Abstract
Problem:
This COVID-19 outpatient randomized controlled trials (RCTs) systematic review compares hospitalization outcomes amongst four treatment classes over pandemic period, geography, variants and vaccine status.
Methods:
Outpatient RCTs with hospitalization endpoint were identified in Pubmed searches through May 2023, excluding RCTs < 30 participants (PROSPERO-CRD42022369181). Risk of bias was extracted from COVID-19-NMA, with odds ratio utilized for pooled comparison.
Results:
Searches identified 281 studies with 61 published RCTs for 33 diverse interventions analyzed. RCTs were largely unvaccinated cohorts with at least one COVID-19 hospitalization risk factor. Grouping by class, monoclonal antibodies (OR=0.31 [95% CI=0.24–0.40]) had highest hospital reduction efficacy, followed by COVID-19 convalescent plasma (CCP) (OR=0.69 [95% CI=0.53 to 0.90]), small molecule antivirals (OR=0.78 [95% CI=0.48–1.33]) and repurposed drugs (OR=0.82 [95% CI- 0.72–0.93]). Earlier in disease onset interventions performed better than later. This meta-analysis allows approximate head-to-head comparisons of diverse outpatient interventions.
Conclusions:
Omicron sublineages (XBB and BQ.1.1) are resistant to monoclonal antibodies. Despite trial heterogeneity, this pooled comparison by intervention class indicated oral antivirals are the preferred outpatient treatment where available, but intravenous interventions from convalescent plasma to remdesivir are also effective and necessary in constrained medical resource settings or for acute and chronic COVID-19 in the immunocompromised.
Keywords: small molecule antivirals, convalescent plasma, monoclonal antibody, COVID-19, outpatients, randomized controlled trial
INTRODUCTION
By May 17, 2023 the world had recorded over 766 million cases and more than 6.9 million deaths from COVID-19. In the US, some 100 million cases have been recorded, with over a million deaths, while six million hospital admissions for COVID took place between August 2020 and December 2022. A pronounced spike in hospitalizations for COVID-19 in the US took place in the first two months of 2022 with the arrival of the Omicron variant of concern (VOC).
Several approaches to reducing the risk of hospitalization have been taken during the pandemic, including administering COVID-19 convalescent plasma (CCP), monoclonal antibodies (mAbs), small molecule antivirals or repurposed drugs. Vaccination and boosters have substantially reduced the hospitalization and death risk, but outpatients at elevated severe COVID risk can still benefit from early treatment to avoid hospitalization. Randomized controlled trials (RCTs) in outpatients have tested therapeutic agents against placebo or standard of care, but very few RCTs has been conducted that compare the main outpatient treatment classes.
The first outpatient treatments for COVID-19 authorized by the FDA were for mAbs (bamlanivimab, bamlanivimab plus etesevimab1 or casirivimab plus imdevimab2), approvals that preceded the introduction of mRNA vaccines3,4. While many small molecules were repurposed as antivirals during the early stages of the pandemic, oral antivirals developed against SARS-CoV-2 for outpatients were not authorized and available until December 2021, when nirmatrelvir/ritonavir5 and molnupiravir6 were approved. The following month, intravenous remdesivir was also approved for outpatient use7. On December 2021, nearly two years after the first use of CCP, the FDA approved CCP outpatient use, but only for immunocompromised patients8,9. The mAbs have been withdrawn due to viral variants BQ.1.* and XBB.* resistance.
The rationale of the study was to assemble in one place all outpatient COVID-19 RCT in the four classes to compare hospitalization outcomes over time, geography in relationship to variants and vaccine status. We were inclusive of smaller studies meeting criteria of RCT with endpoint hospitalization. This systematic review and meta-analysis of outpatient COVID-19 RCTs, sought to compare hospitalization outcomes amongst CCP, mAbs, antivirals or repurposed drugs as grouped classes and individual trials taking into account risk factors for progression, intervention dosage, time between symptom onset and treatment administration, and predominant variants of concern during the RCTs.
2. METHODS
2.1. Registration
The protocol has been registered in PROSPERO, the prospective register of systematic reviews and meta-analyses of the University of York (protocol registration number CRD42022369181).
2.2. Inclusion and Exclusion Criteria and Data Extraction
We included outpatient COVID-19 RCTs with outcome hospitalization or a single CCP study with life-threatening respiratory distress10, by searching MEDLINE (through PubMed), medRxiv and bioRxiv databases for the period of March 1, 2020 to May 22, 2023, with English language as the only restriction. The Medical Subject Heading (MeSH) and search query used were: “(“COVID-19” OR “SARS-CoV-2” OR “coronavirus disease 2019”) AND (“treatment” OR “therapy”) AND (“outpatient”) AND (“hospitalization”)” AND (“randomized clinical trial”). We also screened the reference list of reviewed articles for studies not captured in our initial search. We excluded case reports, case series, retrospective, propensity-matched studies, non-randomized clinical trials, review articles, meta-analyses, guidelines, studies with fewer than 30 participants, studies that did not record or had no hospitalizations, protocol only publications and articles reporting only aggregate data. Trials of COVID prevention11 were excluded, even if hospitalizations were recorded. Inclusion and exclusion reasons are summarized for the 281 citations in Appendix table 1. Articles underwent a blind evaluation for inclusion by two assessors (D.S. and D.F.) and disagreements were resolved by a third senior assessor (A.C.). Figure 1 shows a PRISMA flowchart of the literature reviewing process. The following parameters were extracted by at least one reviewer from studies: baseline SARS-CoV-2 serology status, time from onset of symptoms to treatment, study dates, recruiting countries, gender, age (including the fraction of participants over age 50, 60 and 65), ethnicity, risk factors for COVID-19 progression (systemic arterial hypertension, diabetes mellitus, and obesity), sample size, dosage type of control, hospitalizations and deaths in each arm, and time to symptom resolution (Appendix Table 2). Study dates were used to infer predominant VOCs. The studies were grouped into classes by CCP, mAbs, antivirals or repurposed drugs.
Figure 1.

PRISMA flowchart for randomized controlled trials (RCT) selection in this systematic review.
** All excluded by a human
2.3. Assessment of Risk of Bias and GRADE Assessment
A risk of bias assessment of each selected RCT was performed by COVID-19-NMA initiative12,13. Within-trial risk of bias was assessed, using the Cochrane ROB tool for RCTs14. We explored clinical heterogeneity (e.g., risk factors for progression, time between onset of symptoms and treatment administration, and predominant variants of concern at the time of the interventions) and calculated statistical heterogeneity using τ2, Cochran’s Q and estimated this using the I2 statistic, which examines the total variation percentage across studies due to heterogeneity rather than chance. Each study was evaluated by at least two reviewers.
We used the GRADE (The Grading of Recommendations Assessment, Development and Evaluation) system criteria to assess the quality of the body of evidence associated with specific outcomes, and constructed a ‘Summary of Findings’ table (Appendix Table 3), which defines the certainty of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest14. Publication bias was assessed by visual inspection of funnel plots.
2.4. Statistical Analysis
Descriptive analysis included time-to-treatment, geography (country) of the study, age, sex, race, ethnicity, seropositive, hospital type and medical high-risk conditions. The unweighted pooling ARR, RRR, NNT were calculated based on the arithmetic summation of the total hospitalization or death numbers in each therapeutic category.
Odds ratios (OR) and 95% confidence intervals (CI) were used to show the direction of effect and its significance in comparing treatment group and control groups. The studies were weighted with the Mantel-Haenszel method. The effect heterogeneity was calculated estimating the I-squared (I2) inconsistency index. If significant heterogeneity was detected (I2 > 50% and Cochran’s Q test for heterogeneity was significant (p < 0.10)), a random effect model was performed; otherwise, a fixed (common) effect model was performed. Weight, heterogeneity, between-study variance, and significance level were displayed in forest plots. Robustness of hospital risk reduction utilized a leave the highest enrollment study out. The significance level was 0.05. The figures were created in Prism software, R (version 4.2.1) and its statistical package “meta” (version 6.0–0). All the data manipulation and the analyses were performed in Excel, Prism, MedCalc (version 20.106), R and REVMAN.5.
2.5. Role of the Funding Source
The study sponsors did not contribute to the study design; to the collection, analysis, and interpretation of data; to manuscript preparation, nor to the decision to submit the paper for publication.
3. RESULTS
3.1. Trial Characteristics
We reviewed in detail 61 distinct outpatient RCT publications for 70 trial arms including 33 different interventions, concluded before May 22, 2023, across waves sustained by different SARS-CoV-2 variants of concern (VOC) and different vaccination periods in diverse patient populations (Figure 2). The studies varied in reporting outcomes of hospitalization, whether all-cause or COVID-19 related (Appendix Table 2). The CCP group included 3 RCTs with all-cause hospitalization, 2 trials with COVID-19 related hospitalizations only and one trial with life-threatening respiratory distress in elderly individuals, deemed equivalent to hospital outcome (Appendix Table 2). The mAb RCTs included 4 trials with all-cause hospitalizations and 5 that used COVID-19 related hospitalizations as the outcome. The small molecule antivirals had 12 RCTs with all-cause hospitalizations and 5 with COVID-19 related hospitalizations, while 29 RCT’s of repurposed drugs used all-cause hospitalizations and 10 trials restricted to COVID-19 related hospitalizations. Here we report the hospitalizations as a composite of the two hospital types. Because inclusion criteria varied across the RCTs, control group hospitalization rates varied from 0 to 31% with a mean of 1.6% (Table 1). Three of five CCP RCTs had higher control arm hospitalization rates (11% – 31%) than all other antiviral RCTs, indicating that they studied sicker populations.9 (Table 1 and Figure 3). Seven of nine mAb RCTs had control arm hospitalization rates of 4.6–8.9%, the same range as CCP-CSSC-004 (6.3%)9. Control hospitalization rates in the molnupiravir-MOVE-OUT7, nirmatrelvir/ritonavir5 and remdesivir15 RCTs ranged from 5.3% to 9.7%. Low hospitalization rates were found in RCTs that had many vaccinees (metformin-COVID-OUT – 3.2%16) or in which most participants were seropositive (molnupiravir-PANORAMIC – 0.8%). Low control arm hospitalization rates were also found in two mAb RCTs – the bebtelovimab trial (1.6%)17 and REGN-CoV phase 1/2 (<2%), with the bebtelovimab RCT focusing on low-risk patients 17. Lower control hospitalization rates reduce power to detect absolute risk ratios.
Figure 2.

Duration and calendar months of the RCT in context of dominant variant(s) of concern and seropositivity rates. Study start and end for enrollments are charted with approximate time periods for variants of concern.
Table 1.
Hospital rates, risk reductions, NNT, numbers and symptom resolution
| Study | Control hospitalizations % | hospitalizations % in intervention arm | ARR percent (95% CI) | RRR percent (95% CI) | NNT to prevent 1 hospitalization | Hospitalization (n) in control arm | total pts in control arm (n) | Hospitalization (n) in intervention arm | Total pts (n) in intervention arm | Symptom resolution: median duration-Intervention to control in days | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CCP (5 RCT) % or totals | 12 | 8.8 | 3.2 (0.9, 5.6) | 26.8 (8.1, 41.7) | 31 | 158 | 1315 | 116 | 1319 | ||
| anti-Spike mAbs (8 RCT) % or totals | 5.9 | 1.9 | 4.0 (3.2, 4.8) | 67.8 (59.1, 74.7) | 25 | 242 | 4102 | 88 | 4634 | ||
| Small molecule antiviral (11 RCTs) total or average | 1.8 | 1.3 | 0.5 (0.3, 0.8) | 29.1 (15.9, 40.2) | 187 | 314 | 17079 | 222 | 17025 | ||
| Small molecule antiviral (10 RCTs-w/o Mol-Pan.) total or average | 4.7 | 2.6 | 2.1 (1.3, 2.9) | 44.4 (30.7, 55.3) | 48 | 218 | 4595 | 119 | 4509 | ||
| Repurposed drugs (20 RCTs) total or average | 5.3 | 4.2 | 1.1 (0.5, 1.6) | 20.1 (10.1, 28.9) | 94 | 590 | 11121 | 483 | 11391 | ||
| All (47 RCTs) total or average | 3.9 | 2.6 | 1.2 (1.0, 1.5) | 31.8 (25.9, 37.3) | 81 | 1304 | 33617 | 909 | 34369 | ||
| CCP-CONV-ert24 | 11.2 | 11.7 | −0.5 (−7.0, 5.9) | −4.8 (−83.9, 40.3) | −188 | 21 | 188 | 22 | 188 | NO difference 12 d vs 12 d | |
| CCP-COV-Early35 | 9.3 | 5.9 | 3.4 (−1.8, 8.5) | 36.2 (−27.9, 68.2) | 29 | 19 | 204 | 12 | 202 | NO difference 13 d vs 12 d | |
| CCP-C3PO36 | 22.0 | 20.2 | 1.8 (−5.3, 8.9) | 8.2 (−28.3, 34.4) | 55 | 56 | 254 | 52 | 257 | NO difference | |
| CCP-Argentina10 | 31.3 | 16.3 | 15.0 (2.0, 28.0) | 48.0 (5.8, 71.3) | 7 | 25 | 80 | 13 | 80 | Not reported | |
| CCP-CSSC-0049 | 6.3 | 2.9 | 3.4 (1.0, 5.8) | 54.3 (19.7, 74.0) | 29 | 37 | 589 | 17 | 592 | Not reported | |
| CCP-Argentina (high titer)10 | 31.3 | 8.3 | 22.9 (9.3, 36.5) | 73.3 (17.4, 91.4) | 4 | 25 | 80 | 3 | 36 | Not reported | |
| CCP-CSSC-004 (<= 5 days) 9 | 9.7 | 1.9 | 7.7 (3.7, 11.7) | 79.9 (48.4, 92.2) | 13 | 25 | 259 | 5 | 257 | Not reported | |
| Bamlanivimab-BLAZE-11 | 6.3 | 1.6 | 4.7 (0.5, 8.9) | 74.3 (24.7, 91.2) | 21 | 9 | 143 | 5 | 309 | NO difference 11 d to 11 d | |
| Sotrovimab-COMET-ICE37 | 5.7 | 1.1 | 4.5 (2.4, 6.7) | 80.0 (52.3, 91.6) | 22 | 30 | 529 | 6 | 528 | Not reported | |
| Bamlanivimab/etesevimab-BLAZE-125 | 7.0 | 2.1 | 4.8 (2.3, 7.4) | 69.5 (40.8, 84.3) | 21 | 36 | 517 | 11 | 518 | YES-8d vs 9d p=0.007 | |
| Casirivimab/imdevimab-REGEN-COV Ph 32 | 4.6 | 1.3 | 3.3 (2.0, 4.6) | 71.3 (51.7, 82.9) | 30 | 62 | 1341 | 18 | 1355 | YES-10 d vs 14 p=0.0001 | |
| Casirivimab/imdevimab-REGEN-COV Ph 1/238 | 1.9 | 0.6 | 1.3 (−0.4, 3.1) | 70.1 (−24.4, 92.8) | 76 | 5 | 266 | 3 | 533 | Not reported | |
| Bebtelovimab-BLAZE-417 | 1.6 | 1.6 | −0.04 (−3.1, 3.0) | −2.4 (−615.7, 85.4) | −2667 | 2 | 128 | 2 | 125 | YES-6d to 8d p=0.003 | |
| Regdanvimab-CT-P5926 | 8.7 | 4.4 | 4.2 (−1.9, 10.3) | 48.8 (−25.2, 79.0) | 23 | 9 | 104 | 9 | 203 | YES 6 d vs 9 d p=0.01 | |
| Regdanvimab-CT-P59–227 |
7.9 | 2.4 | 5.4 (3.1, 7.8) | 69.1 (46.4, 82.2) | 18 | 52 | 659 | 16 | 656 | 8 d to 13 d | |
| Tixagevimab–cilgavimab-TACKLE39 | 8.9 | 4.4 | 4.5 (1.1, 7.9) | 50.4 (14.3, 71.3) | 22 | 37 | 415 | 18 | 407 | Not reported | |
| Molnupiravir-MOVe-OUT6 | 9.7 | 6.8 | 3.0 (0.1, 5.8) | 30.4 (0.8, 51.2) | 34 | 68 | 699 | 48 | 709 | NO difference | |
| Molnupiravir-PANORAMIC40 | 0.8 | 0.8 | −0.1 (−0.3, 0.2) | −7.0 (−41.2, 18.9) | −1853 | 96 | 12484 | 103 | 12516 | YES 9 d vs 15 d | |
| Molnupiravir-Aurobindo41 | 0.0 | 0.0 | NC | NC | 0 | 0 | 610 | 0 | 610 | Yes 10 d vs 14 d p<0.001 | |
| Nirmatrelvir/ritonavir-EPIC-HR5 | 6.3 | 0.8 | 5.5 (4.0, 7.1) | 87.8 (74.7, 94.1) | 18 | 66 | 1046 | 8 | 1039 | Not reported | |
| Remdesivir-PINETREE15 | 5.3 | 0.7 | 4.6 (1.8, 7.4) | 86.5 (41.4, 96.9) | 22 | 15 | 283 | 2 | 279 | YES-Alleviation of symptoms by day 14 (rate ratio, 1.92; 95% CI, 1.26 to 2.94) | |
| Interferon Lambda-TOGETHER42 | 3.9 | 2.3 | 1.7 (0.1, 3.2) | 42.6 (3.4, 65.9) | 60 | 40 | 1018 | 21 | 931 | ||
| Interferon Lambda-ILIAD43 | 3.3 | 3.3 | 0 (−9.1, 9.1) | 0 (−1426, 93.4) | 1 | 30 | 1 | 30 | No difference | ||
| Interferon Lambda-COVID-Lambda44 | 3.3 | 3.3 | 0 (−6.4, 6.4) | 0 (−586.9, 85.4) | 2 | 60 | 2 | 60 | NO difference 20 d vs 20 d | ||
| Sofosbuvir and daclatasvir-SOVODAK45 | 14.3 | 3.7 | 10.6 (−4.2, 25.4) | 74.1 (−117, 96.9) | 9 | 4 | 28 | 1 | 27 | NO difference in 7 d symptoms | |
| Favipavir-Avi-Mild-1946 | 1.7 | 5.4 | −3.7 (−8.4, 1.1) | −219 (−1447, 34.3) | −27 | 2 | 119 | 6 | 112 | NO difference 7d vs 7d | |
| Favipiravir-Iran47 | 5.1 | 10.5 | −5.4 (−17.4, 6.6) | −105.3 (−955.6, 60.1) | −19 | 2 | 39 | 4 | 38 | ||
| Favipiravir-FLARE48 | 0.0 | 1.7 | −1.7 (−5.0, 1.6) | NA | −59 | 0 | 60 | 1 | 59 | ||
| Favipiravir/Lopinavir/Ritonavir-FLARE48 | 0.0 | 1.6 | −1.6 (−4.8, 1.5) | NA | −61 | 0 | 60 | 1 | 61 | ||
| Lopinavir/Ritonavir-FLARE48 | 0.0 | 1.7 | −1.7 (−5.0, 1.6) | NA | −60 | 0 | 60 | 1 | 60 | ||
| Lopinavir/ritonavir-TREAT NOW49 | 2.7 | 3.2 | −0.5 (−3.7, 2.6) | −19.8 (−251, 59.1) | −190 | 6 | 226 | 7 | 220 | 6 d to 6 d | |
| Lopinavir/ritonavir-TOGETHER18 | 4.8 | 5.7 | −0.9 (−4.9, 3.1) | −18.4 (−155.4, 45.1) | −112 | 11 | 227 | 14 | 244 | NO difference by Cox proportional HR | |
| Tenofovir Disproxil Fumarate Plus Emtricitabine-AR0-CORONA50 |
3.3 | 6.7 | −3.3 (−14.3, 7.7) | −100 (−1989.8, 80.9) | −30 | 1 | 30 | 2 | 30 | ||
| Metformin-COVID-OUT16 | 3.2 | 1.3 | 1.8 (0.1, 3.5) | 57.5 (3.8, 81.3) | 55 | 19 | 601 | 8 | 596 | NO difference | |
| Metformin-TOGETHER51 |
11.8 | 11.2 | 0.7 (−5.5, 6.8) | 5.6 (−60.8, 44.6) | 152 | 24 | 203 | 24 | 215 | not reported | |
| Fluvoxamine-TOGETHER21 | 12.8 | 10.1 | 2.7 (−0.5, 5.9) | 21.1 (−4.8, 40.6) | 37 | 97 | 756 | 75 | 741 | NO difference-40% resolved by day 14 | |
| Fluvoxamine-STOP COVID52 | 8.3 | 0.0 | 8.3 (1.9, 14.7) | 1 (1, 1) | 12 | 6 | 72 | 0 | 80 | YES (100% vs 91.7% resolved on day 7) p=0.009 | |
| Fluvoxamine-COVID-OUT16 | 1.7 | 2.0 | −0.3 (−2.5, 1.9) | −17.6 (−281, 63.7) | −333 | 5 | 293 | 6 | 299 | No difference (14 symptoms on 4 pt scale over 14 days) | |
| Fluvoxamine ACTIV-653 | 0.3 | 0.1 | 0.18 (−0.4, 0.7) | 54.7 (−398.3, 95.9) | 555 | 2 | 607 | 1 | 670 | 12 d to 13 d | |
| Fluvoxamine/budesonide-TOGETHER54 | 1.1 | 0.9 | 0.1 (−0.9, 1.1) | 12.5 (−140.1, 68.1) | 738 | 8 | 738 | 7 | 738 | not reported | |
| Ivermectin-TOGETHER55 | 14.0 | 11.6 | 2.4 (−1.2, 5.9) | 16.8 (−9.9, 37.1) | 42 | 95 | 679 | 79 | 679 | NO difference-40% resolved by day 14 | |
| Ivermectin-COVID-OUT16 | 1.4 | 1.1 | 0.3 (−1.3, 1.9) | 23.9 (−181, 79.4) | 299 | 5 | 356 | 4 | 374 | No difference (14 symptoms on 4 pt scale over 14 days | |
| Ivermectin Iran56 | 5.0 | 7.1 | −2.1 (−6.1, 1.9) | −42.3 (−178, 27.2) | −47 | 14 | 281 | 19 | 268 | NO difference | |
| Ivermectin-ACTIV-657 | 1.2 | 1.2 | −0.1 (−1.1, 1.0) | −5.3 (−158, 57.0) | −1634 | 9 | 774 | 10 | 817 | No difference (12d vs 13 d) | |
| Ivermectin high dose-ACTIV-658 |
0.3 | 0.8 | −0.5 (−1.4, 0.4) | −150.1 (−1188, 51.1) | −200 | 2 | 604 | 5 | 602 | 11 d vs 11 d no difference | |
| Hydroxychloroquine-TOGETHER18 | 4.8 | 3.7 | 1.1 (−2.7, 4.9) | 22.9 (−88.1, 68.4) | 90 | 11 | 227 | 8 | 214 | NO difference by Cox proportional HR | |
| Hydroxychloroquine-COVID-19 PEP59 | 4.7 | 2.4 | 2.4 (−1.1, 5.9) | 50.2 (−43.1, 82.7) | 42 | 10 | 211 | 5 | 212 | NO Difference in symptom severity score over 14 days | |
| Hydroxychloroquine-AH COVID-1960 | 0.0 | 3.6 | −3.6 (−7.1, −0.1) | NA | −28 | 0 | 37 | 4 | 111 | NO difference 14 d vs 12 d | |
| Hydroxychloroquine-BCN PEP-CoV-261 | 7.0 | 5.9 | 1.1 (−4.5, 6.7) | 16.0 (−103, 65.2) | 89 | 11 | 157 | 8 | 136 | NO difference 10 d vs 12 d | |
| Hydroxychloroquine-BMG62 | 4.8 | 3.4 | 1.4 (−4.0, 6.9) | 29.9 (−154, 80.6) | 69 | 4 | 83 | 5 | 148 | NO difference 11 d vs 12 d | |
| Hydroxychloroquine-Utah63 | 2.6 | 4.6 | −2.0 (−6.2, 2.2) | −73.8 (−481.6, 48.0) | −51 | 4 | 151 | 7 | 152 | 6 d to 6 d | |
| Hydroxychloroquine/Azithromycin-Brazil64 | 2.4 | 2.4 | 0 (−6.5, 6.5) | 0 (−1447, 93.5) | 100 | 1 | 42 | 1 | 42 | ||
| Nitazoxanide-Romark22 | 2.6 | 0.5 | 2.0 (−0.4, 4.5) | 78.8 (−79.7, 97.5) | 49 | 5 | 195 | 1 | 184 | Yes mild illness (13 d vs 18 d, p=0.01), NO difference for moderate illness | |
| Colchicine-COLCORONA20 | 5.8 | 4.7 | 1.2 (−0.1, 2.5) | 20.0 (−2.8, 37.7) | 86 | 131 | 2253 | 104 | 2235 | Not reported | |
| Losartan-MN65 |
1.7 | 5.2 | −3.5 (−10.1, 3.1) | −205 (−2749, 67.3) | −29 | 1 | 59 | 3 | 58 | ||
| Niclosamide66 | 2.9 | 0.0 | 2.9 (−2.7, 8.6) | 1 (1, 1) | 34 | 1 | 34 | 0 | 33 | NO difference 12 d vs 15 d | |
| Aspirin-ACTIV-4B67 | 0.7 | 0.7 | 0.04 (−1.9, 2.0) | 5.6 (−1395, 94) | 2448 | 1 | 136 | 1 | 144 | Not reported | |
| 2.5-mg apixaban-ACTIV-4B67 | 0.7 | 0.7 | −0.01 (−2.0, 2.0) | −0.7 (−1494, 93.6) | −18360 | 1 | 136 | 1 | 135 | Not reported | |
| 5-mg apixaban ACTIV-4B67 | 0.7 | 1.4 | −0.7 (−3.1, 1.7) | −90.2 (−1974, 82.6) | −151 | 1 | 136 | 2 | 143 | Not reported | |
| Sulodexide68 | 29.4 | 17.7 | 11.7 (1.1, 22.3) | 39.7 (3.5, 62.3) | 9 | 35 | 119 | 22 | 124 | Not reported | |
| Enoxaparin-ETHIC69 | 10.5 | 11.4 | −0.9 (−9.2, 7.4) | −8.6 (−131, 49.0) | −111 | 12 | 114 | 12 | 105 | Not reported | |
| Enoxaparin-OVID70 | 3.4 | 3.4 | −0.1 (−3.3, 3.2) | −1.7 (−166, 61.2) | −1740 | 8 | 238 | 8 | 234 | Not reported | |
| Inhaled Ciclesonide-COVIS71 |
3.4 | 1.7 | 1.8 (−1.4, 4.9) | 51.4 (−85.2, 87.2) | 56 | 7 | 203 | 3 | 179 | 19 d to 19 d | |
| Inhaled ciclesonide-COVERAGE72 | 11.2 | 12.7 | −1.5 (−10.1, 7.1) | −13.5 (−134, 45.0) | −66 | 12 | 107 | 14 | 110 | NO difference 13 d vs 12 d | |
| Zinc73 | 6.0 | 8.6 | −2.6 (−12.4, 7.2) | −43.7 (−471.4, 63.9) | −38 | 3 | 50 | 5 | 58 | 11 dto 10 d | |
| Ascorbic acid73 | 6.0 | 4.2 | 1.8 (−6.8, 10.5) | 30.6 (−297.6, 87.9) | 55 | 3 | 50 | 2 | 48 | 12 d to 10 d | |
| Zinc/Ascorbic acid73 | 6.0 | 12.1 | −6.1 (−16.7, 4.6) | −101.1 (−637, 45.1) | −16 | 3 | 50 | 7 | 58 | 10 d too 10 d | |
| Homeopathy-COVID-Simile74 | 6.8 | 2.4 | 4.4 (−4.3, 13.2) | 65.1 (−222.6, 96.2) | 23 | 3 | 44 | 1 | 42 | ||
| Saliravira75 | 28.6 | 0.0 | 28.6 (16.7, 40.4) | 1 (1, 1) | 4 | 16 | 56 | 0 | 87 | YES 9d vs 14 d p<0.05 | |
| Azithromycin-Atomic276 | 11.6 | 10.3 | 1.2 (−5.9, 8.4) | 10.5 (−72.3, 53.6) | 82 | 17 | 147 | 15 | 145 | Not reported | |
| Azithromycin-ACTION28 | 0.0 | 4.0 | −4.0 (−7.4, −0.6) | NA | −25 | 0 | 72 | 5 | 125 | No difference resolution day 14 | |
| Resveratrol77 | 6.0 | 2.0 | 4.0 (−3.6, 11.6) | 66.7 (−210, 96.4) | 25 | 3 | 50 | 1 | 50 | Not reported | |
Figure 3.

