Abstract
Background and Hypothesis
The hippocampus is a heterogenous brain structure that differs between the sexes and has been implicated in the pathophysiology of psychiatric illnesses. Here, we explored sex and diagnostic group differences in hippocampal subfield volumes, in individuals with schizophrenia spectrum disorder (SZ), bipolar disorders (BD), and healthy controls (CTL).
Study Design
One thousand and five hundred and twenty-one participants underwent T1-weighted magnetic resonance imaging (SZ, n = 452, mean age 30.7 ± 9.2 [SD] years, males 59.1%; BD, n = 316, 33.7 ± 11.4, 41.5%; CTL, n = 753, 34.1 ± 9.1, 55.6%). Total hippocampal, subfield, and intracranial volumes were estimated with Freesurfer (v6.0.0). Analysis of covariance and multiple regression models were fitted to examine sex-by-diagnostic (sub)group interactions in volume. In SZ and BD, separately, associations between volumes and clinical as well as cognitive measures were examined between the sexes using regression models.
Study Results
Significant sex-by-group interactions were found for the total hippocampus, dentate gyrus, molecular layer, presubiculum, fimbria, hippocampal-amygdaloid transition area, and CA4, indicating a larger volumetric deficit in male patients relative to female patients when compared with same-sex CTL. Subgroup analyses revealed that this interaction was driven by males with schizophrenia. Effect sizes were overall small (partial η < 0.02). We found no significant sex differences in the associations between hippocampal volumes and clinical or cognitive measures in SZ and BD.
Conclusions
Using a well-powered sample, our findings indicate that the pattern of morphological sex differences in hippocampal subfields is altered in individuals with schizophrenia relative to CTL, due to higher volumetric deficits in males.
Keywords: hippocampus, neuroimaging, schizophrenia, bipolar disorders, sex differences
Introduction
Schizophrenia is a severe, multifactorial brain disorder with prominent sex differences in prevalence and presentation.1 Relative to females, males have a 40% higher likelihood of developing schizophrenia and tend to suffer from more severe forms of the illness, including worse long-term outcomes and more pronounced and persistent negative symptoms.1 Sex differences in the prevalence and presentation of bipolar disorders (BD) have also been reported, but a clear consensus is lacking.2 Despite reported sex differences in disease manifestation, it remains unknown whether and how these sex differences are reflected in the brain. Acquiring such knowledge would constitute a critical step toward mechanistic models explaining sex differences in disease susceptibility.
One brain structure that seems to differ between the sexes and has been implicated in the pathophysiology of both schizophrenia and BD is the hippocampus.3 Located in the medial temporal lobe, the hippocampus is a geminate limbic brain structure with a critical role in learning,4 memory formation,5 and mood regulation.6 These hippocampal-dependent functions differ between the sexes7 and are affected in schizophrenia and BD. For instance, using functional magnetic resonance imaging (MRI), lower hippocampal activation during encoding and recognition tasks has been associated with alterations in declarative memory performance in schizophrenia.8,9 Similarly, structural MRI studies have shown smaller hippocampal volumes in both schizophrenia and BD,10,11 with greater volumetric deficits in schizophrenia.12 Hippocampal volume alterations have also been associated with cognitive impairment,13,14 and thus appear functionally relevant.15 However, sex differences in these associations have scarcely been studied, as sex is frequently used as a covariate and not as a discovery variable.16,17
The hippocampus is, however, not a uniform structure; rather it consists of heterogeneous subfields which serve distinct functions. Using a combination of ultra-high-resolution ex vivo MRI data and conventional in vivo MRI scans, Iglesias and colleagues created a probabilistic labeling algorithm. This method segments 12 hippocampal subfields, namely the parasubiculum, presubiculum, subiculum, cornu ammonis (CA)1, CA3, CA4, granule cells in the molecular layer of the dentate gyrus (GC-ML-DG), hippocampal-amygdaloid transition area (HATA), fimbria, molecular layer, hippocampal fissure, and hippocampal tail.18 Functional separations of hippocampal subfields have been proposed, particularly in the context of declarative memory. For instance, DG and CA3 are thought to play a crucial role in pattern separation, the flexible distinction between multiple, highly similar memories.19 Similarly, mechanisms in CA3, CA1, and subiculum may be critical for pattern completion, the retrieval of full memories from incomplete input. Deficits in these subfields have been proposed to result in spurious or false memory associations, creating a susceptibility to psychosis.19
Meta-analyses in schizophrenia and BD suggest lower volumes in all hippocampal subfields, predominantly in schizophrenia, with the largest effects in the CA1, CA3, CA4, GC-ML-DG, and subiculum.20 A recent large-scale collaborative study in BD found similarly smaller volumes in nine of 12 hippocampal subfields, excluding the fimbria, fissure, and parasubiculum, in individuals with BD relative to healthy controls (CTL).21 Directly comparing both disorders, the left CA2 and right subiculum were smaller in schizophrenia than in BD, suggesting differential patterns of volumetric deficits depending on the diagnosis.20
Although diagnostic group differences in hippocampal subfield volume have been extensively demonstrated, sex differences in hippocampal volumes are still debated. A recent study in over 1,500 healthy adults revealed region-specific sex differences in hippocampal subfield volumes, with larger volumes in males relative to females, most prominently in the fimbria and parasubiculum.22 Similar studies in schizophrenia and BD are currently lacking. While emerging evidence suggests that lower hippocampal volumes are only present in males and absent in females with schizophrenia,23,24 subfield-specific sex differences in schizophrenia are yet to be reported. Investigating sex differences in hippocampal subfield alterations in schizophrenia and BD may advance our understanding of the pathophysiology of these disorders and their relation to biological sex.
Here, we studied sex differences in hippocampal subfield volumes in a large sample of individuals with schizophrenia spectrum disorders (SZ) and BD and CTL. We examined the associations between medication use, symptom profiles as well as cognitive measures on hippocampal volumes by sex and diagnostic groups. Due to a lack of consistent evidence on sex differences in hippocampal volumes in schizophrenia and BD, the analyses were exploratory by nature.
Methods
Participants
The sample of 768 individuals with SZ and BD, and 753 CTL is part of the ongoing Thematically Organized-Psychosis study, Oslo, Norway. Individuals with SZ and BD were recruited from in- and outpatient psychiatric units covering specific catchment areas in the greater Oslo area. CTL were recruited from the national population register in the same catchment area. All participants provided written informed consent. The study was approved by the Regional Committee for Research Ethics and the Norwegian Data Inspectorate and carried out in accordance with the Declaration of Helsinki. For details on inclusion criteria, see supplementary note 1.
Clinical Assessment
Clinical characterization was conducted by trained health personnel. Clinical diagnoses were established according to the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorder IV axis 1 disorder, module A-E.25 The Positive and Negative Syndrome Scale (PANSS)26 was used to assess the presence and severity of psychotic symptoms, and the Inventory for Depressive Symptomatology (IDS)27 and the Young Mania Rating Scale (YMRS)28 to evaluate affective symptoms. General functioning in all participants was measured with the Global Assessment of Function (GAF) scale, split version29 (details see supplementary note 2).
Clinical diagnoses were: (1) SZ, n = 452: Schizophrenia [SCZ, n = 245], schizophreniform [n = 31], schizoaffective [SCZ-AF, n = 59], other psychotic disorders [n = 117], (2) BD, n = 316: Bipolar I [n = 188], bipolar II [n = 113], bipolar not otherwise specified [n = 15].
