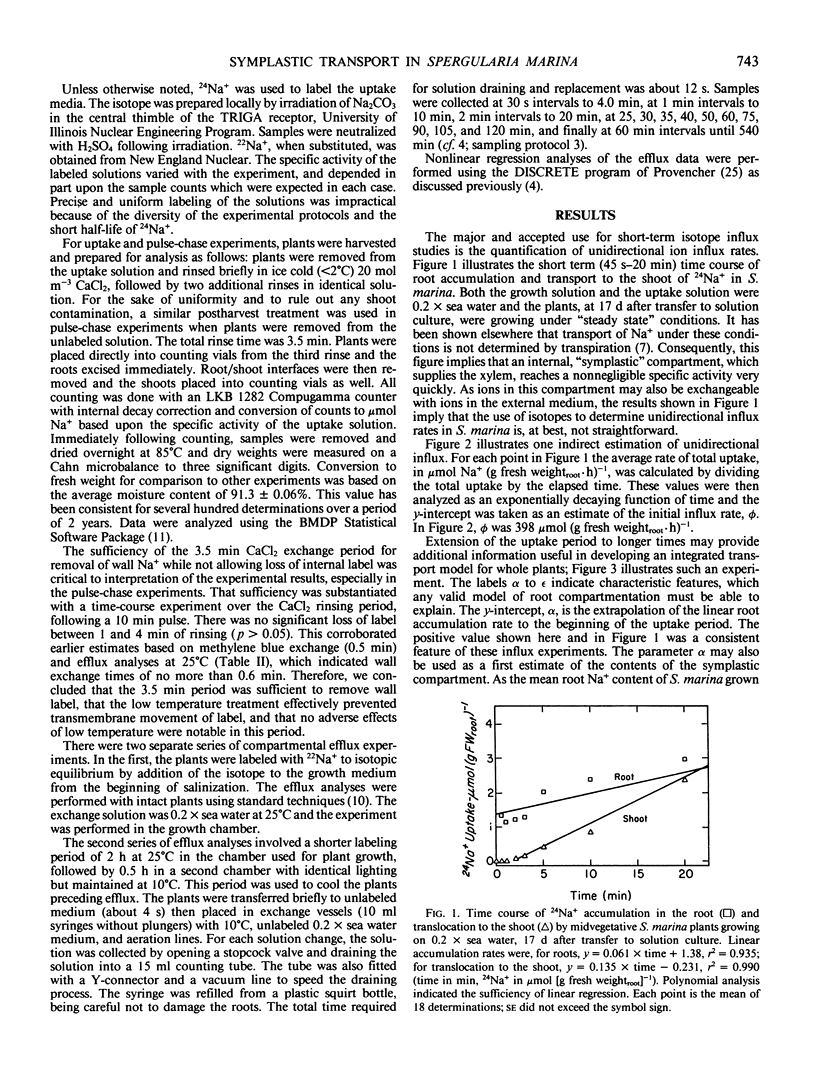
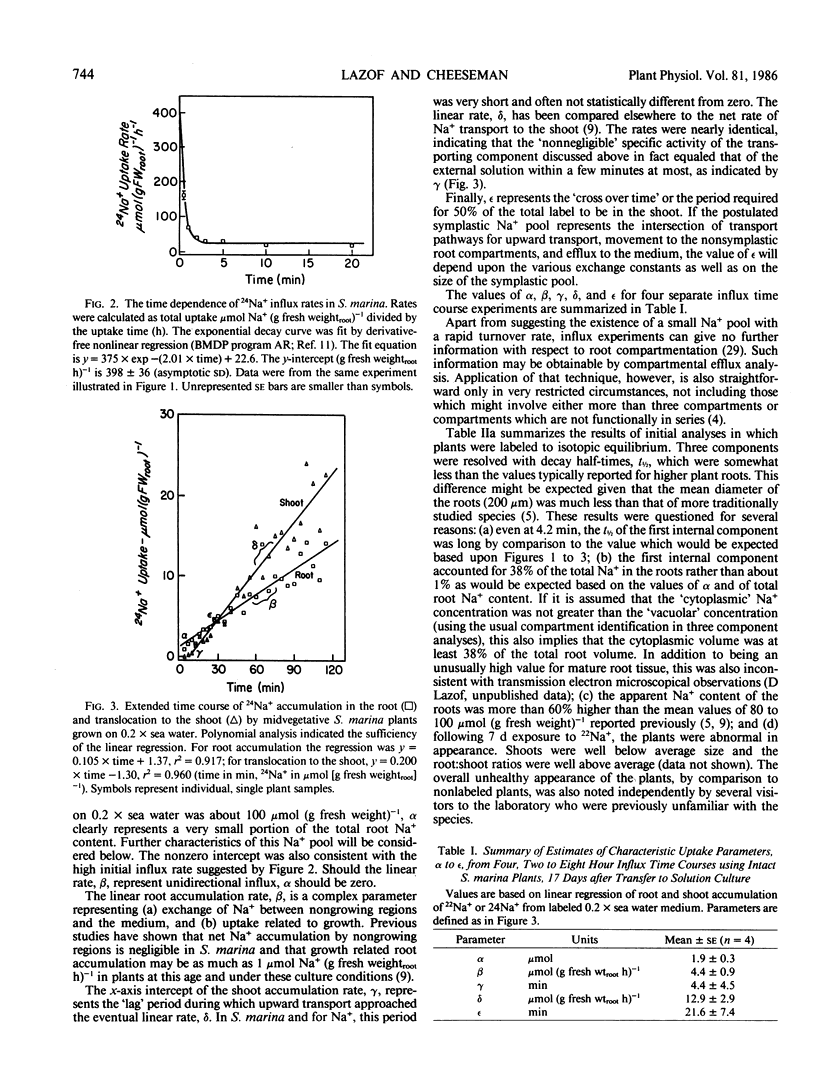
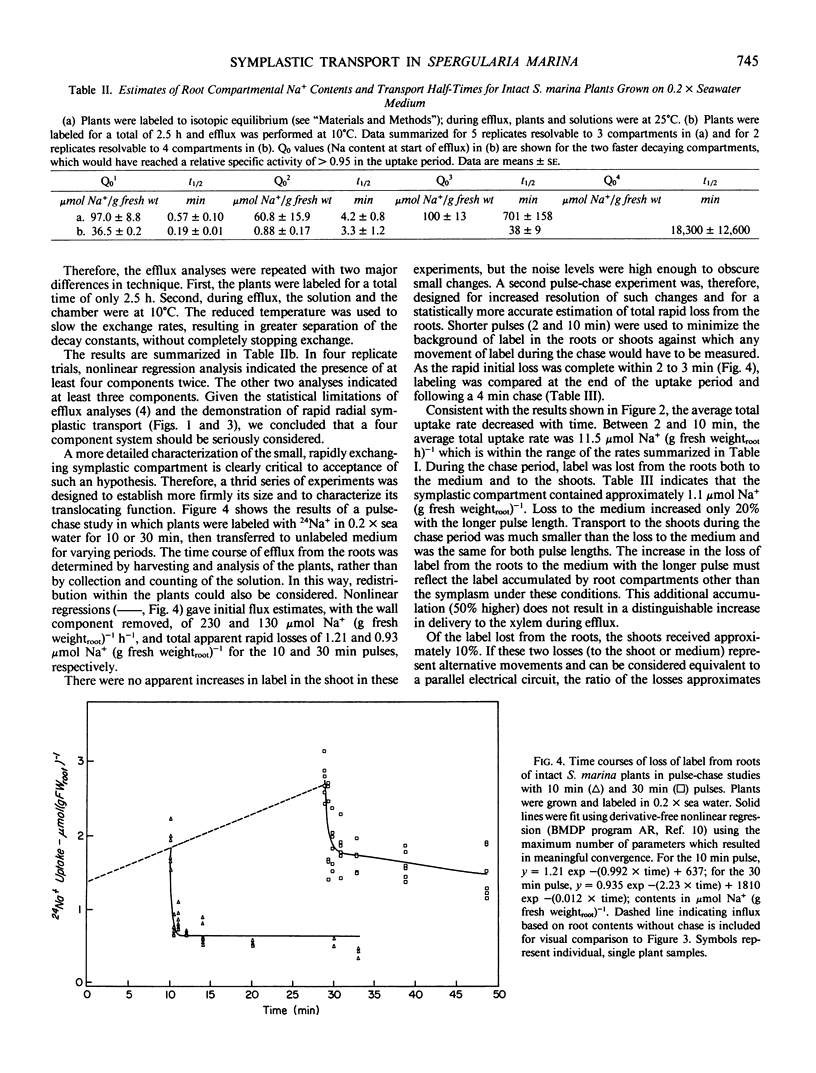
Abstract
In this paper, a combination of tracer uptake, efflux, and pulse-chase techniques is applied to the problem of compartmentation of Na+ (24Na+) in the roots of intact, midvegetative Spergularia marina (L.) Griseb. plants. An approach is presented for conducting useful compartmental analysis when it is known that the assumptions required for straightforward interpretations of influx and efflux studies are invalid. Linear rates of 24Na+ accumulation in both roots and shoots were attained within at most a few minutes following the start of labeling. Shoot 24Na+ contents equaled root contents within about 20 minutes. Analysis of root accumulation rates, and compartmental and pulse-chase efflux studies indicated that the unidirectional flux rates involved were at least an order of magnitude greater than linear rates of root and shoot accumulation. These rapid fluxes involved only a small portion of the total root Na+ (about 1%). The results suggest the existence of a small symplastic compartment, distinct from the `bulk cytoplasm,' rapidly exchanging with the medium, and responsible for delivery of Na+ to the xylem. The physical identity of this compartment and its physiological significance are discussed with respect to precedents in the literature.