Percent hospitalizations in control groups sorted by therapy type and descending control hospitalization rates.
3.2. Trial Outcome Comparison
Examining RCTs by agent class, statistically significant relative risk reductions in hospitalization were found in two of five CCP RCTs, six of nine mAb RCTs, four of 17 small molecule antiviral RCTs, but just 2 of 39 repurposed drug RCTs (Table 1). Except for the bebtelovimab RCT (2 hospitalizations in each arm17), mAb RCTs reduced the risk of hospitalization by 50–80% (average 75%). Two of the three small molecule antiviral drugs (remdesivir15 and nirmatrelvir/ritonavir5) showed very high levels of relative risk reduction - 87% and 88% respectively - but molnupiravir reduced risk of hospitalization by only 30%7 (excluding the large no reduction seen in the PANORAMIC RCT25). The lopinavir/ritonavir combination was associated with a non-significant increase in risk of hospitalization18.
Among repurposed drug RCTs, all except metformin (58%) and sulodexide (40%), showed small and non-significant relative risk reductions of hospitalization - ivermectin19, colchicine20, fluvoxamine21 and hydroxychloroquine18. The nitazoxanide22 RCT found one hospitalization among 184 treated participants compared to five hospitalizations among 195 controls, too few events to achieve significance.
Absolute risk reductions (ARR) and number needed to treat (NNT) to avert hospitalizations varied across studies and treatment classes (Table 1). In general, except for the repurposed drugs the absolute risk difference approximated 3% if one excludes the molnupiravir-Panoramic Study from SMA. The CCP RCTs had an ARR of 3.2% (95%CI-0.9–5.6), mAbs RCTs had an ARR of 4.0% (95%CI-3.2–4.8), small molecule antivirals excluding molnupiravir-Panoramic had ARR of 2.1% (95%CI-1.3–2.9) and the repurposed drugs had a smaller ARR at 1.1% (95%CI-0.5–1.6). The number needed to treat to prevent hospitalizations approximated 30 in the trials, with a few notably low with CCP-Argentina (NNT=7) Nirmatrelvir/ritonavir (NNT=18), except those using repurposed drugs, where NNT averaged 70 (Table 1).
3.3. Pooled Meta-analysis
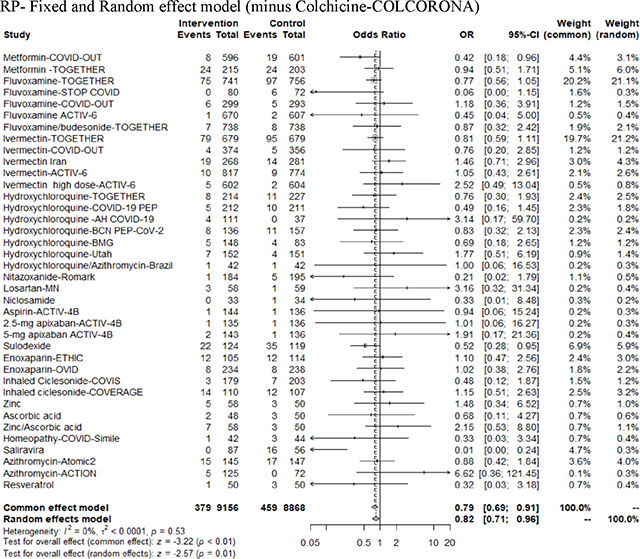
In the pooled meta-analysis by class group, the CCP RCTs had a fixed effect OR of 0.69 (95% CI=0.53 to 0.9) with moderate heterogeneity (I2=43%), the mAbs had a fixed effect OR of 0.31 (95% CI=0.24–0.40) with low heterogeneity (I2=0%), the small molecule antivirals had a random effect OR of 0.78 (95% CI=0.48–1.33) with high heterogeneity (I2=69%) and the repurposed drugs had a random effect OR of 0.82 (95% CI- 0.72–0.93) with low heterogeneity (I2=0) (Figure 4, Appendix Table 4). A biologic reason to use a fixed model for CCP and mAbs is that both formulations have the same active agent as specific antibody and thus there is a high degree of similarity in these two antibody interventions. Both antiviral small molecules and repurposed drugs comparison involve many types and classes of drugs for which the random effect model for the group is biologically more plausible than treating them as fixed. The meta-analysis of all interventions had a random effect OR of 0.67 (95% CI=0.57–0.80) with high heterogeneity (I2=52%) (Appendix Figure 2). Because regional parts of the world might have different thresholds for the hospitalization outcome we grouped by region (USA, Brazil, Europe, Midde East or World) across diverse intervention classes to indicate that the intervention class had more of an effect than region (Appendix Figure 3).
Figure 4.

Odds ratio for hospitalizations with diverse therapeutic interventions, grouped according to mechanism of action (CCP, mAbs, small molecule antivirals and repurposed drugs).
Ten RCTs compared hospitalization rates in early or late interventions (dated from symptom onset) that were extractable from the published papers or supplementary data as a post-hoc analysis (Figure 5). The small molecule drugs showed no significant reduction in the OR with segregation of early treatment from late treatment. In contrast, the antibody therapies showed a difference by treatment timing. Pooling the two classes of antibody treatment, the OR for early treatment was 0.65 (95%CI=0.49–0.85), while the OR for later treatment was 0.86 (95%CI=0.66–1.12).
Figure 5.

Odds ratio for point estimates for hospitalization in RCT subgroups treated A) within 5 days since onset of symptoms and also B) over 5 days within same trial.
A) Early treatment-Fixed and random effect model B) Late treatment-Fixed and random effect model
3.4. Robustness of the Meta-analysis
Within the CCP RCTs excluding either CCP-Argentina for the nonhospital endpoint or CONV-ERT for methylene blue inactivation of antibody function only changed the OR by 0.05 with 95% CI remaining less than 1 for the fixed effect model (Appendix Figure 4). Adding the 7 all-cause hospitalizations to CSSC-004, increased the OR by 0.02 and 95% CI by 0.01, essentially no change (Appendix Figure 4). In a leave out the largest study by participants sensitivity analysis, the OR was not significantly altered in the model effects by more than 0.1 except for SMA where removal of the large Molnupirivir-Panoramic study changed common effect model OR from 0.7 to 0.54. Both the mAb and RP did not change OR results (Appendix Figure 5).
3.5. Certainty of Evidence
All four trial classes showed reduced rates of hospitalization for each group. The final certainty of the available evidence with GRADE assessment (Appendix Table 3) showed a high certainty level within CCP trials, moderate certainty with mAbs, and low certainty with small molecule antivirals and repurposed drugs. The main reason for downgrading individual and pooled studies was imprecision, related to small number of participants and the wide confidence intervals around the effect, followed by ROB (Appendix Figure 6). We did not find concerns in any of the GRADE factors for CCP RCTs, so we graded them as high level of certainty. mAbs were downgraded to moderate certainty due to ROB (in 4 of the 8 included RCTs, ROB for the outcome hospitalization was judged of some concern). In the cumulative analysis, small molecule antivirals were downgraded to low certainty of evidence because of ROB (some/high ROB in 4 RCTs) and inconsistency (due to high heterogeneity), while repurposed drugs were downgraded to low certainty due to ROB (some/high ROB in 5 of the 11 comparisons) and indirectness (due to large difference in mechanism of action of the included drugs). The ROB was independently evaluated by the COVID-19-Network Meta-Analysis (NMA) initiative for most of the RCTs (Appendix Figure 6). Funnel plot analysis with Egger Test shows a low risk of publication bias except for the mAbs, for which either the efficacy of high dose antibodies or non-reporting bias are plausible explanations (Appendix Figure 7).
3.6. Outpatient Intervention Mortality
While several RCTs showed fewer deaths in the treatment arm, no outpatient study was powered to compare differences in mortality. Because of the low rate of deaths during trials the absolute risk reductions amongst the 4 antiviral classes are all below 1% corresponding to relative risk reductions of 20%, 81%, 87% and 22% for CCP, mAbs, small molecule antivirals or repurposed drugs, respectively (Appendix Table 5).
3.7. Time To Symptom Resolution
The two most effective CCP RCTs (Argentine10 and CSSC-0049) did not compare time to symptom resolution, while the COV-Early23 and ConV-ERT24 RCTs reported no difference in the median time of symptom resolution in the two groups24 (Table 1). The mAbs noted faster resolution by 1, 2, 3, 4 or 5 days for bamlanivimab/etesevimab25, bebtelovimab17, regdanvimab26, casirivimab/imdevimab2, or redanvimab27 respectively. The smaller bamlanivimab-only RCT did not show a difference1. Of the three SMAs that noted reductions in hospitalizations, molnupiravir was associated with no difference in time of symptom resolution in MOVe-OUT7 but improvements in both PANORAMIC25 and Aurobindo27 RCTs. The 3-day outpatient remdesivir RCT showed that symptoms were alleviated by day 14 nearly twice as often15. The nirmatrelvir/ritonavir RCT did not report on this parameter5. Seven of 10 RCTs in the antiviral group did not show faster symptom resolution with intervention. The three RCTs largely performed in Brazil for fluvoxamine, ivermectin19 and hydroxychloroquine18 noted no differences in symptom resolution. Metformin did not evidence faster symptom resolution despite reducing hospitalizations. Three of the 25 RCTs reporting symptom resolution in the repurposed drug group noted faster symptom resolution.
3.8. Costs
mAbs and intravenous remdesivir schedules cost about 1000 to 2000 Euros per patient, respectively, while the oral drugs are much less than 1000 Euros per patient (Table 2). By comparison, the cost of CCP approximates 200 Euros per patient, and the cost for repurposed drugs is even lower. Considering the absolute risk reduction in hospitalization, the number needed to treat to prevent a single hospitalization is often very high, as are the associated costs. With the recently patented antivirals, costs for outpatient treatment often exceed the cost of a COVID-19 hospitalization28.
Table 2.
Summary of historical efficacy of different therapeutics against SARS-CoV-2 VOCs.
| approximate cost per patient | average NNT (sourced from Table 2) | cost to prevent a single hospitalization (€) | efficacy against VOC Alpha | efficacy against VOC Delta | efficacy against VOC BA.1 | efficacy against VOC BA.2 | efficacy against BA.4/5 | efficacy against BQ.1.1 | |
|---|---|---|---|---|---|---|---|---|---|
| bamlanivimab+etesesevimab | 2000 | 21 | 42,000 | restricted 04/2021 | |||||
| casirivimab+imdevimab | 2000 | 30 | 60,000 | restricted 01/2022 | |||||
| sotrovimab | 1000 | 22 | 22,000 | restricted 03/2022 | |||||
| tixagevimab+cilgavimab | 1000 | 22 | 22,000 | restricted 10/22 |
|||||
| regdanvimab | 300 | 23 | 6,900 | ||||||
| bebtelovimab | 2000 | Not calculated (low-risk pts) | Not calculated (low-risk pts) | ||||||
| nirmatrelvir | 635 (5 days) | 18 | 11,435 | ||||||
| molnupiravir | 635 (5 days)) | 34 | 21,590 | ||||||
| remdesivir | 1600 (3 days) | 22 (MOVE-Out) | 35,200 | ||||||
| CCP | 200 (600-ml) |
31 | 6,200 | ||||||
| Vax-CCP |
White = drug not available at that time; green = effective; orange = partially effective; red= not effective. Restriction reported refer to initial restrictions by FDA. NNT: number needed to treat.
3.9. Variants
mAbs successively lost efficacy against Delta and Omicron, with cilgavimab (the only Omicron-active ingredient in Evusheld™) and bebtelovimab also failing against BQ.1.1 and XBB.* sublineages (Figure 6). This has led the FDA to withdraw EUAs, while EMA has not restricted usage at all. Small molecule antivirals retain in vitro efficacy against Omicron, but concerns remain: molnupiravir showed low efficacy in vivo6 and is mutagenic for mammals in vitro29, while nirmatrelvir/ritonavir has drug/drug interaction contraindications (CYP3 metabolites especially tacrolimus, anti-cholesterol, anti-migraine or many anti-depressants) and has been associated with early virological and clinical rebounds in immunocompetent patients30. CCP from unvaccinated donors does not inhibit Omicron, but CCP from donors having any sequence of vaccination and recent, within 6 months, COVID-19 or having had boosted mRNA vaccine doses universally has high Omicron-neutralizing activity.
Figure 6.

Venn diagram of mAb efficacy against Omicron sublineages. In vitro activity of currently approved mAbs against Omicron sublineages circulating as of October 2022. Specific Omicron Spike amino acid mutations causing baseline ≥ 4-fold-reduction in neutralization against mAbs are reported. Mutations for which the majority of studies are concordant are reported: the different fold-reductions for each mAb are identified across concordant studies as color coded numbers defining the mean median values of specific reduction in each study. Sourced from https://covdb.stanford.edu/page/susceptibility-data (accessed on January31, 2023
* L452R occurs in all BA.4/BA.5 lineages, but only in several BA.2. sublineages.
R346X and K444X occur in a growing number of BA.2 and BA.4/5 sublineages as a result of convergent evolution.
DISCUSSION
The paucity of head-to-head RCT comparisons amongst outpatient COVID-19 therapies makes the choice of therapy difficult. This meta-analysis allows comparison of placebo controlled RCT interventions amongst four main intervention classes-polyclonal convalescent plasma, monoclonal antibodies, small molecule antivirals and repurposed drugs. Participant features such as risk factors for COVID-19 progression (age, obesity, comorbid conditions), vaccination status and spike protein viral variation impact the hospital outcomes as a component of heterogeniety.
Outpatient RCT data confirm that most antiviral/antimicrobial therapies are more effective when given before rather than after hospital admission. In examining the full assembly of these effective, yet molecularly disparate interventions, we note the consistent importance of early outpatient treatment for patients at risk of progression to hospitalization31. Treatment within 5 days of illness onset was more effective than later treatment, as would be expected for an antiviral mechanism of action. An individual participant meta-analysis of CCP that investigated the effects of early compared to late treatment and of high compared to low dose antibody levels found that both early treatment and high levels of antibody combined to most effectively reduce risk of hospitalizations32.
The relative ease of conducting inpatient RCTs may have led most initial CCP, small molecule antiviral and repurposed trials – which were conducted principally by academic institutions - to be based in hospitals, often in patients treated too late for antiviral treatment to be expected to work, given that antiviral therapy must be given early in disease. The constrained resources available for clinical research by academic medicine during pandemic conditions further interfered with trial work, and several potentially valuable RCT’s with promising findings were terminated before they could provide definitive data. The findings of such trials are reported as null but often viewed as negative, notwithstanding trends towards effectiveness, and are rarely incorporated into clinical recommendations. The pharmaceutical industry – with well-established internal resources for trials and substantial economic support – was able to perform large outpatient trials of mAbs early in the pandemic. Inpatient services are generally more accessible to physician-scientists working in academic medical centers.
The choice, however, has been narrowed in recent months, and the clinical armamentarium has been reduced to small molecule antivirals, repurposed drugs and CCP, because single and double (“cocktail”) MAbs have lost effectiveness against new VOCs33. Both vaccine-elicited and disease-elicited antibodies are polyclonal, meaning that they include various isotypes that provide functional diversity and target numerous epitopes making variant escape much more difficult with CCP. Hence, polyclonal antibody preparations are much more resilient to the relentless evolution of variants. This is in marked contrast to mAbs, which target single epitopes of SARS-CoV-2. The exquisite mAb (and receptor binding domain) specificity renders mAbs susceptible to becoming ineffective with single amino acid changes. Plasma from individuals who have been both vaccinated and boosted is characterized by high amounts of neutralizing antibodies which can be effective against practically any existing VOC, including Omicron34 (so-called “heterologous immunity”, likely due to the well-known phenomenon of “epitope spreading”). Vaccine-boosted CCP has more than ten times the amount of total SARS-CoV-2 specific antibody and viral neutralizing activity compared to the pre-omicron CCP used in the effective outpatient CCP RCTs.
In addition to efficacy, other points to consider in an outpatient pandemic are tolerability, scalability and affordability. Repurposed drugs are generally well tolerated, widely available and relatively inexpensive, but, as we have shown, have limited efficacy. By contrast, small molecule antivirals are often plagued by contraindications and side effects, which make several classes of patients reliant on passive immunotherapies. Both small molecule antivirals and mAbs take time to develop and are unaffordable in low-and-middle income countries (LMIC). CCP is instead a tolerable, scalable, and affordable treatment and is usually provided in a single IV session, in contrast to remdesivir, which requires a three-day intravenous course.
On Dec. 28, 2021 the FDA expanded the authorized emergency use of convalescent plasma with high titers of anti-SARS-CoV-2 antibodies “for the treatment of COVID-19 in patients with immunosuppressive disease or receiving immunosuppressive treatment, in either the outpatient or inpatient setting.” This EUA noted that CCP was safe and effective in immunocompetent individuals and allowed under the emergency measure of the pandemic, but expanded its use in immunosuppressed individuals to outpatient use, notwithstanding the availability of oral drugs and (at that time) two remaining effective mAb treatments for the new omicron variants of concerns.
Limitations of evidence in this review process included general meta-analysis etiologies such as English only publications, limited class subgroup analysis due to low numbers like CCP or mAb, grouping diverse mechanisms of action in the SMA and RP classes which increases heterogeneity, heterogeneity loss of power when evaluating CCP and SMA due to smaller single digit number of RCTs evaluated or reporting bias towards positive successfully executed studies which might miss marginal or futile studies. While risk of bias was independently performed on more than 80% of studies by COVID-19-NMA, the RevMan program determined the missing risk of bias. Unique limitations to outpatient COVID-19 studies are diverse patient population varying in age and comorbidities, changes in outpatient standard of care and vaccination status over both the regions and period covered which led to wide variation in hospital rates which were also influenced by regional accessibility to hospitalization, depending on hospital capacity and other outpatient intervention like mAb early in pandemic. Studies also reported either all cause hospitalization or COVID-19 related hospitalization within the 2 week or 4 week outcome reporting time period as listed in appendix tables. While the pooled OR focused on just hospital rates between studies, the full data set in appendix allowing comparison of just COVID-19 related hospitalizations, pre-Alpha period trials. The outpatient RCTs reviewed here were conducted during different time-periods during the pandemic, thus targeting different variants, and enrolled participants with different vaccination statuses. Further heterogeneity was contributed by variation across the RCTs, in participant age, medical risk factors and serological status.
The published mAbs RCTs assembled here showed better overall class efficacy than other outpatient interventional classes, yet mAb are now clinically ineffective against BQ.1.* and XBB.* Omicron variants. CCP and small molecule antivirals have comparable levels of effectiveness with many individual RCTS, but the latter have many contraindications and side effects. Repurposed drugs are largely either ineffective or mildly effective with just 2 RCT within the class approaching nirmatrelivir or mAbs. CCP is the remaining effective passive antibody therapy, which is especially important in the immunosuppressed but, as the trials show, early treatment with high levels of antibody, has value in other populations as well. Our clinical recommendation from this review is to use CCP on an out-patient basis in regions with no other therapy available regardless of vaccination status for those at high risk of progression to hospitalization.
Acknowledgements
This study was supported by the U.S. Department of Defense’s Joint Program Executive Office for Chemical, Biological, Radiological and Nuclear Defense (JPEO-CBRND), in collaboration with the Defense Health Agency (DHA) (contract number: W911QY2090012) (DS, AC, DH), with additional support from Bloomberg Philanthropies, State of Maryland, the National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases 3R01AI152078–01S1 (DS, AC), NIH National Center for Advancing Translational Sciences U24TR001609-S3 and UL1TR003098 (DH).
Abbreviations:
- CCP
COVID-19 convalescent plasma
- mAb
monoclonal antibody
- RCT
randomized controlled trial
- VOC
variant of concern
Appendix
Description of trial participants
The median age of participants was about 50 years. The CCP group had a nonweighted trial average of median age equal to 58 years, while the anti-Spike mAbs, small molecule antivirals and repurposed drug groups younger average of median age was equal to 45 to 48 years. Most RCTs had more women than men, and 84% of all RCT 60,043 participants had Caucasian ethnicity (Appendix Table 2a,b).
The individual RCTs differed in the percentage of participants with risk factors for progression to severe COVID-19. Of the 37 RCTs reporting aggregated hospitalization risk factors, ten had 100% of participants with at least one hospitalization risk factor, while 5 had fewer than 50%. The bebtelovimab placebo-controlled RCT explicitly focused exclusively on low-risk individuals 1. Individual risk factors such as diabetes mellitus occurred in 10 to 20% of participants in most RCTs. Obesity with BMI over 29 averaged near 40% of RCT participants in the 4 therapy groups after excluding the large single 25,000 molnupiravir-PANORAMIC RCT with 15% of participants with BMI’s over 30 (Appendix Table 2a,b) 25.
Of 18 RCTs reporting seropositivity rates at baseline, 11 had < 25% screening seropositive (Appendix Table 2a,b, main text Figure 2). The molnupiravir-PANORAMIC RCT was an outlier, with 98% participant seropositives25. All but one2 of the RCTs enrolled within 8 days (median) of symptom onset. In RCTs of anti-Spike mAbs and small molecule antivirals, median time from illness onset to intervention was 3.5 to 4 days (Appendix Fig 1, Appendix Table 2a,b). CCP and repurposed antiviral drug RCTs enrolled within 4.5 to 5.1 days from symptom onset.
The CCP RCTs were conducted in the USA3,4, Argentina5, Netherlands6 and Spain7 (Appendix Table 2). The anti-Spike mAb RCTs all had a USA component, but were largely centered in the Americas except for the sotrovimab RCT, which took place in Spain8. Many of the repurposed drugs and nirmatrelvir/ritonavir RCTs recruited worldwide9.
Four of the five CCP RCTs (COV-Early6, CONV-ERT7, Argentina5 and C3PO4), and all eight anti-Spike mAb RCTs took place in the setting of the D614G variant and the Alpha VOC (main text-Figure 2). By contrast, most of the molnupiravir, nirmatrelvir/ritonavir9 and interferon lambda RCTs were conducted in the setting of the Delta VOC. The ivermectin10 and fluvoxamine11 RCTs ended as the Delta VOC wave began in August 2021. The remdesivir RCT spanned D614G, Alpha and Beta VOC but missed Delta12. The CSSC-004 RCT of CCP was the longest RCT reviewed, spanning periods characterized by D614G to Delta VOC infections3.
Appendix Figure 1.

Comparison of mean interval from symptom onset to enrollment/intervention as well as per protocol interval inclusion limit for all participants.
Appendix Figure 2: Odds ratio for hospitalizations from all interventions.

Therapeutic interventions ordered according to mechanism of action (CCP, anti-Spike mAbs, small molecule antivirals and repurposed drugs
Appendix Figure 3: Odds ratio for hospitalizations from all interventions by region.

Therapeutic interventions ordered region including diverse interventions
Appendix Figure 4: Odds ratio for hospitalizations within CCP group.

A) All CCP trials excluding CCP-Argentina with a non hospital endpoint of severe respiratory distress or B) All CCP excluding CONV-ERT because of methylene blue inactivation of antibody function. C) all cause hospitalization for CSSC-004 to match all cause hospitalization for other CCP studies
Appendix Figure 5: Odds ratio for hospitalizations sensitivity analysis.

Odds ratio for hospitalizations with diverse therapeutic interventions, grouped according to mechanism of action (CCP, mAbs, small molecule antivirals and repurposed drugs) with the largest enrollment removed
Appendix Figure 6:

Risk of bias by RCT
Appendix Figure 7: Funnel plots by RCTs class.

Publication bias was examined with Egger Test for Funnel Plot asymmetry. A weighted regression model with multiplicative dispersion used standard error for prediction. A) CCP Funnel Plot Asymmetry (t=1.0879, df=3, p=0.3562). The limit estimate (as sei≥0) b=0.4699 (CI:-2.0201,2.9598) B) anti-Spike mAbs Funnel Plot Asymmetry (t=0.5087, df=7, p=0.6266). The limit estimate (as sei≥0) b=-1.2940 (CI:-2.0658, 0.5221) C) small molecule antivirals Funnel Plot Asymmetry (t=0.0695, df=15, p=0.9455). The limit estimate (as sei≥0) b= −0.2627 (CI:-0.7860, 0.2606) and D) repurposed drugs Funnel Plot Asymmetry (t=0.0645, df=37, p=0.9489). The limit estimate (as sei≥0) b= −0.2080 (CI: −0.4146, −0.0013). For anti-Spike mAbs RCTs, there is a suggestion of missing studies on the right side of the plot, where results would be unfavourable to the experimental intervention, for which either very high efficacy of high-dose anti-Spike mAbs or non-reporting bias is a plausible explanation.
Table 1.
