Abstract
Background and Hypothesis
Aerobic exercise interventions in people with schizophrenia have been demonstrated to improve clinical outcomes, but findings regarding the underlying neural mechanisms are limited and mainly focus on the hippocampal formation. Therefore, we conducted a global exploratory analysis of structural and functional neural adaptations after exercise and explored their clinical implications.
Study Design
In this randomized controlled trial, structural and functional MRI data were available for 91 patients with schizophrenia who performed either aerobic exercise on a bicycle ergometer or underwent a flexibility, strengthening, and balance training as control group. We analyzed clinical and neuroimaging data before and after 6 months of regular exercise. Bayesian linear mixed models and Bayesian logistic regressions were calculated to evaluate effects of exercise on multiple neural outcomes and their potential clinical relevance.
Study Results
Our results indicated that aerobic exercise in people with schizophrenia led to structural and functional adaptations mainly within the default-mode network, the cortico-striato-pallido-thalamo-cortical loop, and the cerebello-thalamo-cortical pathway. We further observed that volume increases in the right posterior cingulate gyrus as a central node of the default-mode network were linked to improvements in disorder severity.
Conclusions
These exploratory findings suggest a positive impact of aerobic exercise on 3 cerebral networks that are involved in the pathophysiology of schizophrenia.
Clinical Trials Registration
The underlying study of this manuscript was registered in the International Clinical Trials Database, ClinicalTrials.gov (NCT number: NCT03466112, https://clinicaltrials.gov/ct2/show/NCT03466112?term=NCT03466112&draw=2&rank=1) and in the German Clinical Trials Register (DRKS-ID: DRKS00009804).
Keywords: schizophrenia, exercise, brain structure, functional connectivity, randomized-controlled trial
Introduction
Exercise interventions represent a promising adjunctive treatment to positively influence the disease course of schizophrenia.1 As outlined by numerous meta-analyses, distinct types of exercise interventions yield the potential to alleviate symptom severity,2–7 to enhance cognitive capacities,4,5 and to elicit improvements in social and occupational functioning.3,5 Among different kinds of exercise treatments in schizophrenia, aerobic exercise interventions have been demonstrated to be particularly beneficial.6–10
Aerobic exercise-induced ameliorations in multiple disorder-related health outcomes are suggested to be mediated by specific cerebral adaptations.11,12 For instance, enhanced levels of aerobic fitness and exercise have been linked to an increased volume of the hippocampal formation and its respective subfields which in turn can lead to improvements in short-term memory, working memory, and general functioning in people with schizophrenia.13–19 Moreover, preliminary findings indicate that a strengthening of hippocampal functional connectivity (FC) with the occipital lobe induced by aerobic exercise is accompanied by improved positive symptoms and cognitive performance.20,21 Given that the hippocampal formation reflects a key region of the pathophysiology of schizophrenia,22–25 these compensatory effects of aerobic exercise appear to be particularly promising.
However, apart from the hippocampal formation, compelling large-scale evidence demonstrates that other areas, embedded in higher-level brain networks, also play a fundamental role in the pathophysiology of schizophrenia: Particularly, volume reductions in the bilateral insula, anterior cingulate cortex, middle frontal cortices, temporal gyrus, and thalamus,26–29 cortical thinning in the prefrontal, temporal and anterior cingulate cortex and the insula30–32 and widespread aberrant patterns of cortical gyrification33,34 have been identified in schizophrenia and other severe mental illnesses. Analogously, core intrinsic connectivity networks, such as the salience network, default-mode network, or fronto-parietal network, reveal consistent deteriorations in FC.27,35,36 Moreover, structural and functional disturbances within several essential key circuits, such as the cortico-striatal-thalamic-cerebellar pathway,37–39 the cortico-striato-pallido-thalamo-cortical circuit,36,40–48 the amygdalocortical circuitry49 or the traditional Papez circuit50 contribute to clinical symptoms in schizophrenia.
To improve general brain health and to counteract neural decline across multiple populations, exercise has been demonstrated to be efficient: Numerous large-scale analyses reveal that aerobic exercise interventions lead to widespread structural and functional neural benefits across the whole brain.51–56 In schizophrenia, only a few studies target the effects of aerobic exercise and fitness on other brain regions than the hippocampal formation.12,57,58 On the structural level, aerobic exercise has been found to increase the volume of the temporal gyrus,59 to strengthen the structural connectivity within brain regions involved in motor functioning60 and to enlarge the cortical thickness of the anterior cingulate cortex, prefrontal cortex, and the entorhinal cortex.61,62 Furthermore, improvements in aerobic fitness are suggested to be associated with decreases in the lateral and third ventricle and a thickening of frontal, temporal, and cingulate cortices in people with schizophrenia.63 Likewise, cross-sectional studies indicate that patients with higher levels of physical activity have larger grey matter volumes and cortical thickness in the prefrontal cortex.64 On the functional level, higher aerobic fitness levels in people with schizophrenia have been linked to an attenuation of cerebello-thalamic hyperconnectivity and to a strengthening of FC within the cerebellum and the default-mode network.65 Importantly, findings regarding the clinical implications of these structural and functional adaptations linked to aerobic exercise and fitness remain scarce and heterogeneous.59–62,64,65
Hence, we aim to identify further potential neural targets besides the hippocampal formation that possibly drive beneficial effects of aerobic exercise on psychiatric symptoms, cognition, and functioning in people with schizophrenia. First, we explored the effects of aerobic exercise on regional brain volumes, cortical thickness, and cortical gyrification, as well as on FC within several core cerebral networks implicated in the pathophysiology of schizophrenia. Second, we examined whether the identified effects on structural and functional brain outcomes are associated with clinical improvements.
Methods
The present project was part of the Enhancing Schizophrenia Prevention and Recovery through Innovative Treatments (ESPRIT) C3 study coordinated by the Central Institute of Mental Health in Mannheim. This multicenter randomized-controlled trial investigates the impact of aerobic exercise compared to a flexibility, strengthening, and balance program on various outcomes of rehabilitation in people with schizophrenia. Data were acquired at the Department of Psychiatry and Psychotherapy of the LMU Hospital in Munich, the Central Institute of Mental Health in Mannheim, and the Departments of Psychiatry and Psychotherapy of the University Hospital Charité in Berlin, University Hospital Duesseldorf and University Hospital RWTH in Aachen. The study is in line with the Declaration of Helsinki and ethical approval was provided by the local ethics committees at each study site. The present work represents a subproject based on a subsample of the ESPRIT C3 study and specifically focuses on the effects of aerobic exercise on multiple neural outcomes and their potential clinical implications.
Study Design and Sample
A total of 180 people with schizophrenia formed the intention-to-treat sample of the ESPRIT C3 study. Subjects were randomized either to an aerobic endurance training (AET) or to a flexibility, strengthening, and balance training (FSBT). Supervised by trained study personnel, patients in both conditions exercised up to 3 times per week between 40 and 50 minutes for a total duration of 6 months. The AET group cycled on a stationary bicycle ergometer at a moderate intensity which was determined individually prior to the study onset using a stepwise lactate threshold test. The FSBT group performed different stretching, mobility, stability, balance, and relaxation exercises.66 Both interventions were standardized in terms of exercise duration and frequency. No exercise-related adverse events occurred. All raters of clinical, cognitive, and functional outcomes were blinded to the group assignment and the study personnel who supervised the exercise trainings were not involved in data acquisition and analysis.
163 participants completed at least one exercise training and thus were part of the ITT2 sample. Out of 163 patients, 99 gave written informed consent to undergo MRI acquisitions. Eight subjects were excluded due to insufficient image quality or missing MRI scans at baseline (Supplementary information). Finally, 91 participants (44 in AET group, 47 in FSBT group) were included in the final analysis.
