Abstract
Polyhydroxy compounds are secondary metabolites that are ubiquitous in plants of higher genera. They possess therapeutic properties against a wide spectrum of diseases, including cancers, neurodegenerative disorders, atherosclerosis, as well as cardiovascular disease. The phyto-chemical flavonol (a type of flavonoid) kaempferol (KMP) (3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4Hchromen-4-one) is abundant in cruciferous vegetables, including broccoli, kale, spinach, and watercress, as well as in herbs like dill, chives, and tarragon. KMP is predominantly hydrophobic in nature due to its diphenylpropane structure (a characteristic feature of flavonoids). Recent findings have indicated the promise of applying KMP in disease prevention due to its potential antioxidant, antimutagenic, antifungal, and antiviral activities. In the literature, there is evidence that KMP exerts its anticancer effects by modulating critical elements in cellular signal transduction pathways linked to apoptosis, inflammation, angiogenesis, and metastasis in cancer cells without affecting the viability of normal cells. It has been shown that KMP triggers cancer cell death by several mechanisms, including cell cycle arrest, caspase activation, metabolic alteration, and impacting human telomerase reverse-transcriptase gene expression. This review is aimed at providing critical insights into the influence of KMP on the intracellular cascades that regulate metabolism and signaling in breast, ovarian, and cervical cancer cells.
Keywords: Natural product, Cancer therapy, Ovarian cancer, Breast cancer, Cervical cancer, Chemoresistance
1. INTRODUCTION
Cell growth and survival are fueled by nutrients that are taken up from the environment and directed into the cellular metabolic pathways to generate energy-rich molecules such as ATP/GTP. In proliferating cells, including tumor/cancer cells, these metabolic processes require additional nutrients to produce ATP and generate all components necessary for continuous cell division. Cellular survival and functionality are heavily contingent on the energy provided through metabolic mechanisms. Minor changes in metabolic pathways have a detrimental impact on the regulation and proliferation of healthy and cancerous cells [1]. Given the increasing prevalence of cancer diagnoses [2] and deaths, a deeper understanding of the fundamental metabolic processes that act upon cellular regulation is required.
Cancer was reported to be responsible for 10 million deaths in 2020 worldwide, making it one of the leading causes of death [3]. Back in the 1920s, Warburg proposed that lactate fermentation from glucose (commonly referred to as aerobic glycolysis) [4] is increased in cancerous and normal proliferating cells. Since then, significant progress has been made in our understanding of the unique relationship between cancer cells and metabolism. Ying et al. and Son et al. [5, 6] found that pancreatic tumor cells function through a KRAS-regulated metabolic pathway. Yang et al. observed that lung cancer cells use the glutathione metabolic pathway [7]. In addition, Sanchez-Vega et al. [8] indicated the role of oncogenes, including Myc, Notch, and Nrf2, in promoting metabolic changes in different types of tumor cells. Studies by DeBerardinis & Chandel [9] and Pavlova & Thompson [10] also showed that changes in the levels of intracellular metabolites, enzymes, and transporters are associated with metabolic alterations leading to genetic modifications in cancer, which differ depending on the tissue type and stage of cancer [11].
Receptor-mediated signal transduction initiates metabolic pathways in mammalian cells to synthesize new nucleic acids, lipids, and proteins, as needed by the proliferating cells [12]. The regulation of cellular metabolism by signaling pathways is tightly coordinated through feedback control on signal transduction cascades, making the relationship bidirectional. Cellular processes, including proliferation, differentiation, apoptosis, and stress responses, are regulated by signaling pathways, most importantly by mitogen-activated protein kinase (MAPK) cascades. Some important genetically altered signaling pathways have been identified in cancer, including RTK/RAS-ERK (extracellular signal-regulated kinases)/MAP-kinase (mitogen-activated protein kinase) and PI3K/Akt signaling [13], which occur heterogeneously depending on the tissue and cancer types [14, 15].
Since 1995, the National Cancer Institute (NCI) has supported researchers in screening novel chemicals as potential anticancer compounds. In 1993, the NCI initiated a campaign to discover therapeutically important natural products [16–18]. An important example is the naturally occurring chemical salicylic acid, which was extracted from the bark of the willow tree and eventually gave rise to the famous drug aspirin [19]. Phytochemicals are non-nutrient metabolites made by common fruits and vegetables to defend against pathogens, competitors, and other threats. There are different categories of phytochemicals based on their activities, such as antifungals [20], antivirals [21], antimutagens [22], antiinflammatories [23], antioxidants [24], and anticancer agents [25]. Some anticancer phytochemicals act across categories [26], and some synergize with other chemotherapeutic agents [27], but perhaps their best attribute is the lack of toxicity to normal cells at therapeutic doses [28].
Flavonoids are a class of polyphenolic phytochemicals that are ubiquitous secondary metabolites in plants of higher genera [29]. This review focuses on the influence of the naturally occurring flavonol kaempferol (KMP, a tetrahydroxyflavone) on the signaling pathways of three common cancers that most often affect women: breast, ovarian, and cervical. The characteristic feature of flavonoids including KMP is the presence of a common skeleton of diphenyl propane (two phenyl rings connected by three carbon linkage). KMP is mostly found in leafy vegetables such as broccoli, kale, and watercress and exhibits antioxidant properties producing significant health benefits [30, 31]. The common sugar-conjugates of KMP include astragalin (kaempferol-3-O-glucoside), kaempferol-3-beta-D-galactoside, kaempferol-3-rutinoside [31]. Research has shown that an increase in the intake of food containing KMP reduces individuals’ risk of developing cancer as well as the risk of cardiovascular disease. KMP can inhibit cancer progression by triggering cell cycle arrest at the G2/M phase, apoptosis, regulation of signaling pathways and protein kinase B, expression of metastasis-related markers, and epithelial–mesenchymal transition-related markers and inhibiting the accumulation of reactive oxygen species [29–31]. The anticancer effects of KMP can also be linked to nutrient delivery, processing, and storage, which create the pathway to starve cancer cells to initiate apoptosis [31]. However, as tumor cells are resilient and adapt well to poor nutrient and hypoxic conditions, under this condition AMP-activated protein kinase (AMPK) promotes the survival mechanism called autophagy, reducing apoptosis and allowing cancer proliferation. In vitro studies have been conducted on combining KMP and AMPK inhibitor to control the growth of cancer cells which showed promise for further exploration [31]. KMP contains four hydroxyl (-OH) groups at 3, 5, 7, and 4′ positions (structure is presented in Schemes 1–3), providing the opportunity of forming co-ordinate bonds between electron donor atom oxygen (of – OH groups) and metal atoms, which can further contribute to its therapeutic activity [31].
Scheme 1.