Included and Excluded trials in search of May 2023 281 studies
| publication number | excluded reason | included in review | class | RCT per study | unique intervention | study name | ref |
| 12 | 1 | CCP | 1 | 1 | CCP-CONV-ert | Alemany A, Millat-Martinez P, Corbacho-Monne M, et al. High-titre methylene blue-treated convalescent plasma as an early treatment for outpatients with COVID-19: a randomised, placebo-controlled trial. Lancet Respir Med 2022; 10(3): 278–88. | |
| 92 | 1 | CCP | 1 | CCP-COV-early | Gharbharan A, Jordans C, Zwaginga L, et al. Outpatient convalescent plasma therapy for high-risk patients with early COVID-19: a randomized placebo-controlled trial. Clin Microbiol Infect 2022. | ||
| 132 | 1 | CCP | 1 | CCP-C3PO | Korley FK, Durkalski-Mauldin V, Yeatts SD, et al. Early Convalescent Plasma for High-Risk Outpatients with Covid-19. N Engl J Med 2021; 385(21): 1951–60. | ||
| 150 | 1 | CCP | 1 | CCP-Argentina | Libster R, Perez Marc G, Wappner D, et al. Early High-Titer Plasma Therapy to Prevent Severe Covid-19 in Older Adults. N Engl J Med 2021; 384(7): 610–8. | ||
| 252 | 1 | CCP | 1 | CCP-CSSC-004 | Sullivan DJ, Gebo KA, Shoham S, et al. Early Outpatient Treatment for Covid-19 with Convalescent Plasma. N Engl J Med 2022; 386(18): 1700–11. | ||
| 54 | 1 | mab | 1 | 1 | Bamlanivimab-BLAZE-1 | Chen P, Nirula A, Heller B, et al. SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19. N Engl J Med 2021; 384(3): 229–37. | |
| 72 | 1 | mab | 1 | 1 | Bebtelovimab-BLAZE-4 | Dougan M, Azizad M, Chen P, et al. Bebtelovimab, alone or together with bamlanivimab and etesevimab, as a broadly neutralizing monoclonal antibody treatment for mild to moderate, ambulatory COVID-19. medRxiv 2022: 2022.03.10.22272100. | |
| 73 | 1 | mab | 1 | 1 | Bamlanivimab/etesevimab-BLAZE-1 | Dougan M, Nirula A, Azizad M, et al. Bamlanivimab plus Etesevimab in Mild or Moderate Covid-19. N Engl J Med 2021; 385(15): 1382–92. | |
| 98 | 1 | mab | 1 | 1 | Sotrovimab-COMET-ICE | Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab. N Engl J Med 2021. | |
| 129 | 1 | mab | 1 | 1 | Regdanvimab-CT-P59–2 | Kim JY, Sandulescu O, Preotescu LL, et al. A Randomized Clinical Trial of Regdanvimab in High-Risk Patients With Mild-to-Moderate Coronavirus Disease 2019. Open Forum Infect Dis 2022; 9(8): ofac406. | |
| 184 | 1 | mab | 1 | 1 | Tixagevimab–cilgavimab-TACKLE | Montgomery H, Hobbs FDR, Padilla F, et al. Efficacy and safety of intramuscular administration of tixagevimab-cilgavimab for early outpatient treatment of COVID-19 (TACKLE): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2022; 10(10): 985–96. | |
| 195 | 1 | mab | 1 | 1 | Casirivimab/imdevimab-REGEN-COV Ph 1/2 | Norton T, Ali S, Sivapalasingam S, et al. REGEN-COV Antibody Combination in Outpatients With COVID-19 – Phase 1/2 Results. medRxiv 2022: 2021.06.09.21257915. | |
| 251 | 1 | mab | 1 | Regdanvimab-CT-P59 | Streinu-Cercel A, Sandulescu O, Preotescu LL, et al. Efficacy and Safety of Regdanvimab (CT-P59): A Phase 2/3 Randomized, Double-Blind, Placebo-Controlled Trial in Outpatients With Mild-to-Moderate Coronavirus Disease 2019. Open Forum Infect Dis 2022; 9(4): ofac053. | ||
| 275 | 1 | mab | 1 | Casirivimab/imdevimab-REGEN-COV Ph 3 | Weinreich DM, Sivapalasingam S, Norton T, et al. REGEN-COV Antibody Combination and Outcomes in Outpatients with Covid-19. N Engl J Med 2021; 385(23): e81. | ||
| 7 | 1 | rp | 1 | 1 | Homeopathy-COVID-Simile | Adler UC, Adler MS, Padula AEM, et al. Homeopathy for COVID-19 in primary care: A randomized, double-blind, placebo-controlled trial (COVID-Simile study). J Integr Med 2022; 20(3): 221–9. | |
| 24 | 1 | rp | 1 | Enoxaparin-OVID | Barco S, Voci D, Held U, et al. Enoxaparin for primary thromboprophylaxis in symptomatic outpatients with COVID-19 (OVID): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet Haematol 2022; 9(8): e585-e93. | ||
| 39 | 1 | rp | 3 | Metformin-COVID-OUT | Bramante CT, Huling JD, Tignanelli CJ, et al. Randomized Trial of Metformin, Ivermectin, and Fluvoxamine for Covid-19. N Engl J Med 2022; 387(7): 599–610. | ||
| 48 | 1 | rp | 1 | 1 | Niclosamide | Cairns DM, Dulko D, Griffiths JK, et al. Efficacy of Niclosamide vs Placebo in SARS-CoV-2 Respiratory Viral Clearance, Viral Shedding, and Duration of Symptoms Among Patients With Mild to Moderate COVID-19: A Phase 2 Randomized Clinical Trial. JAMA Netw Open 2022; 5(2): e2144942. | |
| 58 | 1 | rp | 1 | Inhaled Ciclesonide-COVIS | Clemency BM, Varughese R, Gonzalez-Rojas Y, et al. Efficacy of Inhaled Ciclesonide for Outpatient Treatment of Adolescents and Adults With Symptomatic COVID-19: A Randomized Clinical Trial. JAMA Intern Med 2022; 182(1): 42–9. | ||
| 60 | 1 | rp | 3 | 1 | Aspirin-ACTIV-4B | Connors JM, Brooks MM, Sciurba FC, et al. Effect of Antithrombotic Therapy on Clinical Outcomes in Outpatients With Clinically Stable Symptomatic COVID-19: The ACTIV-4B Randomized Clinical Trial. JAMA 2021; 326(17): 1703–12. | |
| 61 | 1 | rp | 1 | 1 | Enoxaparin-ETHIC | Cools F, Virdone S, Sawhney J, et al. Thromboprophylactic low-molecular-weight heparin versus standard of care in unvaccinated, at-risk outpatients with COVID-19 (ETHIC): an open-label, multicentre, randomised, controlled, phase 3b trial. Lancet Haematol 2022; 9(8): e594-e604. | |
| 76 | 1 | rp | 1 | 1 | Inhaled ciclesonide-COVERAGE | Duvignaud A, Lhomme E, Onaisi R, et al. Inhaled ciclesonide for outpatient treatment of COVID-19 in adults at risk of adverse outcomes: a randomised controlled trial (COVERAGE). Clin Microbiol Infect 2022; 28(7): 1010–6. | |
| 93 | 1 | rp | 1 | 1 | Sulodexide | Gonzalez-Ochoa AJ, Raffetto JD, Hernandez AG, et al. Sulodexide in the Treatment of Patients with Early Stages of COVID-19: A Randomized Controlled Trial. Thromb Haemost 2021; 121(7): 944–54. | |
| 107 | 1 | rp | 1 | Azithromycin-Atomic2 | Hinks TSC, Cureton L, Knight R, et al. Azithromycin versus standard care in patients with mild-to-moderate COVID-19 (ATOMIC2): an open-label, randomised trial. Lancet Respir Med 2021; 9(10): 1130–40. | ||
| 115 | 1 | rp | 1 | Hydroxychloroquine-BMG | Johnston C, Brown ER, Stewart J, et al. Hydroxychloroquine with or without azithromycin for treatment of early SARS-CoV-2 infection among high-risk outpatient adults: A randomized clinical trial. EClinicalMedicine 2021; 33: 100773. | ||
| 128 | 1 | rp | 1 | 1 | Saliravira | Khorshiddoust RR, Khorshiddoust SR, Hosseinabadi T, et al. Efficacy of a multiple-indication antiviral herbal drug (Saliravira(R)) for COVID-19 outpatients: A pre-clinical and randomized clinical trial study. Biomed Pharmacother 2022; 149: 112729. | |
| 145 | 1 | rp | 1 | 1 | Fluvoxamine-STOP COVID | Lenze EJ, Mattar C, Zorumski CF, et al. Fluvoxamine vs Placebo and Clinical Deterioration in Outpatients With Symptomatic COVID-19: A Randomized Clinical Trial. JAMA 2020; 324(22): 2292–300. | |
| 168 | 1 | rp | 1 | Fluvoxamine-ACTIV-6 | McCarthy MW, Naggie S, Boulware DR, et al. Effect of Fluvoxamine vs Placebo on Time to Sustained Recovery in Outpatients With Mild to Moderate COVID-19: A Randomized Clinical Trial. JAMA 2023; 329(4): 296–305. | ||
| 173 | 1 | rp | 1 | 1 | Resveratrol | McCreary MR, Schnell PM, Rhoda DA. Randomized double-blind placebo-controlled proof-of-concept trial of resveratrol for outpatient treatment of mild coronavirus disease (COVID-19). Sci Rep 2022; 12(1): 10978. | |
| 281 | 1 | rp | 1 | Hydroxychloroquine-BCN PEP-CoV-2 | Mitja O, Corbacho-Monne M, Ubals M, et al. Hydroxychloroquine for Early Treatment of Adults With Mild Coronavirus Disease 2019: A Randomized, Controlled Trial. Clin Infect Dis 2021; 73(11): e4073-e81. | ||
| 189 | 1 | rp | 1 | 1 | Ivermectin high dose-ACTIV-6 | Naggie S, Boulware DR, Lindsell CJ, et al. Effect of Higher-Dose Ivermectin for 6 Days vs Placebo on Time to Sustained Recovery in Outpatients With COVID-19: A Randomized Clinical Trial. JAMA 2023; 329(11): 888–97. | |
| 187 | 1 | rp | 1 | Ivermectin-ACTIV-6 | Naggie S, Boulware DR, Lindsell CJ, et al. Effect of Ivermectin vs Placebo on Time to Sustained Recovery in Outpatients With Mild to Moderate COVID-19: A Randomized Clinical Trial. JAMA 2022; 328(16): 1595–603. | ||
| 198 | 1 | rp | 1 | 1 | Azithromycin-ACTION | Oldenburg CE, Pinsky BA, Brogdon J, et al. Effect of Oral Azithromycin vs Placebo on COVID-19 Symptoms in Outpatients With SARS-CoV-2 Infection: A Randomized Clinical Trial. JAMA 2021; 326(6): 490–8. | |
| 214 | 1 | rp | 1 | 1 | Losartan-MN | Puskarich MA, Cummins NW, Ingraham NE, et al. A multi-center phase II randomized clinical trial of losartan on symptomatic outpatients with COVID-19. EClinicalMedicine 2021; 37: 100957. | |
| 219 | 1 | rp | 1 | 1 | Metformin-TOGETHER | Reis G, Dos Santos Moreira Silva EA, Medeiros Silva DC, et al. Effect of early treatment with metformin on risk of emergency care and hospitalization among patients with COVID-19: The TOGETHER randomized platform clinical trial. Lancet Reg Health Am 2022; 6: 100142. | |
| 220 | 1 | rp | 1 | Fluvoxamine/budesonide-TOGETHER | Reis G, Dos Santos Moreira Silva EA, Medeiros Silva DC, et al. Oral Fluvoxamine With Inhaled Budesonide for Treatment of Early-Onset COVID-19 : A Randomized Platform Trial. Ann Intern Med 2023; 176(5): 667–75. | ||
| 221 | 1 | rp | 1 | Fluvoxamine-TOGETHER | Reis G, Dos Santos Moreira-Silva EA, Silva DCM, et al. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob Health 2022; 10(1): e42-e51. | ||
| 224 | 1 | rp | 1 | Ivermectin-TOGETHER | Reis G, Silva E, Silva DCM, et al. Effect of Early Treatment with Ivermectin among Patients with Covid-19. N Engl J Med 2022; 386(18): 1721–31. | ||
| 227 | 1 | rp | 1 | Ivermectin Iran | Rezai MS, Ahangarkani F, Hill A, et al. Non-effectiveness of Ivermectin on Inpatients and Outpatients With COVID-19; Results of Two Randomized, Double-Blinded, Placebo-Controlled Clinical Trials. Front Med (Lausanne) 2022; 9: 919708. | ||
| 229 | 1 | rp | 1 | Hydroxychloroquine/Azithromycin-Brazil | Rodrigues C, Freitas-Santos RS, Levi JE, et al. Hydroxychloroquine plus azithromycin early treatment of mild COVID-19 in an outpatient setting: a randomized, double-blinded, placebo-controlled clinical trial evaluating viral clearance. Int J Antimicrob Agents 2021; 58(5): 106428. | ||
| 233 | 1 | rp | 1 | 1 | Nitazoxanide-Romark | Rossignol JF, Bardin MC, Fulgencio J, Mogelnicki D, Brechot C. A randomized double-blind placebo-controlled clinical trial of nitazoxanide for treatment of mild or moderate COVID-19. EClinicalMedicine 2022; 45: 101310. | |
| 241 | 1 | rp | 1 | 1 | Hydroxychloroquine-AH COVID-19 | Schwartz I, Boesen ME, Cerchiaro G, et al. Assessing the efficacy and safety of hydroxychloroquine as outpatient treatment of COVID-19: a randomized controlled trial. CMAJ Open 2021; 9(2): E693-E702. | |
| 245 | 1 | rp | 1 | Hydroxychloroquine-COVID-19 PEP | Skipper CP, Pastick KA, Engen NW, et al. Hydroxychloroquine in Nonhospitalized Adults With Early COVID-19 : A Randomized Trial. Ann Intern Med 2020; 173(8): 623–31. | ||
| 249 | 1 | rp | 1 | Hydroxychloroquine-Utah | Spivak AM, Barney BJ, Greene T, et al. A Randomized Clinical Trial Testing Hydroxychloroquine for Reduction of SARS-CoV-2 Viral Shedding and Hospitalization in Early Outpatient COVID-19 Infection. Microbiol Spectr 2023; 11(2): e0467422. | ||
| 257 | 1 | rp | 1 | 1 | Colchicine-COLCORONA | Tardif JC, Bouabdallaoui N, ĽAllier PL, et al. Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial. Lancet Respir Med 2021; 9(8): 924–32. | |
| 259 | 1 | rp | 3 | 1 | Zinc | Thomas S, Patel D, Bittel B, et al. Effect of High-Dose Zinc and Ascorbic Acid Supplementation vs Usual Care on Symptom Length and Reduction Among Ambulatory Patients With SARS-CoV-2 Infection: The COVID A to Z Randomized Clinical Trial. JAMA Netw Open 2021; 4(2): e210369. | |
| 36 | 1 | sma | 1 | 1 | Favipiravir-Avi-Mild-19 | Bosaeed M, Alharbi A, Mahmoud E, et al. Efficacy of favipiravir in adults with mild COVID-19: a randomized, double-blind, multicentre, placebo-controlled clinical trial. Clin Microbiol Infect 2022; 28(4): 602–8. | |
| 46 | 1 | sma | 1 | Molnupiravir-PANORAMIC | Butler CC, Hobbs FDR, Gbinigie OA, et al. Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial. Lancet 2023; 401(10373): 281–93. | ||
| 82 | 1 | sma | 1 | 1 | Interferon Lambda-ILIAD | Feld JJ, Kandel C, Biondi MJ, et al. Peginterferon lambda for the treatment of outpatients with COVID-19: a phase 2, placebo-controlled randomised trial. Lancet Respir Med 2021; 9(5): 498–510. | |
| 95 | 1 | sma | 1 | 1 | Remdesivir-PINETREE | Gottlieb RL, Vaca CE, Paredes R, et al. Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients. N Engl J Med 2022; 386(4): 305–15. | |
| 100 | 1 | sma | 1 | 1 | Nirmatrelvir/ritonavir-EPIC-HR | Hammond J, Leister-Tebbe H, Gardner A, et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. N Engl J Med 2022; 386(15): 1397–408. | |
| 112 | 1 | sma | 1 | Interferon Lambda-COVID-Lambda | Jagannathan P, Andrews JR, Bonilla H, et al. Peginterferon Lambda-1a for treatment of outpatients with uncomplicated COVID-19: a randomized placebo-controlled trial. Nature Communications 2021; 12(1): 1967. | ||
| 113 | 1 | sma | 1 | Molnupiravir-MOVe-OUT | Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N Engl J Med 2022; 386(6): 509–20. | ||
| 118 | 1 | sma | 1 | Lopinavir/ritonavir-TREAT NOW | Kaizer AM, Shapiro NI, Wild J, et al. Lopinavir/ritonavir for treatment of non-hospitalized patients with COVID-19: a randomized clinical trial. Int J Infect Dis 2023; 128: 223–9. | ||
| 159 | 1 | sma | 3 | Favipiravir/Lopinavir/Ritonavir-FLARE | Lowe DM, Brown LK, Chowdhury K, et al. Favipiravir, lopinavir-ritonavir, or combination therapy (FLARE): A randomised, double-blind, 2 × 2 factorial placebo-controlled trial of early antiviral therapy in COVID-19. PLoS Med 2022; 19(10): e1004120. | ||
| 203 | 1 | sma | 1 | 1 | Tenofovir/Emtricitabine-AR0-CORONA | Parienti JJ, Prazuck T, Peyro-Saint-Paul L, et al. Effect of Tenofovir Disoproxil Fumarate and Emtricitabine on nasopharyngeal SARS-CoV-2 viral load burden amongst outpatients with COVID-19: A pilot, randomized, open-label phase 2 trial. EClinicalMedicine 2021; 38: 100993. | |
| 222 | 1 | sma | 2 | 1 | Lopinavir/ritonavir-TOGETHER | Reis G, Moreira Silva E, Medeiros Silva DC, et al. Effect of Early Treatment With Hydroxychloroquine or Lopinavir and Ritonavir on Risk of Hospitalization Among Patients With COVID-19: The TOGETHER Randomized Clinical Trial. JAMA Netw Open 2021; 4(4): e216468. | |
| 223 | 1 | sma | 1 | Interferon Lambda-TOGETHER | Reis G, Moreira Silva EAS, Medeiros Silva DC, et al. Early Treatment with Pegylated Interferon Lambda for Covid-19. N Engl J Med 2023; 388(6): 518–28. | ||
| 231 | 1 | sma | 1 | 1 | Sofosbuvir & daclatasvir-SOVODAK | Roozbeh F, Saeedi M, Alizadeh-Navaei R, et al. Sofosbuvir and daclatasvir for the treatment of COVID-19 outpatients: a double-blind, randomized controlled trial. J Antimicrob Chemother 2021; 76(3): 753–7. | |
| 260 | 1 | sma | 1 | 1 | Molnupiravir-Aurobindo | Tippabhotla SK, Lahiri S, Rama Raju D, Kandi C, Prasad VN. Efficacy and Safety of Molnupiravir for the Treatment of Non-Hospitalized Adults With Mild COVID-19: A Randomized, Open-Label, Parallel-Group Phase 3 Trial. SSRN 2022; 4042673. | |
| 264 | 1 | sma | 1 | Favipiravir-Iran | Vaezi A, Salmasi M, Soltaninejad F, Salahi M, Javanmard SH, Amra B. Favipiravir in the Treatment of Outpatient COVID-19: A Multicenter, Randomized, Triple-Blind, Placebo-Controlled Clinical Trial. Adv Respir Med 2023; 91(1): 18–25. | ||
| 25 | guidelines | Bassetti M, Giacobbe DR, Bruzzi P, et al. Clinical Management of Adult Patients with COVID-19 Outside Intensive Care Units: Guidelines from the Italian Society of Anti-Infective Therapy (SITA) and the Italian Society of Pulmonology (SIP). Infect Dis Ther 2021; 10(4): 1837–85. | |||||
| 62 | guidelines | Cuker A, Tseng EK, Nieuwlaat R, et al. American Society of Hematology living guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19: July 2021 update on postdischarge thromboprophylaxis. Blood Adv 2022; 6(2): 664–71. | |||||
| 80 | guidelines | Estcourt LJ, Cohn CS, Pagano MB, et al. Clinical Practice Guidelines From the Association for the Advancement of Blood and Biotherapies (AABB): COVID-19 Convalescent Plasma. Ann Intern Med 2022; 175(9): 1310–21. | |||||
| 139 | guidelines | Kyriakoulis KG, Dimakakos E, Kyriakoulis IG, et al. Practical Recommendations for Optimal Thromboprophylaxis in Patients with COVID-19: A Consensus Statement Based on Available Clinical Trials. J Clin Med 2022; 11(20). | |||||
| 6 | no hospitalizations | Adel Mehraban MS, Shirzad M, Mohammad Taghizadeh Kashani L, et al. Efficacy and safety of add-on Viola odorata L. in the treatment of COVID-19: A randomized double-blind controlled trial. J Ethnopharmacol 2023; 304: 116058. | |||||
| 15 | no hospitalizations | Alizadeh Z, Keyhanian N, Ghaderkhani S, Dashti-Khavidaki S, Shokouhi Shoormasti R, Pourpak Z. A Pilot Study on Controlling Coronavirus Disease 2019 (COVID-19) Inflammation Using Melatonin Supplement. Iran J Allergy Asthma Immunol 2021; 20(4): 494–9. | |||||
| 29 | no hospitalizations | Bechlioulis A, Markozannes G, Chionidi I, et al. The effect of SGLT2 inhibitors, GLP1 agonists, and their sequential combination on cardiometabolic parameters: A randomized, prospective, intervention study. J Diabetes Complications 2023; 37(4): 108436. | |||||
| 31 | no hospitalizations | Ben Abdallah S, Mhalla Y, Trabelsi I, et al. Twice-Daily Oral Zinc in the Treatment of Patients With Coronavirus Disease 2019: A Randomized Double-Blind Controlled Trial. Clin Infect Dis 2023; 76(2): 185–91. | |||||
| 32 | no hospitalizations | Bencheqroun H, Ahmed Y, Kocak M, et al. A Randomized, Double-Blind, Placebo-Controlled, Multicenter Study to Evaluate the Safety and Efficacy of ThymoQuinone Formula (TQF) for Treating Outpatient SARS-CoV-2. Pathogens 2022; 11(5). | |||||
| 41 | no hospitalizations | Brennan CM, Nadella S, Zhao X, et al. Oral famotidine versus placebo in non-hospitalised patients with COVID-19: a randomised, double-blind, data-intense, phase 2 clinical trial. Gut 2022; 71(5): 879–88. | |||||
| 44 | no hospitalizations | Bruno AM, Allshouse AA, Campbell HM, et al. Weight-Based Compared With Fixed-Dose Enoxaparin Prophylaxis After Cesarean Delivery: A Randomized Controlled Trial. Obstet Gynecol 2022; 140(4): 575–83. | |||||
| 68 | no hospitalizations | De Boeck I, Cauwenberghs E, Spacova I, et al. Randomized, Double-Blind, Placebo-Controlled Trial of a Throat Spray with Selected Lactobacilli in COVID-19 Outpatients. Microbiol Spectr 2022; 10(5): e0168222. | |||||
| 103 | no hospitalizations | Hasanpour M, Safari H, Mohammadpour AH, et al. Efficacy of Covexir(R) (Ferula foetida oleo-gum) treatment in symptomatic improvement of patients with mild to moderate COVID-19: A randomized, double-blind, placebo-controlled trial. Phytother Res 2022; 36(12): 4504–15. | |||||
| 125 | no hospitalizations | Khan A, Iqtadar S, Mumtaz SU, et al. Oral Co-Supplementation of Curcumin, Quercetin, and Vitamin D3 as an Adjuvant Therapy for Mild to Moderate Symptoms of COVID-19-Results From a Pilot Open-Label, Randomized Controlled Trial. Front Pharmacol 2022; 13: 898062. | |||||
| 127 | no hospitalizations | Khoo SH, FitzGerald R, Saunders G, et al. Molnupiravir versus placebo in unvaccinated and vaccinated patients with early SARS-CoV-2 infection in the UK (AGILE CST-2): a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Infect Dis 2023; 23(2): 183–95. | |||||
| 133 | no hospitalizations | Kosmopoulos A, Bhatt DL, Meglis G, et al. A randomized trial of icosapent ethyl in ambulatory patients with COVID-19. iScience 2021; 24(9): 103040. | |||||
| 155 | no hospitalizations | Lofgren SM, Nicol MR, Bangdiwala AS, et al. Safety of Hydroxychloroquine Among Outpatient Clinical Trial Participants for COVID-19. Open Forum Infect Dis 2020; 7(11): ofaa500. | |||||
| 230 | no hospitalizations | Rohani M, Mozaffar H, Mesri M, Shokri M, Delaney D, Karimy M. Evaluation and comparison of vitamin A supplementation with standard therapies in the treatment of patients with COVID-19. East Mediterr Health J 2022; 28(9): 673–81. | |||||
| 256 | no hospitalizations | Tandon M, Wu W, Moore K, et al. SARS-CoV-2 accelerated clearance using a novel nitric oxide nasal spray (NONS) treatment: A randomized trial. Lancet Reg Health Southeast Asia 2022; 3: 100036. | |||||
| 1 | not outpt RCT | Aarnio-Peterson CM, Mara CA, Modi AC, Matthews A, Le Grange D, Shaffer A. Augmenting family based treatment with emotion coaching for adolescents with anorexia nervosa and atypical anorexia nervosa: Trial design and methodological report. Contemp Clin Trials Commun 2023; 33: 101118. | |||||
| 2 | not outpt RCT | Abbatecola AM, Incalzi RA, Malara A, et al. Monitoring COVID-19 vaccine use in Italian long term care centers: The GeroCovid VAX study. Vaccine 2022; 40(15): 2324–30. | |||||
| 3 | not outpt RCT | Abena PM, Decloedt EH, Bottieau E, et al. Chloroquine and Hydroxychloroquine for the Prevention or Treatment of COVID-19 in Africa: Caution for Inappropriate Off-label Use in Healthcare Settings. Am J Trop Med Hyg 2020; 102(6): 1184–8. | |||||
| 4 | not outpt RCT | Accelerating C-TI, Vaccines-6 Study G, Naggie S. Ivermectin for Treatment of Mild-to-Moderate COVID-19 in the Outpatient Setting: A Decentralized, Placebo-controlled, Randomized, Platform Clinical Trial. medRxiv 2022. | |||||
| 5 | not outpt RCT | Accelerating Covid-19 Therapeutic I, Vaccines-6 Study G, Naggie S. Inhaled Fluticasone for Outpatient Treatment of Covid-19: A Decentralized, Placebo-controlled, Randomized, Platform Clinical Trial. medRxiv 2022. | |||||
| 9 | not outpt RCT | Agusti A, Guillen E, Ayora A, et al. Efficacy and safety of hydroxychloroquine in healthcare professionals with mild SARS-CoV-2 infection: Prospective, non-randomized trial. Enferm Infecc Microbiol Clin (Engl Ed) 2022; 40(6): 289–95. | |||||
| 10 | not outpt RCT | Ainslie M, Brunette MF, Capozzoli M. Treatment Interruptions and Telemedicine Utilization in Serious Mental Illness: Retrospective Longitudinal Claims Analysis. JMIR Ment Health 2022; 9(3): e33092. | |||||
| 11 | not outpt RCT | Akca Sumengen A, Ocakci AF. Evaluation of the effect of an education program using cartoons and comics on disease management in children with asthma: a randomized controlled study. J Asthma 2023; 60(1): 11–23. | |||||
| 13 | not outpt RCT | Alemany A, Millat-Martinez P, Corbacho-Monne M, et al. Subcutaneous anti-COVID-19 hyperimmune immunoglobulin for prevention of disease in asymptomatic individuals with SARS-CoV-2 infection: a double-blind, placebo-controlled, randomised clinical trial. EClinicalMedicine 2023; 57: 101898. | |||||
| 14 | not outpt RCT | Ali R, Patel A, Waqas MA, Trivedi K, Slim J. Functionality of Monoclonal Antibody Therapy in SARS-CoV-2. J Med Cases 2022; 13(8): 380–5. | |||||
| 16 | not outpt RCT | Aref ZF, Bazeed S, Hassan MH, et al. Possible Role of Ivermectin Mucoadhesive Nanosuspension Nasal Spray in Recovery of Post-COVID-19 Anosmia. Infect Drug Resist 2022; 15: 5483–94. | |||||