Behavioral and neuroimaging data at baseline prior to the intervention (t0) and after 6 months of exercise training (t6) were considered in the present examination. Sample characteristics are summarized in table 1. Further details regarding the study design are described elsewhere.67
Table 1.
Sample Characteristics
| AET n = 44 (48.4 %) (n/mean ± SD) |
FSBT n = 47 (51.6 %) (n/mean ± SD) |
||
|---|---|---|---|
| Study site | pfisher = 0.814 | ||
| Munich | 30 (68.2 %) | 30 (63.8 %) | |
| Mannheim | 10 (22.7 %) | 13 (27.7 %) | |
| Berlin | 4 (9.1 %) | 3 (6.4 %) | |
| Aachen | 0 (0.0 %) | 1 (2.1 %) | |
| Sex | pfisher = 0.268 | ||
| Female | 12 (27.3 %) | 19 (40.4 %) | |
| Male | 32 (72.7 %) | 28 (59.6 %) | |
| Age (years) | 34.55 ± 11.15 | 39.30 ± 12.07 | pwilcox = 0.063 |
| PANSS at baseline (t0) | |||
| Positive scale | 11.18 ± 3.60 | 11.57 ± 4.04 | pwilcox = 0.761 |
| Negative scale | 13.23 ± 5.64 | 11.79 ± 3.59 | pwilcox = 0.420 |
| General scale | 24.89 ± 5.63 | 25.74 ± 5.70 | pwilcox = 0.559 |
| Total scale | 49.30 ± 12.20 | 49.12 ± 10.83 | pwilcox = 1.000 |
| Chlorpromazine equivalents | 458.18 ± 261.44 | 481.68 ± 322.16 | pwilcox = 0.937 |
| Education (years) | 14.68 ± 3.62 | 14.74 ± 4.60 | pwilcox = 0.590 |
| Total number of trainings | 25.73 ± 17.84 | 26.55 ± 21.25 | pwilcox = 0.883 |
| Participants per session | |||
| Baseline (t0) | 44 (100 %) | 47 (100 %) | — |
| After 6 months (t6) | 12 (27.3 %) | 21 (44.7 %) | — |
Descriptive statistics are expressed as mean ± standard deviation. The sample sizes per group refer to the number of participants that were considered for the statistical data analysis. Chlorpromazine equivalents were computed according to the Defined Daily Doses method. AET, aerobic endurance training; FSBT, flexibility, strengthening, and balance training; PANSS, Positive and Negative Syndrome Scale; pfisher, P-value of Fisher´s exact test for categorical data; pwilcox, P-value of Wilcoxon signed-rank test for numeric data; SD, standard deviation.
MRI Data Acquisition and Processing
A 3T Siemens Magnetom Tim Trio scanner was used to acquire MRI data in Mannheim, Berlin, and Aachen, while a 3T Siemens Magnetom Skyra MRI scanner was utilized in Munich. At each site, a 3D T1-weighted magnetization prepared rapid gradient echo (MP RAGE) with an isotropic spatial resolution and a resting-state functional echo planar imaging sequence were measured. Supplementary Table S1 summarizes the scanning parameters. FreeSurfer v7.2 was used to process the structural isotropic 3D T1-weighted MR images and to compute the measures of brain volumes, cortical thickness, and cortical curvature. Processing of the functional EPI images was performed with fMRIPrep v21.0.168 and Nilearn v0.7.1. Figure 1 provides an overview of the analysis procedure. Details regarding pre- and postprocessing, as well as the computation of structural and functional outcome measures are provided in Supplementary information (Supplementary Table S2 and Figure S1).
Fig. 1.
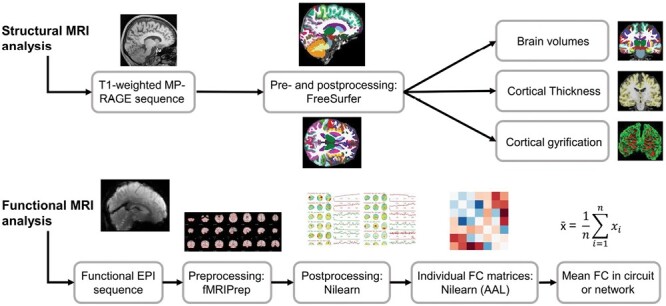
Analysis approach. The analysis workflows for the structural and functional MRI data are illustrated. MP-Rage, magnetization prepared rapid gradient echo; EPI, echo planar imaging; fMRIPrep, functional magnetic resonance imaging preprocessing pipeline; Nilearn, Python library to process, analyze and visualize multimodal MRI data, AAL, Automated Anatomical Labeling; FC, functional connectivity.
Clinical and Cognitive Data
We considered psychiatric symptoms, global disorder severity, cognitive performance, and social and occupational functioning to assess the potential clinical implications of cerebral changes induced by exercise. Positive and negative symptoms, and total symptom severity were assessed by the PANSS.69 Global disorder severity was measured by the Clinical Global Impression Scale.70 A global cognitive composite score that encompassed different cognitive domains was computed based on various validated neuropsychological test batteries (Supplemental Information). The Global Assessment of Functioning Scale (GAF) and the Functional Remission of General Schizophrenia Scale (FROGS) were administered to quantify levels of functioning.71,72 Further details are described in Supplementary information.
Statistical Data Analysis
Statistical data analysis was performed in Rstudio v1.4.1717 based on R v4.2.273,74 and visualizations were created using ggplot2.75
To study the effect of AET in comparison to FSBT on structural (regional volumes, cortical thicknesses, and cortical curvature) and functional (averaged pathway connectivity) brain outcomes, Bayesian linear mixed effect models were computed using the brms package.76 Group (AET, FSBT), session (t0: baseline, t6: 6 months), the group-session interaction, age, sex, chlorpromazine equivalents, number of trainings, outcome at baseline, and study site (Munich, Mannheim, Berlin, Aachen) were included as predictors, while the corresponding structural and functional outcomes served as dependent variables.
Regarding the clinical relevance of individual-specific exercise effects on structural and functional brain outcomes, Bayesian logistic regression models were computed to evaluate if those participants who demonstrated beneficial structural and functional brain changes also revealed improvements in positive, negative, and total symptom severity, cognitive performance, and functioning. Details on the statistical analysis are provided in the Supplementary information.
Results
Exercise Effects on Brain Structure and Function
Figure 2 and Figure 3 depict exercise effects on structural and functional brain outcomes within both groups, respectively. The detailed Bayesian test statistics are provided in Supplementary Table S4.
Fig. 2.