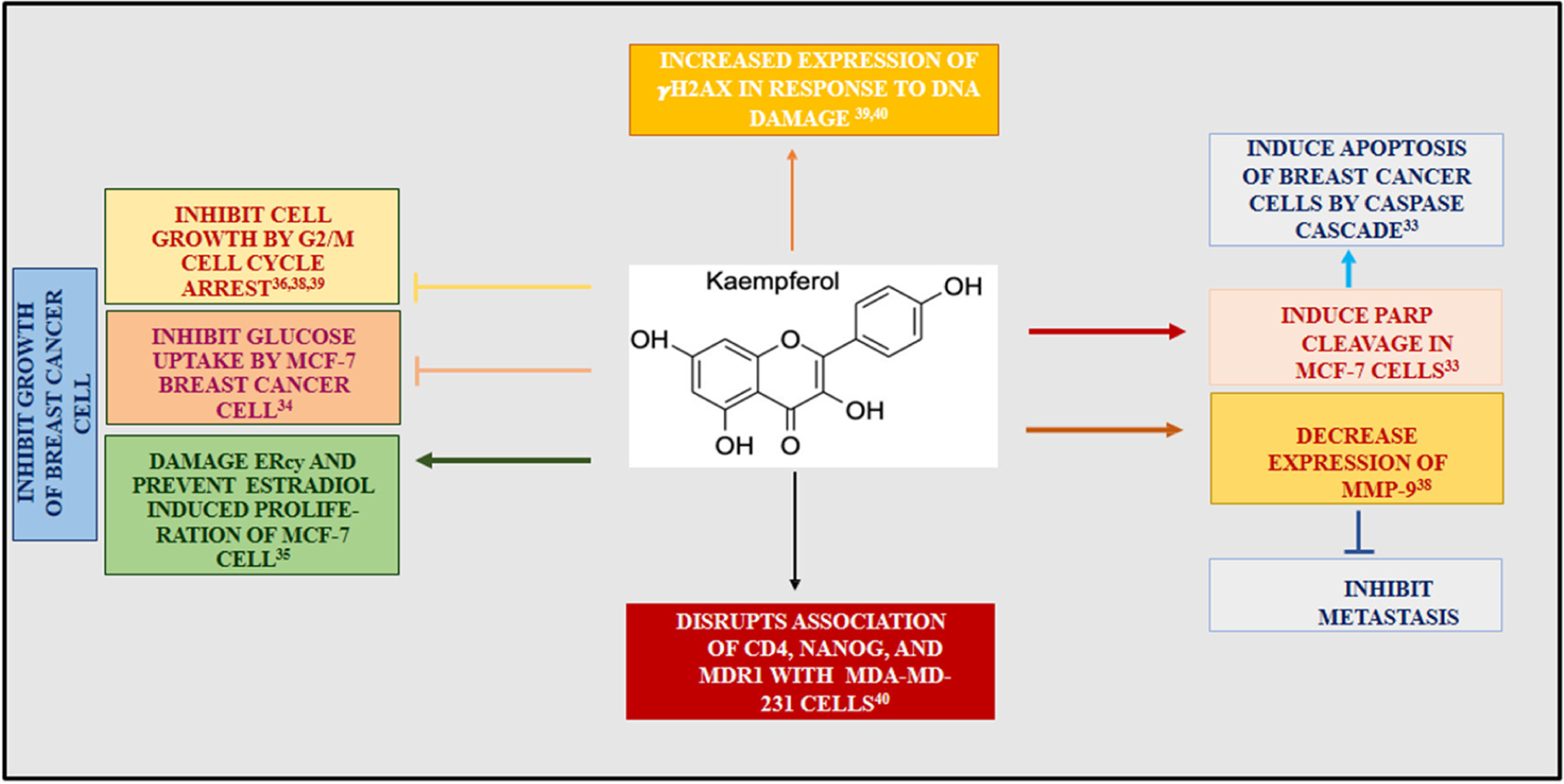
Illustration of the various pathways by which kaempferol (KMP) induces an apoptotic response in breast cancer cells. KMP decreased the uptake of glucose and lactic acid in MCF-7 cells [34]. In addition, KMP damaged the ER in MCF-7 cells inhibiting estradiol-mediated cell growth [35]. Moreover, KMP initiated the caspase cascade by the cleavage of PARP [33]. KMP was shown to induce apoptosis through G2/M cell cycle arrest [36–38]. Furthermore, KMP was shown to have facilitated dsDNA damage, leading to increased expression of γH2AX [39, 40].
Scheme 3.
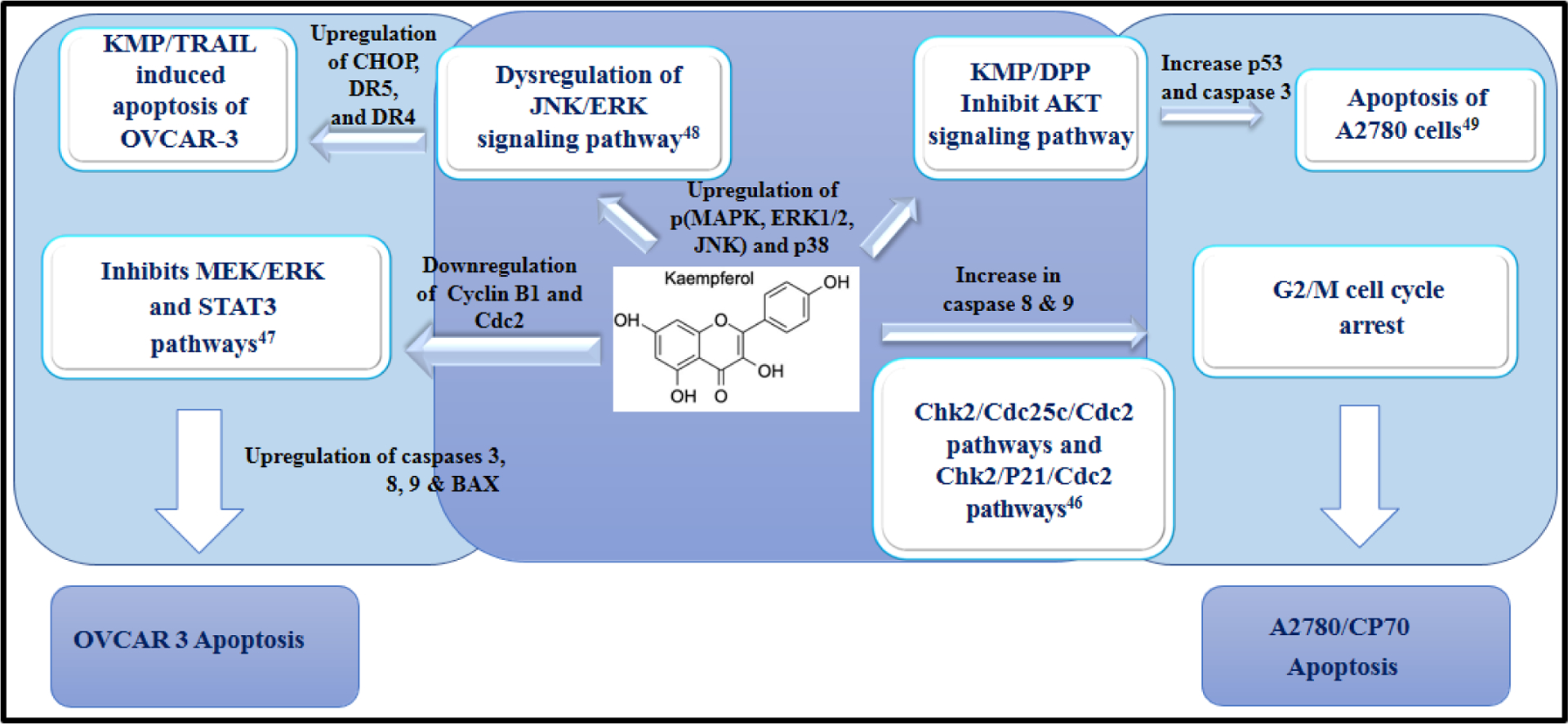
An illustration of the alterations in various cell signaling pathways that are responsible for inducing apoptosis of ovarian cancer cells. KMP causes cell cycle arrest at the G2/M phase through downregulation of the Chk2/p21/Cdc2 pathway [46]. In addition, KMP inhibits the MEK/ERK pathway [47]. Furthermore, KMP dysregulates the JNK/ERK pathway, facilitating KMP/TRAIL-induced apoptosis of OVCAR-3 [48]. Finally, KMP/DPP regulates cell death by inhibiting the AKT signaling pathway [49].