| 17 | not outpt RCT | Avezum A, Oliveira GBF, Oliveira H, et al. Hydroxychloroquine versus placebo in the treatment of non-hospitalised patients with COVID-19 (COPE - Coalition V): A double-blind, multicentre, randomised, controlled trial. Lancet Reg Health Am 2022; 11: 100243. | |||||
| 18 | not outpt RCT | Axfors C, Schmitt AM, Janiaud P, et al. Mortality outcomes with hydroxychloroquine and chloroquine in COVID-19 from an international collaborative meta-analysis of randomized trials. Nat Commun 2021; 12(1): 2349. | |||||
| 20 | not outpt RCT | Bahmer T, Borzikowsky C, Lieb W, et al. Severity, predictors and clinical correlates of Post-COVID syndrome (PCS) in Germany: A prospective, multicentre, population-based cohort study. EClinicalMedicine 2022; 51: 101549. | |||||
| 21 | not outpt RCT | Baksh S, Heath SL, Fukuta Y, et al. Symptom duration and resolution with early outpatient treatment of convalescent plasma for COVID-19: a randomized trial. J Infect Dis 2023. | |||||
| 22 | not outpt RCT | Barati S, Feizabadi F, Khalaj H, et al. Evaluation of noscapine-licorice combination effects on cough relieving in COVID-19 outpatients: A randomized controlled trial. Front Pharmacol 2023; 14: 1102940. | |||||
| 23 | not outpt RCT | Barchuk A, Cherkashin M, Bulina A, et al. Vaccine effectiveness against referral to hospital after SARS-CoV-2 infection in St. Petersburg, Russia, during the Delta variant surge: a test-negative case-control study. BMC Med 2022; 20(1): 312. | |||||
| 26 | not outpt RCT | Batalik L, Dosbaba F, Hartman M, Konecny V, Batalikova K, Spinar J. Long-term exercise effects after cardiac telerehabilitation in patients with coronary artery disease: 1-year follow-up results of the randomized study. Eur J Phys Rehabil Med 2021; 57(5): 807–14. | |||||
| 27 | not outpt RCT | Batioglu-Karaaltin A, Yigit O, Cakan D, et al. Effect of the povidone iodine, hypertonic alkaline solution and saline nasal lavage on nasopharyngeal viral load in COVID-19. Clin Otolaryngol 2023. | |||||
| 28 | not outpt RCT | Bauer A, Schreinlechner M, Sappler N, et al. Discontinuation versus continuation of renin-angiotensin-system inhibitors in COVID-19 (ACEI-COVID): a prospective, parallel group, randomised, controlled, open-label trial. Lancet Respir Med 2021; 9(8): 863–72. | |||||
| 30 | not outpt RCT | Behrouzi B, Bhatt DL, Cannon CP, et al. Association of Influenza Vaccination With Cardiovascular Risk: A Meta-analysis. JAMA Netw Open 2022; 5(4): e228873. | |||||
| 33 | not outpt RCT | Bhatia T, Kumari N, Yadav A, et al. Feasibility, acceptability and evaluation of meditation to augment yoga practice among persons diagnosed with schizophrenia. Acta Neuropsychiatr 2022; 34(6): 330–43. | |||||
| 35 | not outpt RCT | Bledsoe J, Woller SC, Brooks M, et al. Clinically stable covid-19 patients presenting to acute unscheduled episodic care venues have increased risk of hospitalization: secondary analysis of a randomized control trial. BMC Infect Dis 2023; 23(1): 325. | |||||
| 37 | not outpt RCT | Boudreaux ED, Larkin C, Sefair AV, et al. Studying the implementation of Zero Suicide in a large health system: Challenges, adaptations, and lessons learned. Contemp Clin Trials Commun 2022; 30: 100999. | |||||
| 38 | not outpt RCT | Bramante CT, Buse J, Tamaritz L, et al. Outpatient metformin use is associated with reduced severity of COVID-19 disease in adults with overweight or obesity. J Med Virol 2021; 93(7): 4273–9. | |||||
| 40 | not outpt RCT | Bramante CT, Johnson SG, Garcia V, et al. Diabetes medications and associations with Covid-19 outcomes in the N3C database: A national retrospective cohort study. PLoS One 2022; 17(11): e0271574. | |||||
| 47 | not outpt RCT | Cadegiani FA, Goren A, Wambier CG, McCoy J. Early COVID-19 therapy with azithromycin plus nitazoxanide, ivermectin or hydroxychloroquine in outpatient settings significantly improved COVID-19 outcomes compared to known outcomes in untreated patients. New Microbes New Infect 2021; 43: 100915. | |||||
| 50 | not outpt RCT | Cavanna L, Citterio C. Randomised clinical trials on outpatient treatment of SARS-COV-2 infection: Light and shadows. Int J Clin Pract 2021; 75(12): e14896. | |||||
| 52 | not outpt RCT | Chawla A, Birger R, Wan H, et al. Factors Influencing COVID-19 Risk: Insights From Molnupiravir Exposure-Response Modeling of Clinical Outcomes. Clin Pharmacol Ther 2023; 113(6): 1337–45. | |||||
| 53 | not outpt RCT | Chen L, Zhou YZ, Zhou XM, et al. Evaluation of the "safe multidisciplinary app-assisted remote patient-self-testing (SMART) model" for warfarin home management during the COVID-19 pandemic: study protocol of a multi-center randomized controlled trial. BMC Health Serv Res 2021; 21(1): 875. | |||||
| 56 | not outpt RCT | Christie LJ, Fearn N, McCluskey A, et al. Remote constraint induced therapy of the upper extremity (ReCITE): A feasibility study protocol. Front Neurol 2022; 13: 1010449. | |||||
| 57 | not outpt RCT | Clark J, Tong SYC. In outpatients with mild to moderate COVID-19, low-dose fluvoxamine did not reduce time to sustained recovery. Ann Intern Med 2023; 176(5): JC52. | |||||
| 59 | not outpt RCT | Cohen JB, Hanff TC, Corrales-Medina V, et al. Randomized elimination and prolongation of ACE inhibitors and ARBs in coronavirus 2019 (REPLACE COVID) Trial Protocol. J Clin Hypertens (Greenwich) 2020; 22(10): 1780–8. | |||||
| 63 | not outpt RCT | D'Ascanio L, Vitelli F, Cingolani C, Maranzano M, Brenner MJ, Di Stadio A. Randomized clinical trial "olfactory dysfunction after COVID-19: olfactory rehabilitation therapy vs. intervention treatment with Palmitoylethanolamide and Luteolin": preliminary results. Eur Rev Med Pharmacol Sci 2021; 25(11): 4156–62. | |||||
| 64 | not outpt RCT | da Silva RM, Gebe Abreu Cabral P, de Souza SB, et al. Serial viral load analysis by DDPCR to evaluate FNC efficacy and safety in the treatment of mild cases of COVID-19. Front Med (Lausanne) 2023; 10: 1143485. | |||||
| 65 | not outpt RCT | Damery S, Jones J, O'Connell Francischetto E, Jolly K, Lilford R, Ferguson J. Remote Consultations Versus Standard Face-to-Face Appointments for Liver Transplant Patients in Routine Hospital Care: Feasibility Randomized Controlled Trial of myVideoClinic. J Med Internet Res 2021; 23(9): e19232. | |||||
| 66 | not outpt RCT | Davis JS, Ferreira D, Denholm JT, Tong SY. Clinical trials for the prevention and treatment of COVID-19: current state of play. Med J Aust 2020; 213(2): 86–93. | |||||
| 67 | not outpt RCT | Davoodi L, Abedi SM, Salehifar E, et al. Febuxostat therapy in outpatients with suspected COVID-19: A clinical trial. Int J Clin Pract 2020; 74(11): e13600. | |||||
| 70 | not outpt RCT | Di Pierro F, Derosa G, Maffioli P, et al. Possible Therapeutic Effects of Adjuvant Quercetin Supplementation Against Early-Stage COVID-19 Infection: A Prospective, Randomized, Controlled, and Open-Label Study. Int J Gen Med 2021; 14: 2359–66. | |||||
| 74 | not outpt RCT | Dube MP, Lemacon A, Barhdadi A, et al. Genetics of symptom remission in outpatients with COVID-19. Sci Rep 2021; 11(1): 10847. | |||||
| 75 | not outpt RCT | Dugani SB, Kiliaki SA, Nielsen ML, et al. Post-discharge early assessment with remote video link (PEARL) initiative for patients discharged from hospital medicine services. Hosp Pract (1995) 2022; 50(5): 379–86. | |||||
| 77 | not outpt RCT | Duvignaud A, Lhomme E, Pistone T, et al. Home Treatment of Older People with Symptomatic SARS-CoV-2 Infection (COVID-19): A structured Summary of a Study Protocol for a Multi-Arm Multi-Stage (MAMS) Randomized Trial to Evaluate the Efficacy and Tolerability of Several Experimental Treatments to Reduce the Risk of Hospitalisation or Death in outpatients aged 65 years or older (COVERAGE trial). Trials 2020; 21(1): 846. | |||||
| 78 | not outpt RCT | Eikelboom J, Rangarajan S, Jolly SS, et al. The Anti-Coronavirus Therapies (ACT) Trials: Design, Baseline Characteristics, and Challenges. CJC Open 2022; 4(6): 568–76. | |||||
| 81 | not outpt RCT | Fan Y, Shi Y, Zhang J, et al. The effects of narrative exposure therapy on COVID-19 patients with post-traumatic stress symptoms: A randomized controlled trial. J Affect Disord 2021; 293: 141–7. | |||||
| 84 | not outpt RCT | Fink T, Chen Q, Chong L, Hii MW, Knowles B. Telemedicine versus face-to-face follow up in general surgery: a randomized controlled trial. ANZ J Surg 2022; 92(10): 2544–50. | |||||
| 85 | not outpt RCT | Focosi D, Franchini M, Pirofski LA, et al. COVID-19 Convalescent Plasma and Clinical Trials: Understanding Conflicting Outcomes. Clin Microbiol Rev 2022; 35(3): e0020021. | |||||
| 88 | not outpt RCT | Geiger I, Kammerlander C, Hofer C, et al. Implementation of an integrated care programme to avoid fragility fractures of the hip in older adults in 18 Bavarian hospitals - study protocol for the cluster-randomised controlled fracture liaison service FLS-CARE. BMC Geriatr 2021; 21(1): 43. | |||||
| 89 | not outpt RCT | Geriak M, Haddad F, Kullar R, et al. Randomized Prospective Open Label Study Shows No Impact on Clinical Outcome of Adding Losartan to Hospitalized COVID-19 Patients with Mild Hypoxemia. Infect Dis Ther 2021; 10(3): 1323–30. | |||||
| 90 | not outpt RCT | Gerlier C, Pilmis B, Ganansia O, Le Monnier A, Nguyen Van JC. Clinical and operational impact of rapid point-of-care SARS-CoV-2 detection in an emergency department. Am J Emerg Med 2021; 50: 713–8. | |||||
| 91 | not outpt RCT | Ghany R, Palacio A, Dawkins E, et al. Metformin is associated with lower hospitalizations, mortality and severe coronavirus infection among elderly medicare minority patients in 8 states in USA. Diabetes Metab Syndr 2021; 15(2): 513–8. | |||||
| 96 | not outpt RCT | Group A-TL-CS, Lundgren JD, Grund B, et al. A Neutralizing Monoclonal Antibody for Hospitalized Patients with Covid-19. N Engl J Med 2021; 384(10): 905–14. | |||||
| 97 | not outpt RCT | Grundeis F, Ansems K, Dahms K, et al. Remdesivir for the treatment of COVID-19. Cochrane Database Syst Rev 2023; 1(1): CD014962. | |||||
| 99 | not outpt RCT | Gupta A, Madhavan MV, Poterucha TJ, et al. Association Between Antecedent Statin Use and Decreased Mortality in Hospitalized Patients with COVID-19. Res Sq 2020. | |||||
| 101 | not outpt RCT | Hanna CR, Blyth KG, Burley G, et al. Glasgow Early Treatment Arm Favirpiravir (GETAFIX) for adults with early stage COVID-19: A structured summary of a study protocol for a randomised controlled trial. Trials 2020; 21(1): 935. | |||||
| 102 | not outpt RCT | Haran JP, Pinero JC, Zheng Y, Palma NA, Wingertzahn M. Virtualized clinical studies to assess the natural history and impact of gut microbiome modulation in non-hospitalized patients with mild to moderate COVID-19 a randomized, open-label, prospective study with a parallel group study evaluating the physiologic effects of KB109 on gut microbiota structure and function: a structured summary of a study protocol for a randomized controlled study. Trials 2021; 22(1): 245. | |||||
| 104 | not outpt RCT | Hautmann C, Rausch J, Geldermann N, et al. Progress feedback in children and adolescents with internalizing and externalizing symptoms in routine care (OPTIE study): study protocol of a randomized parallel-group trial. BMC Psychiatry 2021; 21(1): 505. | |||||
| 105 | not outpt RCT | Hazan S, Dave S, Gunaratne AW, et al. Effectiveness of ivermectin-based multidrug therapy in severely hypoxic, ambulatory COVID-19 patients. Future Microbiol 2022; 17: 339–50. | |||||
| 106 | not outpt RCT | Helsingen LM, Loberg M, Refsum E, et al. Covid-19 transmission in fitness centers in Norway - a randomized trial. BMC Public Health 2021; 21(1): 2103. | |||||
| 108 | not outpt RCT | Hosseini FS, Malektojari A, Ghazizadeh S, et al. The efficacy and safety of Ivermectin in patients with mild and moderate COVID-19: A structured summary of a study protocol for a randomized controlled trial. Trials 2021; 22(1): 4. | |||||
| 109 | not outpt RCT | Hozayen SM, Zychowski D, Benson S, et al. Outpatient and inpatient anticoagulation therapy and the risk for hospital admission and death among COVID-19 patients. EClinicalMedicine 2021; 41: 101139. | |||||
| 111 | not outpt RCT | Indraratna P, Biswas U, Yu J, et al. Trials and Tribulations: mHealth Clinical Trials in the COVID-19 Pandemic. Yearb Med Inform 2021; 30(1): 272–9. | |||||
| 114 | not outpt RCT | Jering KS, Claggett BL, Pfeffer MA, et al. Prognostic Importance of NT-proBNP (N-Terminal Pro-B-Type Natriuretic Peptide) Following High-Risk Myocardial Infarction in the PARADISE-MI Trial. Circ Heart Fail 2023; 16(5): e010259. | |||||
| 116 | not outpt RCT | Kadali RAK, Janagama R, Peruru S, et al. Non-life-threatening adverse effects with COVID-19 mRNA-1273 vaccine: A randomized, cross-sectional study on healthcare workers with detailed self-reported symptoms. J Med Virol 2021; 93(7): 4420–9. | |||||
| 117 | not outpt RCT | Kaduszkiewicz H, Kochen MM, Kluge S, et al. Recommendations for the Outpatient Drug Treatment of Patients With COVID-19. Dtsch Arztebl Int 2022; 119(19): 342–9. | |||||
| 119 | not outpt RCT | Kaizer AM, Wild J, Lindsell CJ, et al. Trial of Early Antiviral Therapies during Non-hospitalized Outpatient Window (TREAT NOW) for COVID-19: a summary of the protocol and analysis plan for a decentralized randomized controlled trial. Trials 2022; 23(1): 273. | |||||
| 120 | not outpt RCT | Kapepula PM, Kabengele JK, Kingombe M, et al. Artemisia Spp. Derivatives for COVID-19 Treatment: Anecdotal Use, Political Hype, Treatment Potential, Challenges, and Road Map to Randomized Clinical Trials. Am J Trop Med Hyg 2020; 103(3): 960–4. | |||||
| 121 | not outpt RCT | Karaba AH, Johnston TS, Beck E, et al. Endemic Human Coronavirus Antibody Levels Are Unchanged after Convalescent or Control Plasma Transfusion for Early Outpatient COVID-19 Treatment. mBio 2023; 14(1): e0328722. | |||||
| 123 | not outpt RCT | Keitel V, Jensen B, Feldt T, et al. Reconvalescent plasma/camostat mesylate in early SARS-CoV-2 Q-PCR positive high-risk individuals (RES-Q-HR): a structured summary of a study protocol for a randomized controlled trial. Trials 2021; 22(1): 343. | |||||
| 124 | not outpt RCT | Khair A, Cromwell PM, Abdelatif A, et al. Text Messaging, Telephone, or In-Person Outpatient Visit to the Surgical Clinic: A Randomized Trial. J Surg Res 2022; 280: 226–33. | |||||
| 126 | not outpt RCT | Khoo SH, Fitzgerald R, Fletcher T, et al. Optimal dose and safety of molnupiravir in patients with early SARS-CoV-2: a Phase I, open-label, dose-escalating, randomized controlled study. J Antimicrob Chemother 2021; 76(12): 3286–95. | |||||
| 130 | not outpt RCT | Kip KE, McCreary EK, Collins K, et al. Evolving Real-World Effectiveness of Monoclonal Antibodies for Treatment of COVID-19 : A Cohort Study. Ann Intern Med 2023; 176(4): 496–504. | |||||
| 134 | not outpt RCT | Kramer A, Prinz C, Fichtner F, et al. Janus kinase inhibitors for the treatment of COVID-19. Cochrane Database Syst Rev 2022; 6(6): CD015209. | |||||
| 135 | not outpt RCT | Kremsner PG, Ahuad Guerrero RA, Arana-Arri E, et al. Efficacy and safety of the CVnCoV SARS-CoV-2 mRNA vaccine candidate in ten countries in Europe and Latin America (HERALD): a randomised, observer-blinded, placebo-controlled, phase 2b/3 trial. Lancet Infect Dis 2022; 22(3): 329–40. | |||||
| 136 | not outpt RCT | Krzyzanowska MK, Julian JA, Gu CS, et al. Remote, proactive, telephone based management of toxicity in outpatients during adjuvant or neoadjuvant chemotherapy for early stage breast cancer: pragmatic, cluster randomised trial. BMJ 2021; 375: e066588. | |||||
| 138 | not outpt RCT | Kupferschmitt A, Hinterberger T, Montanari I, et al. Relevance of the post-COVID syndrome within rehabilitation (PoCoRe): study protocol of a multicentre study with different specialisations. BMC Psychol 2022; 10(1): 189. | |||||
| 140 | not outpt RCT | Lee MT, George J, Shahab H, Hermel M, Rana JS, Virani SS. Highlights of Cardiovascular Disease Studies Presented at the 2021 American Heart Association Scientific Sessions. Curr Atheroscler Rep 2022; 24(1): 61–72. | |||||
| 141 | not outpt RCT | Lee TC, Bortolussi-Courval E, Belga S, et al. Inhaled corticosteroids for outpatients with COVID-19: a meta-analysis. Eur Respir J 2022; 59(5). | |||||
| 142 | not outpt RCT | Lee TC, Morris AM, Grover SA, Murthy S, McDonald EG. Outpatient Therapies for COVID-19: How Do We Choose? Open Forum Infect Dis 2022; 9(3): ofac008. | |||||
| 144 | not outpt RCT | Legacy M, Seely D, Conte E, et al. Dietary supplements to reduce symptom severity and duration in people with SARS-CoV-2: study protocol for a randomised, double-blind, placebo controlled clinical trial. BMJ Open 2022; 12(3): e057024. | |||||
| 146 | not outpt RCT | Les Bujanda I, Loureiro-Amigo J, Bastons FC, et al. Treatment of COVID-19 pneumonia with glucocorticoids (CORTIVID): a structured summary of a study protocol for a randomised controlled trial. Trials 2021; 22(1): 43. | |||||
| 148 | not outpt RCT | Levine AC, Fukuta Y, Huaman MA, et al. COVID-19 Convalescent Plasma Outpatient Therapy to Prevent Outpatient Hospitalization: A Meta-analysis of Individual Participant Data From Five Randomized Trials. Clin Infect Dis 2023. | |||||
| 149 | not outpt RCT | Li W, Xie L, Zhu X, et al. Effectiveness and safety of Qingfei Dayuan granules for treating influenza and upper respiratory tract infections manifested by the pulmonary heat-toxin syndrome: A multicenter, randomized, double-blind, placebo-controlled trial. Front Pharmacol 2023; 14: 1133560. | |||||
| 151 | not outpt RCT | Licchetta L, Trivisano M, Baldin E, et al. TELEmedicine for EPIlepsy Care (TELE-EPIC): protocol of a randomised, open controlled non-inferiority clinical trial. BMJ Open 2021; 11(12): e053980. | |||||
| 153 | not outpt RCT | Liu HH, Ezekowitz MD, Columbo M, et al. The future is now: our experience starting a remote clinical trial during the beginning of the COVID-19 pandemic. Trials 2021; 22(1): 603. | |||||
| 156 | not outpt RCT | Lokhandwala T, Acharya M, Farrelly E, Coutinho AD, Bell CF, Svedsater H. Within-trial economic analysis of resource use from COMET-ICE: A phase 3 clinical trial evaluating sotrovimab for the treatment of patients with COVID-19 at high risk of progression. J Manag Care Spec Pharm 2022; 28(11): 1261–71. | |||||
| 160 | not outpt RCT | Lui G, Guaraldi G. Drug treatment of COVID-19 infection. Curr Opin Pulm Med 2023; 29(3): 174–83. | |||||
| 162 | not outpt RCT | Manenti L, Maggiore U, Fiaccadori E, et al. Reduced mortality in COVID-19 patients treated with colchicine: Results from a retrospective, observational study. PLoS One 2021; 16(3): e0248276. | |||||
| 163 | not outpt RCT | Mao Z, Li X, Dacosta-Urbieta A, et al. Economic burden and health-related quality-of-life among infants with respiratory syncytial virus infection: A multi-country prospective cohort study in Europe. Vaccine 2023; 41(16): 2707–15. | |||||
| 165 | not outpt RCT | Mason JS, Crowell MS, Brindle RA, et al. The Effect of Blood Flow Restriction Training on Muscle Atrophy Following Meniscal Repair or Chondral Restoration Surgery in Active Duty Military: A Randomized Controlled Trial. J Sport Rehabil 2022; 31(1): 77–84. | |||||
| 166 | not outpt RCT | Mazzaferri F, Mirandola M, Savoldi A, et al. Exploratory data on the clinical efficacy of monoclonal antibodies against SARS-CoV-2 Omicron variant of concern. Elife 2022; 11. | |||||
| 170 | not outpt RCT | McCreary EK, Bariola JR, Minnier T, et al. Launching a comparative effectiveness adaptive platform trial of monoclonal antibodies for COVID-19 in 21 days. Contemp Clin Trials 2022; 113: 106652. | |||||
| 174 | not outpt RCT | McCullough PA, Kelly RJ, Ruocco G, et al. Pathophysiological Basis and Rationale for Early Outpatient Treatment of SARS-CoV-2 (COVID-19) Infection. Am J Med 2021; 134(1): 16–22. | |||||
| 175 | not outpt RCT | McKinnon JE, Wang DD, Zervos M, et al. Safety and tolerability of hydroxychloroquine in health care workers and first responders for the prevention of COVID-19: WHIP COVID-19 Study. Int J Infect Dis 2022; 116: 167–73. | |||||
| 176 | not outpt RCT | Mesri M, Esmaeili Saber SS, Godazi M, et al. The effects of combination of Zingiber officinale and Echinacea on alleviation of clinical symptoms and hospitalization rate of suspected COVID-19 outpatients: a randomized controlled trial. J Complement Integr Med 2021; 18(4): 775–81. | |||||
| 179 | not outpt RCT | Miguel-Cruz A, Ladurner AM, Kohls-Wiebe M, Rawani D. The Effects of 3D Immersion Technology (3Scape) on Mental Health in Outpatients From a Short-Term Assessment, Rehabilitation, and Treatment Program: Feasibility Protocol for a Randomized Controlled Trial. JMIR Res Protoc 2021; 10(9): e25017. | |||||
| 178 | not outpt RCT | Miguel-Cruz A, Sr., Guptill C, Gregson G, et al. Determining the Effectiveness of a New Device for Hand Therapy (The FEPSim Device): Feasibility Protocol for a Randomized Controlled Trial Study. JMIR Res Protoc 2021; 10(5): e22145. | |||||
| 181 | not outpt RCT | Mills FP, Reis G, Wilson LA, et al. Early Treatment with Fluvoxamine among Patients with COVID-19: A Cost-Consequence Model. Am J Trop Med Hyg 2023; 108(1): 101–6. | |||||
| 182 | not outpt RCT | Miryan M, Bagherniya M, Sahebkar A, et al. Effects of curcumin-piperine co-supplementation on clinical signs, duration, severity, and inflammatory factors in patients with COVID-19: a structured summary of a study protocol for a randomised controlled trial. Trials 2020; 21(1): 1027. | |||||
| 183 | not outpt RCT | Levine AC, Fukuta Y, Huaman MA, et al. COVID-19 Convalescent Plasma Outpatient Therapy to Prevent Outpatient Hospitalization: A Meta-analysis of Individual Participant Data From Five Randomized Trials. Clin Infect Dis 2023. | |||||
| 185 | not outpt RCT | Levine AC, Fukuta Y, Huaman MA, et al. COVID-19 Convalescent Plasma Outpatient Therapy to Prevent Outpatient Hospitalization: A Meta-analysis of Individual Participant Data From Five Randomized Trials. medRxiv 2022. | |||||
| 186 | not outpt RCT | Li W, Xie L, Zhu X, et al. Effectiveness and safety of Qingfei Dayuan granules for treating influenza and upper respiratory tract infections manifested by the pulmonary heat-toxin syndrome: A multicenter, randomized, double-blind, placebo-controlled trial. Front Pharmacol 2023; 14: 1133560. | |||||
| 190 | not outpt RCT | Licchetta L, Trivisano M, Baldin E, et al. TELEmedicine for EPIlepsy Care (TELE-EPIC): protocol of a randomised, open controlled non-inferiority clinical trial. BMJ Open 2021; 11(12): e053980. | |||||
| 191 | not outpt RCT | Lin WT, Hung SH, Lai CC, Wang CY, Chen CH. The impact of neutralizing monoclonal antibodies on the outcomes of COVID-19 outpatients: A systematic review and meta-analysis of randomized controlled trials. J Med Virol 2022; 94(5): 2222–9. | |||||
| 193 | not outpt RCT | Liu HH, Ezekowitz MD, Columbo M, et al. The future is now: our experience starting a remote clinical trial during the beginning of the COVID-19 pandemic. Trials 2021; 22(1): 603. | |||||