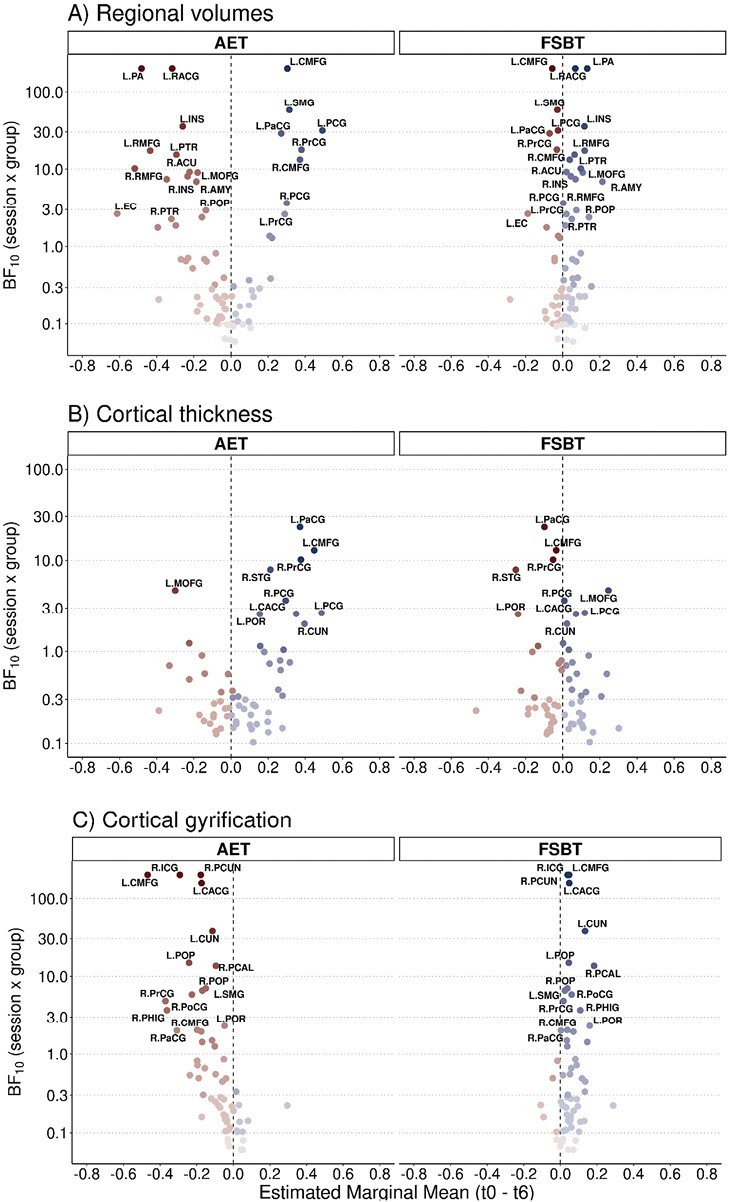
Exercise effects on brain structure. The marginal mean difference of (A) brain volumes (corrected by the intracranial volume), (B) cortical thickness, and (C) cortical gyrification between the baseline session (t0) and the session after 6 months of exercise (t6) extracted from the linear mixed model analysis is depicted on the x-axis. The Bayes factor (BF10) of the interaction term in the linear mixed model analysis is displayed on the y-axis. Effects within the aerobic endurance training (AET) group are illustrated on the left, while effects within the flexibility, strengthening, and balance training (FSBT) group are shown on the right. Regions are labeled if BF10 > 2 and if the MCAR assumption is not violated. Full regions' names with their particular abbreviations are provided in Supplementary Table S6.
Fig. 3.
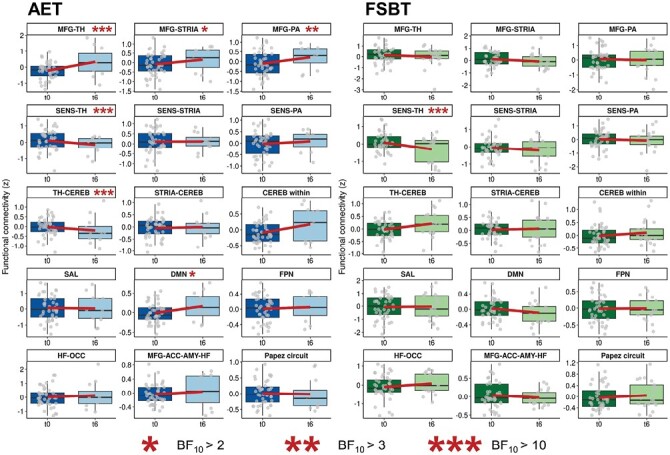
Exercise effects on functional connectivity in schizophrenia-related brain circuits. Boxplots of functional connectivity distributions per session. The regression line illustrates the average change in functional connectivity between the baseline session (t0) and the session after six months of exercise (t6). The aerobic exercise group is illustrated on the left, the control group is displayed on the right. ACC, anterior cingulate cortex; AET, aerobic endurance training; AMY, amygdala; CEREB, cerebellum; DMN, default-mode network; FPN, fronto-parietal network; FSBT, flexibility, strengthening, and balance training; HF, hippocampal formation; MFG, middle frontal gyrus; OCC, occipital lobe; PA, pallidum; SAL, salience network; SENS, sensorimotor cortices; STRIA, striatum; TH, thalamus.
In the AET group, but not in the FSBT group, we identified volume increases in the bilateral caudal middle frontal gyrus, bilateral posterior cingulate gyrus, bilateral precentral gyrus, left supramarginal gyrus, and right paracentral gyrus. Concurrently, we observed volume decreases in the left pallidum, left rostral anterior cingulate gyrus, bilateral insula, bilateral rostral middle frontal gyrus, left pars triangularis, right nucleus accumbens, left medial orbitofrontal gyrus, right amygdala, right pars opercularis, left entorhinal cortex, and right pars triangularis (figure 2A).
We further obtained increases in cortical thickness in the left paracentral gyrus, left caudal middle frontal gyrus, right precentral gyrus, right superior temporal gyrus, left caudal anterior cingulate gyrus, the bilateral posterior cingulate gyrus, left pars orbitalis, and right cuneus only in the AET group. The left medial orbitofrontal gyrus was the only region showing a decrease in cortical thickness (figure 2B).
With respect to the cortical gyrification, decreases were present in the bilateral caudal middle frontal gyrus, right isthmus cingulate gyrus, right precuneus, left caudal anterior cingulate gyrus, left cuneus, bilateral pars opercularis, left supramarginal gyrus, right postcentral gyrus, right precentral gyrus, right parahippocampal gyrus, right caudal middle frontal gyrus, left pars orbitalis, and right paracentral gyrus in the AET group, but not in the FSBT group. No increases in cortical gyrification in the AET group existed (figure 2C).
On the functional level, FC between the middle frontal gyrus and the thalamus, striatum, and pallidum and FC within the DMN increased from session t0 to session t6 in the AET group, but not in the FSBT group. FC between the thalamus and the cerebellum decreased only in the AET group, whereas decreases in FC between sensorimotor cortices and the thalamus were identified in both exercise groups (figure 3).
Clinical Implication of Structural and Functional Brain Changes Induced by Aerobic Exercise
We investigated whether the identified effects of aerobic exercise on the neural level are associated with improvements on the clinical level, using the PANSS scales to assess schizophrenic symptoms, the clinical global impression to cover general disorder severity, a global cognition score that encompasses different cognitive domains and the GAF and FROGS score to measure levels of functioning. The full test statistics of these results are provided in Supplementary Table S5.
We found that an increase in the volume of the right posterior cingulate gyrus was associated with an improvement in the clinical global impression (BF10 = 10.66). On the functional level, a decrease in FC between the thalamus and the cerebellum was linked to an improvement in the FROGS (BF10 = 4.39). There were several other associations with a BF10 higher than 2, but no clear and consistent pattern for a whole network and overlapping clinical outcomes could be identified.
Discussion
The current study is the first that comprehensively explores the effects of aerobic exercise on widespread structural and functional brain patterns and their clinical relevance in people with schizophrenia.
We provide evidence that aerobic exercise in people with schizophrenia can lead to increases in the volume and cortical thickness of the middle frontal and posterior cingulate gyrus, as well as decreases of the cortical gyrification of the middle frontal gyrus and precuneus. These regions are central nodes of the default-mode network.77,78 Additionally, our findings suggest that aerobic exercise enhances FC within the default-mode network. These results are in line with previous evidence in people with schizophrenia, indicating that aerobic exercise interventions, enhanced aerobic fitness levels, or higher amounts of physical activity, in general, are associated with increases in cortical thickness in middle frontal areas and with increased FC within the default-mode network.61,63–65 Importantly, volume reductions, cortical thinning, increased gyrification, and reduced FC within the default-mode network have been widely reported in people with schizophrenia and other psychiatric conditions.27–30,33–36 Hence, our findings may indicate that aerobic exercise has the potential to mitigate disruptions of the default-mode network in people with schizophrenia.