1.1. Kaempferol’s Role in Breast Cancer
Breast cancer is one of the most prevalent forms of cancer found in women. According to the GLOBOCAN 2020 report, globally 7.8 million women have been diagnosed with breast cancer in the last 5 years [3]. Although advances in diagnostics and early prevention have increased survival rates since the 1980s, breast cancer is still the second largest cause of death in women [32]. Yi et al. [33] and others used estrogen receptor-positive mammary cancer cells (MCF-7) to investigate the mechanism by which KMP produces an apoptotic response. In addition, Azevedo et al. (2015) showed that KMP induced apoptosis in MCF-7 cells by disrupting the uptake of glucose, which is an essential sugar in the production of cellular energy [34]. They observed that the inability of MCF-7 to take up glucose was detrimental to cellular function and led to programmed cell death. They also found that KMP prevented the uptake of lactic acid, thereby inhibiting cell survival [34]. Moreover, research by Hung et al. [35] on MCF-7 cells demonstrated that KMP damages the endoplasmic reticulum (ER), thereby inhibiting MCF-7 cells from undergoing estradiol-mediated growth. Yi et al. [33] also found that KMP facilitated the inhibition of cancer cell growth by activating the caspase pathway. Caspases are essential enzymes involved in apoptosis. The cleavage of poly(ADP-ribose) polymerase (PARP) leads to a caspase pathway cascade that eventually results in programmed cell death. Yi et al. also found that KMP effectively induced the cleavage of PARP in MCF-7 cells. Moreover, they showed that KMP has the ability to reduce the expression of antiapoptotic proteins (e.g., Bcl12) and promote the expression of proapoptotic ones (e.g., BAX). Furthermore, Liao et al. [36] showed that KMP elicits strong antiproliferative effects on different breast cancer cell types by disrupting mitochondrial function.
Research by Choi et al. (2008) showed that KMP induced apoptosis of breast cancer cells through G2/M cell cycle arrest [37]. They found that KMP-treated cancer cells had a decrease in numbers at the G1 phase of the cell cycle and increased cell numbers at the G2/M phase. They showed that this cell cycle arrest occurred via the inhibition of cyclin-dependent kinase 1 (CDK1), which is an important protein involved in cell division. Their findings indicated the preventive role of KMP on the growth of human breast cancer cells by inhibiting the production of CDK1 at the G2/M phase of the cell cycle. Furthermore, Li et al. (2015) [38] observed that KMP has the ability to suppress the expression of matrix metalloproteinases (MMP), which are protein markers of malignancy in breast cancer cells. For example, increased expression of MMP-3 has been shown to correlate with more aggressive breast cancer. Among all polymorphs of MMP, MMP-1, MMP-3, and MMP-9 have been shown to play critical roles in the migration of breast cancer cells. Li et al. also found that KMP prevented the progression of breast cancer metastasis by decreasing the expression and overall activity of MMP-9.
Zhu et al. [39] treated MDA-MB-231 (triple-negative breast cancer) cells with KMP and monitored the cell cycle via flow cytometry. Their study showed that, at the G1 phase of the cell cycle, the total number of cells decreased by 30%, whereas that at the G2 phase increased by 28%. This led to the finding that KMP initiated cell cycle arrest at the G2/M phase, leading to apoptosis of MDA-MB-231, which also agrees with the findings of Choi et al. [37] Moreover, Zhu and colleagues found that KMP-treated cells exhibited increased expression of γH2AX, a molecular marker that is expressed due to DNA double-strand breaks.
In a more recent study, Nandi et al. (2022) [40] investigated the anticancer properties of KMP along with a calcium channel blocker drug, verapamil, on breast cancer stem cells (BCSCs). Verapamil is used in the treatment of Peyronie’s disease and has a significant impact on fibroblast function (e.g., cell proliferation). They treated the BCSCs MDA-MB-231 with KMP, verapamil, and KMP & verapamil simultaneously. They found that the treatment of MDA-MB-231 cells with KMP had an antiproliferative effect and deregulated specific chemo-evasion markers, such as CD44, NANOG, and multidrug resistance mutation 1 (MDR1). CD44 is a cell surface adhesion receptor that has been shown to be strongly associated with multiple cancer types and assists in the promotion of cancer cell metastasis. Meanwhile, NANOG is a homeobox protein that is responsible for maintaining the pluripotency of stem cells. The above study suggested that KMP mediates G2/M cell cycle arrest, thus disrupting the association of CD44, NANOG, and MDR1 with MDA-MD-231. KMP-treated cells also showed increased expression of γH2AX in response to DNA damage, a finding similar to that of Zhu [39]. Moreover, Nandi’s team discovered that the treatment of cells with both KMP and verapamil had higher efficacy at preventing the regulation of chemo-evasive proteins as well as the proliferation of BCSCs. Scheme 1 demonstrates the effect of KMP on pathways associated with breast cancer.
KMP was shown to prevent the proliferation of breast cancer cells by the upregulation of pro-apoptotic genes leading to G2/M cell cycle arrest [37, 39, 40]. KMP has been reported to trigger ER stress mediated ROS production in estrogen receptor positive cells due to KMP’s ability to bind to estrogen receptor alpha [33, 41]. Likewise, KMP utilized the caspase pathway which is a mitochondrial-mediated apoptotic pathway in response to cellular stress [33, 41, 42]. Moreover, KMP prevented the uptake of glucose disrupting mitochondria from producing cellular ATP [34].
1.2. Kaempferol’s Role in Cervical Cancer
Over the past 30 years, the number of young women affected by cervical cancer has increased by 30% [41]. Kashafi et al. used HeLa cells (a human epithelial cell line that plays an integral role in the investigation of various human pathologies) to study the mechanism by which KMP induces apoptosis in cervical cancer cells [43]. They compared HeLa cells and HFF cells (i.e., healthy cells) that were treated with KMP. In the experiment, cellular viability and telomerase expression were measured using assays such as MTT and RT-PCR. They found that KMP induced apoptosis by a process of downregulating the PI3K/AKT pathway, an essential cellular signaling pathway responsible for regulating the cell cycle and promoting cellular proliferation. This pathway is initiated by the phosphorylation of PI3K activating AKT, in turn leading to various downstream effects. Kashafi et al. [43] discovered that the downregulation of this pathway resulted in a decrease in cellular growth and an increase in the expression of apoptotic genes such as p53, thereby triggering death in cervical cancer cells. They also found that KMP-induced apoptosis of cervical cancer cells occurs through suppression of the expression of the human telomerase reverse transcriptase gene (hTERT). The rate-limiting step in telomerase activity is hTERT, which has been shown to be involved in cellular proliferation. This study demonstrated that KMP has the ability to impede hTERT activity, thereby dysregulating the metastasis of cervical cancer cells.
Tu et al. (2016) investigated KMP-induced apoptosis based on changes in cellular topography at the subcellular and nanolevels [44]. Specifically, they used human cervical squamous cells (SiHa) treated with KMP for flow cytometry studies, which showed that KMP increased the intracellular Ca2+ concentration as well as decreased mitochondrial potential. Tu et al. proposed that apoptosis is initiated by the release of cytochrome c from mitochondria, leading to the expression of various apoptotic caspases. This then leads to an increase in free Ca2+ ions in the intracellular space, which has been identified as the cause of cell death. Moreover, Tu et al. confirmed cervical cell inhibition using laser confocal microscopy and atomic force microscopy, showing a decrease in cervical cell activity by observing the changes in cellular membrane topography. Therefore, they demonstrated that KMP prevents the metastasis of cervical cancer cells through the misregulation of essential metabolic pathways, producing a cellular apoptotic response. Scheme 2 provides a summary of the effect of KMP on signaling pathways associated with cervical cancer.
Scheme 2.