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheeseman J. M. Compartmental efflux analysis: an evaluation of the technique and its limitations. Plant Physiol. 1986 Apr;80(4):1006–1011. doi: 10.1104/pp.80.4.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. F., Higinbotham N. Electrochemical gradients and k and cl fluxes in excised corn roots. Plant Physiol. 1976 Feb;57(2):129–136. doi: 10.1104/pp.57.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACROBBIE E. A. THE NATURE OF THE COUPLING BETWEEN LIGHT ENERGY AND ACTIVE ION TRANSPORT IN NITELLA TRANSLUCENS. Biochim Biophys Acta. 1965 Jan 25;94:64–73. doi: 10.1016/0926-6585(65)90008-7. [DOI] [PubMed] [Google Scholar]
- MacRobbie E. A. The active transport of ions in plant cells. Q Rev Biophys. 1970 Aug;3(3):251–294. doi: 10.1017/s0033583500004741. [DOI] [PubMed] [Google Scholar]
- Provencher S. W. A Fourier method for the analysis of exponential decay curves. Biophys J. 1976 Jan;16(1):27–41. doi: 10.1016/S0006-3495(76)85660-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush D. W., Epstein E. Comparative studies on the sodium, potassium, and chloride relations of a wild halophytic and a domestic salt-sensitive tomato species. Plant Physiol. 1981 Dec;68(6):1308–1313. doi: 10.1104/pp.68.6.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]


