejection fraction. Heart Rhythm 2018;15:651–7. [DOI] [PubMed] [Google Scholar]
- 15. Aldaas OM, Malladi CL, Mylavarapu PS, Lupercio F, Darden D, Han FT et al. Comparison of outcomes after ablation of atrial fibrillation in patients with heart failure with preserved versus reduced ejection fraction. Am J Cardiol 2020;136:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yamauchi R, Morishima I, Okumura K, Kanzaki Y, Morita Y, Takagi K et al. Catheter ablation for non-paroxysmal atrial fibrillation accompanied by heart failure with preserved ejection fraction: feasibility and benefits in functions and B-type natriuretic peptide. Europace 2021;23:1252–61. [DOI] [PubMed] [Google Scholar]
- 17. Krishnamurthy A, Goyal P, Markowitz SM, Liu CF, Thomas G, Ip JE et al. Outcomes of patients with heart failure with preserved ejection fraction undergoing catheter ablation of atrial fibrillation. Heart Rhythm O2 2022;3:501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fujimoto H, Doi N, Okayama S, Naito M, Kobori A, Kaitani K et al. Long-term prognosis of patients undergoing radiofrequency catheter ablation for atrial fibrillation: comparison between heart failure subtypes based on left ventricular ejection fraction. Europace 2022;24:576–86. [DOI] [PubMed] [Google Scholar]
- 19. Machino-Ohtsuka T, Seo Y, Ishizu T, Sugano A, Atsumi A, Yamamoto M et al. Efficacy, safety, and outcomes of catheter ablation of atrial fibrillation in patients with heart failure with preserved ejection fraction. J Am Coll Cardiol 2013;62:1857–65. [DOI] [PubMed] [Google Scholar]
- 20. Rattka M, Pott A, Kühberger A, Weinmann K, Scharnbeck D, Stephan T et al. Restoration of sinus rhythm by pulmonary vein isolation improves heart failure with preserved ejection fraction in atrial fibrillation patients. Europace 2020;22:1328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sugumar H, Nanayakkara S, Vizi D, Wright L, Chieng D, Leet A et al. A prospective STudy using invAsive haemodynamic measurements foLLowing catheter ablation for AF and early HFpEF: STALL AF-HFpEF. Eur J Heart Fail 2021;23:785–96. [DOI] [PubMed] [Google Scholar]
- 22. Zylla MM, Leiner J, Rahm AK, Hoffmann T, Lugenbiel P, Schweizer P et al. Catheter ablation of atrial fibrillation in patients with heart failure and preserved ejection fraction. Circ Heart Fail 2022;15:e009281. [DOI] [PubMed] [Google Scholar]
- 23. Packer DL, Piccini JP, Monahan KH, Al-Khalidi HR, Silverstein AP, Noseworthy PA et al. Ablation versus drug therapy for atrial fibrillation in heart failure: results from the CABANA trial. Circulation 2021;143:1377–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Parkash R, Wells GA, Rouleau J, Talajic M, Essebag V, Skanes A et al. Randomized ablation-based rhythm-control versus rate-control trial in patients with heart failure and atrial fibrillation: results from the RAFT-AF trial. Circulation 2022;145:1693–704. [DOI] [PubMed] [Google Scholar]
- 25. von Olshausen G, Benson L, Dahlstrom U, Lund LH, Savarese G, Braunschweig F. Catheter ablation for patients with atrial fibrillation and heart failure: insights from the Swedish Heart Failure Registry. Eur J Heart Fail 2022;24:1636–46. [DOI] [PubMed] [Google Scholar]
- 26. Shiraishi Y, Kohsaka S, Ikemura N, Kimura T, Katsumata Y, Tanimoto K et al. Catheter ablation for patients with atrial fibrillation and heart failure with reduced and preserved ejection fraction: insights from the KiCS-AF multicentre cohort study. Europace 2023;25:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fukui A, Tanino T, Yamaguchi T, Hirota K, Saito S, Okada N et al. Catheter ablation of atrial fibrillation reduces heart failure rehospitalization in patients with heart failure with preserved ejection fraction. J Cardiovasc Electrophysiol 2020;31:682–8. [DOI] [PubMed] [Google Scholar]
- 28. Rattka M, Kuhberger A, Pott A, Stephan T, Weinmann K, Baumhardt M et al. Catheter ablation for atrial fibrillation in HFpEF patients—a propensity-score-matched analysis. J Cardiovasc Electrophysiol 2021;32:2357–67. [DOI] [PubMed] [Google Scholar]
- 29. Parkash R, Wells G, Rouleau J, Talajic M, Essebag V, Skanes A et al. A randomized ablation-based atrial fibrillation rhythm control versus rate control trial in patients with heart failure and high burden atrial fibrillation: the RAFT-AF trial rationale and design. Am Heart J 2021;234:90–100. [DOI] [PubMed] [Google Scholar]
- 30. Yamane S. Catheter Ablation of Atrial Fibrillation. Shenyang, China: Liaoning Science and Technology Press; 2018. [Google Scholar]
- 31. Edgerton JR, Jackman WM, Mack MJ. A new epicardial lesion set for minimal access left atrial maze: the Dallas lesion set. Ann Thorac Surg 2009;88:1655–7. [DOI] [PubMed] [Google Scholar]
- 32. Edgerton JR, Jackman WM, Mahoney C, Mack MJ. Totally thorascopic surgical ablation of persistent AF and long-standing persistent atrial fibrillation using the “Dallas” lesion set. Heart Rhythm 2009;6:S64–70. [DOI] [PubMed] [Google Scholar]
- 33. Chinese Society of Cardiology of Chinese Medical Association; Editorial Board of Chinese Journal of Cardiology . Chinese guidelines for the diagnosis and treatment of heart failure 2014. Zhonghua Xin Xue Guan Bing Za Zhi 2014;42:98–122. [PubMed] [Google Scholar]