The default-mode network reflects a self-referential introspective state and is associated with theory of mind and social cognition.77,79 In the psychiatric context, deteriorations of the default-mode network have been associated with more severe negative symptoms in schizophrenia80 and impaired inhibition control across multiple psychiatric disorders.27 We observe that an exercise-induced volume increase of a central node of the default-mode network—the posterior cingulate gyrus—is linked to an improvement in general disorder severity. Hence, the beneficial effects of aerobic exercise on the default-mode network may represent a potential neural pathway of exercise-induced improvements in clinical outcomes. However, other structural and functional adaptations in the default-mode network caused by aerobic exercise do not show consistent clinical implications. Taken together, we conclude that aerobic exercise specifically promotes beneficial adaptations in the default-mode network on the structural and functional level, but the clinical relevance of these cerebral changes remains to be determined.
In addition to exercise-induced adaptations in the default-mode network, our findings suggest that aerobic exercise can lead to structural changes of the middle frontal gyrus, the central sensorimotor gyri, and the pallidum. On the functional level, we observe a strengthening of FC between the thalamus, striatum, and pallidum and the middle frontal gyrus, whereas FC between the thalamus and the central sensorimotor gyri decreases in response to exercise. These structures are part of the cortico-striato-pallido-thalamo-cortical loop in which disruptions have been frequently demonstrated in schizophrenia. Specifically, the thalamus, striatum, and pallidum reveal reduced FC with the prefrontal cortex and a hyperconnectivity with central sensorimotor cortices.40–47 Aberrant FC within the cortico-striato-pallido-thalamo-cortical circuit is assumed to be modulated by schizophrenia-related striatal dopamine dysfunction.81 Striatal dopamine dysfunction is seen as the interface between the dopamine, glutamate, and serotonin hypothesis of psychosis.82 In particular, hypofunctional NMDA receptors in the prefrontal cortex and hyperactivated cortical 5-HT2A receptors are assumed to impair downstream glutamate signaling which in turn is considered to cause an overactivation of striatal dopamine synapses.82 Importantly, pathological alterations on the microscopic level and corresponding hyper- and hypo-connectivity patterns on the macroscopic level are interrelated.83 For instance, pharmaco-fMRI studies demonstrate that ketamine-induced blockage of NMDA receptors in healthy individuals induces thalamic hyperconnectivity with central sensorimotor cortices and thus leads to macroscale dysconnectivity patterns similar to those observed in manifested schizophrenia.84,85 Accordingly, both, the dopaminergic, glutamatergic, and serotonergic dysregulations in schizophrenia and the dysconnectivity patterns within the cortico-striato-pallido-thalamo-cortical loop are suggested to contribute to psychotic symptom severity and cognitive deficits.40–42,46,82,83
Our findings indicate that aerobic exercise counteracts structural alterations and aberrant connectivity patterns of the cortico-striato-pallido-thalamo-cortical circuit and thus may also yield compensatory effects on relevant neurotransmitter pathways. Despite these benefits on the cerebral level, we do not find stable clinical implications of such structural and functional adaptations within the cortico-striato-pallido-thalamo-cortical loop in the current analysis.
Apart from the effects of aerobic exercise on the default-mode network and the cortico-striato-pallido-thalamo-cortical circuit, we observe that aerobic exercise reduces FC between the thalamus and the cerebellum in our sample of people with schizophrenia. This is in line with our previous analysis, demonstrating that patients with higher aerobic fitness levels have lower FC between the thalamus and the cerebellum.65 As described by the cognitive dysmetria hypothesis, schizophrenia is characterized by deteriorations in the cerebello-thalamo-cortical circuit, leading to an impaired ability to coordinate mental processes and thus accounting for a broad range of clinical symptoms.86 Compelling fMRI evidence corroborates the cognitive dysmetria hypothesis by showing that cerebello-thalamo-cortical hyperconnectivity can be regarded as a heritable functional neural trait in schizophrenia that may reflect an increased need for mental capacities to fulfill respective cognitive demands.37,38 Similar to the previously described dysconnectivity within the cortico-striato-pallido-thalamo-cortical circuit in schizophrenia, cerebello-thalamo-cortical hyperconnectivity is expected to result from NMDA receptor hypofunction and the accompanied disrupted glutamate system downstream from the cortex.37,38 On the clinical level, cerebello-thalamo-cortical hyperconnectivity is associated with symptoms of disorganization such as bizarre thoughts and behavior.37 We observe that an attenuation of the hyperconnectivity between the thalamus and the cerebellum was related to improvements in social and occupational functioning, but not to benefits in clinical symptoms or cognition. Thus, both the current results and our previous cross-sectional study65 indicate that aerobic exercise and increased fitness levels may alleviate hyperconnectivity within the cerebello-thalamo-cortical loop, but the clinical implications of these effects remain inconclusive.
With respect to the underlying mechanisms, beneficial effects of aerobic exercise on brain structure and function may result from several neural adaptations that occur in response to regular engagement in aerobic exercise. These cerebral adjustments consist of upregulations of neurotrophic factors (eg, BDNF), facilitated neuroplastic processes (eg, neurogenesis, angiogenesis, and gliogenesis) and increases in dendritic density and length.11,87,88 Importantly, these processes may be explained by exercise-induced improvements in physical health.89 In particular, the prevalence of physical comorbidities such as obesity is substantially higher in people with schizophrenia.90–92 Besides the well-described bidirectional interrelation between obesity and mental health on the behavioral level,93 obesity is also associated with widespread structural and functional deteriorations on the neural level.94–97 Hence, exercise-induced improvements in physical health may represent a potential factor that also counteracts neural disruptions in people with schizophrenia.
In contrast to the beneficial effects on structural and functional outcomes on the cerebral level, we also observe volume decreases in the aerobic exercise group in several key regions such as the insula, the anterior cingulate gyrus, the rostral middle frontal gyrus, or the amygdala. Volume reductions in these areas are a central hallmark of schizophrenia and other psychiatric disorders.26–29 Therefore, our findings reveal that aerobic exercise may not yield the potential to mitigate volume decline across the whole brain, but rather targets specific neural circuits as outlined above.
Our study has some important limitations: First, the drop-out rates during the study course were very high. Only 12 patients (27.3 %) in the AET group and 21 (44.7 %) in the FSBT group completed the MRI session after 6 months of exercise. We ensured that the drop-outs did not bias the identified longitudinal effects of exercise on brain outcomes by interpreting results only in case of a non-violated Missing-Completely-at-Random assumption (Supplementary Table S3). Moreover, we did not find significant differences in relevant demographic or clinical baseline data between subjects that had MRI data available after 6 months of exercise and those who only had a baseline MRI scan (Supplementary Table S7 and S8). However, regarding the clinical implications of exercise-induced neural adaptations, only between 23 and 33 participants (depending on the availability of the clinical scores) across both groups could be considered. This pronounced reduction of the initial sample size of 91 participants led to a decreased statistical power which may explain the current heterogeneous results regarding the clinical relevance of neural findings. Future exercise trials in people with schizophrenia should identify subgroups of patients at high risk for drop-out to provide additional support for these patients aiming to maintain their participation. Moreover, future exercise studies are required to investigate the efficiency of certain study characteristics such as continuous supervision during trainings or reward systems for regular participation that may improve adherence.