Cognitive Assessment
Clinical psychologists and trained personnel administered cognitive assessments of the clinical groups and CTL, respectively. Between 2004 and 2019, the cognitive assessment was performed using 2 different cognitive test batteries. Seven hundred and twenty-nine participants were evaluated with battery 1, a standardized battery described in detail elsewhere.30 Starting from 2012, participants (n = 722) were assessed using a licensed translated version of the Measurement and Treatment Research to Improve Cognition in Schizophrenia Consensus Cognitive Battery (MCCB).31 In the current study, we focused on four cognitive domains, that have been linked to hippocampal functioning,13,14 may vary between the sexes30,32,33 and may be impaired in schizophrenia and BD34: (1) processing speed, (2) working memory, (3) verbal learning, and (4) verbal memory (for details see supplementary note 3). The tests included in battery 1 and 2 are detailed in table 1. A total of 67 participants did not have cognitive data. Current intelligence quotient was assessed using the Wechsler Abbreviated Scale of Intelligence (4 subtests).
Table 1.
Overview of Cognitive Domains and Corresponding Tests, Stratified by Diagnostic Group and Sex
| BD | SZ | ||||||
|---|---|---|---|---|---|---|---|
| Female | Male | P | Female | Male | P | ||
| N | Battery | 173 | 126 | 172 | 250 | ||
| Cognitive measures, z-scores | |||||||
| Verbal learning | 0.16 ± 0.95 | −0.45 ± 1.31 | <0.001 | −0.62 ± 1.25 | −0.97 ± 1.22 | 0.005 | |
| CVLT, list A total correct | 1 | 0.20 ± 0.94 | −0.48 ± 1.38 | <0.001 | −0.49 ± 1.13 | −0.92 ± 1.20 | 0.004 |
| HVLT-R, immediate recall | 2 | 0.08 ± 0.97 | −0.38 ± 1.18 | 0.037 | −0.83 ± 1.41 | −1.05 ± 1.27 | 0.306 |
| Verbal memory | 0.11 ± 0.94 | −0.57 ± 1.37 | <0.001 | −0.77 ± 1.34 | −0.93 ± 1.32 | 0.244 | |
| CVLT, long delay free recall | 1 | 0.12 ± 0.96 | −0.65 ± 1.45 | <0.001 | −0.55 ± 1.20 | −0.87 ± 1.27 | 0.042 |
| HVLT-R, delayed recall | 2 | 0.07 ± 0.90 | −0.42 ± 1.16 | 0.022 | −1.21 ± 1.51 | −1.05 ± 1.41 | 0.540 |
| Processing speed | −0.44 ± 1.15 | −0.90 ± 1.11 | 0.001 | −0.97 ± 1.16 | −1.39 ± 1.21 | <0.001 | |
| Digit Symbol Coding Test from WAIS-III | 1 | −0.55 ± 1.20 | −0.89 ± 1.12 | 0.044 | −0.99 ± 1.10 | −1.29 ± 1.19 | 0.038 |
| Brief Assessment of Cognition in Schizophrenia | 2 | −0.23 ± 1.02 | −0.93 ± 1.12 | 0.002 | −0.93 ± 1.27 | −1.56 ± 1.23 | 0.002 |
| Working memory | −0.51 ± 0.95 | −0.54 ± 0.94 | 0.787 | −0.82 ± 0.99 | −0.77 ± 1.01 | 0.625 | |
| Letter-Number Sequencing Test from WAIS-III | 1 | −0.64 ± 0.98 | −0.65 ± 0.93 | 0.951 | −0.89 ± 0.99 | −0.80 ± 1.03 | 0.517 |
| Letter-Number Sequencing Test from the MCCB | 2 | −0.28 ± 0.86 | −0.35 ± 0.95 | 0.687 | −0.69 ± 0.99 | −0.71 ± 0.97 | 0.946 |
Mean ± standard deviation. Abbreviation: BD, bipolar disorders, SZ, schizophrenia spectrum disorders, N, sample size; CVLT, California verbal learning test; HVLT-R, Hopkins verbal learning test revised; WAIS, Wechsler adult intelligence scale; MCCB, MATRICS consensus cognitive battery. Kruskal–Wallis tests were used to compare cognitive scores between females and male by diagnostic group. Significant group differences are highlighted in bold.
To merge SZ and BD test scores from both batteries for each cognitive domain, we computed z-scores for both sexes combined based on the performance of the CTL group. Z-scores were calculated using the following formula:
where X is the raw cognitive score, μ is the mean and σ is standard deviation of the CTL group. Combined z-scores for each cognitive domain and separate z-scores per tests, stratified by sex and diagnostic group, are highlighted in table 1. Raw scores for each cognitive test are summarized in supplementary table S1, stratified by sex and diagnostic group, including values from CTL.
Medication Use
In the clinical groups, current use of the following medication was recorded and converted into defined daily dose (DDD)35: Antipsychotics, antidepressants, and antiepileptics. In individuals with BD, lithium user status (yes/no) and serum concentration were also assessed (details published elsewhere36).
Neuroimaging Data Acquisition
T1-weighted images were acquired either at 1.5T (2004–2009, n = 745) or at 3T (2011–2019, n = 776). At 1.5T, images were acquired on a Siemens Magnetom Sonata scanner. At 3T, participants were scanned either on General Electric Signa HDxt scanner (n = 438) or a General Electric Discovery 750 scanner (n = 338). For details about the MRI acquisition parameters, see supplementary note 4.
Neuroimaging Data Processing
The FreeSurfer software package (v6.0.0) was used to process T1-weighted images to extract volume estimates for left and right hippocampal total and subfield volumes as well as intracranial volume (ICV); estimated based on the Talairach transform. The automated segmentation of the hippocampus is based on a probabilistic atlas, and segmentation quality was visually inspected. If necessary, manual editing of the surface reconstruction was performed by trained assistants following standard FreeSurfer procedures.37 No individuals were excluded based on the inspection of the hippocampal segmentation. We used ComBat to remove unwanted variation associated with scanner while preserving biological associations in the data38,39 (see supplementary note 5, figure S1–S2).
Statistical Analysis
All statistical tests were conducted in R, v4.2.2. Model formulas in R are specified in the text (1-9). Main effects of the interaction terms are automatically included in the model. Continuous variables in interaction terms were standardized (subtracting the mean and dividing by the SD) before the regression analysis. All analyses included combined hippocampal volumes (left + right) as dependent variables (DV). Results from the main analyses for the left and right hippocampal subfields are reported in supplementary table S2. To account for multiple comparisons, false discovery rate (FDR) correction40 was applied across all hippocampal volumes (total + subfield volumes) for each set of analyses testing a hypothesis. The sets of FDR corrections are reflected in the corresponding results tables.
Sex and Diagnostic Group Differences in Hippocampal Volume
To examine sex-by-diagnostic group differences in hippocampal volumes, we used analysis of covariance (ANCOVA, type III, car package). Total hippocampal volume and each subfield volume were entered as DV in separate models, which included diagnostic group-by-sex interaction as a fixed factor and age, age2, and ICV as covariates:
| (1) |
Post-hoc Tukey tests were performed to contrast volume differences between females vs. males, females vs. females, and males vs. males for each diagnostic group. Sex-adjusted case–control differences in hippocampal volumes were also assessed in additional models, correcting for sex, age, age2, and ICV:
| (2) |
To explore the absolute differences in hippocampal volumes by sex and diagnostic group, we re-ran the main analyses without adjustment for ICV:
| (3) |
Effect sizes were calculated as partial eta-squared (partial η2) based on the F-statistic.