- 34. Heart Failure Group of Chinese Society of Cardiology of Chinese Medical Association; Chinese Heart Failure Association of Chinese Medical Doctor Association; Editorial Board of Chinese Journal of Cardiology . Chinese guidelines for the diagnosis and treatment of heart failure 2018. Zhonghua Xin Xue Guan Bing Za Zhi 2018;46:760–89. [DOI] [PubMed] [Google Scholar]
- 35. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787–847. [DOI] [PubMed] [Google Scholar]
- 36. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 37. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018;378:417–27. [DOI] [PubMed] [Google Scholar]
- 38. Thomas L, Li F, Pencina M. Using propensity score methods to create target populations in observational clinical research. JAMA 2020;323:466–7. [DOI] [PubMed] [Google Scholar]
- 39. Elze MC, Gregson J, Baber U, Williamson E, Sartori S, Mehran R et al. Comparison of propensity score methods and covariate adjustment: evaluation in 4 cardiovascular studies. J Am Coll Cardiol 2017;69:345–57. [DOI] [PubMed] [Google Scholar]
- 40. Putter H, Schumacher M, van Houwelingen HC. On the relation between the cause-specific hazard and the subdistribution rate for competing risks data: the Fine–Gray model revisited. Biom J 2020;62:790–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wood AM, White IR, Royston P. How should variable selection be performed with multiply imputed data? Stat Med 2008;27:3227–46. [DOI] [PubMed] [Google Scholar]
- 42. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med 2017;167:268–74. [DOI] [PubMed] [Google Scholar]
- 43. Di Biase L, Mohanty P, Mohanty S, Santangeli P, Trivedi C, Lakkireddy D et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation 2016;133:1637–44. [DOI] [PubMed] [Google Scholar]
- 44. Huang HD, Waks JW, Contreras-Valdes FM, Haffajee C, Buxton AE, Josephson ME. Incidence and risk factors for symptomatic heart failure after catheter ablation of atrial fibrillation and atrial flutter. Europace 2016;18:521–30. [DOI] [PubMed] [Google Scholar]
- 45. Padala SK, Gunda S, Sharma PS, Kang L, Koneru JN, Ellenbogen KA. Risk model for predicting complications in patients undergoing atrial fibrillation ablation. Heart Rhythm 2017;14:1336–43. [DOI] [PubMed] [Google Scholar]
- 46. Srivatsa UN, Danielsen B, Anderson I, Amsterdam E, Pezeshkian N, Yang Y et al. Risk predictors of stroke and mortality after ablation for atrial fibrillation: the California experience 2005–2009. Heart Rhythm 2014;11:1898–903. [DOI] [PubMed] [Google Scholar]
- 47. Leening MJ, Heeringa J, Deckers JW, Franco OH, Hofman A, Witteman JC et al. Healthy volunteer effect and cardiovascular risk. Epidemiology 2014;25:470–1. [DOI] [PubMed] [Google Scholar]
- 48. Shrank WH, Patrick AR, Brookhart MA. Healthy user and related biases in observational studies of preventive interventions: a primer for physicians. J Gen Intern Med 2011;26:546–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Packer M. Effect of catheter ablation on pre-existing abnormalities of left atrial systolic, diastolic, and neurohormonal functions in patients with chronic heart failure and atrial fibrillation. Eur Heart J 2019;40:1873–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med 2020;383:1305–16. [DOI] [PubMed] [Google Scholar]
- 51. Messerli FH, Rimoldi SF, Bangalore S. The transition from hypertension to heart failure: contemporary update. JACC Heart Fail 2017;5:543–51. [DOI] [PubMed] [Google Scholar]
- 52. Kowallick JT, Staab W, Schuster A, Backhaus SJ, Weber-Krüger M, Bauer L et al. Reverse left ventricular structural remodeling after catheter ablation of atrial fibrillation in patients with preserved left ventricular function: insights from cardiovascular magnetic resonance native T1 mapping. Heart Rhythm 2019;16:424–32. [DOI] [PubMed] [Google Scholar]
- 53. Nagai T, Arakawa J, Hamabe A, Tabata H. Improvement of left ventricular function after successful radiofrequency catheter ablation in persistent atrial fibrillation with preserved left ventricular ejection fraction: a comprehensive echocardiographic assessment using two-dimensional speckle tracking analysis. J Echocardiogr 2019;17:95–103. [DOI] [PubMed] [Google Scholar]
- 54. Tops LF, Den Uijl DW, Delgado V, Marsan NA, Zeppenfeld K, Holman E et al. Long-term improvement in left ventricular strain after successful catheter ablation for atrial fibrillation in patients with preserved left ventricular systolic function. Circ Arrhythm Electrophysiol 2009;2:249–57. [DOI] [PubMed] [Google Scholar]
- 55. Cha YM, Wokhlu A, Asirvatham SJ, Shen WK, Friedman PA, Munger TM et al. Success of ablation for atrial fibrillation in isolated left ventricular diastolic dysfunction: a comparison to systolic dysfunction and normal ventricular function. Circ Arrhythm Electrophysiol 2011;4:724–32. [DOI] [PubMed] [Google Scholar]
- 56. Chieng D, Sugumar H, Segan L, Tan C, Vizi D, Nanayakkara S et al. Atrial fibrillation ablation for heart failure with preserved ejection fraction: a randomized controlled trial. JACC Heart Fail 2023;11:646–58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.