Second, this study represents a global and primarily exploratory analysis that considered effects of aerobic exercise on multiple structural and functional brain outcomes. Despite the identified promising effects of aerobic exercise on key brain networks in people with schizophrenia, we emphasize that these findings require a further hypothesis-driven replication in an independent sample.
Third, due to ethical reasons, we did not include a control condition without any exercise treatment. Thus, the differences between both exercise groups on the neural level could also result from the control intervention that might have prevented beneficial adaptations in the default-mode network, the cortico-striato-pallido-thalamo-cortical loop, and the cerebello-thalamo-cortical circuit. However, we estimate the probability of such an effect to be rather low, because different types of exercise interventions, including strength trainings, have been shown to be beneficial without any relevant side effects.2–7
Lastly, our sample consisted largely of males (65.9 %), impeding the generalizability of the current findings.
In summary, we provide first exploratory evidence that aerobic exercise may positively affect disruptions of the default-mode network, the cortico-striato-pallido-thalamo-cortical circuit, and the cerebello-thalamo-cortical pathways in people with schizophrenia. Volume increases within the default-mode network induced by aerobic exercise may drive improvements in disorder severity, but the clinical implications of exercise effects on the brain should be further clarified in future large-scale randomized controlled trials.
Supplementary Material
Acknowledgments
The authors thank the Clinical Trials Center Cologne (CTC Cologne) and the Institute for Medical Statistics and Computational Biology of the Medical Faculty of the University of Cologne for developing the database and the secure web-based randomization system and performing data management and monitoring. Furthermore, they express their appreciation to the Clinical Open Research Engine (CORE) at the University Hospital LMU (Munich, Germany) for providing the computational infrastructure to run the CPU-intensive MRI analysis pipelines.
Contributor Information
Lukas Roell, Department of Psychiatry and Psychotherapy, University Hospital, LMU Munich, Munich, Germany; Neuroimaging Core Unit Munich (NICUM), University Hospital, LMU Munich, Munich, Germany.
Daniel Keeser, Department of Psychiatry and Psychotherapy, University Hospital, LMU Munich, Munich, Germany; Neuroimaging Core Unit Munich (NICUM), University Hospital, LMU Munich, Munich, Germany; Department of Radiology, University Hospital, LMU Munich, Munich, Germany.
Boris Papazov, Department of Radiology, University Hospital, LMU Munich, Munich, Germany.
Moritz Lembeck, Department of Psychiatry and Psychotherapy, University Hospital, LMU Munich, Munich, Germany.
Irina Papazova, Department of Psychiatry, Psychotherapy and Psychosomatics of the University Augsburg, Medical Faculty, University of Augsburg, Bezirkskrankenhaus Augsburg, Augsburg, Germany.
David Greska, Department of Psychiatry and Psychotherapy, University Hospital, LMU Munich, Munich, Germany.
Susanne Muenz, Department of Psychiatry and Psychotherapy, University Hospital, LMU Munich, Munich, Germany.
Thomas Schneider-Axmann, Department of Psychiatry and Psychotherapy, University Hospital, LMU Munich, Munich, Germany.
Eliska B Sykorova, Central Institute of Mental Health, Medical Faculty Mannheim, Heidelberg University, Germany.
Christina E Thieme, Central Institute of Mental Health, Medical Faculty Mannheim, Heidelberg University, Germany.
Bob O Vogel, Department of Psychiatry and Psychotherapy, University Hospital Charité Berlin, Berlin, Germany.
Sebastian Mohnke, Department of Psychiatry and Psychotherapy, University Hospital Charité Berlin, Berlin, Germany.
Charlotte Huppertz, Department of Psychiatry, Psychotherapy and Psychosomatics, RWTH Aachen University, Aachen, Germany.
Astrid Roeh, Department of Psychiatry, Psychotherapy and Psychosomatics of the University Augsburg, Medical Faculty, University of Augsburg, Bezirkskrankenhaus Augsburg, Augsburg, Germany.
Katriona Keller-Varady, Hannover Medical School, Department of Rehabilitation and Sports Medicine, Hannover, Germany.
Berend Malchow, Department of Psychiatry and Psychotherapy, University Hospital Göttingen, Göttingen, Germany.
Sophia Stoecklein, Department of Radiology, University Hospital, LMU Munich, Munich, Germany.
Birgit Ertl-Wagner, Department of Radiology, University Hospital, LMU Munich, Munich, Germany; Division of Neuroradiology, Department of Diagnostic Imaging, The Hospital for Sick Children, Toronto, Canada; Department of Medical Imaging, University of Toronto, Toronto, Canada.
Karsten Henkel, Department of Psychiatry, Psychotherapy and Psychosomatics, RWTH Aachen University, Aachen, Germany.
Bernd Wolfarth, Department of Sports Medicine, University Hospital Charité Berlin, Berlin, Germany.
Wladimir Tantchik, Department of Psychiatry and Psychotherapy, University Hospital Charité Berlin, Berlin, Germany.
Henrik Walter, Department of Psychiatry and Psychotherapy, University Hospital Charité Berlin, Berlin, Germany.
Dusan Hirjak, Central Institute of Mental Health, Medical Faculty Mannheim, Heidelberg University, Germany.
Andrea Schmitt, Department of Psychiatry and Psychotherapy, University Hospital, LMU Munich, Munich, Germany; Laboratory of Neuroscience (LIM27), Institute of Psychiatry, University of Sao Paulo, São Paulo, Brazil.
Alkomiet Hasan, Department of Psychiatry, Psychotherapy and Psychosomatics of the University Augsburg, Medical Faculty, University of Augsburg, Bezirkskrankenhaus Augsburg, Augsburg, Germany.
Andreas Meyer-Lindenberg, Central Institute of Mental Health, Medical Faculty Mannheim, Heidelberg University, Germany.
Peter Falkai, Department of Psychiatry and Psychotherapy, University Hospital, LMU Munich, Munich, Germany; Max Planck Institute of Psychiatry, Munich, Germany.
Isabel Maurus, Department of Psychiatry and Psychotherapy, University Hospital, LMU Munich, Munich, Germany.
Funding
The work was supported by the German Federal Ministry of Education and Research (BMBF) through the research network on psychiatric diseases ESPRIT (Enhancing Schizophrenia Prevention and Recovery through Innovative Treatments; coordinator, Andreas Meyer-Lindenberg; grant number, 01EE1407E). The study was supported by the Else Kröner-Fresenius Foundation.