To further evaluate hippocampal volume differences between diagnostic groups by age, we fitted regression models (lm function) with total hippocampal and subfield volumes as DV and group-by-age interaction, group-by-age2 interaction, sex, and ICV as independent variables:
| (4) |
Additional regression models were fitted to further examine sex-by-diagnostic subgroup differences (ie, bipolar I, bipolar II, SCZ-AF, SCZ, and other psychotic disorders, relative to CTL) in total hippocampal and subfield volumes, adjusting for age, age2, and ICV:
| (5) |
Effect sizes for regression results were calculated as Cohen’s d based on the t-statistic (see supplementary note 6 for additional considerations).
Sex-Specific Associations Between Medication, Clinical Measures, and Hippocampal Volumes
To test if putative associations between medication use (antipsychotics, antidepressants, and antiepileptics) and hippocampal volumes (DV) differed between the sexes, additional regression models were fitted using an interaction term (sex-by-DDD) in SZ and BD separately:
| (6) |
In BD, we further explored the effects of lithium use status (yes/no) and serum concentration levels, on hippocampal volumes, in separate regression models.
Sex-Specific Associations Between Clinical Measures and Hippocampal Volumes
The aforementioned model specifications were used to examine the associations between hippocampal volumes and psychotic symptoms (PANSS, , negative/positive- subscale) in SZ, affective symptoms (YMRS and IDS) in BD, age of onset, duration of illness, and general functioning (GAF symptoms/function) between the sexes:
| (7) |
As both age of onset (BD r = 0.58, SZ r = 0.66) and duration of illness (BD r = 0.66, SZ r = 0.62) had high Pearson correlations with age, these linear models were only adjusted for ICV, whereas the other models were adjusted for age, age2, and ICV.
Sex-Specific Associations Between Cognitive Measures and Hippocampal Volumes
To investigate associations between cognitive measures (verbal learning, verbal memory, processing speed, and working memory) and hippocampal volumes between females and males, regression models with a sex-by-cognitive measure interaction term were fitted. These models were adjusted for age, age2, ICV, and years of education:
| (8) |
The sex-adjusted main effects of medication, clinical measures as well as cognitive measures on hippocampal volumes were tested in separate models, covarying for the beforementioned covariates and sex:
| (9) |
Results
Demographic and Clinical Variables
Sample demographics and clinical characteristics, stratified by sex and diagnostic group, are summarized in table 2, and supplementary note 7. Cognitive measures, stratified by diagnostic group and sex, are displayed in table 1 and supplementary table S1, and described in supplementary note 8.
Table 2.
Demographics and Clinical Measures of the Study Sample
| CTL | BD | SZ | Dx Differences | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Female N = 334 |
Male N = 419 |
P | Female N = 185 |
Male N = 131 |
P | Female N = 185 |
Male N = 267 |
P | Female P |
Male P |
|
| Age at Scan (years) | 33.4 [26.3, 40.5] | 33.1 [27.7, 40.2] | .864 | 30.3 [24.4, 41.0] | 32.1 [25.5, 40.7] | .317 | 29.0 [23.9, 38.4] | 28.2 [23.2, 34.9] | .243 | .004 | <.001 |
| Education (years) | 15.0 [12.0, 16.0] | 15.0 [12.0, 17.0] | .663 | 15.0 [12.3, 16.5] | 14.5 [13.0, 16.0] | .823 | 13.0 [12.0, 16.0] | 13.0 [12.0, 15.0] | .323 | <.001 | <.001 |
| BMI (kg/m2) | 23.3 [21.4, 26.3] | 24.7 [23.2, 27.3] | <.001 | 24.0 [21.8, 27.2] | 26.0 [22.9, 28.1] | .004 | 23.9 [21.6, 27.2] | 25.8 [23.1, 29.3] | <.001 | .271 | .029 |
| IQ | 112.0 [107.0, 119.0] | 115.0 [108.8, 120.0] | .006 | 108.0 [101.0, 116.0] | 109.0 [103.0, 117.0] | .347 | 104.0 [95.0, 112.0] | 107.0 [94.3, 114.0] | .283 | <.001 | <.001 |
| Age of Onset (years) | 18.0 [15.0, 23.0] | 19.0 [16.0, 26.8] | .018 | 22.0 [18.0, 27.5] | 22.0 [19.0, 27.0] | .489 | <.001 | .003 | |||
| DOI (years) | 11.0 [6.4, 17.9] | 9.8 [5.4, 16.8] | .203 | 5.7 [2.3, 11.5] | 4.4 [1.6, 9.1] | .011 | <.001 | <.001 | |||
| GAF, symptom | 60.0 [51.0, 67.0] | 60.0 [52.0, 65.0] | .932 | 45.0 [38.0, 55.0] | 41.0 [37.0, 51.0] | .025 | <.001 | <.001 | |||
| GAF, function | 56.5 [48.0, 65.0] | 54.0 [47.0, 67.0] | .654 | 45.0 [40.0, 55.0] | 44.0 [38.0, 52.0] | .044 | <.001 | <.001 | |||
| PANSS, total | 43.0 [37.0, 49.0] | 43.0 [38.3, 50.0] | .333 | 54.5 [44.0, 66.3] | 60.0 [51.0, 71.0] | <.001 | <.001 | <.001 | |||
| PANSS, negative | 9.0 [7.0, 11.0] | 10.0 [8.0, 12.0] | .008 | 12.0 [9.0, 17.0] | 15.0 [11.0, 20.0] | <.001 | <.001 | <.001 | |||
| PANSS, positive | 9.0 [7.0, 10.0] | 9.0 [7.0, 11.0] | .569 | 13.0 [9.0, 16.0] | 14.0 [11.0, 17.0] | .019 | <.001 | <.001 | |||
| YMRS | 2.0 [0.0, 4.8] | 2.0 [0.0, 4.0] | .964 | 1.0 [0.0, 5.0] | 2.5 [0.0, 8.0] | .021 | .611 | .054 | |||
| IDS | 16.0 [9.0, 26.0] | 13.0 [7.0, 20.3] | .014 | 15.0 [7.0, 25.0] | 15.0 [7.0, 25.0] | .699 | .180 | .154 | |||
| AP, user N (%) | 113 (61.7) | 96 (73.3) | .044 | 171 (92.4) | 259 (97.0) | .046 | <.001 | <.001 | |||
| DDD | 0.5 [0.3, 1.0] | 0.9 [0.5, 1.3] | .007 | 1.0 [0.7, 1.8] | 1.2 [0.8, 1.8] | .296 | <.001 | <.001 | |||
| AD, user N (%) | 77 (41.6) | 34 (26.0) | .006 | 65 (35.1) | 74 (27.7) | .115 | <.001 | <.001 | |||
| DDD | 1.0 [1.0, 2.0] | 1.5 [1.0, 2.0] | .265 | 1.0 [1.0, 1.6] | 1.5 [1.0, 2.0] | .113 | .695 | .828 | |||
| Lithium, user N (%) | 35 (18.9) | 20 (15.3) | .488 | 6 (3.2) | 5 (1.9) | .536 | <.001 | <.001 | |||
| DDD | 1.0 [1.0, 1.3] | 1.0 [0.8, 1.4] | .918 | 0.6 [0.5, 0.8] | 0.8 [0.5, 1.0] | .400 | .002 | .121 | |||
| AE, user N (%) | 71 (38.4) | 44 (33.6) | .451 | 29 (15.7) | 29 (10.9) | .173 | <.001 | <.001 | |||
| DDD | 0.7 [0.3, 1.0] | 0.8 [0.6, 1.1] | .150 | 0.7 [0.6, 0.8] | 0.7 [0.5, 0.9] | .660 | 0.924 | .242 | |||
| ICV (liter) | 1.5 [1.4, 1.5] | 1.6 [1.5, 1.7] | <.001 | 1.5 [1.4, 1.6] | 1.7 [1.6, 1.8] | <.001 | 1.5 [1.4, 1.5] | 1.6 [1.5, 1.8] | <.001 | 0.072 | .117 |
*Non-normal continuous data in median [Interquartile range] and categorical data as number %. Abbreviations: CTL, healthy controls; BD, bipolar disorders; SZ, schizophrenia disorders; N, number; R, right; L, left; A, ambidextrous; M, missing; y, year; BMI, body mass index; IQ, intelligence quotient; DOI, duration of illness; GAF, global assessment of functioning; PANSS, positive and negative syndrome scale; YMRS, young mania rating scale; IDS, inventory for depressive symptomatology; AP, antipsychotics; DDD, defined daily dose; AD, antidepressants; AE, antiepileptics; ICV, intracranial volume; Dx, Diagnosis. Chi2 test for categorial data and Kruskal-Wallis test for continuous data were used to test for group differences in demographic and clinical measures. Significant results are highlighted in bold.