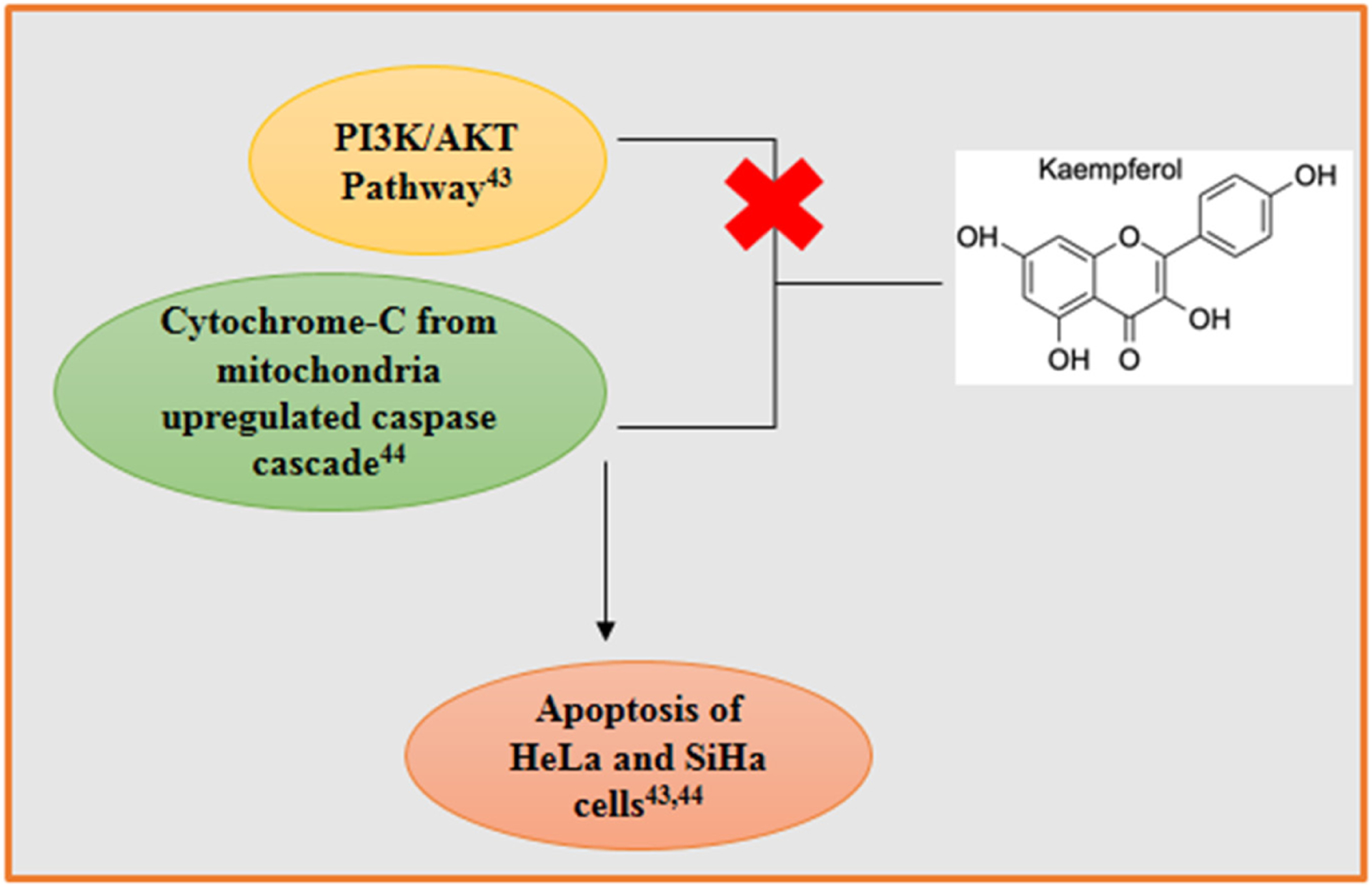
An illustration of kaempferol (KMP) inhibiting the PI3K/AKT signaling pathway, leading to downstream apoptotic effects [43]. KMP was shown to have an effect of decreasing mitochondrial potential, releasing cytochrome c, and thus upregulating the caspase cascade [44].
KMP treatment in cervical cancer cells also initiates G2/M phase arrest followed by apoptotic cell death. KMP mediated apoptosis is assisted by p53 activation, intracellular calcium accumulation and disruption of mitochondrial membrane potential [43]. KMP downregulated the PI3K/AKT pathway which is essential for cellular proliferation [44].
1.3. Kaempferol’s Role in Ovarian Cancer
Ovarian cancer (OVCAR) is the third leading cause of cancer-related death among females. In 2020–2021, 21,750 new ovarian cancer cases emerged. Many OVCAR cases are diagnosed at late disease stages, making treatment difficult and invasive [45]. The deletion or mutation of Checkpoint Kinase 2 (CHEK2) has been linked to the development of numerous cancers, including OVCAR [46]. CHEK2 is an essential regulatory gene that is associated with tumor suppression and cell cycle arrest in response to damage in the DNA sequence. Mutations can lead to the abnormal accumulation of cancer cells and the promotion of proto-oncogenes. Gao et al. used KMP-induced ovarian cancer cells A2780/CP70 to study the apoptotic mechanism [46]. They showed that KMP triggered apoptosis of A2780/CP70 cells through activating both intrinsic and extrinsic pathways. They proposed that the mechanism involved KMP-initiated cell cycle arrest at the G2/M phase through the upregulation of p21 by activating CHEK2. Interestingly, Gao et al. found in previous studies that KMP activated apoptosis through a p53-dependent pathway initiated by CHEK2. However, one study has shown that the upregulation of p53 was not affected by the deletion of the CHEK2 gene. Similarly, using flow cytometry, Gao and colleagues showed that KMP activated apoptosis of A2780/CP70 through the caspase 8 and caspase 9 pathways. To replicate their findings, Gao et al. used a high-grade ovarian cancer cell line (OVCAR-3). They found that KMP induced apoptosis in OVCAR-3 via cell cycle arrest at the G2/M phase. Furthermore, Yang et al. [47] observed that KMP induced apoptosis of OVCAR-3 cells through cell cycle arrest at the G0/G1 phase. This was shown to occur via the activation of caspases 3, 8, and 9 and BAX, and the downregulation of Cyclin B1 and Cdc2, as confirmed by immunoblot assay. Similarly, Zhao et al. showed that KMP sensitized OVCAR-3 and SKOV-3 cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) [48]. They found that cells treated with KMP in conjunction with TRAIL showed increased expression of CHOP, which is involved in the regulation of death regulator 5 (DR5). DR5 plays an essential role in the TRAIL mechanism and caspase activation. Furthermore, cells stimulated with TRAIL and subsequently treated with KMP showed the upregulation of phosphorylated MAP-kinases ERK1/2, JNK, and p38 as well as DR4 and DR5. Some ovarian cancer cell lines are resistant to TRAIL. However, Zhao and colleagues showed that KMP increased TRAIL-mediated cell death in a dose-dependent manner by upregulating death receptors on the cell membrane [48]. Similarly, KMP-treated cells that were stimulated by TRAIL showed increased expression of phosphorylated MAP kinases (i.e., ERK1/2, JNK, p38). These results clarify that KMP can assist TRAIL-mediated apoptosis through dysregulation of the JNK/ERK signaling pathway in OVCAR-3 and SKOV-3 ovarian cancer cells.
El-Kott et al. [49] found that KMP inhibited the proliferation of A2780 ovarian cancer cells through ER stress-mediated autophagy. Their study showed that KMP induced ER stress and autophagy by altering the concentration of intracellular Ca2+. Increased expression of markers of ER stress and autophagy in KMP-treated A2780 cells was also observed. These cells also had increased sensitivity to cisplatin (DPP). This group also studied the effect of DPP in the presence of KMP and found that KMP/DPP-treated cells had a greater decrease in phosphorylated AKT and greater increases in p53 and caspase 3 than KMP- and DPP-treated cells. These studies confirmed that KMP facilitated DPP-mediated cell death through inhibition of the AKT signaling pathway. It is also evident that KMP enhances chemosensitization in drug-resistant OVCAR. Scheme 3 shows the role of KMP in regulating the pathways associated with OVCAR.
KMP induced apoptosis of ovarian cancer cells through a G2/M cell cycle arrest and the downregulation of essential signaling pathways such as MEK/ERK, JNK/ERK, and AKT pathway [46–49]. The downregulation of such pathways resulted in the apoptosis of ovarian cancer cells via caspases 8 and 9 released by the mitochondria [42, 48]. Secondly, the dysregulation of the JNK/ERK pathway also produced an upregulation of CHOP which is released in response to ER stress [49]. Moreover, KMP increased intracellular Ca2+ concentrations leading to cell autophagy in response to ER stress. KMP inhibits ovarian tumor cell growth by G2/M phase arrest along with upregulation of p53, Chk2, Cdc25C/Cdc2 [48, 50]. KMP attenuates ER stress mediated cell death in non-cancerous cells whereas it can trigger ER mediated cell death via various mechanisms including autophagy in a selective manner in a range of cancer cells [49, 51].
