Abstract
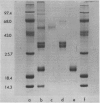
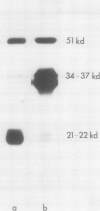
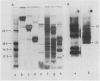
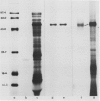
When the glutelin protein fraction of rice (Oryza sativa L.) seeds was fractionated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, three size classes of proteins, 51 kilodaltons (kD), 34 to 37 kD, and 21 to 22 kD, as well as a contaminating prolamine polypeptide of 14 kD were detected. Antibodies were raised against these proteins and employed in studies to determine whether a precursor-product relationship existed among the glutelin components. Antibodies of the 34 to 37 kD and 21 to 22 kD polypeptides strongly reacted with the 51 kD protein, and conversely, anti-51 kD protein cross reacted with both of the putative subunits. Immunoprecipitation of in vitro translated products resulted in the synthesis of only the precursor form, indicating that the α and β subunits are proteolytic products of the 51 kD precursor protein. The poly(A)+ RNA directed in vitro translated product was about 2000 daltons larger than both the authentic glutelin precursor and the in vitro translated product from polysome run-off synthesis. Western blot analysis of the 34 to 37 kD and 21 to 22 kD polypeptides partially digested with Staphylococcus aureus V8 protease revealed distinct patterns indicating that these proteins are structurally unrelated. As observed for the glutelins, the rice prolamines are also synthesized as a precursor of 16 kD, 2000 daltons larger than the mature polypeptide. Addition of dog pancreatic microsomal membranes to a wheat germ protein translation system resulted in the processing of the prolamine preprotein but not the preproglutelin to the mature form.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adeli K., Allan-Wojtas P., Altosaar I. Intracellular transport and posttranslational cleavage of oat globulin precursors. Plant Physiol. 1984 Sep;76(1):16–20. doi: 10.1104/pp.76.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adeli K., Altosaar I. Characterization of oat vicilin-like polypeptides. Plant Physiol. 1984 May;75(1):225–227. doi: 10.1104/pp.75.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinegar A. C., Peterson D. M. Synthesis of Oat Globulin Precursors : Analogy to Legume 11S Storage Protein Synthesisa. Plant Physiol. 1982 Dec;70(6):1767–1769. doi: 10.1104/pp.70.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Okita T. W., Decaleya R., Rappaport L. Synthesis of a possible precursor of alpha-amylase in wheat aleurone cells. Plant Physiol. 1979 Jan;63(1):195–200. doi: 10.1104/pp.63.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita T. W., Greene F. C. Wheat Storage Proteins : ISOLATION AND CHARACTERIZATION OF THE GLIADIN MESSENGER RNAs. Plant Physiol. 1982 Apr;69(4):834–839. doi: 10.1104/pp.69.4.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert L. S., Adeli K., Altosaar I. Homology among 3S and 7S Globulins from Cereals and Pea. Plant Physiol. 1985 Aug;78(4):812–816. doi: 10.1104/pp.78.4.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walburg G., Larkins B. A. Oat seed globulin: subunit characterization and demonstration of its synthesis as a precursor. Plant Physiol. 1983 May;72(1):161–165. doi: 10.1104/pp.72.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen T. N., Luthe D. S. Biochemical characterization of rice glutelin. Plant Physiol. 1985 May;78(1):172–177. doi: 10.1104/pp.78.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata H., Sugimoto T., Tanaka K., Kasai Z. Biosynthesis of storage proteins in developing rice seeds. Plant Physiol. 1982 Oct;70(4):1094–1100. doi: 10.1104/pp.70.4.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]