Sex and Diagnostic Group Differences in Hippocampal Volumes
Including a sex-by-diagnostic group interaction term, total hippocampal, CA1, CA4, GC-ML-DG, HATA, and molecular layer volumes were significantly smaller in individuals with SZ and BD relative to CTL (model1, see Table 3, figure 1). All hippocampal volumes were smaller in SZ and BD relative to CTL, when not accounting for potential sex-by-diagnostic group interactions (model2, see supplementary table S3).
Table 3.
Sex, Diagnostic Group, and Sex-by-Diagnostic Group Effects on Total Hippocampal and Subfield Volumes Assessed Using Analysis of Covariance
| Subfield | Variable | F-value | partial η2 | P-value | P FDR-value |
|---|---|---|---|---|---|
| CA1 | Diagnostic group | 8.575 | 0.011 | 1.98e-04 | .001 |
| Sex | 16.114 | 0.011 | 6.26e-05 | .000 | |
| Group-by-sex interaction | 2.334 | 0.003 | .097 | .119 | |
| CA3 | Diagnostic group | 3.098 | 0.004 | .045 | .066 |
| Sex | 3.704 | 0.002 | .054 | .071 | |
| Group-by-sex interaction | 3.126 | 0.004 | .044 | .066 | |
| CA4 | Diagnostic group | 5.237 | 0.007 | .005 | .012 |
| Sex | 3.853 | 0.003 | .050 | .069 | |
| Group-by-sex interaction | 4.944 | 0.006 | .007 | .016 | |
| Fimbria | Diagnostic group | 0.103 | 1.36e-04 | .902 | .902 |
| Sex | 23.009 | 0.015 | 1.77e-06 | 3.45e-05 | |
| Group-by-sex interaction | 3.914 | 0.005 | .020 | .033 | |
| GC-ML-DG | Diagnostic group | 6.666 | 0.009 | .001 | .005 |
| Sex | 6.455 | 0.004 | .011 | .021 | |
| Group-by-sex interaction | 5.866 | 0.008 | .003 | .008 | |
| HATA | Diagnostic group | 8.937 | 0.012 | 1.39e-04 | .001 |
| Sex | 15.237 | 0.010 | 9.90e-05 | .001 | |
| Group-by-sex interaction | 4.012 | 0.005 | .018 | .031 | |
| Hippocampal tail | Diagnostic group | 1.584 | 0.002 | .205 | .229 |
| Sex | 9.354 | 0.006 | .002 | .006 | |
| Group-by-sex interaction | 2.298 | 0.003 | .101 | .119 | |
| Hippocampal fissure | Diagnostic group | 1.899 | 0.003 | .150 | .172 |
| Sex | 28.125 | 0.018 | 1.31e-07 | 5.09e-06 | |
| Group-by-sex interaction | 0.188 | 2.49e-04 | .828 | .850 | |
| Molecular layer | Diagnostic group | 8.118 | 0.011 | 3.11e-04 | .001 |
| Sex | 8.679 | 0.006 | .003 | .008 | |
| Group-by-sex interaction | 4.749 | 0.006 | .009 | .018 | |
| Parasubiculum | Diagnostic group | 0.696 | 0.001 | .499 | .526 |
| Sex | 11.692 | 0.008 | .001 | .003 | |
| Group-by-sex interaction | 2.921 | 0.004 | .054 | .071 | |
| Presubiculum | Diagnostic group | 2.561 | 0.003 | .078 | .098 |
| Sex | 17.224 | 0.011 | 3.51e-05 | 3.42e-04 | |
| Group-by-sex interaction | 4.092 | 0.005 | .017 | .030 | |
| Subiculum | Diagnostic group | 1.169 | 0.002 | .311 | .337 |
| Sex | 6.708 | 0.004 | .010 | .019 | |
| Group-by-sex interaction | 3.266 | 0.004 | .038 | .060 | |
| Hippocampus | Diagnostic group | 6.608 | 0.009 | .001 | .005 |
| Sex | 19.027 | 0.012 | 1.38e-05 | 1.79e-04 | |
| Group-by-sex interaction | 6.114 | 0.008 | .002 | .006 |
Note The statistical results are based on analysis of covariance (adjusted for age, age2, and intracranial volume). Significant results are highlighted in bold. Abbreviation: CA, cornu ammonis; GC-ML-DG, granule cells in the molecular layer of the dentate gyrus; HATA, hippocampal-amygdaloid transition area; FDR, false discovery rate. FDR-correction was applied across all volumes and tests listed in this table.
Fig. 1.
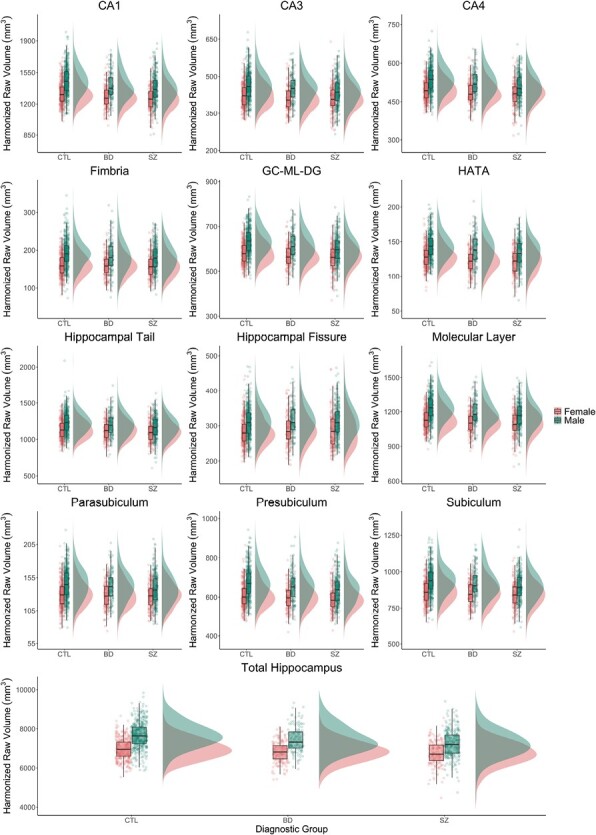
Hippocampal volumes stratified by diagnostic group and sex. ComBat-harmonized volumetric data is displayed as raincloud plots, which combines boxplots, unadjusted raw data points (scatterplot), and the distributions of the data (histogram) using split-half violins. CTL, healthy controls, BD, bipolar disorders, SZ, schizophrenia spectrum disorders; CA, cornu ammonis; GC-ML-DG, granule cells in the molecular layer of the dentate gyrus; HATA, hippocampal-amygdaloid transition area.