1.4. Cytotoxic Role of Kaempferol
The dietary ingredient KMP has been found to be mutagenic in several studies on cell culture and animal models [52–58], with no reports on its tumerogenic activity. Carver et. al. (1982) [52] performed studies on hamster ovarian cells showing flavonols such as quercetin and KMP were involved with DNA damage and chromosomal aberrations without point mutation. Furthermore, Sahu et al., (1994) [56] reported that polyphenolic flavonoid such as KMP induced nuclear DNA damage and lipid peroxidation in isolated rat liver nuclei, which are suggestive of pro-oxidative role of KMP. Takanashi et al. (1983) [57] conducted oral administration of KMP in rats over 540 days which triggered mutagenic activities without causing any tumor formation. In a more recent study by Yangzom et al. (2022) [58], oral administration of KMP over a 28-day period revealed no toxic effect on the physiological function of mice. Over this time period, no significant alterations in oxidative stress and lipid profiles were observed. Studies by Arif et al. [52] showed that flavonoids facilitate the copper-dependent oxidative DNA breakage, which can be exploited to treat cancer.
CONCLUSION
Different cancer cells and subtypes clearly rely on a variety of strategies to promote cell survival and metastasis. Chemotherapeutic agents or targeted therapeutics do not always exhibit their full potential for inhibiting cancer. It is thus worthwhile to seek alternative strategies to resolve cellular abnormalities. Recent evidence has indicated that kaempferol is a valuable anticancer therapeutic due to its low systemic toxicity and high potency for inhibiting multiple pro-cancerous pathways in a selective manner. Kaempferol has also been found to target cancer stem cells and could thus be a potent choice to treat recurrent tumors, which are a particular concern in cancer therapy. Kaempferol treatment has been proven to enhance the rate of apoptosis, making it a suitable candidate for treating cancer. Pharmaceutical applications of kaempferol either alone or in combination with conventional treatments, such as radio/chemotherapy, could be exploited to establish a new paradigm in cancer treatment.
ACKNOWLEDGEMENTS
We thank Edanz (www.edanz.com/ac) for editing this manuscript.
FUNDING
This work was supported by a Research and Creative Activity Grant (150030-26214-150) and a Welch Foundation Grant (AN-0008) at the Department of Chemistry and Bio-chemistry of Stephen F. Austin State University. DR thanks Mississippi INBRE, funded by NIGMS of NIH, under grant number P20GM103476 at Alcorn State University.
LIST OF ABBREVIATIONS
- AMPK
AMP-activated Protein Kinase
- KMP
Kaempferol
- MAPK
Mitogen-activated Protein Kinase
- RTK/RAS-ERK
Extracellular Signal-regulated Kinases
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- [1].Vander Heiden MG; Lunt SY; Dayton TL; Fiske BP; Israelsen WJ; Mattaini KR; Vokes NI; Stephanopoulos G; Cantley LC; Metallo CM; Locasale JW Metabolic pathway alterations that support cell proliferation. Cold Spring Harb. Symp. Quant. Biol, 2011, 76(0), 325–334. 10.1101/sqb.2012.76.010900 [DOI] [PubMed] [Google Scholar]
- [2].Velders MA; Hagström E; James SK Temporal trends in the prevalence of cancer and its impact on outcome in patients with first myocardial infarction: A nationwide study. J. Am. Heart Assoc, 2020, 9(4), e014383. 10.1161/JAHA.119.014383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sung H; Ferlay J; Siegel RL; Laversanne M; Soerjomataram I; Jemal A; Bray F Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin, 2021, 71(3), 209–249. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- [4].Warburg O The metabolism of carcinoma cells. J. Cancer Res, 1925, 9(1), 148–163. 10.1158/jcr.1925.148 [DOI] [Google Scholar]
- [5].Ying H; Kimmelman AC; Lyssiotis CA; Hua S; Chu GC; Fletcher-Sananikone E; Locasale JW; Son J; Zhang H; Col-off JL; Yan H; Wang W; Chen S; Viale A; Zheng H; Paik J; Lim C; Guimaraes AR; Martin ES; Chang J; Hezel AF; Perry SR; Hu J; Gan B; Xiao Y; Asara JM; Weissleder R; Wang YA; Chin L; Cantley LC; DePinho RA Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell, 2012, 149(3), 656–670. 10.1016/j.cell.2012.01.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Son J; Lyssiotis CA; Ying H; Wang X; Hua S; Ligorio M; Perera RM; Ferrone CR; Mullarky E; Shyh-Chang N; Kang Y; Fleming JB; Bardeesy N; Asara JM; Haigis MC; DePinho RA; Cantley LC; Kimmelman AC Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature, 2013, 496(7443), 101–105. 10.1038/nature12040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yang P; Ebbert JO; Sun Z; Weinshilboum RM Role of the glutathione metabolic pathway in lung cancer treatment and prognosis: A review. J. Clin. Oncol, 2006, 24(11), 1761–1769. 10.1200/JCO.2005.02.7110 [DOI] [PubMed] [Google Scholar]
- [8].Sanchez-Vega F; Mina M; Armenia J; Chatila WK; Luna A; La KC; Dimitriadoy S; Liu DL; Kantheti HS; Saghafinia S; Chakravarty D; Daian F; Gao Q; Bailey MH; Liang WW; Foltz SM; Shmulevich I; Ding L; Heins Z; Ochoa A; Gross B; Gao J; Zhang H; Kundra R; Kandoth C; Bahceci I; Dervishi L; Dogrusoz U; Zhou W; Shen H; Laird PW; Way GP; Greene CS; Liang H; Xiao Y; Wang C; Iavarone A; Berger AH; Bivona TG; Lazar AJ; Hammer GD; Giordano T; Kwong LN; McArthur G; Huang C; Tward AD; Frederick MJ; McCormick F; Meyerson M; Van Allen EM; Cherniack AD; Ciriello G; Sander C; Schultz N; Caesar-Johnson SJ; Demchok JA; Felau I; Kasapi M; Ferguson ML; Hutter CM; Sofia HJ; Tarnuzzer R; Wang Z; Yang L; Zenklusen JC; Zhang JJ; Chudamani S; Liu J; Lolla L; Naresh R; Pihl T; Sun Q; Wan Y; Wu Y; Cho J; DeFreitas T; Frazer S; Gehlenborg N; Getz G; Heiman DI; Kim J; Lawrence MS; Lin P; Meier S; Noble MS; Saksena G; Voet D; Zhang H; Bernard B; Chambwe N; Dhankani V; Knijnenburg T; Kramer R; Leinonen K; Liu Y; Miller M; Reynolds S; Shmulevich I; Thorsson V; Zhang W; Akbani R; Broom BM; Hegde AM; Ju Z; Kanchi RS; Korkut A; Li J; Liang H; Ling S; Liu W; Lu Y; Mills GB; Ng K-S; Rao A; Ryan M; Wang J; Weinstein JN; Zhang J; Abeshouse A; Armenia J; Chakravarty D; Chatila WK; de Bruijn I; Gao J; Gross BE; Heins ZJ; Kundra R; La K; Ladanyi M; Luna A; Nissan MG; Ochoa A; Phillips SM; Reznik E; Sanchez-Vega F; Sander C; Schultz N; Sheridan R; Sumer SO; Sun Y; Taylor BS; Wang J; Zhang H; Anur P; Peto M; Spellman P; Benz C; Stuart JM; Wong CK; Yau C; Hayes DN; Parker JS; Wilkerson MD; Ally A; Balasundaram M; Bowlby R; Brooks D; Carlsen R; Chuah E; Dhalla N; Holt R; Jones SJM; Kasaian K; Lee D; Ma Y; Marra MA; Mayo M; Moore RA; Mungall AJ; Mungall K; Robertson AG; Sadeghi S; Schein JE; Sipahimalani P; Tam A; Thiessen N; Tse K; Wong T; Berger AC; Beroukhim R; Cherniack AD; Cibulskis C; Gabriel SB; Gao GF; Ha G; Meyerson M; Schumacher SE; Shih J; Kucherlapati MH; Kucherlapati RS; Baylin S; Cope L; Danilova L; Bootwalla MS; Lai PH; Maglinte DT; Van Den Berg DJ; Weisenberger DJ; Auman JT; Balu S; Bodenheimer T; Fan C; Hoadley KA; Hoyle AP; Jefferys SR; Jones CD; Meng S; Mieczkowski PA; Mose LE; Perou AH; Perou CM; Roach J; Shi Y; Simons JV; Skelly