| 196 | not outpt RCT | Lofgren SM, Nicol MR, Bangdiwala AS, et al. Safety of Hydroxychloroquine among Outpatient Clinical Trial Participants for COVID-19. medRxiv 2020. | |||||
| 197 | not outpt RCT | Lofgren SM, Nicol MR, Bangdiwala AS, et al. Safety of Hydroxychloroquine Among Outpatient Clinical Trial Participants for COVID-19. Open Forum Infect Dis 2020; 7(11): ofaa500. | |||||
| 200 | not outpt RCT | Lokhandwala T, Acharya M, Farrelly E, Coutinho AD, Bell CF, Svedsater H. Within-trial economic analysis of resource use from COMET-ICE: A phase 3 clinical trial evaluating sotrovimab for the treatment of patients with COVID-19 at high risk of progression. J Manag Care Spec Pharm 2022; 28(11): 1261–71. | |||||
| 201 | not outpt RCT | Lopes RD, de Barros ESPGM, Furtado RHM, et al. Randomized clinical trial to evaluate a routine full anticoagulation Strategy in Patients with Coronavirus Infection (SARS-CoV2) admitted to hospital: Rationale and design of the ACTION (AntiCoagulaTlon cOroNavirus)-Coalition IV trial. Am Heart J 2021; 238: 1–11. | |||||
| 202 | not outpt RCT | Lother SA, Abassi M, Agostinis A, et al. Post-exposure prophylaxis or pre-emptive therapy for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): study protocol for a pragmatic randomized-controlled trial. Can J Anaesth 2020; 67(9): 1201–11. | |||||
| 204 | not outpt RCT | Lui G, Guaraldi G. Drug treatment of COVID-19 infection. Curr Opin Pulm Med 2023; 29(3): 174–83. | |||||
| 206 | not outpt RCT | Malin JJ, Weibel S, Gruell H, Kreuzberger N, Stegemann M, Skoetz N. Efficacy and safety of molnupiravir for the treatment of SARS-CoV-2 infection: a systematic review and meta-analysis. J Antimicrob Chemother 2023. | |||||
| 208 | not outpt RCT | Manenti L, Maggiore U, Fiaccadori E, et al. Reduced mortality in COVID-19 patients treated with colchicine: Results from a retrospective, observational study. PLoS One 2021; 16(3): e0248276. | |||||
| 209 | not outpt RCT | Mao Z, Li X, Dacosta-Urbieta A, et al. Economic burden and health-related quality-of-life among infants with respiratory syncytial virus infection: A multi-country prospective cohort study in Europe. Vaccine 2023; 41(16): 2707–15. | |||||
| 210 | not outpt RCT | Martins-Filho PR, Ferreira LC, Heimfarth L, Araujo AAS, Quintans-Junior LJ. Efficacy and safety of hydroxychloroquine as pre-and post-exposure prophylaxis and treatment of COVID-19: A systematic review and meta-analysis of blinded, placebo-controlled, randomized clinical trials. Lancet Reg Health Am 2021; 2: 100062. | |||||
| 211 | not outpt RCT | Mason JS, Crowell MS, Brindle RA, et al. The Effect of Blood Flow Restriction Training on Muscle Atrophy Following Meniscal Repair or Chondral Restoration Surgery in Active Duty Military: A Randomized Controlled Trial. J Sport Rehabil 2022; 31(1): 77–84. | |||||
| 212 | not outpt RCT | Mazzaferri F, Mirandola M, Savoldi A, et al. Exploratory data on the clinical efficacy of monoclonal antibodies against SARS-CoV-2 Omicron variant of concern. Elife 2022; 11. | |||||
| 213 | not outpt RCT | McCarthy MW, Naggie S, Boulware DR, et al. Fluvoxamine for Outpatient Treatment of COVID-19: A Decentralized, Placebo-controlled, Randomized, Platform Clinical Trial. medRxiv 2022. | |||||
| 215 | not outpt RCT | McCoy J, Goren A, Cadegiani FA, et al. Proxalutamide Reduces the Rate of Hospitalization for COVID-19 Male Outpatients: A Randomized Double-Blinded Placebo-Controlled Trial. Front Med (Lausanne) 2021; 8: 668698. | |||||
| 218 | not outpt RCT | McCreary EK, Bariola JR, Minnier T, et al. Launching a comparative effectiveness adaptive platform trial of monoclonal antibodies for COVID-19 in 21 days. Contemp Clin Trials 2022; 113: 106652. | |||||
| 225 | not outpt RCT | McCreary EK, Bariola JR, Wadas RJ, et al. Association of Subcutaneous or Intravenous Administration of Casirivimab and Imdevimab Monoclonal Antibodies With Clinical Outcomes in Adults With COVID-19. JAMA Netw Open 2022; 5(4): e226920. | |||||
| 228 | not outpt RCT | McCreary MR, Schnell PM, Rhoda DA. Randomized Double-blind Placebo-controlled Proof-of-concept Trial of Resveratrol for Outpatient Treatment of Mild Coronavirus Disease (COVID-19). Res Sq 2021. | |||||
| 232 | not outpt RCT | McCullough PA, Kelly RJ, Ruocco G, et al. Pathophysiological Basis and Rationale for Early Outpatient Treatment of SARS-CoV-2 (COVID-19) Infection. Am J Med 2021; 134(1): 16–22. | |||||
| 234 | not outpt RCT | McKinnon JE, Wang DD, Zervos M, et al. Safety and tolerability of hydroxychloroquine in health care workers and first responders for the prevention of COVID-19: WHIP COVID-19 Study. Int J Infect Dis 2022; 116: 167–73. | |||||
| 235 | not outpt RCT | Mesri M, Esmaeili Saber SS, Godazi M, et al. The effects of combination of Zingiber officinale and Echinacea on alleviation of clinical symptoms and hospitalization rate of suspected COVID-19 outpatients: a randomized controlled trial. J Complement Integr Med 2021; 18(4): 775–81. | |||||
| 237 | not outpt RCT | Migliorini F, Vaishya R, Eschweiler J, Oliva F, Hildebrand F, Maffulli N. Vitamins C and D and COVID-19 Susceptibility, Severity and Progression: An Evidence Based Systematic Review. Medicina (Kaunas) 2022; 58(7). | |||||
| 238 | not outpt RCT | Miguel-Cruz A, Ladurner AM, Kohls-Wiebe M, Rawani D. The Effects of 3D Immersion Technology (3Scape) on Mental Health in Outpatients From a Short-Term Assessment, Rehabilitation, and Treatment Program: Feasibility Protocol for a Randomized Controlled Trial. JMIR Res Protoc 2021; 10(9): e25017. | |||||
| 240 | not outpt RCT | Miguel-Cruz A, Sr., Guptill C, Gregson G, et al. Determining the Effectiveness of a New Device for Hand Therapy (The FEPSim Device): Feasibility Protocol for a Randomized Controlled Trial Study. JMIR Res Protoc 2021; 10(5): e22145. | |||||
| 242 | not outpt RCT | Millat-Martinez P, Gharbharan A, Alemany A, et al. Prospective individual patient data meta-analysis of two randomized trials on convalescent plasma for COVID-19 outpatients. Nat Commun 2022; 13(1): 2583. | |||||
| 243 | not outpt RCT | Mills FP, Reis G, Wilson LA, et al. Early Treatment with Fluvoxamine among Patients with COVID-19: A Cost-Consequence Model. Am J Trop Med Hyg 2023; 108(1): 101–6. | |||||
| 244 | not outpt RCT | Miryan M, Bagherniya M, Sahebkar A, et al. Effects of curcumin-piperine co-supplementation on clinical signs, duration, severity, and inflammatory factors in patients with COVID-19: a structured summary of a study protocol for a randomised controlled trial. Trials 2020; 21(1): 1027. | |||||
| 246 | not outpt RCT | Mitja O, Reis G, Boulware DR, et al. Hydroxychloroquine for treatment of non-hospitalized adults with COVID-19: A meta-analysis of individual participant data of randomized trials. Clin Transl Sci 2023; 16(3): 524–35. | |||||
| 247 | not outpt RCT | Muschol J, Heinrich M, Heiss C, et al. Economic and Environmental Impact of Digital Health App Video Consultations in Follow-up Care for Patients in Orthopedic and Trauma Surgery in Germany: Randomized Controlled Trial. J Med Internet Res 2022; 24(11): e42839. | |||||
| 250 | not outpt RCT | Nachega JB, Leisegang R, Kallay O, Mills EJ, Zumla A, Lester RT. Mobile Health Technology for Enhancing the COVID-19 Response in Africa: A Potential Game Changer? Am J Trop Med Hyg 2020; 103(1): 3–5. | |||||
| 253 | not outpt RCT | Naggie S, Boulware DR, Lindsell CJ, et al. Effect of Ivermectin 600 mug/kg for 6 days vs Placebo on Time to Sustained Recovery in Outpatients with Mild to Moderate COVID-19: A Randomized Clinical Trial. medRxiv 2022. | |||||
| 258 | not outpt RCT | Nappi F, Iervolino A, Avtaar Singh SS. Molecular Insights of SARS-CoV-2 Antivirals Administration: A Balance between Safety Profiles and Impact on Cardiovascular Phenotypes. Biomedicines 2022; 10(2). | |||||
| 262 | not outpt RCT | Narayanan D, Parimon T. Current Therapeutics for COVID-19, What We Know about the Molecular Mechanism and Efficacy of Treatments for This Novel Virus. Int J Mol Sci 2022; 23(14). | |||||
| 263 | not outpt RCT | Nascimento L, Mendes LA, Torres-Castro R, et al. Physical performance testing in post-COVID-19 patients: protocol for a systematic review of psychometric measurement properties. BMJ Open 2023; 13(4): e067392. | |||||
| 265 | not outpt RCT | Ngo BT, Marik P, Kory P, et al. The time to offer treatments for COVID-19. Expert Opin Investig Drugs 2021; 30(5): 505–18. | |||||
| 267 | not outpt RCT | Nicastri E, Marinangeli F, Pivetta E, et al. A phase 2 randomized, double-blinded, placebo-controlled, multicenter trial evaluating the efficacy and safety of raloxifene for patients with mild to moderate COVID-19. EClinicalMedicine 2022; 48: 101450. | |||||
| 268 | not outpt RCT | Nyirenda JL, Sofroniou M, Toews I, et al. Fluvoxamine for the treatment of COVID-19. Cochrane Database Syst Rev 2022; 9(9): CD015391. | |||||
| 269 | not outpt RCT | Ogletree ML, Chander Chiang K, Kulshrestha R, Agarwal A, Agarwal A, Gupta A. Treatment of COVID-19 Pneumonia and Acute Respiratory Distress With Ramatroban, a Thromboxane A(2) and Prostaglandin D(2) Receptor Antagonist: A Four-Patient Case Series Report. Front Pharmacol 2022; 13: 904020. | |||||
| 271 | not outpt RCT | Oliveira GBF, Neves P, Oliveira HA, et al. Rivaroxaban in Outpatients with Mild or Moderate COVID-19: Rationale and Design of the Study CARE (CARE - Coalition COVID-19 Brazil VIII). Arq Bras Cardiol 2023; 120(3): e20220431. | |||||
| 272 | not outpt RCT | Oliveira Junior HA, Ferri CP, Boszczowski I, et al. Rationale and Design of the COVID-19 Outpatient Prevention Evaluation (COPE - Coalition V) Randomized Clinical Trial: Hydroxychloroquine vs. Placebo in Non-Hospitalized Patients. Arq Bras Cardiol 2022; 118(2): 378–87. | |||||
| 273 | not outpt RCT | Olson SM, Newhams MM, Halasa NB, et al. Effectiveness of Pfizer-BioNTech mRNA Vaccination Against COVID-19 Hospitalization Among Persons Aged 12–18 Years - United States, June-September 2021. MMWR Morb Mortal Wkly Rep 2021; 70(42): 1483–8. | |||||
| 276 | not outpt RCT | Pan DZ, Odorizzi PM, Schoenichen A, et al. Remdesivir improves biomarkers associated with disease severity in COVID-19 patients treated in an outpatient setting. Commun Med (Lond) 2023; 3(1): 2. | |||||
| 278 | not outpt RCT | Pembroke S, Rogerson S, Coyne I. Conducting a randomised controlled trial of a psychosocial intervention for adolescents with type 1 diabetes during COVID-19: recommendations to overcome the challenges complicated by inconsistent public health guidelines on research. Trials 2022; 23(1): 362. | |||||
| 279 | not outpt RCT | Pham B, Rios P, Radhakrishnan A, et al. Comparative-effectiveness research of COVID-19 treatment: a rapid scoping review. BMJ Open 2022; 12(6): e045115. | |||||
| 280 | not outpt RCT | Pinzon MA, Ortiz S, Holguin H, et al. Dexamethasone vs methylprednisolone high dose for Covid-19 pneumonia. PLoS One 2021; 16(5): e0252057. | |||||
| 51 | open label | Plasse TF, Delgado B, Potts J, et al. A randomized, placebo-controlled pilot study of upamostat, a host-directed serine protease inhibitor, for outpatient treatment of COVID-19. Int J Infect Dis 2023; 128: 148–56. | |||||
| 79 | open label | Popp M, Reis S, Schiesser S, et al. Ivermectin for preventing and treating COVID-19. Cochrane Database Syst Rev 2022; 6(6): CD015017. | |||||
| 157 | open label | Popp M, Stegemann M, Metzendorf MI, et al. Ivermectin for preventing and treating COVID-19. Cochrane Database Syst Rev 2021; 7(7): CD015017. | |||||
| 248 | open label | Popp M, Stegemann M, Riemer M, et al. Antibiotics for the treatment of COVID-19. Cochrane Database Syst Rev 2021; 10(10): CD015025. | |||||
| 254 | open label | Portal-Celhay C, Forleo-Neto E, Eagan W, et al. Virologic Efficacy of Casirivimab and Imdevimab COVID-19 Antibody Combination in Outpatients With SARS-CoV-2 Infection: A Phase 2 Dose-Ranging Randomized Clinical Trial. JAMA Netw Open 2022; 5(8): e2225411. | |||||
| 110 | propensity matcch | Powell-Jackson T, King JJC, Makungu C, et al. Infection prevention and control compliance in Tanzanian outpatient facilities: a cross-sectional study with implications for the control of COVID-19. Lancet Glob Health 2020; 8(6): e780-e9. | |||||
| 171 | propensity matcch | Procter BC, Ross C, Pickard V, Smith E, Hanson C, McCullough PA. Clinical outcomes after early ambulatory multidrug therapy for high-risk SARS-CoV-2 (COVID-19) infection. Rev Cardiovasc Med 2020; 21(4): 611–4. | |||||
| 42 | protocol | Puspitasari AJ, Heredia D, Coombes BJ, et al. Feasibility and Initial Outcomes of a Group-Based Teletherapy Psychiatric Day Program for Adults With Serious Mental Illness: Open, Nonrandomized Trial in the Context of COVID-19. JMIR Ment Health 2021; 8(3): e25542. | |||||
| 49 | protocol | Rahman AE, Hossain AT, Nair H, et al. Prevalence of hypoxaemia in children with pneumonia in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Glob Health 2022; 10(3): e348-e59. | |||||
| 94 | protocol | Rahmati M, Molanouri Shamsi M, Woo W, et al. Effects of physical rehabilitation interventions in COVID-19 patients following discharge from hospital: A systematic review. J Integr Med 2023; 21(2): 149–58. | |||||
| 158 | protocol | Rajkumar T, Freyne J, Varnfield M, et al. Remote blood pressure monitoring in high risk pregnancy - study protocol for a randomised controlled trial (REMOTE CONTROL trial). Trials 2023; 24(1): 334. | |||||
| 199 | protocol | Reis S, Metzendorf MI, Kuehn R, et al. Nirmatrelvir combined with ritonavir for preventing and treating COVID-19. Cochrane Database Syst Rev 2022; 9(9): CD015395. | |||||
| 236 | protocol | Reis S, Popp M, Schiesser S, et al. Anticoagulation in COVID-19 patients - An updated systematic review and meta-analysis. Thromb Res 2022; 219: 40–8. | |||||
| 169 | retracted | Rizk JG, Gupta A, Lazo JG, Jr., et al. To Anticoagulate or Not to Anticoagulate in COVID-19: Lessons after 2 Years. Semin Thromb Hemost 2023; 49(1): 62–72. | |||||
| 19 | review | Rohani M, Mozaffar H, Mesri M, Shokri M, Delaney D, Karimy M. Evaluation and comparison of vitamin A supplementation with standard therapies in the treatment of patients with COVID-19. East Mediterr Health J 2022; 28(9): 673–81. | |||||
| 34 | review | Rosenthal N, Cao Z, Gundrum J, Sianis J, Safo S. Risk Factors Associated With In-Hospital Mortality in a US National Sample of Patients With COVID-19. JAMA Netw Open 2020; 3(12): e2029058. | |||||
| 45 | review | Rubin DJ, Shah AA. Predicting and Preventing Acute Care Re-Utilization by Patients with Diabetes. Curr Diab Rep 2021; 21(9): 34. | |||||
| 55 | review | Ruzhentsova TA, Oseshnyuk RA, Soluyanova TN, et al. Phase 3 trial of coronavir (favipiravir) in patients with mild to moderate COVID-19. Am J Transl Res 2021; 13(11): 12575–87. | |||||
| 69 | review | Saiz-Rodriguez M, Pena T, Lazaro L, et al. Outpatient treatment of COVID-19 with steroids in the phase of mild pneumonia without the need for admission as an opportunity to modify the course of the disease: A structured summary of a randomised controlled trial. Trials 2020; 21(1): 632. | |||||
| 71 | review | Salovaara PK, Li C, Nicholson A, Lipsitz SR, Natarajan S. Navigating COVID-19 and related challenges to completing clinical trials: Lessons from the PATRIOT and STEP-UP randomized prevention trials. Clin Trials 2023; 20(2): 153–65. | |||||
| 83 | review | Sanchez-Rico M, Limosin F, Vernet R, et al. Hydroxyzine Use and Mortality in Patients Hospitalized for COVID-19: A Multicenter Observational Study. J Clin Med 2021; 10(24). | |||||
| 86 | review | Sanghavi D, Bansal P, Kaur IP, et al. Impact of colchicine on mortality and morbidity in COVID-19: a systematic review. Ann Med 2022; 54(1): 775–89. | |||||
| 87 | review | Savoldi A, Morra M, De Nardo P, et al. Clinical efficacy of different monoclonal antibody regimens among non-hospitalised patients with mild to moderate COVID-19 at high risk for disease progression: a prospective cohort study. Eur J Clin Microbiol Infect Dis 2022; 41(7): 1065–76. | |||||
| 122 | review | Schwartz RA, Suskind RM. Azithromycin and COVID-19: Prompt early use at first signs of this infection in adults and children, an approach worthy of consideration. Dermatol Ther 2020; 33(4): e13785. | |||||
| 131 | review | Shim MS, Kim S, Choi M, Choi JY, Park CG, Kim GS. Developing an app-based self-management program for people living with HIV: a randomized controlled pilot study during the COVID-19 pandemic. Sci Rep 2022; 12(1): 19401. | |||||
| 137 | review | Siami Z, Aghajanian S, Mansouri S, et al. Effect of Ammonium Chloride in addition to standard of care in outpatients and hospitalized COVID-19 patients: A randomized clinical trial. Int J Infect Dis 2021; 108: 306–8. | |||||
| 143 | review | Skovsgaard CV, Kruse M, Hjollund N, Maribo T, de Thurah A. Cost-effectiveness of a telehealth intervention in rheumatoid arthritis: economic evaluation of the Telehealth in RA (TeRA) randomized controlled trial. Scand J Rheumatol 2023; 52(2): 118–28. | |||||
| 152 | review | So H, Chow E, Cheng IT, et al. Use of telemedicine for follow-up of lupus nephritis in the COVID-19 outbreak: The 6-month results of a randomized controlled trial. Lupus 2022; 31(4): 488–94. | |||||
| 161 | review | Song JY, Yoon JG, Seo YB, et al. Ciclesonide Inhaler Treatment for Mild-to-Moderate COVID-19: A Randomized, Open-Label, Phase 2 Trial. J Clin Med 2021; 10(16). | |||||
| 164 | review | Spyropoulos AC, Connors JM, Douketis JD, et al. Good practice statements for antithrombotic therapy in the management of COVID-19: Guidance from the SSC of the ISTH. J Thromb Haemost 2022; 20(10): 2226–36. | |||||
| 177 | review | Tafler L, Danilevsky A, Seth D. Azithromycin in the Successful Management of COVID-19: A Family Physician's Perspective. Cureus 2021; 13(4): e14574. | |||||
| 192 | review | Takayama S, Namiki T, Ito T, et al. A multi-center, randomized controlled trial by the Integrative Management in Japan for Epidemic Disease (IMJEDI study-RCT) on the use of Kampo medicine, kakkonto with shosaikotokakikyosekko, in mild-to-moderate COVID-19 patients for symptomatic relief and prevention of severe stage: a structured summary of a study protocol for a randomized controlled trial. Trials 2020; 21(1): 827. | |||||
| 205 | review | Talasaz AH, Sadeghipour P, Kakavand H, et al. Recent Randomized Trials of Antithrombotic Therapy for Patients With COVID-19: JACC State-of-the-Art Review. J Am Coll Cardiol 2021; 77(15): 1903–21. | |||||
| 216 | review | Tandon M, Wu W, Moore K, et al. SARS-CoV-2 accelerated clearance using a novel nitric oxide nasal spray (NONS) treatment: A randomized trial. Lancet Reg Health Southeast Asia 2022; 3: 100036. | |||||
| 217 | review | Thomas JJ, Becker KR, Breithaupt L, et al. Cognitive-behavioral therapy for adults with avoidant/restrictive food intake disorder. J Behav Cogn Ther 2021; 31(1): 47–55. | |||||
| 226 | review | Tunjungputri RN, Tetrasiwi EN, Mulansari NA, Harimurti K, Nelwan EJ. Parenteral and Oral Anticoagulant Treatment for Hospitalized and Post-Discharge COVID-19 Patients: A Systematic Review and Meta-Analysis. Acta Med Indones 2022; 54(2): 190–209. | |||||
| 239 | review | Turan B, Akinci MA. Changing Trends of Diagnoses in a Child and Adolescent Psychiatry Outpatient Clinic Before and During COVID-19: An Analysis of Registered Data. Psychiatr Danub 2023; 35(1): 92–6. | |||||
| 255 | review | Turkia M. The History of Methylprednisolone, Ascorbic Acid, Thiamine, and Heparin Protocol and I-MASK+ Ivermectin Protocol for COVID-19. Cureus 2020; 12(12): e12403. | |||||
| 261 | review | Vainio PJ, Hietasalo P, Koivisto AL, et al. Hydroxychloroquine in the treatment of adult patients with Covid-19 infection in a primary care setting (LIBERTY): A structured summary of a study protocol for a randomised controlled trial. Trials 2021; 22(1): 44. | |||||
| 266 | review | Vatvani AD, Kurniawan A, Hariyanto TI. Efficacy and Safety of Fluvoxamine as Outpatient Treatment for Patients With Covid-19: A Systematic Review and Meta-analysis of Clinical Trials. Ann Pharmacother 2023: 10600280231162243. | |||||
| 277 | review | Venkatesh N, Paldus B, Lee MH, MacIsaac RJ, Jenkins AJ, O'Neal DN. COVID-19, Type 1 Diabetes Clinical Practice, Research, and Remote Medical Care: A View From the Land Down-Under. J Diabetes Sci Technol 2020; 14(4): 803–4. | |||||
| 43 | sub study | Verma N, Buch B, Taralekar R, Acharya S. Diagnostic concordance of telemedicine as compared to face-to-face care in primary health care clinics in rural India: a randomized crossover trial. JMIR Form Res 2023. | |||||
| 180 | sub study | Vlake JH, Van Bommel J, Wils EJ, et al. Effect of intensive care unit-specific virtual reality (ICU-VR) to improve psychological well-being and quality of life in COVID-19 ICU survivors: a study protocol for a multicentre, randomized controlled trial. Trials 2021; 22(1): 328. | |||||
| 270 | sub study | Voci D, Gotschi A, Held U, et al. Enoxaparin for outpatients with COVID-19: 90-day results from the randomised, open-label, parallel-group, multinational, phase III OVID trial. Thromb Res 2023; 221: 157–63. | |||||
| 274 | sub study | Vollmuth C, Miljukov O, Abu-Mugheisib M, et al. Impact of the coronavirus disease 2019 pandemic on stroke teleconsultations in Germany in the first half of 2020. Eur J Neurol 2021; 28(10): 3267–78. | |||||
| 194 | under 30 in arm | Vuorio A, Brinck J, Kovanen PT. Continuation of fibrate therapy in patients with metabolic syndrome and COVID-19: a beneficial regime worth pursuing. Ann Med 2022; 54(1): 1952–5. | |||||
| 207 | under 30 in arm | Wagner C, Griesel M, Mikolajewska A, et al. Systemic corticosteroids for the treatment of COVID-19: Equity-related analyses and update on evidence. Cochrane Database Syst Rev 2022; 11(11): CD014963. | |||||
| 8 | duplicate citation | Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19. N Engl J Med 2021; 384(3): 238–51. | |||||
| 147 | duplicate citation | Xiang HR, He B, Li Y, Cheng X, Zhang QZ, Peng WX. Bamlanivimab plus etesevimab treatment have a better outcome against COVID-19: A meta-analysis. J Med Virol 2022; 94(5): 1893–905. | |||||
| 154 | duplicate citation | Xu C, Yi T, Tan S, et al. Association of Oral or Intravenous Vitamin C Supplementation with Mortality: A Systematic Review and Meta-Analysis. Nutrients 2023; 15(8). | |||||
| 167 | duplicate citation | Yasein N, Shroukh W, Barghouti F, et al. The potential counter effect of COVID-19 outbreak on an antimicrobial agents prescribing educational intervention. J Infect Dev Ctries 2021; 15(11): 1653–60. | |||||
| 172 | duplicate citation | Yin W, Liu Y, Hu H, Sun J, Liu Y, Wang Z. Telemedicine management of type 2 diabetes mellitus in obese and overweight young and middle-aged patients during COVID-19 outbreak: A single-center, prospective, randomized control study. PLoS One 2022; 17(9): e0275251. | |||||
| 188 | duplicate citation | Zarabanda D, Vukkadala N, Phillips KM, et al. The Effect of Povidone-Iodine Nasal Spray on Nasopharyngeal SARS-CoV-2 Viral Load: A Randomized Control Trial. Laryngoscope 2022; 132(11): 2089–95. |
Table 2a.