We found significant main effects of sex for the total hippocampus and for 10 of the 12 hippocampal subfields, not including CA3 and CA4 (model1). Significant sex-by-group interactions were found for the total hippocampus, CA4, fimbria, GC-ML-DG, HATA, presubiculum, and molecular layer (model1). Effect sizes were overall small (partial η2 ≤ 0.018). When not adjusting for ICV, we found significant sex-by-group interactions for total hippocampus volume and GC-ML-DG after FDR correction (model 3, see supplementary table S4). Post-hoc Tukey tests revealed higher volumes in males than females in the CTL group in most subfields, but not in BD or SZ (details for subfields, see supplementary table S5).
Age and age2 showed a significant main effect on all volumes, except the presubiculum and parasubiculum, based on the ANCOVA models with sex-by-group interactions (model 4).
The diagnostic subgroup analysis revealed significant sex-by-group effects for GC-ML-DG, molecular layer, fimbria, CA4, and total hippocampus volumes in schizophrenia only (model 5, figure 2, supplementary table S6). In detail, females with schizophrenia showed higher volumes in these subfields than males with schizophrenia relative to CTL, where volumes were higher in males than in females.
Fig. 2.
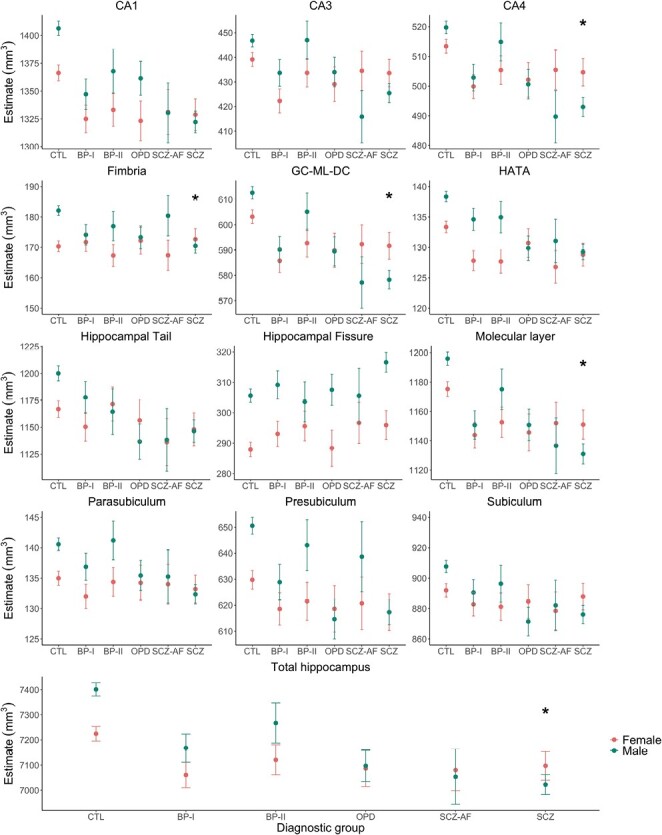
Estimates of total hippocampus and subfield volumes stratified by diagnostic subgroup and sex. The model is adjusted for age, age2, and intracranial volume. Estimates are displayed with upper and lower confidence intervals. Stars represent significant group difference relative to healthy controls (CTL), after false discovery rate correction. Significance codes: P < .01 “*.” Abbreviation: BP, bipolar; SCZ, schizophrenia; SCZ-AF, schizoaffective disorder, OPD, other psychotic disorders; CA, cornu ammonis; GC-ML-DG, granule cells in the molecular layer of the dentate gyrus; HATA, hippocampal-amygdaloid transition area.
Sex-Specific Associations Between Medication Use and Hippocampal Volumes
After FDR correction, we found no significant main effects or sex-by-medication interaction effects between DDD measures, lithium use, and hippocampal volumes in SZ and BD (model 6 and 9, see supplementary table S7).
Sex-Specific Associations Between Clinical Characteristics and Hippocampal Volumes
We found a significant main effect for the association between fissure volumes and age of onset (t = 4.149, P = 4.32e-05, PFDR = .007) as well as duration of illness (t = 3.713, P = 2.43e-04, PFDR = .025) in BD (model 7). Similarly, in SZ, we also found a significant association between age of onset and duration of illness and fissure volume. Only the association with duration of illness was significant after FDR correction (t = 4.432, P = 1.18e-05, PFDR = .004). No other main or interaction effects survived FDR correction (model 7 and 9). All associations are detailed in supplementary table S8.
Sex-Specific Associations Between Cognitive Measures and Hippocampal Volumes
We found no significant associations between cognitive measures (z-scores) and hippocampal volumes in SZ and BD, after FDR correction (model 8 and 9, see supplementary table S9).
Discussion
Using a well-powered sample, our findings indicate that the pattern of morphological sex differences in hippocampal subfields is altered in individuals with schizophrenia relative to CTL, due to higher volumetric deficits in males.
Alterations in hippocampal morphology in psychiatric illnesses have been widely reported, both on a macro- and microscopic level. Here, we found significantly lower total hippocampal, CA1, CA4, GC-ML-DG, molecular layer, and HATA volumes in individuals with SZ and BD relative to CTL, when accounting for sex-by-diagnostic group interactions and ICV. When not adjusting for this interaction, all 12 hippocampal subfields were smaller in SZ and BD relative to CTL, which is in line with previous results.20 When not accounting for ICV, significant sex-by-group interactions for total hippocampus volume and the GC-ML-DG remained after FDR correction. Effect sizes were overall small.
While diagnostic group differences in hippocampal subfield volumes have been extensively demonstrated, sex differences in hippocampal volumes are still debated. In CTL, region-specific sex differences in hippocampal subfield volumes have been demonstrated, with larger volumes in males relative to females, most prominently in the fimbria and parasubiculum.22 Our post-hoc Tukey pair-wise comparison revealed significantly higher volumes in healthy males relative to healthy females for the total hippocampus, parasubiculum, presubiculum, CA1, molecular layer, HATA, fimbria, hippocampal tail, and fissure, after adjustment for ICV. We found no evidence for larger volumes in healthy female CTL as compared to males for any of the subfields. Although the mechanisms behind larger hippocampal volumes in human males compared to females are currently unclear, animal work suggests sex and sex hormone differences in hippocampus structure and plasticity that may contribute to the observed volumetric sex difference.7 However, it should be noted that sex differences in neuroanatomical structure may be dependent on ICV estimation41 and choice of statistical method for adjusting for ICV.42–44 Here, we estimated ICV as estimated total ICV via Freesurfer v6.0.0 and used the ANCOVA method, which has been shown to more effectively remove ICV-related variation than the proportions method.42
While we found higher volumes for most subfields in healthy males relative to females, this sex difference was largely absent or even reversed in individuals with SZ and BD. Directly comparing SZ and BD, we found lower total hippocampal, GC-ML-DG, and HATA volumes in males with SZ relative to males with BD before FDR correction (see supplementary table S5). No differences were found between females with BD and SZ. This finding is in line with previous studies showing greater magnitudes of hippocampal subfield volume reductions in schizophrenia than in BD.12,45 However, our results suggest that this differential pattern of volume deficits is not just dependent on diagnosis but also on sex.