T; Soloway MG; Tan D; Veluvolu U; Fan H; Hinoue T; Laird PW; Shen H; Zhou W; Bellair M; Chang K; Covington K; Creighton CJ; Dinh H; Doddapaneni HV; Donehower LA; Drummond J; Gibbs RA; Glenn R; Hale W; Han Y; Hu J; Korchina V; Lee S; Lewis L; Li W; Liu X; Morgan M; Morton D; Muzny D; Santibanez J; Sheth M; Shinbrot E; Wang L; Wang M; Wheeler DA; Xi L; Zhao F; Hess J; Appelbaum EL; Bailey M; Cordes MG; Ding L; Fronick CC; Fulton LA; Fulton RS; Kandoth C; Mardis ER; McLellan MD; Miller CA; Schmidt HK; Wilson RK; Crain D; Curley E; Gardner J; Lau K; Mallery D; Morris S; Paulauskis J; Penny R; Shelton C; Shelton T; Sherman M; Thompson E; Yena P; Bowen J; Gastier-Foster JM; Gerken M; Leraas KM; Lichtenberg TM; Ramirez NC; Wise L; Zmuda E; Corcoran N; Costello T; Hovens C; Carvalho AL; de Carvalho AC; Fregnani JH; Longatto-Filho A; Reis RM; Scapulatempo-Neto C; Silveira HCS; Vidal DO; Burnette A; Eschbacher J; Hermes B; Noss A; Singh R; Anderson ML; Castro PD; Ittmann M; Huntsman D; Kohl B; Le X; Thorp R; Andry C; Duffy ER; Lyadov V; Paklina O; Setdikova G; Shabunin A; Tavobilov M; McPherson C; Warnick R; Berkowitz R; Cramer D; Feltmate C; Horowitz N; Kibel A; Muto M; Raut CP; Malykh A; Barnholtz-Sloan JS; Barrett W; Devine K; Fulop J; Ostrom QT; Shimmel K; Wolinsky Y; Sloan AE; De Rose A; Giuliante F; Goodman M; Karlan BY; Hagedorn CH; Eckman J; Harr J; Myers J; Tucker K; Zach LA; Deyarmin B; Hu H; Kvecher L; Larson C; Mural RJ; Somiari S; Vicha A; Zelinka T; Bennett J; Iacocca M; Rabeno B; Swanson P; Latour M; Lacombe L; Têtu B; Bergeron A; McGraw M; Staugaitis SM; Chabot J; Hibshoosh H; Sepulveda A; Su T; Wang T; Potapova O; Voronina O; Desjardins L; Mariani O; Roman-Roman S; Sastre X; Stern M-H; Cheng F; Signoretti S; Berchuck A; Bigner D; Lipp E; Marks J; McCall S; McLendon R; Secord A; Sharp A; Behera M; Brat DJ; Chen A; Delman K; Force S; Khuri F; Magliocca K; Maithel S; Olson JJ; Owonikoko T; Pickens A; Ramalingam S; Shin DM; Sica G; Van Meir EG; Zhang H; Eijckenboom W; Gillis A; Korpershoek E; Looijenga L; Oosterhuis W; Stoop H; van Kessel KE; Zwarthoff EC; Calatozzolo C; Cuppini L; Cuzzubbo S; DiMeco F; Finocchiaro G; Mattei L; Perin A; Pollo B; Chen C; Houck J; Lohavanichbutr P; Hartmann A; Stoehr C; Stoehr R; Taubert H; Wach S; Wullich B; Kycler W; Murawa D; Wiznerowicz M; Chung K; Edenfield WJ; Martin J; Baudin E; Bubley G; Bueno R; De Rienzo A; Richards WG; Kalkanis S; Mikkelsen T; Noushmehr H; Scarpace L; Girard N; Aymerich M; Campo E; Giné E; Guillermo AL; Van Bang N; Hanh PT; Phu BD; Tang Y; Colman H; Evason K; Dottino PR; Martignetti JA; Gabra H; Juhl H; Akeredolu T; Stepa S; Hoon D; Ahn K; Kang KJ; Beuschlein F; Breggia A; Birrer M; Bell D; Borad M; Bryce AH; Castle E; Chandan V; Cheville J; Copland JA; Farnell M; Flotte T; Giama N; Ho T; Kendrick M; Kocher J-P; Kopp K; Moser C; Nagorney D; O’Brien D; O’Neill BP; Patel T; Petersen G; Que F; Rivera M; Roberts L; Smallridge R; Smyrk T; Stanton M; Thompson RH; Torbenson M; Yang JD; Zhang L; Brimo F; Ajani JA; Gonzalez AMA; Behrens C; Bondaruk J; Broaddus R; Czerniak B; Esmaeli B; Fujimoto J; Gershenwald J; Guo C; Lazar AJ; Logothetis C; Meric-Bernstam F; Moran C; Ramondetta L; Rice D; Sood A; Tamboli P; Thompson T; Troncoso P; Tsao A; Wistuba I; Carter C; Haydu L; Hersey P; Jakrot V; Kakavand H; Kefford R; Lee K; Long G; Mann G; Quinn M; Saw R; Scolyer R; Shannon K; Spillane A; Stretch J; Synott M; Thompson J; Wilmott J; Al-Ahmadie H; Chan TA; Ghossein R; Gopalan A; Levine DA; Reuter V; Singer S; Singh B; Tien NV; Broudy T; Mirsaidi C; Nair P; Drwiega P; Miller J; Smith J; Zaren H; Park J-W; Hung NP; Kebebew E; Linehan WM; Metwalli AR; Pacak K; Pinto PA; Schiffman M; Schmidt LS; Vocke CD; Wentzensen N; Worrell R; Yang H; Moncrieff M; Goparaju C; Melamed J; Pass H; Botnariuc N; Caraman I; Cernat M; Chemencedji I; Clipca A; Doruc S; Gorincioi G; Mura S; Pirtac M; Stancul I; Tcaciuc D; Albert M; Alexopoulou I; Arnaout A; Bartlett J; Engel J; Gilbert S; Parfitt J; Sekhon H; Thomas G; Rassl DM; Rintoul RC; Bifulco C; Tamakawa R; Urba W; Hayward N; Timmers H; Antenucci A; Facciolo F; Grazi G; Marino M; Merola R; de Krijger R; Gimenez-Roqueplo A-P; Piché A; Chevalier S; McKercher G; Birsoy K; Barnett G; Brewer C; Farver C; Naska T; Pennell NA; Raymond D; Schilero C; Smolenski K; Williams F; Morrison C; Borgia JA; Liptay MJ; Pool M; Seder CW; Junker K; Omberg L; Dinkin M; Manikhas G; Alvaro D; Bragazzi MC; Cardinale V; Carpino G; Gaudio E; Chesla D; Cottingham S; Dubina M; Moiseenko F; Dhanasekaran R; Becker K-F; Janssen K-P; Slotta-Huspenina J; Abdel-Rahman MH; Aziz D; Bell S; Cebulla CM; Davis A; Duell R; Elder JB; Hilty J; Kumar B; Lang J; Lehman NL; Mandt R; Nguyen P; Pilarski R; Rai K; Schoenfield L; Senecal K; Wakely P; Hansen P; Lechan R; Powers J; Tischler A; Grizzle WE; Sexton KC; Kastl A; Henderson J; Porten S; Waldmann J; Fassnacht M; Asa SL; Schadendorf D; Couce M; Graefen M; Huland H; Sauter G; Schlomm T; Simon R; Tennstedt P; Olabode O; Nelson M; Bathe O; Carroll PR; Chan JM; Disaia P; Glenn P; Kelley RK; Landen CN; Phillips J; Prados M; Simko J; Smith-McCune K; VandenBerg S; Roggin K; Fehrenbach A; Kendler A; Sifri S; Steele R; Jimeno A; Carey F; Forgie I; Mannelli M; Carney M; Hernandez B; Campos B; Herold-Mende C; Jungk C; Unterberg A; von Deimling A; Bossler A; Galbraith J; Jacobus L; Knudson M; Knutson T; Ma D; Milhem M; Sigmund R; Godwin AK; Madan R; Rosenthal HG; Adebamowo C; Adebamowo SN; Boussioutas A; Beer D; Giordano T; Mes-Masson A-M; Saad F; Bocklage T; Landrum L; Mannel R; Moore K; Moxley K; Postier R; Walker J; Zuna R; Feldman M; Valdivieso F; Dhir R; Luketich J; Pinero EMM; Quintero-Aguilo M; Carlotti CG Jr; Dos Santos JS; Kemp R; Sankarankuty A; Tirapelli D; Catto J; Agnew K; Swisher E; Creaney J; Robinson B; Shelley CS; Godwin EM; Kendall S; Shipman C; Bradford C; Carey T; Haddad A; Moyer J; Peterson L; Prince M; Rozek L; Wolf G; Bowman R; Fong KM; Yang I; Korst R; Rathmell WK; Fantacone-Campbell JL; Hooke JA; Kovatich AJ; Shriver CD; DiPersio J; Drake B; Govindan R; Heath S; Ley T; Van Tine B; Westervelt P; Rubin MA; Lee JI; Aredes ND; Mariamidze A Oncogenic signaling pathways in the cancer genome atlas. Cell, 2018, 173(2), 321–337.e10. 10.1016/j.cell.2018.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].DeBerardinis RJ; Chandel NS Fundamentals of cancer metabolism. Sci. Adv, 2016, 2(5), e1600200. 10.1126/sciadv.1600200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pavlova NN; Thompson CB The emerging hallmarks of cancer metabolism. Cell Metab, 2016, 23(1), 27–47. 10.1016/j.cmet.2015.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Reznik E; Luna A; Aksoy BA; Liu EM; La K; Ostrovnaya I; Creighton CJ; Hakimi AA; Sander C A landscape of metabolic variation across tumor types. Cell Syst, 2018, 6(3), 301–313.e3. 10.1016/j.cels.2017.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ward PS; Thompson CB Signaling in control of cell growth and metabolism. Cold Spring Harb. Perspect. Biol, 2012, 4(7), a006783–a006783. 10.1101/cshperspect.a006783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Vogelstein B; Kinzler KW Cancer genes and the pathways they control. Nat. Med, 2004, 10(8), 789–799. 10.1038/nm1087 [DOI] [PubMed] [Google Scholar]