Disclosure Statement
AS was an honorary speaker for TAD Pharma and Roche and a member of Roche advisory boards. AH is an editor of the German (DGPPN) schizophrenia treatment guidelines and first author of the WFSBP schizophrenia treatment guidelines; he has been on the advisory boards of and/or has received speaker fees from AbbVie, Boehringer Ingelheim, Janssen-Cilag, Lundbeck, Recordati, Rovi, and Otsuka. PF is a coeditor of the German (DGPPN) schizophrenia treatment guidelines and a coauthor of the WFSBP schizophrenia treatment guidelines; he is on the advisory boards and receives speaker fees from Janssen, Lundbeck, Otsuka, Servier, and Richter. AML has received consultant fees from Boehringer Ingelheim, Elsevier, Brainsway, Lundbeck Int. Neuroscience Foundation, Lundbeck A/S, Sumitomo Dainippon Pharma Co., Academic Medical Center of the University of Amsterdam, Synapsis Foundation-Alzheimer Research Switzerland, IBS Center for Synaptic Brain Dysfunction, Blueprint Partnership, University of Cambridge, Dt. Zentrum für Neurodegenerative Erkrankungen, Zürich University, Brain Mind Institute, L.E.K. Consulting, ICARE Schizophrenia, Science Advances, Foundation FondaMental, v Behring Röntgen Stiftung, The Wolfson Foundation, and Sage Therapeutics; in addition, he has received speaker fees from Lundbeck International Foundation, Paul-Martini-Stiftung, Lilly Deutschland, Atheneum, Fama Public Relations, Institut d’investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Janssen-Cilag, Hertie Stiftung, Bodelschwingh-Klinik, Pfizer, Atheneum, University of Freiburg, Schizophrenia Academy, Hong Kong Society of Biological Psychiatry, Fama Public Relations, Spanish Society of Psychiatry, Italian Society of Biological Psychiatry, Reunions I Ciencia S.L., and Brain Center Rudolf Magnus UMC Utrecht and was awarded the Prix Roger de Spoelberch grant and the CINP Lilly Neuroscience Clinical Research Award 2016. BEW is a central radiology reader for Bayer Healthcare and her spouse is an employee of Siemens Healthineers. LR, DK, BP, ML, IP, DG, SM, TSA, ES, CT, BV, SM, CH, AR, KKV, BW, SS, KH, BW, WT, HW, DH, and IM report no conflicts of interest.
Data Availability
All analysis scripts and documentation sheets will be published on OSF. Additional data can be made available upon request to the corresponding author.
References
- 1. Schmitt A, Reich-Erkelenz D, Hasan A, Falkai P.. Aerobic exercise in mental disorders: from basic mechanisms to treatment recommendations. Eur Arch Psychiatry Clin Neurosci. 2019;269(5):483–484. [DOI] [PubMed] [Google Scholar]
- 2. Ashdown-Franks G, Firth J, Carney R, et al. Exercise as medicine for mental and substance use disorders: a meta-review of the benefits for neuropsychiatric and cognitive outcomes. Sports Med. 2020;50(1):151–170. [DOI] [PubMed] [Google Scholar]
- 3. Dauwan M, Begemann MJ, Heringa SM, Sommer IE.. exercise improves clinical symptoms, quality of life, global functioning, and depression in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2016;42(3):588–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Firth J, Cotter J, Elliott R, French P, Yung AR.. A systematic review and meta-analysis of exercise interventions in schizophrenia patients. Psychol Med. 2015;45(7):1343–1361. [DOI] [PubMed] [Google Scholar]
- 5. Fernández-Abascal B, Suárez-Pinilla P, Cobo-Corrales C, Crespo-Facorro B, Suárez-Pinilla M.. In- and outpatient lifestyle interventions on diet and exercise and their effect on physical and psychological health: a systematic review and meta-analysis of randomised controlled trials in patients with schizophrenia spectrum disorders and first episode of psychosis. Neurosci Biobehav Rev. 2021;125:535–568. [DOI] [PubMed] [Google Scholar]
- 6. Vogel JS, van der Gaag M, Slofstra C, Knegtering H, Bruins J, Castelein S.. The effect of mind-body and aerobic exercise on negative symptoms in schizophrenia: a meta-analysis. Psychiatry Res. 2019;279:295–305. [DOI] [PubMed] [Google Scholar]
- 7. Sabe M, Kaiser S, Sentissi O.. Physical exercise for negative symptoms of schizophrenia: systematic review of randomized controlled trials and meta-analysis. Gen Hosp Psychiatry. 2020;62:13–20. [DOI] [PubMed] [Google Scholar]
- 8. Firth J, Stubbs B, Rosenbaum S, et al. Aerobic exercise improves cognitive functioning in people with schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2017;43(3):546–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xu Y, Cai Z, Fang C, Zheng J, Shan J, Yang Y.. Impact of aerobic exercise on cognitive function in patients with schizophrenia during daily care: a meta-analysis. Psychiatry Res. 2022;312:114560. [DOI] [PubMed] [Google Scholar]
- 10. Shimada T, Ito S, Makabe A, et al. Aerobic exercise and cognitive functioning in schizophrenia: an updated systematic review and meta-analysis. Psychiatry Res. 2022;314:114656. [DOI] [PubMed] [Google Scholar]
- 11. Maurus I, Hasan A, Röh A, et al. Neurobiological effects of aerobic exercise, with a focus on patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2019;269(5):499–515. [DOI] [PubMed] [Google Scholar]
- 12. Firth J, Cotter J, Carney R, Yung AR.. The pro-cognitive mechanisms of physical exercise in people with schizophrenia. Br J Pharmacol. 2017;174(19):3161–3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pajonk FG, Wobrock T, Gruber O, et al. Hippocampal plasticity in response to exercise in schizophrenia. Arch Gen Psychiatry. 2010;67(2):133–143. [DOI] [PubMed] [Google Scholar]
- 14. Lin J, Chan SK, Lee EH, et al. Aerobic exercise and yoga improve neurocognitive function in women with early psychosis. NPJ Schizophr. 2015;1(0):15047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Falkai P, Maurus I, Schmitt A, et al. Improvement in daily functioning after aerobic exercise training in schizophrenia is sustained after exercise cessation. Eur Arch Psychiatry Clin Neurosci. 2021;271(7):1201–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Khonsari NM, Badrfam R, Mohammdi MR, et al. Effect of aerobic exercise as adjunct therapy on the improvement of negative symptoms and cognitive impairment in patients with schizophrenia: a randomized, case-control clinical trial. J Psychosoc Nurs Ment Health Serv. 2022;60(5):38–43. [DOI] [PubMed] [Google Scholar]
- 17. Woodward ML, Gicas KM, Warburton DE, et al. Hippocampal volume and vasculature before and after exercise in treatment-resistant schizophrenia. Schizophr Res. 2018;202:158–165. [DOI] [PubMed] [Google Scholar]
- 18. Maurus I, Roell L, Keeser D, et al. Fitness is positively associated with hippocampal formation subfield volumes in schizophrenia: a multiparametric magnetic resonance imaging study. Transl Psychiatry. 2022;12(1):388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maurus I, Röll L, Keeser D, et al. Associations between aerobic fitness, negative symptoms, cognitive deficits and brain structure in schizophrenia-a cross-sectional study. Schizophr. 2022;8(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Damme KSF, Gupta T, Ristanovic I, Kimhy D, Bryan AD, Mittal VA.. Exercise intervention in individuals at clinical high risk for psychosis: benefits to fitness, symptoms, hippocampal volumes, and functional connectivity. Schizophr Bull. 2022;48(6):1394–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dean DJ, Bryan AD, Newberry R, Gupta T, Carol E, Mittal VA.. A supervised exercise intervention for youth at risk for psychosis: an open-label pilot study. J Clin Psychiatry. 2017;78(9):e1167–e1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Erp TG, Hibar DP, Rasmussen JM, et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. 2016;21(4):547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Adriano F, Caltagirone C, Spalletta G.. Hippocampal volume reduction in first-episode and chronic schizophrenia: a review and meta-analysis. Neuroscientist. 2012;18(2):180–200. [DOI] [PubMed] [Google Scholar]
- 24. Honea R, Crow TJ, Passingham D, Mackay CE.. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry. 2005;162(12):2233–2245. [DOI] [PubMed] [Google Scholar]