In a previous study using the same study sample, we found no significant group-by-sex interaction for bilateral total amygdala volume and amygdala nuclei volumes,36 suggesting that sex-by-diagnostic group differences may be specific to the hippocampus within the amygdala-hippocampus formation. The diagnostic subgroup analysis revealed significant sex-by-group effect on hippocampal volume in schizophrenia only, particularly for the GC-ML-DG, molecular layer, fimbria, CA4, and total hippocampus. In these subfields, volumes were significantly lower in males with schizophrenia as compared with CTL, but not in females with schizophrenia. This finding is in line with previous studies, showing lower total hippocampal or medial temporal lobe volume in males with schizophrenia, but not in females with schizophrenia relative to same-sex CTL.46,47
Our findings suggest that this male-specific deficit in total hippocampal volume may be driven by lower volumes in the CA4, GC-ML-DG, molecular layer, and fimbria. The CA4 is considered a starting point for axonal fibers of the fornix, and fornix fibers traverse through the fimbria, which connects the hippocampus with several cortical and subcortical regions. As the hippocampus and fornix are anatomically tightly connected, lower CA4 and fimbria volume may contribute to impaired fornix integrity, as reported in schizophrenia.48 Interestingly, impaired fornix-hippocampus integrity has previously been linked both to early psychosis49 and cognitive disturbances and memory function in schizophrenia.50 Furthermore, the molecular layer has also been linked to memory function.51 Based on these previous findings, one might speculate that the observed male-specific volume reductions in the CA4, GC-ML-DG, fimbria, and molecular layer in schizophrenia could be linked to cognitive impairments, which have been shown to be more pronounced in males than females with schizophrenia.33 Even though we found sex differences in SZ on verbal learning, verbal memory, and processing speed tasks, where females outperformed males, we did not find any significant interaction effects between cognitive measures and volumes in the aforementioned subfields by sex. Similarly, none of the observed main effects of cognitive measures on subfield-specific volumes survived FDR correction. This finding is not in line with previous results.13,52,53 However, previous studies have either focused on a few cognitive tests,13,52 not multiple domains, or have studied the whole hippocampus and not hippocampal subfields,53 reducing the number of multiple comparisons. Furthermore, while we selected tasks that have previously been linked to hippocampal volumes and have been shown to be impaired in SZ and BD, these cognitive tasks are not specific to the hippocampus; but also rely on other brain regions such as the dorsolateral prefrontal cortex.54 More research, including cognitive batteries particularly sensitive to hippocampal functioning,7 may elucidate the relationship between cognitive functioning, hippocampal morphology, and sex in SZ and BD. However, it should be noted that sex differences in structure may not translate to or cause sex differences in cognition. It could be equally possible that sex differences in structure prevent sex differences in behavior by compensating for sex differences in physiological conditions such as sex hormone levels.55
Although medication, including antipsychotics,56 antidepressants,57 and lithium58 have been associated with hippocampal volumes, we found no association between current use of psychotropic drugs or mood stabilizers and volumes in SZ and BD after FDR-correction. However, as medication effects might depend on the specific types of drugs and duration of exposure which may not necessarily be linear, the use of defined daily dose alone to address this confound has limitations. Similarly, we found no associations between most clinical measures and hippocampal volumes after FDR correction. The lack of an association between psychotic symptoms and volumes in SZ may result from the relatively small variation in psychotic symptoms in our sample. It may also relate to issues such as variability of symptom states over time, or currently unknown confounders. We did, however, find a significant main effect of age of onset and duration of illness on hippocampal fissure volume in BD and SZ, suggesting a positive association. The hippocampal fissure does not represent hippocampal tissue, but rather a cerebral spinal fluid cleft that forms during early brain development.59 The presence of enlarged hippocampal fissures has been shown in first-episode individuals with schizophrenia, and may be a sign of disrupted hippocampal development in schizophrenia.60
A major strength of the current study is the large sample size with a balanced sex distribution, and detailed clinical and cognitive assessment of individuals with SZ and BD. This allowed for a comprehensive study of sex differences in hippocampal subfield volumes in SZ, BD, and CTL, currently unmatched in the literature. However, the cross-sectional nature of the data precludes causal inferences, and longitudinal studies are needed to determine the timing of changes in hippocampal subfields by diagnostic group and sex. Furthermore, automated hippocampal subfield segmentation using MRI is challenging. The segmentation method used here is based on ultra-high-resolution ex vivo MRI data which allows for the precise delineation of hippocampal subfields to determine tissue priors.18 In the current study, however, subfields are probabilistically labeled based on conventional MRI data with 1 mm isotropic resolution. As such, the volumes of subfields contained within the interior of the hippocampal formation must be interpreted with caution, and a replication of our results using high-resolution MRI data is warranted. Furthermore, the reliability of automated volumetry is inversely associated with volume size.61 The volumes of smaller hippocampal subfields, such as the hippocampal fissure, fimbria, parasubiculum, and HATA, may thus be less reliable. In addition, although automated volumetry has been shown to be generally reliable in multicenter MRI studies,61 and we successfully harmonized volumes and ICV across scanners using ComBat, residual effects of scanner may still be present. Besides the effect of sex, we also found significant main effects of age and age2 on all volumes, except the presubiculum and parasubiculum. This finding is partly in line with a study in CTL, reporting age-related volume changes in all subfields, except in the subiculum complex (subiculum proper, presubiculum, and parasubiculum).60,62 However, before FDR correction, we found a significant age-by-diagnostic group interaction for individuals with SZ relative to CTL in the presubiculum and parasubiculum, potentially suggesting abnormal aging processes in these subfields in SZ.
In summary, the pattern of morphological sex differences in the hippocampus appears to be altered in individuals with schizophrenia relative to CTL, due to higher volumetric deficits in males. Our findings highlight that sex should be considered when studying individuals with psychiatric illnesses, not just as a covariate, which might mask potential sex differences, but as a discovery variable.
Supplementary Material
Contributor Information
Claudia Barth, Department of Psychiatric Research, Diakonhjemmet Hospital, Oslo, Norway; Institute of Clinical Medicine, University of Oslo, NORMENT, Oslo, Norway.
Stener Nerland, Department of Psychiatric Research, Diakonhjemmet Hospital, Oslo, Norway; Institute of Clinical Medicine, University of Oslo, NORMENT, Oslo, Norway.
Kjetil N Jørgensen, Institute of Clinical Medicine, University of Oslo, NORMENT, Oslo, Norway; Department of Psychiatry, Telemark Hospital, Skien, Norway.
Beathe Haatveit, Institute of Clinical Medicine, University of Oslo, NORMENT, Oslo, Norway; Division of Mental Health and Addiction, Oslo University Hospital, NORMENT, Oslo, Norway.
Laura A Wortinger, Department of Psychiatric Research, Diakonhjemmet Hospital, Oslo, Norway; Institute of Clinical Medicine, University of Oslo, NORMENT, Oslo, Norway.