- [14].Magee JA; Piskounova E; Morrison SJ Cancer stem cells: Impact, heterogeneity, and uncertainty. Cancer Cell, 2012, 21(3), 283–296. 10.1016/j.ccr.2012.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hemmings BA; Restuccia DF PI3K-PKB/Akt pathway. Cold Spring Harb. Perspect. Biol, 2012, 4(9), a011189–a011189. 10.1101/cshperspect.a011189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wall ME Camptothecin and taxol: Discovery to clinic. Med. Res. Rev, 1998, 18(5), 299–314. [DOI] [PubMed] [Google Scholar]
- [17].Ottaviani A; Iacovelli F; Fiorani P; Desideri A Natural compounds as therapeutic agents: The case of human topoisomerase IB. Int. J. Mol. Sci, 2021, 22(8), 4138. 10.3390/ijms22084138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hendriks HR; Govaerts AS; Fichtner I; Burtles S; Westwell AD; Peters GJ Pharmacologically directed strategies in academic anticancer drug discovery based on the European NCI compounds initiative. Br. J. Cancer, 2017, 117(2), 195–202. 10.1038/bjc.2017.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Limmroth V; Katsarava Z; Diener H-C Acetylsalicylic acid in the treatment of headache. Cephalalgia, 1999, 19(6), 545–551. 10.1046/j.1468-2982.1999.019006545.x [DOI] [PubMed] [Google Scholar]
- [20].Salehi B; Mishra A; Nigam M; Sener B; Kilic M; Sharifi-Rad M; Fokou P; Martins N; Sharifi-Rad J Resveratrol: A double-edged sword in health benefits. Biomedicines, 2018, 6(3), 91. 10.3390/biomedicines6030091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Akinwumi B; Bordun KA; Anderson H Biological activities of stilbenoids. Int. J. Mol. Sci, 2018, 19(3), 792. 10.3390/ijms19030792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Soundararajan P; Kim J Anti-carcinogenic glucosinolates in cruciferous vegetables and their antagonistic effects on prevention of cancers. Molecules, 2018, 23(11), 2983. 10.3390/molecules23112983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Teng J; Li J; Zhao Y; Wang M Hesperetin, a dietary flavonoid, inhibits AGEs-induced oxidative stress and inflammation in RAW264.7 cells. J. Funct. Foods, 2021, 81, 104480. 10.1016/j.jff.2021.104480 [DOI] [Google Scholar]
- [24].Piletz JE; Mao Y; Roy D; Qizilbash B; Nkamssi E; Weir E; Graham J; Emmanuel M; Iqbal S; Brue K; Sengupta B Transepithelial anti-neuroblastoma response to kale among four vegetable juices using in vitro model co-culture system. Nutrients, 2021, 13(2), 488. 10.3390/nu13020488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Khuda-Bukhsh AR; Das S; Saha SK Molecular approaches toward targeted cancer prevention with some food plants and their products: Inflammatory and other signal pathways. Nutr. Cancer, 2014, 66(2), 194–205. 10.1080/01635581.2014.864420 [DOI] [PubMed] [Google Scholar]
- [26].Lipinski B Hydroxyl radical and its scavengers in health and disease. Oxid. Med. Cell. Longev, 2011, 2011, 809696. 10.1155/2011/809696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Singh SK; Banerjee S; Acosta EP; Lillard JW; Singh R Resveratrol induces cell cycle arrest and apoptosis with docetaxel in prostate cancer cells via a p53/p21WAF1/CIP1 and p27KIP1 pathway. Oncotarget, 2017, 8(10), 17216–17228. 10.18632/oncotarget.15303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].NavaneethaKrishnan S; Rosales JL; Lee KYS; Rosales JL; Lee K-Y ROS-mediated cancer cell killing through dietary phytochemicals. Oxid. Med. Cell. Longev, 2019, 2019, 1–16. 10.1155/2019/9051542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yang L; Gao Y; Bajpai VK; El-Kammar HA; Simal-Gandara J; Cao H; Cheng KW; Wang M; Arroo RRJ; Zou L; Farag MA; Zhao Y; Xiao J Advance toward isolation, extraction, metabolism and health benefits of kaempferol, a major dietary flavonoid with future perspectives. Crit. Rev. Food Sci. Nutr, 2021, 1980762. 10.1080/10408398.2021.1980762 [DOI] [PubMed] [Google Scholar]
- [30].Chen AY; Chen YC A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem, 2013, 138(4), 2099–2107. 10.1016/j.foodchem.2012.11.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kashyap D; Sharma A; Tuli HS; Sak K; Punia S; Mukherjee TK Kaempferol–A dietary anticancer molecule with multiple mechanisms of action: Recent trends and advancements. J. Funct. Foods, 2017, 30, 203–219. 10.1016/j.jff.2017.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Breast Cancer: Statistics. Conquer Cancer, 2022. [Google Scholar]
- [33].Yi X; Zuo J; Tan C; Xian S; Luo C; Chen S; Yu L; Luo Y Kaempferol, a flavonoid compound from gynura medica induced apoptosis and growth inhibition in MCF-7 breast cancer cell. Afr. J. Tradit. Complement. Altern. Med, 2016, 13(4), 210–215. 10.21010/ajtcam.v13i4.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Azevedo C; Correia-Branco A; Araújo JR; Guimarães JT; Keating E; Martel F The chemopreventive effect of the dietary compound kaempferol on the MCF-7 human breast cancer cell line is dependent on inhibition of glucose cellular uptake. Nutr. Cancer, 2015, 67(3), 504–513. 10.1080/01635581.2015.1002625 [DOI] [PubMed] [Google Scholar]
- [35].Hung H Inhibition of estrogen receptor alpha expression and function in MCF-7 cells by kaempferol. J. Cell. Physiol, 2004, 198(2), 197–208. 10.1002/jcp.10398 [DOI] [PubMed] [Google Scholar]
- [36].Liao W; Chen L; Ma X; Jiao R; Li X; Wang Y Protective effects of kaempferol against reactive oxygen species-induced hemolysis and its antiproliferative activity on human cancer cells. Eur. J. Med. Chem, 2016, 114, 24–32. 10.1016/j.ejmech.2016.02.045 [DOI] [PubMed] [Google Scholar]
- [37].Choi EJ; Ahn WS Kaempferol induced the apoptosis via cell cycle arrest in human breast cancer MDA-MB-453 cells. Nutr. Res. Pract, 2008, 2(4), 322–325. 10.4162/nrp.2008.2.4.322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Li C; Zhao Y; Yang D; Yu Y; Guo H; Zhao Z; Zhang B; Yin X Inhibitory effects of kaempferol on the invasion of human breast carcinoma cells by downregulating the expression and activity of matrix metalloproteinase-9. Biochem. Cell Biol, 2015, 93(1), 16–27. 10.1139/bcb-2014-0067 [DOI] [PubMed] [Google Scholar]
- [39].Zhu L; Xue L Kaempferol suppresses proliferation and induces cell cycle arrest, apoptosis, and DNA damage in breast cancer cells. Oncol. Res, 2019, 27(6), 629–634. 10.3727/096504018X15228018559434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Nandi SK; Roychowdhury T; Chattopadhyay S; Basu S; Chatterjee K; Choudhury P; Banerjee N; Saha P; Mukhopadhyay S; Mukhopadhyay A; Bhattacharya R Deregulation of the CD44-NANOG-MDR1 associated chemoresistance pathways of breast cancer stem cells potentiates the anti-cancer effect of Kaempferol in synergism with Verapamil. Toxicol. Appl. Pharmacol, 2022, 437, 115887. 10.1016/j.taap.2022.115887 [DOI] [PubMed] [Google Scholar]
- [41].American Cancer Society. Cancer Facts & Figures 2022. Available form: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2022.html.