Demographic and clinical characteristics of recruits in the RCTs analyzed in this review.
| Study | mITT | median age (range) | total female n(%) | White n(%) | Black n(%) | Hispanic n(%) | 1 or more medical high risk conditons for COVID-19 progession | diabetes n(%) | hypertension n(%) | obesity or BMI >30 n(%) | median duration symptoms | Seropositive at baseline n(%) | Hospital type | Endpoint days for hosp |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CCP (5 RCTs) totals or averages | 2634 | 58 | 1409 (53) | 2213 (84) | 266 (10) | 862 (33) | 2074 (79) | 326 (15) | 606 (33) | 854 (38) | 4.5 | 73 (9) | ||
| anti-Spike mAbs (8 RCTs) totals or averages | 7421 | 47 | 3944 (53) | 6214 (84) | 455 (6) | 3113 (42) | 6562 (88) | 1067 (14) | 1249 (17) | 3197 (43) | 3.5 | 1087 (15) | ||
| Small molecule antivirals (11 RCTs) totals or averages | 33148 | 45.4 | 18116 (55) | 28726 (87) | 399 (1) | 2458 (7) | 22400 (68) | 3150 (10) | 6954 (21) | 6271 (19) | 4 | 25710 (77) {710/8148=9%w/o-Mol-Pan.) | ||
| Repurposed drugs (27 RCTs) totals or averages | 16840 | 48 | 9595 (57) | 14752 (89) | 815 (5) | 4212 (32) | 8669 (88) | 2174 (13) | 4318 (27) | 6615 (46) | 5.1 | 2303 (51) | ||
| CCP-CONV-ert7 | 376 | 56 | 173 (46) | 0 | 0 | 376 (100) | 278 (74) | 49 (13) | not reported | 96 (26) | 4.4 | 43 (11) | All cause | 28–30 |
| CCP-COV-Early13 | 406 | 58 | 187 (46) | 406 (100) | 0 | 0 | 278 (68) | not reported | not reported | not reported | 5 (iqr4–6) | 30 (8) | All cause | 28–30 |
| CCP-C3PO4 | 511 | 54 | 274 (54) | 237 (46) | 103 (20) | 156 (31) | 511 (100) | 142 (28) | 216 (42) | 302 (59) | 4 | not reported | All cause | 15 |
| CCP-Argentina5 | 160 | 77 (65–90+) | 100 (62) | 0 | 0 | 160 (100) | 131 (82) | 36 (23) | 114 (71) | 12 (8) | 3 | not reported | hypoxia resp rate def | 28–30 |
| CCP-CSSC-0043 | 1181 | 43 (18–85) | 675 (57) | 934 (79) | 163 (14) | 170 (14) | 470 (40) | 99 (8) | 276 (23) | 444 (38) | 6 | not reported | COVID-19 related | 28–30 |
| Bamlanivimab-BLAZE-114 | 452 | 45 (18–86) | 249 (55) | 389 (86) | 29 (6) | 198 (44) | 310 (69) | not reported | 201 (44) | 4 | not reported | COVID-19 related + ED visit | 28–30 | |
| Sotrovimab-COMET-ICE8 | 1057 | 53(17–96) | 571 (54) | 919 (87) | 42 (4) | 687 (65) | 1055 (99.9) | 233 (23) | not reported | 665 (63) | 3 | not reported | All cause | 28–30 |
| Bamlanivimab/etesevimab-BLAZE-115 | 1035 | 54 (12–77+) | 538 (52) | 896 (87) | 83 (8) | 304 (29) | 983 (95) | 285 (28) | not reported | median 34 bmi | 4 | not reported | COVID-19 related | 28–30 |
| Casirivimab/imdevimab-REGEN-COV Ph 316 | 2696 | 50 (iqr 39–50) | 1407 (52) | 2297 (85) | 143 (5) | 935 (35) | 2696 (100) | 412 (15) | 993 (37) | 1559 (58) | 3 | 620 (23) | COVID-19 related | 28–30 |
| Casirivimab/imdevimab-REGEN-COV Ph 1/217 | 799 | 42 (iqr 31–52) | 423 (53) | 681 (85) | 74 (9) | 403 (50) | 483 (61) | 298 (37) | 3 | 304 (38) | All cause | 28–30 | ||
| Bebtelovimab-BLAZE-41 | 253 | 34 | 135 (53) | 187 (74) | 48 (19) | 91 (36) | 0 (0) | not reported | not reported | 3 | 27 (11) | COVID-19 related | 28–30 | |
| Regdanvimab-CT-P5918 | 307 | 51 (iqr40–60) | 166 (51) | 286 (87) | 0 | 27 (8) | 226 (69) | 29 (9) | not reported | 52 (16) | 3 | 9 (3) | All cause | 28–30 |
| Regdanvimab-CT-P59–219 |
1315 | 48 (iqr38–59) | 641 (49) | 1132 (86) | 7 (1) | 276 (21) | 880 (67) | 120 (9) | 443 (34) | 415 (32) | 4 | 148 (11) | All cause | 28 |
| Tixagevimab–cilgavimab-TACKLE20 | 822 | 46 (sd 15.2) | 455 (50) | 559 (62) | 36 (4) | 468 (52) | 809 (90) | 108 (12) | 256 (28) | 388 (43) | 5 | 127 (14) | COVID-19 related | 28–30 |
| Molnupiravir-MOVe-OUT21 | 1408 | 43 (18–90) | 735 (51.3) | 813 (56) | 75 (5) | 711 (49) | 1424 (99.4) | 228 (15.9)% | not reported | 1056(73) | 3 | 620 (23) | All cause | 28–30 |
| Molnupiravir-PANORAMIC22 | 25000 | 57 (18–99) | 15101 (59) | 24270 (94) | 155 (0.6) | 17759 (69) | 2195 (9)% | 5782 (22) | 3912 (15)% | 3 | 25333 (98) 2+ doses of vaccine | All cause | 28–30 | |
| Molnupiravir-Aurobindo23 | 1220 | 36 (18–60) | 468 (38) | 1220 (100) | 0 | 0 | 90 (7.3) | 3 | not reported | All cause | 28–30 | |||
| Nirmatrelvir/ritonavir-EPIC-HR9 | 2085 | 46 (18–88) | 1098 (49) | 1607 (72) | 110 (4.9) | 1010 (45) | 2085 (100) | 252 (11) | 739 (33) | 744 (36) | 3 | 27 (11) | COVID-19 related | 28–30 |
| Remdesivir-PINETREE12 | 562 | 50 (12–77+) | 269 (48) | 452 (80) | 42 (7.5) | 235 (41) | 562 (100) | 346 (62) | 268 (48) | 310 (55) | 5 | 9 (3) | COVID-19 related | 28–30 |
| Interferon Lambda-TOGETHER24 | 1949 | 43 (18–92) | 1113 (57) | 58 (3) | 28 (1) | 1853 (95) | 1124 (58) | 181 (9) | 581 (30) | 719 (37) | 3 | 1107 (58) FV | COVID-19 related | 28 |
| Interferon Lambda-ILIAD25 | 60 | 46 (iqr32–54) | 35 (60) | 31 | 6 | 9 | 12 | 5 | 5/51 (10) | COVID-19 related | 14 | |||
| Interferon Lambda-COVID-Lambda26 | 120 | 36 (18–71) | 50 (42) | 33 (28) | 74 (63) | 12 (10) | 14 (12) | 5 (iqr3–6) | 49 (41) | All cause | 28–30 | |||
| Sofosbuvir and daclatasvir-SOVODAK27 | 55 | <50 | 29 (53) | 55 (100) | not reported | not reported | All cause | 28–30 | ||||||
| Favipavir-Avi-Mild-1928 | 231 | 37 (iqr32–44) | 76 (33) | 231 (100) | 0 | 0 | 25 (11) | 14 (6) | 39 (17) | 3 | not reported | All cause | 28–30 | |
| Favipiravir-Iran29 | 77 | 41 | 34 | 77 (100) | 0 | 0 | 11 (14) | nr | nr | nr | 4 | All cause | 28 | |
| Favipiravir-FLARE30 | 119 | 40 | 56 (47) | 98 (82) | 17 (14) | 20 (17) | nr | 74 (62) | All cause | 28 | ||||
| Favipiravir/Lopinavir/Ritonavir-FLARE30 | 121 | 40 | 59 (49) | 99 (82) | 19 (16) | 20 (17) | nr | 77 (64) | All cause | 28 | ||||
| Lopinavir/Ritonavir-FLARE30 | 120 | 40 | 60 (50) | 98 (82) | 16 (13) | 20 (17) | nr | 74 (62) | All cause | 28 | ||||
| Lopinavir/ritonavir-TREAT NOW31 | 446 | 41 (19–75) | 261 (59) | 354 (79) | 35 (8) | 33 (7) | 347 (78) | 17 (4) | 77 (17) | 149 (35) | 4 | 93 (21) | All cause | 29 |
| Lopinavir/ritonavir-TOGETHER32 | 471 | 53 (IQR 18–94) | 255 (54) | 14 (3) | 11 (2) | 428 (91) | 471 (100) | 92 (20) | 137 (29) | 198 (42) | 6 | not reported | COVID-19 related | 90 |
| Tenofovir Disproxil Fumarate Plus Emtricitabine-AR0-CORONA33 |
60 | 42 | 34 (57) | 2 | 3 | 4 | All cause | 14 | ||||||
| Metformin-COVID-OUT34 | 1197 | 46 (iqr 37–55) | 741 (56) | 1091 (82) | 90 (7) | 26 (2) | 353 (27) cvd | 646 (49) | 5 | 690 (52fv) | COVID-19 related | 28–30 | ||
| Metformin-TOGETHER35 |
418 | 52 (18–90) | 239 (57) | 8 (2) | 6 (1) | 381 (91) | 418 (100) | 61 (15) | 167 (40) | 188 (45) | 3 | All cause | 28–30 | |
| Fluvoxamine-TOGETHER11 | 1497 | <50 | 862 (58) | 1486 (99) | 5 (1) | 1486 (99) | 1497 (100) | 243 (16) | 194 (13) | 751 (50) | 4 | not reported | COVID-19 related | 28–30 |
| Fluvoxamine-STOP COVID36 | 152 | 46 | 109 (72) | 106 (70) | 38 (25) | 5 (3) | 17 (11) | 30 (20) | 75 (49) | 4 | not reported | COVID-19 related | 15 (2 noncovid after day 15 to day 28 | |
| Fluvoxamine-COVID-OUT34 | 592 | 44 (iqr37–53) | 358 (54) | 539 (82) | 51 (8) | 7 (1) | 172 (26) cvd | 302 (46) | 5 | 373 (56fv) | COVID-19 related | 28–30 | ||
| Fluvoxamine ACTIV-637 | 1288 | 48 (iqr39–58) | 734 (57) | 1038 (81) | 96 (7) | 221 (17) | 115 (9) | 304 (24) | 469 (36) | 5 | 861 (67) | All cause | 28 | |
| Fluvoxamine/budesonide-TOGETHER38 | 1476 | 51 (18–102) | 898 (61) | 36 (2) | 20 (1) | 1419 (96) | 1476 (100) | 278 (19) | 656 (44) | 597 (42) | 3 | 1377 (94) | All cause | 28–30 |
| Ivermectin-TOGETHER39 | 1349 | 49 | 791 (58) | 1310 (98) | 12 (1) | 1310 (98) | 1349 (100) | 180 (13) | 114 (8) | 675 (50) | 4 | not reported | COVID-19 related | 28–30 |
| Ivermectin-COVID-OUT34 | 730 | 46 (iqr37–56) | 442 (55) | 662 (82) | 59 (7) | 13 (2) | 184 (23) cvd | 383 (47) | 5 | 449 (56fv) | COVID-19 related | 28–30 | ||
| Ivermectin Iran40 | 549 | 35 (5–87) | 294 (48) | 582 (100) | 0 | 0 | 112 (20) | 42 (7.3) | 46 (7.8) | 101 (21) | 3 | not reported | All cause | not stated |
| Ivermectin-ACTIV-641 | 1591 | 47 (iqr39–56) | 932 (59) | 1286 (81) | 113(7) | 163 (10) | 184 | 415 | 648 | 6 | 753 (fv47) | All cause | 28–30 | |
| Ivermectin high dose-ACTIV-642 |
1206 | 48 (iqr38–58) | 713 (59) | 909 (75) | 93 (8) | 160 13) | 109 (9) | 317 (27) | 259 (21) | 5 | 1008 (84) | All cause | 28 | |
| Hydroxychloroquine-TOGETHER32 | 441 | 53 (IQR 18–81) | 243 (55) | 422 (96) | 7 (1) | 422 (96) | 441 (100) | 89 (20) | 210 (48) | 177 (40) | 6 | not reported | COVID-19 related | 90 |
| Hydroxychloroquine-COVID-19 PEP43 | 423 | 40 (iqr 32–50) | 238 (56) | 235 (48) | 15 (3) | 28 (6) | 15 (3) | 46 (11) | 2 | not reported | All cause | 14 | ||
| Hydroxychloroquine-AH COVID-1944 | 148 | 47 | 66 (45) | 51 | 12 | 29 | 41 | 7 (iqr5–8) | not reported | All cause | 28–30 | |||
| Hydroxychloroquine-BCN PEP-CoV-245 | 293 | 42 (12 sd) | 201 (69) | 156 (53) | 20 (7) | 3 (iqr 2–4) | not reported | All cause | 28–30 | |||||
| Hydroxychloroquine-BMG46 | 231 | 37 (18–78) | 131 (57) | 117 (51) | 26 (11) | 71 (31) | 129 (56) | 17 (7) | 27 (12) | 98 (42) | 6 | not reported | COVID-19 related | 28–30 |
| Hydroxychloroquine-Utah47 | 303 | 42 | 176 (48) | 165 (45) | 2 (1) | 158 (43) | 90 (25) | 28 (8) | 52 914) | nr | nr | All cause | 28 | |
| Hydroxychloroquine/Azithromycin-Brazil48 | 84 | 37 | 34 | 44 | 5 | 79 | nr | nr | nr | 4 | nr | All cause | 21 | |
| Nitazoxanide-Romark49 | 379 | 40 (12–83) | 214 (57) | 233 (61) | 8 (2) | 130 (34) | 238 (63%) | 2 | 38 (10) | COVID-19 related | 28–30 | |||
| Colchicine-COLCORONA2 | 4488 | 54 (iqr 47–61) | 2421 (54) | 4182 (93) | 233 (5) | <10% | 4488 (100) | 894 (20) | 1629 (36) | 2052 (46) | 5.3 | not reported | COVID-19 related | 28–30 |
| Losartan-MN50 |
117 | 38 | 58 | 84 | 8 | 10 | 7 | 9 | 42 | 2 | All cause | 28 | ||
| Niclosamide51 | 67 | 36 mean | 26 (39) | 53 (79) | 4 (6) | 7 (10) | 5 (8) | 4 (7) | not reported | not reported | All cause | 28–30 | ||
| Aspirin-ACTIV-4B52 | 280 | 54 (iqr 46–59) | 191 (58) | 250 (76) | 36 (11) | 93 (28) | 53 (16) | 109 (33) | 164 (50) | 10 (diagnosis) | not reported | All cause | 45 | |
| 2.5-mg apixaban-ACTIV-4B52 | 271 | 54 (iqr 46–59) | 191 (58) | 255 (78) | 38 (12) | 91 (28) | 60 (18) | 120 (37) | 164 (50) | 10 (diagnosis) | not reported | All cause | 45 | |
| 5-mg apixaban ACTIV-4B52 | 279 | 54 (iqr 46–59) | 198 (6 | 251 (77) | 36 (11) | 80 (24) | 55 (17) | 111 (34) | 164 (50) | 10 (diagnosis) | not reported | All cause | 45 | |
| Sulodexide53 | 243 | 55 | 128 (53) | 243 (100) | 243 (100) | 50 (21) | 83 (43) | 3 | not reported | All cause | 21 | |||
| Enoxaparin-ETHIC54 | 219 | 59 (iqr51–66) | 96 (44) | 129 (59) | 5 (2) | 12 (5) | 50/152 (33) | 114/152 (75) | 109 (49) | 5 | not reported | All cause | 21 | |
| Enoxaparin-OVID55 | 572 | 56 (iqr53–62) | 217 (38) | 446 (78) | 3 (1) | 38 (7) | 115 (20) | 3 (dx) | not reported | All cause | 28–30 | |||
| Inhaled Ciclesonide-COVIS56 |
400 | 43 (13–87) | 221 (55) | 345 (86) | 47 (12) | 172 (43) | 30 (8) | 89 (22) | 200 (50) | NR | All cause | 30 | ||
| Inhaled ciclesonide-COVERAGE57 | 217 | 63 (50–86) | 111 (51) | 217 (100) | 0 | 0 | 157 (72) | 33(16) | 89 (41) | 52 (24) | 4 | not reported | All cause | 28–30 |
| Zinc58 | 108 | 43 | 68 | 73 | 28 | 10 | 35 | 54 | nr | nr | All cause | 28 | ||
| Ascorbic acid58 | 98 | 44 | 64 | 70 | 23 | 5 | 22 | 49 | nr | nr | All cause | 28 | ||
| Zinc/Ascorbic acid58 | 108 | 46 | 62 | 71 | 32 | 20 | 41 | 54 | nr | nr | All cause | 28 | ||
| Homeopathy-COVID-Simile59 | 86 | 41 | 56 | 86 (100) | 8 | 21 | 9 | nr | All cause | 27 | ||||
| Saliravira60 | 143 | 50 (24–80) | 59 (41) | 143 (100) | 33 (23) | not reported | not reported | All cause | 23 | |||||
| Azithromycin-Atomic261 | 292 | 46 | 143 (49) | 201 (68) | 11 (4) | 70 (24) | 25 (9) | 52 (18) | 6 | not reported | All cause | 28–30 | ||
| Azithromycin-ACTION62 | 197 | 43 | 130 (66) | 169 (86) | 9 (5) | 59 (30) | 24 (12) | 26 (13) | 6 | not reported | All cause | 21 | ||
| Resveratrol63 | 100 | 55 (45–84) | 62 (59) | 93 (89) | 4 (4) | 2 (2) | 32 (30) | 10 (10) | 50 (50) | 5 | not reported | All cause | 21 |
Appendix Table 2b:
Additional baseline data from RCTs-Geography, age symptom onset
| Study | Enrollment Period | Study months | Geography | Enrolled | age over 65 n(%) | age over 60 n(%) | age over 50 n(%) | symptoms <= 8 days n(%) | symptoms <=7 days n(%) | symptoms <= 5 days n(%) | symptoms <= 3 days n(%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CCP-CONV-ert7 | Nov 10 2020–July 28 2021 | 9 | Spain | 376 | 376 (100) | 376 (100) | |||||
| CCP-COV-Early13 | Netherlands | 406 | 351 (86) | 406 (100) | |||||||
| CCP-C3PO4 | Aug 2020-Feb 2021 | 7 | USA | 511 | 511 (100) | 511 (100) | 246 (48) | ||||
| CCP-Argentina5 | Jun 4 2020 – Oct 25 2020 | 5 | Argentina | 160 | 160 (100) | 160 (100) | |||||
| CCP-CSSC-0043 | June 3 2020-Oct 2021 | 16 | USA | 1225 | 80 (7) | 410 (35) | 1181 (100) | 517 (44) | |||
| Bamlanivimab-BLAZE-114 | June 2020-Aug 2020 | 3 | USA | 467 | 53 (12) | 226 (50)mean | |||||
| Sotrovimab-COMET-ICE8 | Aug 27 2020-March 2021 | 6 | United States, Canada, Brazil, and Spain | 1057 | 211(20) | 1057 (100) | 624 (59) | ||||
| Bamlanivimab/etesevimab-BLAZE-115 | Sept 2020-Dec 2020 | 3 | USA | 1035 | 323 (31) | 979 (95) | |||||
| Casirivimab/imdevimab-REGEN-COV Ph 316 | Sept 24 2020-Jan 17 2021 | 4 | USA Mexico | 2696 | 358 (13) | 2696 (100) | 1489 (66) | ||||
| Casirivimab/imdevimab-REGEN-COV Ph 1/217 | June 16, 2020 – Sept 23, 2020 | 3 | USA | 799 | 799 (100) | 599 (75) | 400 (50) | ||||
| Bebtelovimab-BLAZE-41 | May 2021-July 2021 | 3 | USA | 253 | 1 (<1) | 253 (100) | |||||
| Regdanvimab-CT-P5918 | Oct 2020-Dec 2020 | 2 | South Korea, Romania, Spain, USA | 327 | 85 (26) | 327 (100) | |||||
| Regdanvimab-CT-P59–219 |
Jan 18 2021 to april 24 2021 | 3 | world wide | 1315 | 297 (23) | 986 (75) | 329 (25) | ||||
| Tixagevimab–cilgavimab-TACKLE20 | Jan 28, 2021–July 22, 2021, | 6 | USA, Latin America, Europe, and Japan. | 1014 | 116 (13) | 910 (100) | |||||
| Molnupiravir-MOVe-OUT21 | May 2021–Oct 2021 | 6 | worldwide | 1433 | 246 (17) | 1408 (100) | 674 (48) | ||||
| Molnupiravir-PANORAMIC22 | Dec 8–2021 – April 27, 2022 | 5 | UK | 25783 | 6838 (27) | 22510 (87) | |||||
| Molnupiravir-Aurobindo23 | July 1, 2021 – Aug 24, 2021 | 2 | India | 1220 | 661 (54) | ||||||
| Nirmatrelvir/ritonavir-EPIC-HR9 | July 1 2021 – Dec 2021 | 6 | worldwide | 2246 | 287(12.8) | 2246 (100) | 1489 (66.3) | ||||
| Remdesivir-PINETREE12 | Sept 2020-Apr 2021 | 8 | USA, Spain, Denmark UK | 562 | 170 (30) | 562 (100) | |||||
| Interferon Lambda-TOGETHER24 | Jun 24 2021-feb 7 2022 | 8 | Brazil | 1949 | 752 (39) | 1949 (100) | 1158 (59) | ||||
| Interferon Lambda-ILIAD25 | May 18, 2020–Sep 4 2020 | 4 | Canada | 60 | 60 (100) | ||||||
| Interferon Lambda-COVID-Lambda26 | Apil 25 2020-July 7 2020 | 2 | USA | 120 | |||||||
| Sofosbuvir and daclatasvir-SOVODAK27 | April 8 2020-May 19 2020 | 1 | Iran | 55 | |||||||
| Favipavir-Avi-Mild-1928 | July 23, 2020–Aug 4 2021 | 12 | Saudi Arabia | 245 | 30 (13) | 231 (100) | |||||
| Favipiravir-Iran29 | Dec 5 2020-mar 31 2021 | 4 | Iran | 77 | 77 (100) | ||||||
| Favipiravir-FLARE30 | Oct 6 2020–Nov 4 2021 | 13 | United Kingdom | 119 | 119 (100) | 76 (64) | |||||
| Favipiravir/Lopinavir/Ritonavir-FLARE30 | Oct 6 2020–Nov 4 2021 | 13 | United Kingdom | 121 | 121 (100) | 80 (66) | |||||
| Lopinavir/Ritonavir-FLARE30 | Oct 6 2020–Nov 4 2021 | 13 | United Kingdom | 120 | 120 (100) | 75 (63) | |||||
| Lopinavir/ritonavir-TREAT NOW31 | Jun 2020-Dec 2021 | 18 | USA | 446 | 13 (3) | 112 (25) | 446 (100) | 349 (78) | 124 (28) | ||
| Lopinavir/ritonavir-TOGETHER32 | June 2 2020-Oct 9 2020 | 4 | Brazil | 471 | 275 | 471 (100) | 74 (16) | ||||
| Tenofovir Disproxil Fumarate Plus Emtricitabine-AR0-CORONA33 |
Nov 20 2020-Mar 19 2021 | 4 | France | 60 | 60 (100) | 45 (75) | |||||
| Metformin-COVID-OUT34 | Dec 30 2020 – Jan 28 2022 | 13 | USA | 1323 | 1197 (100) | ||||||
| Metformin-TOGETHER35 |
jan 15 2021–April 13 2021 | 3 | Brazil | 418 | 234 (56) | 418 (100) | 184 (44) | ||||
| Fluvoxamine-TOGETHER11 | Jan 2021 – Aug 2021 | 8 | Brazil | 1497 | 655 (44) | 1497 (100) | 638 (43) | ||||
| Fluvoxamine-STOP COVID36 | April 10 2020 – Aug 5 2020 | 4 | USA | 152 | 152 (100) | 114 (75) | |||||
| Fluvoxamine-COVID-OUT34 | Dec 30 2020 – Jan 28 2022 | 13 | USA | 661 | 733 (100) | ||||||
| Fluvoxamine ACTIV-637 | Au 6 2021-May 27 2022 | 10 | USA | 1288 | 541 (42) | 966 (75) | 644 (50) | ||||
| Fluvoxamine/budesonide-TOGETHER38 | Jan 15 2022-July 6 2022 | 6 | Brazil | 1476 | 829 (56) | 1476 (100) | 917 (62) | ||||
| Ivermectin-TOGETHER39 | March 23 – Aug 2 2021 | 5 | Brazil | 1358 | 1358 (100) | 597 (44) | |||||
| Ivermectin-COVID-OUT34 | Dec 30 2020 – Jan 28 2022 | 13 | USA | 808 | 592 (100) | ||||||
| Ivermectin Iran40 | Feb 19 21 – Aug 30 21 | 7 | Iran | 582 | 291 (50) | ||||||
| Ivermectin-ACTIV-641 | June 23 2021 – Feb 4 2022 | 7 | USA | 1591 | 680 (43) | 1193 (75) | |||||
| Ivermectin high dose-ACTIV-642 |
feb 16 2022-July 22 2022 | 5 | USA | 1206 | 905 (75) | 603 (50) | 302 (25) | ||||
| Hydroxychloroquine-TOGETHER32 | June 2 2020-Oct 9 2020 | 4 | Brazil | 441 | 262 (59) | 441 (100) | 77 (17) | ||||
| Hydroxychloroquine-COVID-19 PEP43 | March 22 2020 – May 20 2020 | 2 | USA Canada | 491 | 99 (20) | 423 (100) | |||||
| Hydroxychloroquine-AH COVID-1944 | April 15 2020–May 22 2020 | 1 | Canada | 148 | |||||||
| Hydroxychloroquine-BCN PEP-CoV-245 | March 17 2020-May 26 2020 | 2 | Spain | 293 | 293 (100) | ||||||
| Hydroxychloroquine-BMG46 | April 15 2020-July 27 2020 | 3 | USA | 231 | 23 (10) | 143 (62) | 85 (37) | ||||
| Hydroxychloroquine-Utah47 | Apr 2020-Apr 2021 | 12 | USA | 303 | |||||||
| Hydroxychloroquine/Azithromycin-Brazil48 | Apr 12 2020-May 13 2020 | 1 | Brazil | 83 | 84 (100) | ||||||
| Nitazoxanide-Romark49 | Aug 2020–Jan 2021 | 5 | USA Peurto rico | 379 | 379 (100) | ||||||
| Colchicine-COLCORONA2 | March 2020-Dec 2020 | 9 | Brazil, Canada, Greece, South Africa, Spain, and the USA | 4488 | 1122 (25) | 3590 (80) | |||||
| Losartan-MN50 |
Apr 2020-Nov 2020 | 8 | USA | 117 | 3 | 117 (100) | 106 | ||||
| Niclosamide51 | Oct 1 2020-April 20 2021 | 7 | USA | 73 | 67 (100) | ||||||
| Aspirin-ACTIV-4B52 | Sept 1 2020 – June 17 2021 | 10 | USA | 328 | ~82 (25) | 82 (25) | |||||
| 2.5-mg apixaban-ACTIV-4B52 | Sept 1 2020 – June 17 2021 | 10 | USA | 329 | ~82 (25) | 82 (25) | |||||
| 5-mg apixaban ACTIV-4B52 | Sept 1 2020 – June 17 2021 | 10 | USA | 328 | ~82 (25) | 82 (25) | |||||
| Sulodexide53 | June 5 2020 – August 5 2020 | 2 | Mexico | 243 | 243 (100) | ||||||
| Enoxaparin-ETHIC54 | Oct 27 2020 – Nov 8 2021 | 12 | Belgium, Brazil, India, South Africa, Spain, and the UK). | 219 | 164 (75) | 121 (50) | |||||
| Enoxaparin-OVID55 | Aug 5 2020-Jan 14 2022 | 17 | Switzerland and Germany | 572 | 572 (100) | 429 (dx 75) | |||||
| Inhaled Ciclesonide-COVIS56 |
jun 11 2020-nov 3 2020 | 5 | USA | 400 | |||||||
| Inhaled ciclesonide-COVERAGE57 | Dec 29 2020-July 22 2021 | 7 | France | 217 | 151 (70) | 217 (100) | 217 (100) | ||||
| Zinc58 | Apr 27 2020-Oct 14 2020 | 6 | USA | 108 | |||||||
| Ascorbic acid58 | Apr 27 2020-Oct 14 2020 | 6 | USA | 98 | |||||||
| Zinc/Ascorbic acid58 | Apr 27 2020-Oct 14 2020 | 6 | USA | 108 | |||||||
| Homeopathy-COVID-Simile59 | Jun 29 2020-Ap 6 2021 | 10 | Brazil | 86 | |||||||
| Saliravira60 | Dec 21 2020 – March 1 2021 | 3 | Iran | 143 | |||||||
| Azithromycin-Atomic261 | June 3, 2020–Jan 29, 2021, | 8 | UK | 292 | |||||||
| Azithromycin-ACTION62 | May 22 2020 – March 16 2021 | 9 | USA | 197 | 18 (9) | 197 (100) | |||||
| Resveratrol63 | September 13, 2020 – December 11, 2020, | 3 | USA | 100 | 16 (16) | 50 (50) |
Appendix Table 3.