Ingrid Melle, Institute of Clinical Medicine, University of Oslo, NORMENT, Oslo, Norway; Division of Mental Health and Addiction, Oslo University Hospital, NORMENT, Oslo, Norway.
Unn K Haukvik, Division of Mental Health and Addiction, Oslo University Hospital, NORMENT, Oslo, Norway; Department of Adult Mental Health, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; Centre of Research and Education in Forensic Psychiatry, Oslo University Hospital, Oslo, Norway.
Torill Ueland, Division of Mental Health and Addiction, Oslo University Hospital, NORMENT, Oslo, Norway; Department of Psychology, University of Oslo, Oslo, Norway.
Ole A Andreassen, Institute of Clinical Medicine, University of Oslo, NORMENT, Oslo, Norway; Division of Mental Health and Addiction, Oslo University Hospital, NORMENT, Oslo, Norway.
Ingrid Agartz, Department of Psychiatric Research, Diakonhjemmet Hospital, Oslo, Norway; Institute of Clinical Medicine, University of Oslo, NORMENT, Oslo, Norway; Department of Clinical Neuroscience, Centre for Psychiatry Research, Karolinska Institutet and Stockholm Health Care Services, Stockholm County Council, Stockholm, Sweden.
Funding
This work was supported by the Research Council of Norway (223273, 250358, and 286838), the South–Eastern Norway Regional Health Authority (2017097, 2019104, and 2020020), and the Kristian Gerhard Jebsen Foundation (SKGJ-MED-008).
Conflict of Interest
For work unrelated to the content of this manuscript, OAA and IA received speaker's honorarium from Lundbeck. OAA also received speaker's honorarium from Sunovion and Janssen, and works as a consultant for Cortechs.ai. The other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1. Sommer IE, Tiihonen J, van Mourik A, Tanskanen A, Taipale H.. The clinical course of schizophrenia in women and men-a nation-wide cohort study. npj Schizophr. 2020;6(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Menculini G, SteardoL, Jr, Sciarma T, et al. Sex differences in bipolar disorders: impact on psychopathological features and treatment response. Front Psychiatry. 2022;13:926594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Galea LA, Wainwright SR, Roes MM, Duarte-Guterman P, Chow C, Hamson DK.. Sex, hormones and neurogenesis in the hippocampus: hormonal modulation of neurogenesis and potential functional implications. J Neuroendocrinol. 2013;25(11):1039–1061. [DOI] [PubMed] [Google Scholar]
- 4. Duarte-Guterman P, Yagi S, Chow C, Galea LA.. Hippocampal learning, memory, and neurogenesis: effects of sex and estrogens across the lifespan in adults. Horm Behav. 2015;74:37–52. [DOI] [PubMed] [Google Scholar]
- 5. Bliss TV, Collingridge GL.. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361(6407):31–39. [DOI] [PubMed] [Google Scholar]
- 6. MacQueen GM, Campbell S, McEwen BS, et al. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci U S A. 2003;100(3):1387–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yagi S, Galea LAM.. Sex differences in hippocampal cognition and neurogenesis. Neuropsychopharmacology. 2019;44(1):200–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weiss AP, Zalesak M, DeWitt I, Goff D, Kunkel L, Heckers S.. Impaired hippocampal function during the detection of novel words in schizophrenia. Biol Psychiatry. 2004;55(7):668–675. [DOI] [PubMed] [Google Scholar]
- 9. Jessen F, Scheef L, Germeshausen L, et al. Reduced hippocampal activation during encoding and recognition of words in schizophrenia patients. Am J Psychiatry. 2003;160(7):1305–1312. [DOI] [PubMed] [Google Scholar]
- 10. van Erp TGM, Hibar DP, Rasmussen JM, et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium (vol 21, pg 547, 2016). Mol Psychiatry. 2016;21(4):585–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hibar DP, Westlye LT, van Erp TG, et al. ; Costa Rica/Colombia Consortium for Genetic Investigation of Bipolar Endophenotypes. Subcortical volumetric abnormalities in bipolar disorder. Mol Psychiatry. 2016;21(12):1710–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arnold SJ, Ivleva EI, Gopal TA, et al. Hippocampal volume is reduced in schizophrenia and schizoaffective disorder but not in psychotic bipolar I disorder demonstrated by both manual tracing and automated parcellation (FreeSurfer). Schizophr Bull. 2015;41(1):233–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haukvik UK, Westlye LT, Morch-Johnsen L, et al. In vivo hippocampal subfield volumes in schizophrenia and bipolar disorder. Biol Psychiatry. 2015;77(6):581–588. [DOI] [PubMed] [Google Scholar]
- 14. Valdes Hernandez MDC, Cox SR, Kim J, et al. Hippocampal morphology and cognitive functions in community-dwelling older people: the Lothian Birth Cohort 1936. Neurobiol Aging. 2017;52:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Falkai P, Schmitt A.. The need to develop personalized interventions to improve cognition in schizophrenia. World Psychiatry. 2019;18(2):170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rechlin RK, Splinter TFL, Hodges TE, Albert AY, Galea LAM.. An analysis of neuroscience and psychiatry papers published from 2009 and 2019 outlines opportunities for increasing discovery of sex differences. Nat Commun. 2022;13(1):2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garcia-Sifuentes Y, Maney DL.. Reporting and misreporting of sex differences in the biological sciences. . Elife. 2021;10:e70817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Iglesias JE, Augustinack JC, Nguyen K, et al. ; Alzheimer's Disease Neuroimaging Initiative. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. Neuroimage. 2015;115:117–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tamminga CA, Stan AD, Wagner AD.. The hippocampal formation in schizophrenia. Am J Psychiatry. 2010;167(10):1178–1193. [DOI] [PubMed] [Google Scholar]
- 20. Haukvik UK, Tamnes CK, Soderman E, Agartz I.. Neuroimaging hippocampal subfields in schizophrenia and bipolar disorder: a systematic review and meta-analysis. J Psychiatr Res. 2018;104:217–226. [DOI] [PubMed] [Google Scholar]
- 21. Haukvik UK, Gurholt TP, Nerland S, et al. In vivo hippocampal subfield volumes in bipolar disorder-a mega-analysis from the enhancing neuro imaging genetics through meta-analysis Bipolar Disorder Working Group. Hum Brain Mapp. 2020;43:385–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Eijk L, Hansell NK, Strike LT, et al. Region-specific sex differences in the hippocampus. Neuroimage. 2020;215:116781. [DOI] [PubMed] [Google Scholar]
- 23. Exner C, Nehrkorn B, Martin V, Huber M, Shiratori K, Rief W.. Sex-dependent hippocampal volume reductions in schizophrenia relate to episodic memory deficits. J Neuropsychiatry Clin Neurosci. 2008;20(2):227–230. [DOI] [PubMed] [Google Scholar]
- 24. Narr KL, Thompson PM, Sharma T, et al. Three-dimensional mapping of temporo-limbic regions and the lateral ventricles in schizophrenia: gender effects. Biol Psychiatry. 2001;50(2):84–97. [DOI] [PubMed] [Google Scholar]
- 25. Spitzer RL, Williams JBW, Gibbon M, First MB.. Structured Clinical Interview for DSM-III-R - Patient Version (SCID-P). New York: Biometrics Research Department, New York State Psychiatric Institute; 1988. [Google Scholar]
- 26. Kay SR, Fiszbein A, Opler LA.. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 27. Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH.. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26(3):477–486. [DOI] [PubMed] [Google Scholar]