- [42].Lee GA; Choi KC; Hwang KA Treatment with phytoestrogens reversed triclosan and bisphenol A-induced anti-apoptosis in breast cancer cells. Biomol. Ther. (Seoul), 2018, 26(5), 503–511. 10.4062/biomolther.2017.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kashafi E; Moradzadeh M; Mohamadkhani A; Erfanian S Kaempferol increases apoptosis in human cervical cancer HeLa cells via PI3K/AKT and telomerase pathways. Biomed. Pharmacother, 2017, 89, 573–577. 10.1016/j.biopha.2017.02.061 [DOI] [PubMed] [Google Scholar]
- [44].Tu LY; Bai HH; Cai JY; Deng SP The mechanism of kaempferol induced apoptosis and inhibited proliferation in human cervical cancer SiHa cell: From macro to nano. Scanning, 2016, 38(6), 644–653. 10.1002/sca.21312 [DOI] [PubMed] [Google Scholar]
- [45].Cancer.Net. Ovarian, Fallopian Tube; Cancer, Peritoneal Ovarian, fallopian tube, and peritoneal cancer: Statistics. 2022. Available form: https://www.cancer.net/cancer-types/ovarian-fallopian-tube-and-peritoneal-cancer/statistics
- [46].Gao Y; Yin J; Rankin G; Chen Y Kaempferol induces G2/M cell cycle arrest via checkpoint kinase 2 and promotes apoptosis via death receptors in human ovarian carcinoma A2780/CP70 cells. Molecules, 2018, 23(5), 1095. 10.3390/molecules23051095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Yang S; Si L; Jia Y; Jian W; Yu Q; Wang M; Lin R Kaempferol exerts anti-proliferative effects on human ovarian cancer cells by inducing apoptosis, G0/G1 cell cycle arrest and modulation of MEK/ERK and STAT3 pathways. J. BUON, 2019, 24(3), 975–981. [PubMed] [Google Scholar]
- [48].Zhao Y; Tian B; Wang Y; Ding H Kaempferol sensitizes human ovarian cancer cells OVCAR-3 and SKOV-3 to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis via JNK/ERK-CHOP pathway and up-regulation of death receptors 4 and 5. Med. Sci. Monit, 2017, 23, 5096–5105. 10.12659/MSM.903552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].El-Kott AF; Shati AA; Al-Kahtani MA; Alharbi SA Kaempferol induces cell death in A2780 ovarian cancer cells and increases their sensitivity to cisplatin by activation of cytotoxic endoplasmic reticulum-mediated autophagy and inhibition of protein kinase B. Folia Biol. (Praha), 2020, 66(1), 36–46. [DOI] [PubMed] [Google Scholar]
- [50].Luo H; Rankin GO; Li Z; DePriest L; Chen YC Kaempferol induces apoptosis in ovarian cancer cells through activating p53 in the intrinsic pathway. Food Chem, 2011, 128(2), 513–519. 10.1016/j.foodchem.2011.03.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ashrafizadeh M; Tavakol S; Ahmadi Z; Roomiani S; Mohammadinejad R; Samarghandian S Therapeutic effects of kaempferol affecting autophagy and endoplasmic reticulum stress. Phytother. Res, 2020, 34(5), 911–923. 10.1002/ptr.6577 [DOI] [PubMed] [Google Scholar]
- [52].Arif H; Sohail A; Farhan M; Rehman AA; Ahmad A; Hadi SM Flavonoids-induced redox cycling of copper ions leads to generation of reactive oxygen species: A potential role in cancer chemoprevention. Int. J. Biol. Macromol, 2018, 106, 569–578. 10.1016/j.ijbiomac.2017.08.049 [DOI] [PubMed] [Google Scholar]
- [53].Carver JH; Carrano AV; MacGregor JT Genetic effects of the flavonols quercetin, kaempferol, and galangin on Chinese hamster ovary cells in vitro. Mutat. Res. Envir. Mutag. Relat. Subj, 1983, 113(1), 45–60. 10.1016/0165-1161(83)90240-6 [DOI] [PubMed] [Google Scholar]
- [54].Hu Y; Cheng Z; Heller LI; Krasnoff SB; Glahn RP; Welch RM Kaempferol in red and pinto bean seed (Phaseolus vulgaris L.) coats inhibits iron bioavailability using an in vitro digestion/human Caco-2 cell model. J. Agric. Food Chem, 2006, 54(24), 9254–9261. 10.1021/jf0612981 [DOI] [PubMed] [Google Scholar]
- [55].Ren J; Lu Y; Qian Y; Chen B; Wu T; Ji G Recent progress regarding kaempferol for the treatment of various diseases (Review). Exp. Ther. Med, 2019, 18(4), 2759–2776. 10.3892/etm.2019.7886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Sahu SC; Gray GC Kaempferol-induced nuclear DNA damage and lipid peroxidation. Cancer Lett, 1994, 85(2), 159–164. 10.1016/0304-3835(94)90269-0 [DOI] [PubMed] [Google Scholar]
- [57].Takanashi H; Aiso S; Hirono I; Matsushima T; Sugimura T Carcinogenecity test of quercetin and kaempferol in rats by oral administration. J. Food Saf, 1983, 5(2), 55–60. 10.1111/j.1745-4565.1983.tb00455.x [DOI] [Google Scholar]
- [58].Yangzom P; Amruthanand S; Sharma M; Mahajan S; Linga-raju MC; Parida S; Sahoo M; Kumar D; Singh TU Subacute 28 days oral toxicity study of kaempferol and biochanin-A in the mouse model. J. Biochem. Mol. Toxicol, 2022, 36(8)e23090 10.1002/jbt.23090 [DOI] [PubMed] [Google Scholar]