Summary of findings table GRADE evaluation by RCT.
Patient or population: COVID-19 outpatients
Settings: Ambulatory patients with COVID-19
Intervention: COVID-19 convalescent plasma, anti-Spike mAbs, small molecule antivirals and repurposed drugs Comparison: standard of care, placebo
| Study | Assumed risk-controls Illustrative comparative risks* (95% CI) | Corresponding risk-Intervention Illustrative comparative risks* (95% CI) | Effect size: OR (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments |
|---|---|---|---|---|---|---|
| CCP-mITT all cause hospitalization: cumulative results | 120 per 1000 | 82 per 1000 (from 63 to 108) | 0.69(0.53/0.90) | 2634 participants (5 RCTs) | ⊕⊕⊕⊕ high (there are no concerns in any of the GRADE factors) |
CCP reduces significantly need of hospitalization compared to placebo. Most information is from results at low risk of bias or with some concerns, but unlikely to lower confidence in the estimate of effect. |
| Anti-spike mAbs: combined results | 58.9 per 1000 | 18.8 per 1000 (from 14.7to 22.1) | 0.32(0.25/0.41) | 8736 (9 trials) | ⊕⊕⊕⊝ moderate (downgraded for ROB) |
Anti-Spike mAbs reduce hospitalization compared to placebo |
| Small molecule antivirals: combined results | 18.3 per 1000 | 14.3 per 1000 (from 8.2 to 24.3) | 0.78(0.45/1.33) | 34104 (17) | ⊕⊝⊝⊝ very-low (downgraded for imprecision, inconsistency (I2=69) and ROB) |
-It is unclear if antivirals reduce rate of hospitalization compared to placebo |
| Repurposed drugs combined results | 53.05 per 1000 | 41.9 per 1000 (from 37.1 to 47.7) | 0.79(0.70/0.90) | 22512 (39 arms, 21 comparisons) | ⊕⊕⊕⊝ moderate (downgraded for ROB) |
Repurposed treatments reduce rate of hospitalization compared to placebo |
| CCP | ||||||
| CCP-CONV-ert7 | 111 per 1000 | 116 per 1000 (from 61 to 219) | 1.05 (0.55/1.98) | 376 (1) | ⊕⊕⊕⊝ moderate (downgraded for imprecision-95% CI includes line of no effect) |
CCP does not reduce hospitalization compared to placebo |
| CCP-COV-Early13 | 93 per 1000 | 57.6 per 1000 (from 26.9 to 120.9) | 0.62 (0.29/1.30) | 406 (1) | ⊕⊕⊕⊝ moderate (downgraded for imprecision-95% CI includes line of no effect) |
It is unclear if CCP reduces hospitalization compared to placebo |
| CCP-C3PO4 | 220 per 1000 | 198 per 1000 (from 127 to 301) | 0.9 (0.58/1.37) | 511 (1) | ⊕⊕⊕⊝ moderate (downgraded for imprecision-95% CI includes line of no effect) |
It is unclear if CCP reduces hospitalization compared to placebo |
| CCP-Argentina5 | 312 per 1000 | 133 per 1000 (from 62 to 180) | 0.43 (0.20/0.91) | 160 (1) | ⊕⊕⊕⊝ moderate (downgraded for imprecision due to low number of participants) |
CCP reduces rate of hospitalization compared to placebo |
| CCP-CSSC-0043 | 62.8 per 1000 | 27.6 per 1000 (from 15.7 to 49.6) | 0.44 (0.25/0.79) | 1181 (1) | ⊕⊕⊕⊕ high (there are no concerns in any of the GRADE factors) |
CCP reduces rate of hospitalization compared to placebo |
| Anti-Spike mAbs | ||||||
| Bamlanivimab14 | 62.9 per 1000 | 15 per 1000 (from 5 to 46.5) | 0.24 (0.08/0.74) | 919 (1 RCT) | ⊕⊕⊕⊝ moderate (downgraded for imprecision) |
Bamlanivimab reduces need of hospitalization compared to placebo |
| Sotrovimab-COMET-ICE23 | 56.7 per 1000 | 10.7 per 1000 (from 4.5 to 26) | 0.19 (0.08/0.46) | 1061 (1 RCT) | ⊕⊕⊕⊝ moderate (downgraded for ROB) |
Sotrovimab reduces need of hospitalization compared to placebo |
| Bamlanivimab/etesevimab15 | 69.3 per 1000 | 20 per 1000 (from 10.3 to 40.1) | 0.29 (0.15/0.58) | 1035 (1 RCT) | ⊕⊕⊝⊝ low(downgraded for imprecision and ROB) |
Bamlanivimab/etesevimab in combination reduce need of hospitalization compared to placebo |
| Casirivimab/imdevimab16 | 41.6 per 1000 | 11.6 per 1000 (from 7.0 to 19.1) | 0.28 (0.17/0.46) | 3495 (2 RCT) | ⊕⊕⊝⊝ low (downgraded for ROB and imprecision due to low number of events) |
Casirivimab/imdevimab in combination reduce need of hospitalization compared to placebo |
| Bebtelovimab-BLAZE-41 | 15.6 per 1000 | 15.9 per 1000 (from 2.1 to 115) | 1.02 (0.14/7.39) | 253 (1 RCT) | ⊕⊕⊝⊝ low (downgraded twice for serious imprecision) | Bebtelovimab does not reduce need of hospitalization compared to placebo |
| Regdanvimab18,19 | 79.9 per 1000 | 26.3 per 1000 (from 5.9 to 42.3) | 0.33 (0.20/0.53) | 1622 (2 RCTs) | ⊕⊕⊕⊝ moderate (downgraded for ROB) |
Regdanviman reduces hospitalization compared to placebo |
| Tixagevimab–cilgavimab-TACKLE20 | 89.1 per 1000 | 41.8 per 1000 (from 24 to 74.7) | 0.47 (0.27/0.84) | 822 (1) | ⊕⊕⊕⊝ moderate (downgraded for imprecision) |
Tixagevimab-cilgavimab reduces hospitalization compared to placebo in unvaccinated adults |
| Small molecule antivirals | ||||||
| Molnupiravir21–23 | 11.8 per 1000 | 10.8 per 1000 (from 8.6 to 13.4) | 0.91 (0.73/1.14) | 27628 (3 RCTs) | ⊕⊕⊝⊝ low (downgraded for inconsistency and imprecision) | It is unclear if Molnupiravir reduces hospitalization compared to placebo |
| Nirmatrelvir/ritonavir9 | 63 per 1000 | 7.5 per 1000 (from 3.7 to 15.1) | 0.12 (0.06/0.24) | 2085 (1) | ⊕⊕⊝⊝ low (downgraded for ROB and imprecision). | Nirmatrelvir/ritonavir reduces hospitalization compared to placebo in unvaccinated adults |
| Remdesivir12 | 53 per 1000 | 6.8 per 1000 (from 1.5 to 50.2) | 0.13 (0.03/0.57) | 562 (1) | ⊕⊕⊝⊝ low (downgraded for ROB and imprecision) |
Remdesivir reduces hospitalization compared to placebo |
| Favipiravir28–30 | 18.3 per 1000 | 51.2 per 1000 (from 16.8 to 155.9) | 2.80 (0.92/18.52) | 427 (3) | ⊕⊕⊝⊝ low (downgraded for serious imprecision) | Favipiravir does not reduce need of hospitalization compared to placebo |
| Favipiravir-Lopinavir/r-FLARE30 | Not calculable | - | 3.05 (0.12/76.39) | 120 (1) | ⊕⊕⊝⊝ low (downgraded for serious imprecision) | Favipiravir+Lopinavir/r does not reduce need of hospitalization compared to placebo |
| Peginterferon lambda24–26 | 38.8 per 1000 | 23.2 per 1000 (from 13.9 to 38.4) | 0.60 (0.36/0.99) | 2129 (3 RCTs) | ⊕⊕⊕⊝ moderate (downgraded for ROB) | -Peginterferon lambda reduces hospitalization compared to placebo. |
| Sofosbuvir and daclatasvir-SOVODAK27 | 142.8 per 1000 | 32.8 per 1000 (from 2.8 to 315) | 0.23 (0.02/2.21) | 55 (1) | ⊕⊕⊝⊝ low (downgraded for serious imprecision) | It is unclear if sofosbuvir/daclatasvir reduces hospitalization compared to placebo |
| Lopinavir/ritonavir30–32 | 33.1 per 1000 | 41.4 per 1000 (from 21.8 to 78.4) | 1.25 (0.66/2.37) | 1037 (3) | ⊕⊕⊝⊝ low (downgraded for serious imprecision) | Lopinavir/ritonavir does not reduce need of hospitalization compared to placebo |
| Tenofovir Disproxil Fumarate Plus Emtricitabine-AR0-CORONA | 33.3 per 1000 | 68.9 per 1000 (from 5.9 to 804.1 | 2.07 (0.18/24.5) | 60 (1 RCT) | ⊕⊝⊝⊝ very-low (downgraded for serious imprecision and ROB) |
TDF+Emtricitabine does not reduce need of hospitalization compared to standard of care |
| Repurposed | ||||||
| Metformin-COVID-OUT34,35 | 53.4 per 1000 | 37.4 per 1000 (from 22.9 to 60.3) | 0.70 (0.43/1.13 | 1615 (2 RCTs) | ⊕⊕⊝⊝ low (downgraded for imprecision and inconsistency (I2=58) |
It is unclear if metformin reduces hospitalization compared to placebo. |
| Fluvoxamine11,34,36,37 | 63.6 per 1000 | 46.4 per 1000 (from 34.3 to 62.9) | 0.73 (0.54/0.99) | 3518 (4 RCTs) | ⊕⊕⊕⊝ moderate (downgraded for ROB) |
Fluvoxamine reduces hospitalization compared to placebo |
| Fluvoxamine/budesonide-TOGETHER38 | 10.8 per 1000 | 9.4 per 1000 (from 3.4 to 26.1) | 0.87 (0.32/2.42) | 1476 (1 RCT) | ⊕⊕⊕⊝ moderate (downgraded for imprecision) |
It is unclear if fluvoxamine/budenoside reduces hospitalization compared to placebo. |
| Ivermectin10,34,40–42 | 46.3 per 1000 | 43.1 per 1000 (from 32.8 to 56.0) | 0.93 (0.71/1.221 | 5434 (5 RCTs) | ⊕⊕⊕⊝ moderate (downgraded for imprecision) |
-It is unclear if Ivermectin reduces rate of hospitalization compared to placebo |
| Hydroxychloroquine32,43,64–66 | 44.0per 1000 | 38.7 per 1000 (from 22.8 to 65.1) | 0.74 (0.45/1.23) | 1536 (6 RCTs) | ⊕⊕⊕⊝ moderate (downgraded for imprecision-95% CI includes line of no effect) |
It is unclear if hydroxychloroquine reduces hospitalization compared to placebo |
| Hydroxychloroquine/azithromycin48 | 23.8 per 1000 | 23.8 per 1000 (from 1.42 to 39.2) | 1.00 (0.06/16.5) | 84 (1 RCT) | ⊕⊕⊝⊝ low (downgraded for imprecision and ROB) |
It is unclear if hydroxychloroquine/azithromycin reduces hospitalization compared to placebo |
| Nitazoxanide-Romark49 | 25.6 per 1000 | 5.3 per 1000 (from 0.5 to 45.8) | 0.21 (0.02/1.79) | 379 (1) | ⊕⊝⊝⊝ very-low (downgraded for serious imprecision and ROB) |
It is unclear if nitazoxanide reduces hospitalization compared to placebo |
| Colchicine-COLCORONA2 | 58.1 per 1000 | 45.8 per 1000 (from 35.4 to 59.8) | 0.79 (0.61/1.03) | 379 (1) | ⊕⊕⊕⊝ moderate (downgraded for imprecision-95% CI includes line of no effect) |
It is unclear if colchicine reduces hospitalization compared to placebo |
| Losartan-MN50 | 16.9 per 1000 | 53.5 per 1000 (from 5.4 to 529.6) | 3.16 (0.32/31.34) | 117 (1 RCT) | ⊕⊝⊝⊝ very-low (downgraded for serious imprecision and ROB) |
It is unclear if losartan reduces rate of hospitalization compared to placebo |
| Niclosamide51 | 29.4 per 1000 | 9.5 per 1000 (from 0.29 to 249) | 0.33 (0.01/8.48) | 67 (1) | ⊕⊕⊝⊝ low (downgraded for serious imprecision) |
It is unclear if niclosamide reduces hospitalization compared to placebo |
| aspirin52 | 7.3 per 1000 | 6.8 per 1000 (from 0.4 to 11) | 0.94 (0.06/15.2) | 280 (1) | ⊕⊝⊝⊝ very-low (downgraded for serious imprecision and indirectness) |
Aspirin does not reduce need of hospitalization compared to placebo |
| apibaxan52 | 7.3 per 1000 | 7.3 per 1000 (from 1 to 52) | 1.0 (0.14/7.18) | 414 (2 arms) | ⊕⊝⊝⊝ very-low (downgraded for serious imprecision and indirectness) |
Apibaxan 2.5–5 mg does not reduce need of hospitalization compared to placebo |
| Sulodexide53 | 294 per 1000 | 223.4 per 1000 (from 82.3 to 279.3) | 0.52 (0.28/0.95) | 243 (1) | ⊕⊕⊕⊝ moderate (downgraded for imprecision) |
Sulodexide reduces hospitalization compared to placebo |
| Enoxaparin-LMW heparin54,55 | 56.8 per 1000 | 60.2 per 1000 (from 31.8 to 115.3) | 1.06 (0.56/2.03) | 691 (2) | ⊕⊕⊝⊝ low (downgraded for serious imprecision) |
LMW heparin does not reduce hospitalization compared to placebo |
| Inhaled ciclesonide56,57 | 61.2 per 1000 | 55.1 per 1000 (from 27.5 to 105.2) | 0.90 (0.45/1.72) | 599 (2) | ⊕⊝⊝⊝ very-low (downgraded for serious imprecision and ROB) |
Inhaled ciclesonide does not reduce need of hospitalization compared to placebo |
| Zinc58 | 60 per 1000 | 88.8 per 1000 (from 20.4 to 391.2) | 1.48 (0.34/6.52) | 108 (1) | ⊕⊝⊝⊝ very-low (downgraded for serious imprecision and ROB) |
Zinc, ascorbic acid anc combination of both did not reduce rate of hospitalization compared with usual care. |
| Ascorbic acid58 | 60 per 1000 | 40.8 per 1000 (from 6.6 to 256.2) | 0.68 (0.11/4.27) | 98 (1 RCT) | ⊕⊝⊝⊝ very-low (downgraded for serious imprecision and ROB) |
|
| Zinc/Ascorbic acid58 | 60 per 1000 | 129 per 1000 (from 31.8 to 528) | 2.15 (0.53/8.80) | 108 (1 RCT) | ⊕⊝⊝⊝ very-low (downgraded for serious imprecision and ROB) |
|
| Homeopathy-COVID-Simile59 | 68.1 per 1000 | 22.5 per 1000 (from 2.0 to 227.4) | 0.33 (0.03/3.34) | 86 (1 RCT) | ⊕⊝⊝⊝ very-low (downgraded for imprecision and serious ROB) |
It is unclear if homeopathy reduces hospitalization compared to placebo |
| Saliravira60 | 285 per 1000 | 133.9 per 1000 (from 82.6 to 220.2) | 0.47 (0.29/0.77) | 143 (1) | ⊕⊝⊝⊝ very-low (downgraded for serious imprecision and serious ROB) |
Saliravira reduces hospitalization compared to control |
| Azithromycin61,62 | 77.6 per 1000 | 85.3 per 1000 (from 43.4 to 169.1) | 1.10 (0.56/2.18) | 489 (2) | ⊕⊕⊝⊝ low (downgraded for serious imprecision) |
azithromycin does not reduce hospitalization compared to placebo |
| Resveratrol63 | 60 per 1000 | 19.2 per 1000 (from 1.8 to 190.8) | 0.32 (0.03/3.18) | 100 (1) | ⊕⊕⊝⊝ low (downgraded for serious imprecision) |
It is unclear if resveratrol reduces hospitalization compared to placebo |
The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect (the Risk Difference, also called ARR, absolute risk reduction)of the intervention (and its 95% CI). GRADE Working Group grades of evidenceHigh quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate.
OR, Odds Ratio; CIs, confidence intervals; ROB, risk of bias. GRADE, Grading of Recommendations, Assessment, Development and Evaluations
Appendix Table 4:
Hospitalized Odds Ratio statistics
| Study | Hospitalization Odds ratio | Hospitalization 95 CI low | Hospitalization 95 CI high | Hospitalization significance (p) | Hospitalization z statistic |
|---|---|---|---|---|---|
| Total CCP | 0.69 | 0.53 | 0.90 | 0.0035 | 2.697265 |
| Total mAb | 0.31 | 0.24 | 0.4 | P<0.001 | 9.29792 |
| Total antivirals | 0.78 | 0.45 | 1.33 | P<0.001 | 3.9493 |
| Total repurposed | 0.82 | 0.72 | 0.93 | P<0.001 | 3.74264 |
| Total | 0.67 | 0.57 | 0.80 | P<0.001 | 9.01288 |
| CCP-CONV-ert7 | 1.05 | 0.56 | 1.99 | 0.4356 | 0.162033 |
| CCP-COV-Early13 | 0.61 | 0.29 | 1.30 | 0.1021 | 1.269694 |
| CCP-C3PO4 | 0.90 | 0.59 | 1.37 | 0.3078 | 0.502004 |
| CCP-Argentina5 | 0.43 | 0.20 | 0.91 | 0.0140 | 2.197789 |
| CCP-CSSC-0043 | 0.44 | 0.25 | 0.79 | 0.0031 | 2.737543 |
| CCP-Argentina (high titer)5 | 0.20 | 0.06 | 0.71 | 0.0132 | 2.478 |
| CCP-CSSC-004 (<= 5 days) 3 | 0.18 | 0.07 | 0.49 | 0.0007 | 3.38 |
| Bamlanivimab-BLAZE-114 | 0.24 | 0.08 | 0.74 | 0.0066 | 2.479993 |
| Sotrovimab-COMET-ICE8 | 0.19 | 0.08 | 0.46 | 0.0001 | 3.663844 |
| Bamlanivimab/etesevimab-BLAZE-115 | 0.29 | 0.15 | 0.58 | 0.0002 | 3.534471 |
| Casirivimab/imdevimab-REGEN-COV Ph 316 | 0.28 | 0.16 | 0.47 | 0.0000 | 4.734662 |
| Casirivimab/imdevimab-REGEN-COV Ph 1/217 | 0.30 | 0.07 | 1.25 | 0.0484 | 1.660556 |
| Bebtelovimab-BLAZE-41 | 1.02 | 0.14 | 7.39 | 0.4905 | 0.023906 |
| Regdanvimab-CT-P5918 | 0.49 | 0.19 | 1.27 | 0.0716 | 1.463817 |
| Regdanvimab-CT-P59–219 |
0.29 | 0.16 | 0.52 | 0.00001 | 4.22581 |
| Tixagevimab–cilgavimab-TACKLE20 | 0.47 | 0.26 | 0.84 | 0.0057 | 2.528551 |
| Molnupiravir-MOVe-OUT21 | 0.67 | 0.46 | 0.99 | 0.0223 | 2.00829 |
| Molnupiravir-PANORAMIC22 | 1.07 | 0.81 | 1.42 | 0.3156 | 0.479984 |
| Molnupiravir-Aurobindo23 | NA | NA | NA | NA | NA |
| Nirmatrelvir/ritonavir-EPIC-HR9 | 0.12 | 0.06 | 0.24 | 0.0000 | 5.731691 |
| Remdesivir-PINETREE12 | 0.13 | 0.03 | 0.57 | 0.0034 | 2.703067 |
| Interferon Lambda-TOGETHER24 | 0.47 | 0.29 | 0.77 | 0.0011 | 3.054966 |
| Interferon Lambda-ILIAD25 | 1.00 | 0.06 | 16.76 | 0.5000 | 0 |
| Interferon Lambda-COVID-Lambda26 | 1.00 | 0.14 | 7.34 | 0.5000 | 0 |
| Sofosbuvir and daclatasvir-SOVODAK27 | 0.23 | 0.02 | 2.21 | 0.1018 | 1.271414 |
| Favipavir-Avi-Mild-1928 | 3.31 | 0.65 | 16.76 | 0.0739 | 1.44706 |
| Favipiravir-Iran29 | 2.18 | 0.37 | 12.65 | 0.19324 | 0.86602 |
| Favipiravir-FLARE30 | 3.1 | 0.12 | 77.71 | 0.24541 | 0.68902 |
| Favipiravir/Lopinavir/Ritonavir-FLARE30 | 3 | 0.12 | 75.11 | 0.25187 | 0.66863 |
| Lopinavir/Ritonavir-FLARE30 | 3.05 | 0.12 | 76.39 | 0.24865 | 0.67874 |
| Lopinavir/ritonavir-TREAT NOW31 | 1.21 | 0.4 | 3.64 | 0.37059 | 0.3303 |
| Lopinavir/ritonavir-TOGETHER32 | 1.20 | 0.53 | 2.69 | 0.3333 | 0.430926 |
| Tenofovir Disproxil Fumarate Plus Emtricitabine-AR0-CORONA33 |
2.07 | 0.18 | 24.15 | 0.28057 | 0.58116 |
| Metformin-COVID-OUT34 | 0.42 | 0.18 | 0.96 | 0.0198 | 2.057 |
| Metformin-TOGETHER35 |
0.94 | 0.51 | 1.71 | 0.41626 | 0.21147 |
| Fluvoxamine-TOGETHER11 | 0.77 | 0.56 | 1.05 | 0.0505 | 1.640023 |
| Fluvoxamine-STOP COVID36 | 0.06 | 0.00 | 1.15 | 0.0310 | 1.866043 |
| Fluvoxamine-COVID-OUT34 | 1.18 | 0.36 | 3.91 | 0.3935 | 0.270145 |
| Fluvoxamine ACTIV-637 | 0.45 | 0.04 | 5 | 0.25869 | 0.64738 |
| Fluvoxamine/budesonide-TOGETHER38 | 0.87 | 0.32 | 2.42 | 0.39769 | 0.25933 |
| Ivermectin-TOGETHER39 | 0.81 | 0.59 | 1.11 | 0.09724 | 1.29742 |
| Ivermectin-COVID-OUT34 | 0.76 | 0.20 | 2.85 | 0.3414 | 0.408715 |
| Ivermectin Iran40 | 1.46 | 0.71 | 2.96 | 0.1507 | 1.033341 |
| Ivermectin-ACTIV-641 | 1.05 | 0.43 | 2.61 | 0.4553 | 0.112309 |
| Ivermectin high dose-ACTIV-642 |
2.52 | 0.49 | 13.04 | 0.13512 | 1.10252 |
| Hydroxychloroquine-TOGETHER32 | 0.76 | 0.30 | 1.93 | 0.2840 | 0.570928 |
| Hydroxychloroquine-COVID-19 PEP43 | 0.49 | 0.16 | 1.45 | 0.0971 | 1.298164 |
| Hydroxychloroquine-AH COVID-1944 | 3.14 | 0.17 | 59.70 | 0.2232 | 0.761332 |
| Hydroxychloroquine-BCN PEP-CoV-245 | 0.83 | 0.32 | 2.13 | 0.3486 | 0.389184 |
| Hydroxychloroquine-BMG46 | 0.69 | 0.18 | 2.65 | 0.2945 | 0.54027 |
| Hydroxychloroquine-Utah47 | 1.77 | 0.51 | 6.19 | 0.18429 | 0.89915 |
| Hydroxychloroquine/Azithromycin-Brazil48 | 1 | 0.06 | 16.5 | 0.5 | 0 |
| Nitazoxanide-Romark49 | 0.21 | 0.02 | 1.79 | 0.0766 | 1.428571 |
| Colchicine-COLCORONA2 | 0.79 | 0.61 | 1.03 | 0.0407 | 1.742726 |
| Losartan-MN50 |
3.16 | 0.32 | 31.34 | 0.16245 | 0.98443 |
| Niclosamide51 | 0.33 | 0.01 | 8.48 | 0.2529 | 0.665353 |
| Aspirin-ACTIV-4B52 | 0.94 | 0.06 | 15.24 | 0.4838 | 0.040562 |
| 2.5-mg apixaban-ACTIV-4B52 | 1.01 | 0.06 | 16.27 | 0.4979 | 0.005238 |
| 5-mg apixaban ACTIV-4B52 | 1.91 | 0.17 | 21.36 | 0.2988 | 0.527902 |
| Sulodexide53 | 0.52 | 0.28 | 0.95 | 0.0167 | 2.128119 |
| Enoxaparin-ETHIC54 | 1.10 | 0.47 | 2.56 | 0.4155 | 0.213485 |
| Enoxaparin-OVID55 | 1.02 | 0.38 | 2.76 | 0.4862 | 0.034489 |
| Inhaled Ciclesonide-COVIS56 |
0.48 | 0.12 | 1.87 | 0.14459 | 1.05994 |
| Inhaled ciclesonide-COVERAGE57 | 1.15 | 0.51 | 2.63 | 0.3659 | 0.34277 |
| Zinc58 | 1.48 | 0.36 | 6.52 | 0.30296 | 0.5159 |
| Ascorbic acid58 | 0.68 | 0.11 | 4.27 | 0.34085 | 0.41015 |
| Zinc/Ascorbic acid58 | 2.15 | 0.53 | 8.8 | 0.1435 | 1.06472 |
| Homeopathy-COVID-Simile59 | 0.33 | 0.03 | 3.34 | 0.17503 | 0.93449 |
| Saliravira60 | 0.01 | 0.00 | 0.24 | 0.0016 | 2.9467 |
| Azithromycin-Atomic261 | 0.88 | 0.42 | 1.84 | 0.3694 | 0.333472 |
| Azithromycin-ACTION62 | 6.62 | 0.36 | 121.45 | 0.1015 | 1.272994 |
| Resveratrol63 | 0.32 | 0.03 | 3.18 | 0.1654 | 0.972432 |
Appendix Table 5: Deaths during RCTs.