- 28. Young RC, Biggs JT, Ziegler VE, Meyer DA.. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. [DOI] [PubMed] [Google Scholar]
- 29. Pedersen G, Hagtvet KA, Karterud S.. Generalizability studies of the Global Assessment of Functioning-Split version. Compr Psychiatry. 2007;48(1):88–94. [DOI] [PubMed] [Google Scholar]
- 30. Simonsen C, Sundet K, Vaskinn A, et al. Psychosocial function in schizophrenia and bipolar disorder: relationship to neurocognition and clinical symptoms. J Int Neuropsychol Soc. 2010;16(5):771–783. [DOI] [PubMed] [Google Scholar]
- 31. Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165(2):203–213. [DOI] [PubMed] [Google Scholar]
- 32. Vaskinn A, Sundet K, Simonsen C, Hellvin T, Melle I, Andreassen OA.. Sex differences in neuropsychological performance and social functioning in schizophrenia and bipolar disorder. Neuropsychology. 2011;25(4):499–510. [DOI] [PubMed] [Google Scholar]
- 33. Zhang B, Han M, Tan S, et al. Gender differences measured by the MATRICS consensus cognitive battery in chronic schizophrenia patients. Sci Rep. 2017;7(1):11821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tregellas JR, Smucny J, Harris JG, et al. Intrinsic hippocampal activity as a biomarker for cognition and symptoms in schizophrenia. Am J Psychiatry. 2014;171(5):549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. WHO Collaborating Centre for Drug Statistics Methodology. Guidelines for ATC Classification and DDD Assignment. Oslo, Norway; 2022. [Google Scholar]
- 36. Barth C, Nerland S, de Lange AG, et al. In vivo amygdala nuclei volumes in schizophrenia and bipolar disorders. Schizophr Bull. 2021;47:1431–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McCarthy CS, Ramprashad A, Thompson C, Botti JA, Coman IL, Kates WR.. A comparison of FreeSurfer-generated data with and without manual intervention. Front Neurosci. 2015;9:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fortin JP, Cullen N, Sheline YI, et al. Harmonization of cortical thickness measurements across scanners and sites. Neuroimage. 2018;167:104–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Johnson WE, Li C, Rabinovic A.. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8(1):118–127. [DOI] [PubMed] [Google Scholar]
- 40. Benjamini Y, Hochberg Y.. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57(1):289–300. [Google Scholar]
- 41. Nerland S, Stokkan TS, Jorgensen KN, et al. A comparison of intracranial volume estimation methods and their cross-sectional and longitudinal associations with age. Hum Brain Mapp. 2022;43(15):4620–4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pintzka CW, Hansen TI, Evensmoen HR, Haberg AK.. Marked effects of intracranial volume correction methods on sex differences in neuroanatomical structures: a HUNT MRI study. Front Neurosci. 2015;9:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nordenskjold R, Malmberg F, Larsson EM, et al. Intracranial volume normalization methods: considerations when investigating gender differences in regional brain volume. Psychiatry Res. 2015;231(3):227–235. [DOI] [PubMed] [Google Scholar]
- 44. Sanchis-Segura C, Ibanez-Gual MV, Aguirre N, Cruz-Gomez AJ, Forn C.. Effects of different intracranial volume correction methods on univariate sex differences in grey matter volume and multivariate sex prediction. Sci Rep. 2020;10(1):12953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rimol LM, Hartberg CB, Nesvag R, et al. Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biol Psychiatry. 2010;68(1):41–50. [DOI] [PubMed] [Google Scholar]
- 46. Bryant NL, Buchanan RW, Vladar K, Breier A, Rothman M.. Gender differences in temporal lobe structures of patients with schizophrenia: a volumetric MRI study. Am J Psychiatry. 1999;156(4):603–609. [DOI] [PubMed] [Google Scholar]
- 47. Pruessner M, Lepage M, Collins DL, Pruessner JC, Joober R, Malla AK.. Reduced hippocampal volume and hypothalamus-pituitary-adrenal axis function in first episode psychosis: evidence for sex differences. Neuroimage Clin. 2015;7:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fitzsimmons J, Kubicki M, Smith K, et al. Diffusion tractography of the fornix in schizophrenia. Schizophr Res. 2009;107(1):39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Baumann PS, Griffa A, Fournier M, et al. Impaired fornix-hippocampus integrity is linked to peripheral glutathione peroxidase in early psychosis. Transl Psychiatry. 2016;6(7):e859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kuroki N, Kubicki M, Nestor PG, et al. Fornix integrity and hippocampal volume in male schizophrenic patients. Biol Psychiatry. 2006;60(1):22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wan M, Ye Y, Lin H, et al. Deviations in hippocampal subregion in older adults with cognitive frailty. Front Aging Neurosci. 2020;12:615852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Huang J, Zhu Y, Fan F, et al. Hippocampus and cognitive domain deficits in treatment-resistant schizophrenia: a comparison with matched treatment-responsive patients and healthy controls(☆,☆☆, bigstar, bigstar bigstar). Psychiatry Res Neuroimaging. 2020;297:111043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pujol N, Penades R, Junque C, et al. Hippocampal abnormalities and age in chronic schizophrenia: morphometric study across the adult lifespan. Br J Psychiatry. 2014;205(5):369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Molina V, Solera S, Sanz J, et al. Association between cerebral metabolic and structural abnormalities and cognitive performance in schizophrenia. Psychiatry Res. 2009;173(2):88–93. [DOI] [PubMed] [Google Scholar]
- 55. De Vries GJ. Minireview: sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology. 2004;145(3):1063–1068. [DOI] [PubMed] [Google Scholar]
- 56. Balu DT, Lucki I.. Adult hippocampal neurogenesis: regulation, functional implications, and contribution to disease pathology. Neurosci Biobehav Rev. 2009;33(3):232–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Boldrini M, Hen R, Underwood MD, et al. Hippocampal angiogenesis and progenitor cell proliferation are increased with antidepressant use in major depression. Biol Psychiatry. 2012;72(7):562–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Simonetti A, Sani G, Dacquino C, et al. Hippocampal subfield volumes in short- and long-term lithium-treated patients with bipolar I disorder. Bipolar Disord. 2016;18(4):352–362. [DOI] [PubMed] [Google Scholar]
- 59. Samann PG, Iglesias JE, Gutman B, et al. FreeSurfer-based segmentation of hippocampal subfields: a review of methods and applications, with a novel quality control procedure for ENIGMA studies and other collaborative efforts. Hum Brain Mapp. 2020;43:207–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Smith GN, Lang DJ, Kopala LC, Lapointe JS, Falkai P, Honer WG.. Developmental abnormalities of the hippocampus in first-episode schizophrenia. Biol Psychiatry. 2003;53(7):555–561. [DOI] [PubMed] [Google Scholar]
- 61. Quattrini G, Pievani M, Jovicich J, et al. ; PharmaCog Consortium. Amygdalar nuclei and hippocampal subfields on MRI: test-retest reliability of automated volumetry across different MRI sites and vendors. Neuroimage. 2020;218:116932. [DOI] [PubMed] [Google Scholar]
- 62. Daugherty AM, Bender AR, Raz N, Ofen N.. Age differences in hippocampal subfield volumes from childhood to late adulthood. Hippocampus. 2016;26(2):220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