- 25. Brugger SP, Howes OD.. Heterogeneity and homogeneity of regional brain structure in schizophrenia: a meta-analysis. JAMA Psychiatry. 2017;74(11):1104–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goodkind M, Eickhoff SB, Oathes DJ, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72(4):305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sha Z, Wager TD, Mechelli A, He Y.. Common dysfunction of large-scale neurocognitive networks across psychiatric disorders. Biol Psychiatry. 2019;85(5):379–388. [DOI] [PubMed] [Google Scholar]
- 28. Cui Y, Li C, Liu B, et al. Consistent brain structural abnormalities and multisite individualised classification of schizophrenia using deep neural networks. Br J Psychiatry. 2022;221(6):732–739. [DOI] [PubMed] [Google Scholar]
- 29. Kuo SS, Pogue-Geile MF.. Variation in fourteen brain structure volumes in schizophrenia: a comprehensive meta-analysis of 246 studies. Neurosci Biobehav Rev. 2019;98:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Erp TGM, Walton E, Hibar DP, et al. ; Karolinska Schizophrenia Project. Cortical brain abnormalities in 4474 individuals with schizophrenia and 5098 control subjects via the enhancing neuro imaging genetics through meta Analysis (ENIGMA) consortium. Biol Psychiatry. 2018;84(9):644–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhao Y, Zhang Q, Shah C, et al. Cortical thickness abnormalities at different stages of the illness course in schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry. 2022;79(6):560–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jalbrzikowski M, Hayes RA, Wood SJ, et al. ; ENIGMA Clinical High Risk for Psychosis Working Group. Association of structural magnetic resonance imaging measures with psychosis onset in individuals at clinical high risk for developing psychosis: an enigma working group mega-analysis. JAMA Psychiatry. 2021;78(7):753–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Matsuda Y, Ohi K.. Cortical gyrification in schizophrenia: current perspectives. Neuropsychiatr Dis Treat. 2018;14:1861–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sasabayashi D, Takahashi T, Takayanagi Y, Suzuki M.. Anomalous brain gyrification patterns in major psychiatric disorders: a systematic review and transdiagnostic integration. Transl Psychiatry. 2021;11(1):176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dong D, Wang Y, Chang X, Luo C, Yao D.. Dysfunction of large-scale brain networks in schizophrenia: a meta-analysis of resting-state functional connectivity. Schizophr Bull. 2018;44(1):168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brandl F, Avram M, Weise B, et al. Specific substantial dysconnectivity in schizophrenia: a transdiagnostic multimodal meta-analysis of resting-state functional and structural magnetic resonance imaging studies. Biol Psychiatry. 2019;85(7):573–583. [DOI] [PubMed] [Google Scholar]
- 37. Cao H, Chén OY, Chung Y, et al. Cerebello-thalamo-cortical hyperconnectivity as a state-independent functional neural signature for psychosis prediction and characterization. Nat Commun. 2018;9(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cao H, Ingvar M, Hultman CM, Cannon T.. Evidence for cerebello-thalamo-cortical hyperconnectivity as a heritable trait for schizophrenia. Transl Psychiatry. 2019;9(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ji JL, Diehl C, Schleifer C, et al. Schizophrenia exhibits bi-directional brain-wide alterations in cortico-striato-cerebellar circuits. Cereb Cortex. 2019;29(11):4463–4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Avram M, Brandl F, Bauml J, Sorg C.. Cortico-thalamic hypo- and hyperconnectivity extend consistently to basal ganglia in schizophrenia. Neuropsychopharmacology. 2018;43(11):2239–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Anticevic A, Cole MW, Repovs G, et al. Characterizing thalamo-cortical disturbances in schizophrenia and bipolar illness. Cereb Cortex. 2014;24(12):3116–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Anticevic A, Haut K, Murray JD, et al. Association of thalamic dysconnectivity and conversion to psychosis in youth and young adults at elevated clinical risk. JAMA Psychiatry. 2015;72(9):882–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Anticevic A, Yang G, Savic A, et al. Mediodorsal and visual thalamic connectivity differ in schizophrenia and bipolar disorder with and without psychosis history. Schizophr Bull. 2014;40(6):1227–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tu PC, Lee YC, Chen YS, Hsu J-W, Li C-T, Su T-P.. Network-specific cortico-thalamic dysconnection in schizophrenia revealed by intrinsic functional connectivity analyses. Schizophr Res. 2015;166(1-3):137–143. [DOI] [PubMed] [Google Scholar]
- 45. Welsh RC, Chen AC, Taylor SF.. Low-frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in schizophrenia. Schizophr Bull. 2010;36(4):713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Woodward ND, Heckers S.. Mapping thalamocortical functional connectivity in chronic and early stages of psychotic disorders. Biol Psychiatry. 2016;79(12):1016–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Woodward ND, Karbasforoushan H, Heckers S.. Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry. 2012;169(10):1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lui S, Yao L, Xiao Y, et al. Resting-state brain function in schizophrenia and psychotic bipolar probands and their first-degree relatives. Psychol Med. 2015;45(1):97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Benes FM. Amygdalocortical circuitry in schizophrenia: from circuits to molecules. Neuropsychopharmacology. 2010;35(1):239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Falkai P, Raabe F, Bogerts B, et al. Association between altered hippocampal oligodendrocyte number and neuronal circuit structures in schizophrenia: a postmortem analysis. Eur Arch Psychiatry Clin Neurosci. 2020;270(4):413–424. [DOI] [PubMed] [Google Scholar]
- 51. Farhani F, Shahrbanian S, Auais M, Hekmatikar AHA, Suzuki K.. Effects of aerobic training on brain plasticity in patients with mild cognitive impairment: a systematic review of randomized controlled trials. Brain Sci. 2022;12(6):732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bray NW, Pieruccini-Faria F, Bartha R, Doherty TJ, Nagamatsu LS, Montero-Odasso M.. The effect of physical exercise on functional brain network connectivity in older adults with and without cognitive impairment. A systematic review. Mech Ageing Dev. 2021;196:111493. [DOI] [PubMed] [Google Scholar]
- 53. Vorkapic C, Leal S, Alves H, Douglas M, Britto A, Dantas EHM.. Born to move: a review on the impact of physical exercise on brain health and the evidence from human controlled trials. Arq Neuropsiquiatr. 2021;79(6):536–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ji L, Steffens DC, Wang L.. Effects of physical exercise on the aging brain across imaging modalities: a meta-analysis of neuroimaging studies in randomized controlled trials. Int J Geriatr Psychiatry. 2021;36(8):1148–1157. [DOI] [PubMed] [Google Scholar]
- 55. Domingos C, Pêgo JM, Santos NC.. Effects of physical activity on brain function and structure in older adults: a systematic review. Behav Brain Res. 2021;402:113061. [DOI] [PubMed] [Google Scholar]
- 56. Li MY, Huang MM, Li SZ, Tao J, Zheng G-H, Chen L-D.. The effects of aerobic exercise on the structure and function of DMN-related brain regions: a systematic review. Int J Neurosci. 2017;127(7):634–649. [DOI] [PubMed] [Google Scholar]
- 57. van der Stouwe ECD, van Busschbach JT, de Vries B, Cahn W, Aleman A, Pijnenborg GHM.. Neural correlates of exercise training in individuals with schizophrenia and in healthy individuals: a systematic review. NeuroImage Clin. 2018;19:287–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vancampfort D, Probst M, De Hert M, et al. Neurobiological effects of physical exercise in schizophrenia: a systematic review. Disabil Rehabil. 2014;36(21):1749–1754. [DOI] [PubMed] [Google Scholar]
- 59. Malchow B, Keeser D, Keller K, et al. Effects of endurance training on brain structures in chronic schizophrenia patients and healthy controls. Schizophr Res. 2016;173(3):182–191. [DOI] [PubMed] [Google Scholar]
- 60. Svatkova A, Mandl RC, Scheewe TW, Cahn W, Kahn RS, Hulshoff Pol HE.. Physical exercise keeps the brain connected: biking increases white matter integrity in patients with schizophrenia and healthy controls. Schizophr Bull. 2015;41(4):869–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. McEwen SC, Jarrahi B, Ventura J, et al. A combined exercise and cognitive training intervention induces fronto-cingulate cortical plasticity in first-episode psychosis patients. Schizophr Res. 2023;251:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Takahashi S, Keeser D, Rauchmann BS, et al. Effect of aerobic exercise combined with cognitive remediation on cortical thickness and prediction of social adaptation in patients with schizophrenia. Schizophr Res. 2020;216:397–407. [DOI] [PubMed] [Google Scholar]
- 63. Scheewe TW, van Haren NE, Sarkisyan G, et al. Exercise therapy, cardiorespiratory fitness and their effect on brain volumes: a randomised controlled trial in patients with schizophrenia and healthy controls. Eur Neuropsychopharmacol. 2013;23(7):675–685. [DOI] [PubMed] [Google Scholar]
- 64. McEwen SC, Hardy A, Ellingson BM, et al. Prefrontal and hippocampal brain volume deficits: role of low physical activity on brain plasticity in first-episode schizophrenia patients. J Int Neuropsychol Soc. 2015;21(10):868–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Roell L, Maurus I, Keeser D, et al. Association between aerobic fitness and the functional connectome in patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2022;272(7):1253–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Liu-Ambrose T, Nagamatsu LS, Graf P, Beattie BL, Ashe MC, Handy TC.. Resistance training and executive functions: a 12-month randomized controlled trial. Arch Intern Med. 2010;170(2):170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Maurus I, Hasan A, Schmitt A, et al. Aerobic endurance training to improve cognition and enhance recovery in schizophrenia: design and methodology of a multicenter randomized controlled trial. Eur Arch Psychiatry Clin Neurosci. 2020;271:315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Esteban O, Markiewicz CJ, Blair RW, et al. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods. 2019;16(1):111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kay SR, Fiszbein A, Opler LA.. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 70. Busner J, Targum SD.. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont). 2007;4(7):28–37. [PMC free article] [PubMed] [Google Scholar]
- 71. Endicott J, Spitzer RL, Fleiss JL, Cohen J.. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976;33(6):766–771. [DOI] [PubMed] [Google Scholar]
- 72. Llorca PM, Lançon C, Lancrenon S, et al. The “Functional Remission of General Schizophrenia” (FROGS) scale: development and validation of a new questionnaire. Schizophr Res. 2009;113(2-3):218–225. [DOI] [PubMed] [Google Scholar]
- 73. Rstudio Team. RStudio: Integrated development environment for R [computer program]. Version 1.4.1717. Boston, MA: RStudio, PBC; 2020. [Google Scholar]
- 74. R Core Team. R: A language and environment for statistical computing. [computer program]. Version 4.2.2. Vienna, Austria: R Foundation for Statistical Computing; 2021. [Google Scholar]
- 75. Wickham H. ggplot2: Elegant Graphics for Data Analysis [computer program]. Version 3.4.1. New York: Springer; 2016. [Google Scholar]
- 76. Bürkner PC. brms: an R package for bayesian multilevel models using stan. J Stat Softw. 2017;80(1):1–28. [Google Scholar]
- 77. Laird AR, Fox PM, Eickhoff SB, et al. Behavioral interpretations of intrinsic connectivity networks. J Cogn Neurosci. 2011;23(12):4022–4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yeo BT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(3):1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mak LE, Minuzzi L, MacQueen G, Hall G, Kennedy SH, Milev R.. The default mode network in healthy individuals: a systematic review and meta-analysis. Brain Connect. 2017;7(1):25–33. [DOI] [PubMed] [Google Scholar]
- 80. O’Neill A, Mechelli A, Bhattacharyya S.. Dysconnectivity of large-scale functional networks in early psychosis: a meta-analysis. Schizophr Bull. 2019;45(3):579–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Avram M, Brandl F, Knolle F, et al. Aberrant striatal dopamine links topographically with cortico-thalamic dysconnectivity in schizophrenia. Brain. 2020;143(11):3495–3505. [DOI] [PubMed] [Google Scholar]
- 82. Stahl SM. Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: dopamine, serotonin, and glutamate. CNS Spectr. 2018;23(3):187–191. [DOI] [PubMed] [Google Scholar]
- 83. Friston K, Brown HR, Siemerkus J, Stephan KE.. The dysconnection hypothesis (2016). Schizophr Res. 2016;176(2–3):83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Höflich A, Hahn A, Küblböck M, et al. Ketamine-induced modulation of the thalamo-cortical network in healthy volunteers as a model for schizophrenia. Int J Neuropsychopharmacol. 2015;18(9):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Abram SV, Roach BJ, Fryer SL, et al. Validation of ketamine as a pharmacological model of thalamic dysconnectivity across the illness course of schizophrenia. Mol Psychiatry. 2022;27(5):2448–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Andreasen NC, Paradiso S, O’Leary DS.. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24(2):203–218. [DOI] [PubMed] [Google Scholar]
- 87. Kandola A, Hendrikse J, Lucassen PJ, Yücel M.. Aerobic exercise as a tool to improve hippocampal plasticity and function in humans: practical implications for mental health treatment. Front Hum Neurosci. 2016;10:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Liu PZ, Nusslock R.. Exercise-mediated neurogenesis in the hippocampus via BDNF. Front Neurosci. 2018;12:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Vancampfort D, Rosenbaum S, Ward PB, Stubbs B.. Exercise improves cardiorespiratory fitness in people with schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2015;169(1-3):453–457. [DOI] [PubMed] [Google Scholar]
- 90. Manu P, Dima L, Shulman M, Vancampfort D, De Hert M, Correll CU.. Weight gain and obesity in schizophrenia: epidemiology, pathobiology, and management. Acta Psychiatr Scand. 2015;132(2):97–108. [DOI] [PubMed] [Google Scholar]
- 91. Rege S. Antipsychotic induced weight gain in schizophrenia: mechanisms and management. Aust N Z J Psychiatry. 2008;42(5):369–381. [DOI] [PubMed] [Google Scholar]
- 92. Tek C, Kucukgoncu S, Guloksuz S, Woods SW, Srihari VH, Annamalai A.. Antipsychotic-induced weight gain in first-episode psychosis patients: a meta-analysis of differential effects of antipsychotic medications. Early Intervent Psychiatry. 2016;10(3):193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Avila C, Holloway AC, Hahn MK, et al. An overview of links between obesity and mental health. Curr Obes Rep. 2015;4(3):303–310. [DOI] [PubMed] [Google Scholar]
- 94. Li L, Yu H, Zhong M, et al. Gray matter volume alterations in subjects with overweight and obesity: evidence from a voxel-based meta-analysis. Front Psychiatry. 2022;13:955741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Herrmann MJ, Tesar AK, Beier J, Berg M, Warrings B.. Grey matter alterations in obesity: a meta-analysis of whole-brain studies. Obes Rev. 2019;20(3):464–471. [DOI] [PubMed] [Google Scholar]
- 96. Syan SK, McIntyre-Wood C, Minuzzi L, Hall G, McCabe RE, MacKillop J.. Dysregulated resting state functional connectivity and obesity: a systematic review. Neurosci Biobehav Rev. 2021;131:270–292. [DOI] [PubMed] [Google Scholar]
- 97. Parsons N, Steward T, Clohesy R, Almgren H, Duehlmeyer L.. A systematic review of resting-state functional connectivity in obesity: refining current neurobiological frameworks and methodological considerations moving forward. Rev Endocr Metab Disord. 2022;23(4):861–879. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All analysis scripts and documentation sheets will be published on OSF. Additional data can be made available upon request to the corresponding author.