Cumulatively, the CCP RCTs noted 10 deaths in the control arm versus 8 in CCP arm. The anti-Spike mAbs RCTs had 21 total deaths among controls and 4 in the intervention arm. The total for all small molecule antiviral RCTs was 28 in the controls and 7 in the interventions. The repurposed drugs RCTs recorded 72 deaths in the control groups and 53 in the intervention groups.
| Study | ARR% | RRR% | 95% CI ARR | 95% CI RRR | Odds ratio | 95 CI low | 95 CI high | z statistic | significance (p) |
| Total CCP | 0.15 | 20.2 | (−0.48, 0.78) | (−101.5, 68.4) | 0.8 | 0.31 | 2.02 | 0.47848 | 0.31616 |
| Total mAb | 0.45 | 80.8 | (0.21, 0.7) | (49.4, 92.7) | 0.19 | 0.07 | 0.5 | 3.3459 | 0.00041 |
| Total antivirals | 0.16 | 87.1 | (0.1, 0.23) | (63.4, 95.4) | 0.13 | 0.05 | 0.37 | 3.85181 | 0.00006 |
| Total repurposed | 0.16 | 21.8 | (−0.1, 0.4) | (−8.3, 43.5) | 0.78 | 0.56 | 1.08 | 1.4795 | 0.0695 |
| Total | 0.19 | 44.7 | (0.11, 0.28) | (27.6, 57.8) | 0.55 | 0.42 | 0.72 | 4.29906 | 0.00001 |
| Study | Deaths control | Total control | Deaths intervent. | Total intervent. | Total both arms | % death control | % death intervent. | ||
| Total CCP | 10 | 1315 | 8 | 1319 | 2634 | 0.76 | 0.61 | ||
| Total mAb | 23 | 4102 | 5 | 4634 | 8736 | 0.56 | 0.11 | ||
| Total antivirals | 31 | 17073 | 4 | 17031 | 34104 | 0.18 | 0.02 | ||
| Total repurposed | 81 | 11109 | 65 | 11396 | 22505 | 0.73 | 0.57 | ||
| Total | 131 | 29680 | 72 | 30363 | 60043 | 0.44 | 0.24 | ||
| CCP-CONV-ert7 | 2 | 188 | 0 | 188 | 376 | 1.06 | 0 | ||
| CCP-COV-Early13 | 0 | 204 | 1 | 202 | 406 | 0 | 0.5 | ||
| CCP-C3PO4 | 1 | 254 | 5 | 257 | 511 | 0.39 | 1.95 | ||
| CCP-Argentina5 | 4 | 80 | 2 | 80 | 160 | 5 | 2.5 | ||
| CCP-CSSC-0043 | 3 | 589 | 0 | 592 | 1181 | 0.51 | 0 | ||
| Bamlanivimab-BLAZE-114 | 0 | 143 | 0 | 309 | 452 | 0 | 0 | ||
| Sotrovimab-COMET-ICE8 | 2 | 529 | 0 | 528 | 1057 | 0.38 | 0 | ||
| Bamlanivimab/etesevimab-BLAZE-115 | 10 | 517 | 0 | 518 | 1035 | 1.93 | 0 | ||
| Casirivimab/imdevimab-REGEN-COV Ph 316 | 3 | 1341 | 1 | 1355 | 2696 | 0.22 | 0.07 | ||
| Casirivimab/imdevimab-REGEN-COV Ph 1/217 | 0 | 266 | 0 | 533 | 799 | 0 | 0 | ||
| Bebtelovimab-BLAZE-41 | 0 | 128 | 0 | 125 | 253 | 0 | 0 | ||
| Regdanvimab-CT-P5918 | 0 | 104 | 0 | 203 | 307 | 0 | 0 | ||
| Regdanvimab-CT-P59–219 |
2 | 659 | 1 | 656 | 1315 | 0.3 | 0.15 | ||
| Tixagevimab–cilgavimab-TACKLE20 | 6 | 415 | 3 | 407 | 822 | 1.45 | 0.74 | ||
| Molnupiravir-MOVe-OUT21 | 9 | 699 | 1 | 709 | 1408 | 1.29 | 0.14 | ||
| Molnupiravir-PANORAMIC22 | 5 | 12484 | 2 | 12516 | 25000 | 0.04 | 0.02 | ||
| Molnupiravir-Aurobindo23 | 0 | 610 | 0 | 610 | 1220 | 0 | 0 | ||
| Nirmatrelvir/ritonavir-EPIC-HR9 | 12 | 1046 | 0 | 1039 | 2085 | 1.15 | 0 | ||
| Remdesivir-PINETREE12 | 1 | 283 | 0 | 279 | 562 | 0.35 | 0 | ||
| Interferon Lambda-TOGETHER24 | 4 | 1018 | 1 | 931 | 1949 | 0.4 | 0.11 | ||
| Interferon Lambda-ILIAD25 | 0 | 30 | 0 | 30 | 60 | 0 | 0 | ||
| Interferon Lambda-COVID-Lambda26 | 0 | 60 | 0 | 60 | 120 | 0 | 0 | ||
| Sofosbuvir and daclatasvir-SOVODAK27 | 0 | 28 | 0 | 27 | 55 | 0 | 0 | ||
| Favipavir-Avi-Mild-1928 | 0 | 119 | 0 | 112 | 231 | 0 | 0 | ||
| Favipiravir-Iran29 | 0 | 39 | 0 | 38 | 77 | 0 | 0 | ||
| Favipiravir-FLARE30 | 0 | 60 | 0 | 59 | 119 | 0 | 0 | ||
| Favipiravir/Lopinavir/Ritonavir-FLARE30 | 0 | 60 | 0 | 61 | 121 | 0 | 0 | ||
| Lopinavir/Ritonavir-FLARE30 | 0 | 60 | 0 | 60 | 120 | 0 | 0 | ||
| Lopinavir/ritonavir-TREAT NOW31 | 0 | 220 | 0 | 226 | 446 | 0 | 0 | ||
| Lopinavir/ritonavir-TOGETHER32 | 0 | 227 | 0 | 244 | 471 | 0 | 0 | ||
| Tenofovir Disproxil Fumarate Plus Emtricitabine-AR0-CORONA33 |
0 | 30 | 0 | 30 | 60 | 0 | 0 | ||
| Metformin-COVID-OUT34 | 1 | 601 | 1 | 596 | 1197 | 0.17 | 0.17 | ||
| Metformin-TOGETHER35 |
9 | 203 | 7 | 215 | 418 | 4.4 | 3.2 | ||
| Fluvoxamine-TOGETHER11 | 25 | 756 | 17 | 741 | 1497 | 3.31 | 2.29 | ||
| Fluvoxamine-STOP COVID36 | 0 | 72 | 0 | 80 | 152 | 0 | 0 | ||
| Fluvoxamine-COVID-OUT34 | 0 | 293 | 0 | 299 | 592 | 0 | 0 | ||
| Fluvoxamine ACTIV-637 | 0 | 599 | 0 | 662 | 1261 | 0 | 0 | ||
| Fluvoxamine/budesonide-TOGETHER38 | 0 | 738 | 1 | 738 | 1476 | 0 | 0.14 | ||
| Ivermectin-TOGETHER39 | 24 | 675 | 21 | 674 | 1349 | 3.56 | 3.12 | ||
| Ivermectin-COVID-OUT34 | 0 | 356 | 1 | 374 | 730 | 0 | 0.27 | ||
| Ivermectin Iran40 | 1 | 281 | 1 | 268 | 549 | 0.36 | 0.37 | ||
| Ivermectin-ACTIV-641 | 0 | 774 | 1 | 817 | 1591 | 0 | 0.12 | ||
| Ivermectin high dose-ACTIV-642 |
0 | 604 | 1 | 602 | 1206 | 0 | 0.17 | ||
| Hydroxychloroquine-TOGETHER32 | 1 | 227 | 0 | 214 | 441 | 0.44 | 0 | ||
| Hydroxychloroquine-COVID-19 PEP43 | 1 | 211 | 1 | 212 | 423 | 0.47 | 0.47 | ||
| Hydroxychloroquine-AH COVID-1944 | 0 | 37 | 0 | 111 | 148 | 0 | 0 | ||
| Hydroxychloroquine-BCN PEP-CoV-245 | 0 | 157 | 0 | 136 | 293 | 0 | 0 | ||
| Hydroxychloroquine-BMG46 | 0 | 83 | 0 | 148 | 231 | 0 | 0 | ||
| Hydroxychloroquine-Utah47 | 0 | 151 | 0 | 152 | 303 | 0 | 0 | ||
| Hydroxychloroquine/Azithromycin-Brazil48 | 0 | 42 | 0 | 42 | 84 | 0 | 0 | ||
| Nitazoxanide-Romark49 | 0 | 195 | 0 | 184 | 379 | 0 | 0 | ||
| Colchicine-COLCORONA2 | 9 | 2253 | 5 | 2235 | 4488 | 0.4 | 0.22 | ||
| Losartan-MN50 |
0 | 59 | 0 | 58 | 117 | 0 | 0 | ||
| Niclosamide51 | 0 | 34 | 0 | 33 | 67 | 0 | 0 | ||
| Aspirin-ACTIV-4B52 | 0 | 136 | 0 | 144 | 280 | 0 | 0 | ||
| 2.5-mg apixaban-ACTIV-4B52 | 0 | 136 | 0 | 135 | 271 | 0 | 0 | ||
| 5-mg apixaban ACTIV-4B52 | 0 | 136 | 0 | 143 | 279 | 0 | 0 | ||
| Sulodexide53 | 7 | 119 | 3 | 124 | 243 | 5.88 | 2.42 | ||
| Enoxaparin-ETHIC54 | 0 | 114 | 1 | 105 | 219 | 0 | 0.95 | ||
| Enoxaparin-OVID55 | 0 | 238 | 0 | 234 | 472 | 0 | 0 | ||
| Inhaled Ciclesonide-COVIS56 |
0 | 203 | 0 | 197 | 400 | 0 | 0 | ||
| Inhaled ciclesonide-COVERAGE57 | 2 | 107 | 0 | 110 | 217 | 1.87 | 0 | ||
| Zinc58 | 0 | 50 | 0 | 58 | 108 | 0 | 0 | ||
| Ascorbic acid58 | 0 | 50 | 1 | 48 | 98 | 0 | 2.08 | ||
| Zinc/Ascorbic acid58 | 0 | 50 | 2 | 58 | 108 | 0 | 3.45 | ||
| Homeopathy-COVID-Simile59 | 0 | 44 | 0 | 42 | 86 | 0 | 0 | ||
| Saliravira60 | 0 | 56 | 0 | 87 | 143 | 0 | 0 | ||
| Azithromycin-Atomic261 | 1 | 147 | 1 | 145 | 292 | 0.68 | 0.69 | ||
| Azithromycin-ACTION62 | 0 | 72 | 0 | 125 | 197 | 0 | 0 | ||
| Resveratrol63 | 0 | 50 | 0 | 50 | 100 | 0 | 0 |
Appendix References
- 1.Dougan M, Azizad M, Chen P, et al. Bebtelovimab, alone or together with bamlanivimab and etesevimab, as a broadly neutralizing monoclonal antibody treatment for mild to moderate, ambulatory COVID-19. medRxiv 2022: 2022.03.10.22272100. [Google Scholar]
- 2.Tardif JC, Bouabdallaoui N, ĽAllier PL, et al. Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial. Lancet Respir Med 2021; 9(8): 924–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sullivan DJ, Gebo KA, Shoham S, et al. Early Outpatient Treatment for Covid-19 with Convalescent Plasma. N Engl J Med 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korley FK, Durkalski-Mauldin V, Yeatts SD, et al. Early Convalescent Plasma for High-Risk Outpatients with Covid-19. N Engl J Med 2021; 385(21): 1951–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Libster R, Perez Marc G, Wappner D, et al. Early High-Titer Plasma Therapy to Prevent Severe Covid-19 in Older Adults. N Engl J Med 2021; 384(7): 610–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Millat-Martinez P, Gharbharan A, Alemany A, et al. Prospective individual patient data meta-analysis of two randomized trials on convalescent plasma for COVID-19 outpatients. Nature Communications 2022; 13(1): 2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alemany A, Millat-Martinez P, Corbacho-Monne M, et al. High-titre methylene blue-treated convalescent plasma as an early treatment for outpatients with COVID-19: a randomised, placebo-controlled trial. Lancet Respir Med 2022; 10(3): 278–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab. N Engl J Med 2021. [DOI] [PubMed] [Google Scholar]
- 9.Hammond J, Leister-Tebbe H, Gardner A, et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. N Engl J Med 2022; 386(15): 1397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reis G, Silva E, Silva DCM, et al. Effect of Early Treatment with Ivermectin among Patients with Covid-19. N Engl J Med 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reis G, Dos Santos Moreira-Silva EA, Silva DCM, et al. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob Health 2022; 10(1): e42–e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gottlieb RL, Vaca CE, Paredes R, et al. Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients. N Engl J Med 2022; 386(4): 305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gharbharan A, Jordans C, Zwaginga L, et al. Outpatient convalescent plasma therapy for high-risk patients with early COVID-19. A randomized placebo-controlled trial. Clin Microbiol Infect 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen P, Nirula A, Heller B, et al. SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19. N Engl J Med 2021; 384(3): 229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dougan M, Nirula A, Azizad M, et al. Bamlanivimab plus Etesevimab in Mild or Moderate Covid-19. N Engl J Med 2021; 385(15): 1382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weinreich DM, Sivapalasingam S, Norton T, et al. REGEN-COV Antibody Combination and Outcomes in Outpatients with Covid-19. N Engl J Med 2021; 385(23): e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norton T, Ali S, Sivapalasingam S, et al. REGEN-COV Antibody Combination in Outpatients With COVID-19 – Phase 1/2 Results. medRxiv 2022: 2021.06.09.21257915. [Google Scholar]
- 18.Streinu-Cercel A, Sandulescu O, Preotescu LL, et al. Efficacy and Safety of Regdanvimab (CT-P59): A Phase 2/3 Randomized, Double-Blind, Placebo-Controlled Trial in Outpatients With Mild-to-Moderate Coronavirus Disease 2019. Open Forum Infect Dis 2022; 9(4): ofac053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JY, Sandulescu O, Preotescu LL, et al. A Randomized Clinical Trial of Regdanvimab in High-Risk Patients With Mild-to-Moderate Coronavirus Disease 2019. Open Forum Infect Dis 2022; 9(8): ofac406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montgomery H, Hobbs FDR, Padilla F, et al. Efficacy and safety of intramuscular administration of tixagevimab-cilgavimab for early outpatient treatment of COVID-19 (TACKLE): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2022; 10(10): 985–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N Engl J Med 2022; 386(6): 509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butler C, Hobbs FDR, Gbinigie O, et al. Molnupiravir Plus Usual Care Versus Usual Care Alone as Early Treatment for Adults with COVID-19 at Increased Risk of Adverse Outcomes (PANORAMIC): Preliminary Analysis from the United Kingdom Randomised, Controlled Open-Label, Platform Adaptive Trial. SSRN 2022; ssrn.4237902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tippabhotla SK, Lahiri S, Rama Raju D, Kandi C, Prasad VN. Efficacy and Safety of Molnupiravir for the Treatment of Non-Hospitalized Adults With Mild COVID-19: A Randomized, Open-Label, Parallel-Group Phase 3 Trial. SSRN 2022; 4042673. [Google Scholar]
- 24.Reis G, Moreira Silva EAS, Medeiros Silva DC, et al. Early Treatment with Pegylated Interferon Lambda for Covid-19. N Engl J Med 2023; 388(6): 518–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feld JJ, Kandel C, Biondi MJ, et al. Peginterferon lambda for the treatment of outpatients with COVID-19: a phase 2, placebo-controlled randomised trial. Lancet Respir Med 2021; 9(5): 498–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jagannathan P, Andrews JR, Bonilla H, et al. Peginterferon Lambda-1a for treatment of outpatients with uncomplicated COVID-19: a randomized placebo-controlled trial. Nature Communications 2021; 12(1): 1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roozbeh F, Saeedi M, Alizadeh-Navaei R, et al. Sofosbuvir and daclatasvir for the treatment of COVID-19 outpatients: a double-blind, randomized controlled trial. Journal of Antimicrobial Chemotherapy 2020; 76(3): 753–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bosaeed M, Alharbi A, Mahmoud E, et al. Efficacy of favipiravir in adults with mild COVID-19: a randomized, double-blind, multicentre, placebo-controlled clinical trial. Clin Microbiol Infect 2022; 28(4): 602–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaezi A, Salmasi M, Soltaninejad F, Salahi M, Javanmard SH, Amra B. Favipiravir in the Treatment of Outpatient COVID-19: A Multicenter, Randomized, Triple-Blind, Placebo-Controlled Clinical Trial. Adv Respir Med 2023; 91(1): 18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lowe DM, Brown LK, Chowdhury K, et al. Favipiravir, lopinavir-ritonavir, or combination therapy (FLARE): A randomised, double-blind, 2 × 2 factorial placebo-controlled trial of early antiviral therapy in COVID-19. PLoS Med 2022; 19(10): e1004120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaizer AM, Shapiro NI, Wild J, et al. Lopinavir/ritonavir for treatment of non-hospitalized patients with COVID-19: a randomized clinical trial. Int J Infect Dis 2023; 128: 223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reis G, Moreira Silva E, Medeiros Silva DC, et al. Effect of Early Treatment With Hydroxychloroquine or Lopinavir and Ritonavir on Risk of Hospitalization Among Patients With COVID-19: The TOGETHER Randomized Clinical Trial. JAMA Netw Open 2021; 4(4): e216468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parienti JJ, Prazuck T, Peyro-Saint-Paul L, et al. Effect of Tenofovir Disoproxil Fumarate and Emtricitabine on nasopharyngeal SARS-CoV-2 viral load burden amongst outpatients with COVID-19: A pilot, randomized, open-label phase 2 trial. EClinicalMedicine 2021; 38: 100993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bramante CT, Huling JD, Tignanelli CJ, et al. Randomized Trial of Metformin, Ivermectin, and Fluvoxamine for Covid-19. N Engl J Med 2022; 387(7): 599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reis G, Dos Santos Moreira Silva EA, Medeiros Silva DC, et al. Effect of early treatment with metformin on risk of emergency care and hospitalization among patients with COVID-19: The TOGETHER randomized platform clinical trial. Lancet Reg Health Am 2022; 6: 100142. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Lenze EJ, Mattar C, Zorumski CF, et al. Fluvoxamine vs Placebo and Clinical Deterioration in Outpatients With Symptomatic COVID-19: A Randomized Clinical Trial. JAMA 2020; 324(22): 2292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCarthy MW, Naggie S, Boulware DR, et al. Effect of Fluvoxamine vs Placebo on Time to Sustained Recovery in Outpatients With Mild to Moderate COVID-19: A Randomized Clinical Trial. JAMA 2023; 329(4): 296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reis G, Dos Santos Moreira Silva EA, Medeiros Silva DC, et al. Oral Fluvoxamine With Inhaled Budesonide for Treatment of Early-Onset COVID-19 : A Randomized Platform Trial. Ann Intern Med 2023; 176(5): 667–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reis G, Silva E, Silva DCM, et al. Effect of Early Treatment with Ivermectin among Patients with Covid-19. N Engl J Med 2022; 386(18): 1721–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rezai MS, Ahangarkani F, Hill A, et al. Non-effectiveness of Ivermectin on Inpatients and Outpatients With COVID-19; Results of Two Randomized, Double-Blinded, Placebo-Controlled Clinical Trials. Front Med (Lausanne) 2022; 9: 919708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naggie S, Boulware DR, Lindsell CJ, et al. Effect of Ivermectin vs Placebo on Time to Sustained Recovery in Outpatients With Mild to Moderate COVID-19: A Randomized Clinical Trial. JAMA 2022; 328(16): 1595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naggie S, Boulware DR, Lindsell CJ, et al. Effect of Higher-Dose Ivermectin for 6 Days vs Placebo on Time to Sustained Recovery in Outpatients With COVID-19: A Randomized Clinical Trial. JAMA 2023; 329(11): 888–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skipper CP, Pastick KA, Engen NW, et al. Hydroxychloroquine in Nonhospitalized Adults With Early COVID-19 : A Randomized Trial. Ann Intern Med 2020; 173(8): 623–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwartz I, Boesen ME, Cerchiaro G, et al. Assessing the efficacy and safety of hydroxychloroquine as outpatient treatment of COVID-19: a randomized controlled trial. CMAJ Open 2021; 9(2): E693–E702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitja O, Reis G, Boulware DR, et al. Hydroxychloroquine for treatment of non-hospitalized adults with COVID-19: A meta-analysis of individual participant data of randomized trials. Clin Transl Sci 2023; 16(3): 524–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnston C, Brown ER, Stewart J, et al. Hydroxychloroquine with or without azithromycin for treatment of early SARS-CoV-2 infection among high-risk outpatient adults: A randomized clinical trial. EClinicalMedicine 2021; 33: 100773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spivak AM, Barney BJ, Greene T, et al. A Randomized Clinical Trial Testing Hydroxychloroquine for Reduction of SARS-CoV-2 Viral Shedding and Hospitalization in Early Outpatient COVID-19 Infection. Microbiol Spectr 2023; 11(2): e0467422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodrigues C, Freitas-Santos RS, Levi JE, et al. Hydroxychloroquine plus azithromycin early treatment of mild COVID-19 in an outpatient setting: a randomized, double-blinded, placebo-controlled clinical trial evaluating viral clearance. Int J Antimicrob Agents 2021; 58(5): 106428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rossignol JF, Bardin MC, Fulgencio J, Mogelnicki D, Brechot C. A randomized double-blind placebo-controlled clinical trial of nitazoxanide for treatment of mild or moderate COVID-19. EClinicalMedicine 2022; 45: 101310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Puskarich MA, Cummins NW, Ingraham NE, et al. A multi-center phase II randomized clinical trial of losartan on symptomatic outpatients with COVID-19. EClinicalMedicine 2021; 37: 100957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cairns DM, Dulko D, Griffiths JK, et al. Efficacy of Niclosamide vs Placebo in SARS-CoV-2 Respiratory Viral Clearance, Viral Shedding, and Duration of Symptoms Among Patients With Mild to Moderate COVID-19: A Phase 2 Randomized Clinical Trial. JAMA Network Open 2022; 5(2): e2144942–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Connors JM, Brooks MM, Sciurba FC, et al. Effect of Antithrombotic Therapy on Clinical Outcomes in Outpatients With Clinically Stable Symptomatic COVID-19: The ACTIV-4B Randomized Clinical Trial. JAMA 2021; 326(17): 1703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gonzalez-Ochoa AJ, Raffetto JD, Hernandez AG, et al. Sulodexide in the Treatment of Patients with Early Stages of COVID-19: A Randomized Controlled Trial. Thromb Haemost 2021; 121(7): 944–54. [DOI] [PubMed] [Google Scholar]
- 54.Cools F, Virdone S, Sawhney J, et al. Thromboprophylactic low-molecular-weight heparin versus standard of care in unvaccinated, at-risk outpatients with COVID-19 (ETHIC): an open-label, multicentre, randomised, controlled, phase 3b trial. Lancet Haematol 2022; 9(8): e594–e604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barco S, Voci D, Held U, et al. Enoxaparin for primary thromboprophylaxis in symptomatic outpatients with COVID-19 (OVID): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet Haematol 2022; 9(8): e585–e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clemency BM, Varughese R, Gonzalez-Rojas Y, et al. Efficacy of Inhaled Ciclesonide for Outpatient Treatment of Adolescents and Adults With Symptomatic COVID-19: A Randomized Clinical Trial. JAMA Intern Med 2022; 182(1): 42–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duvignaud A, Lhomme E, Onaisi R, et al. Inhaled ciclesonide for outpatient treatment of COVID-19 in adults at risk of adverse outcomes: a randomised controlled trial (COVERAGE). Clin Microbiol Infect 2022; 28(7): 1010–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thomas S, Patel D, Bittel B, et al. Effect of High-Dose Zinc and Ascorbic Acid Supplementation vs Usual Care on Symptom Length and Reduction Among Ambulatory Patients With SARS-CoV-2 Infection: The COVID A to Z Randomized Clinical Trial. JAMA Netw Open 2021; 4(2): e210369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adler UC, Adler MS, Padula AEM, et al. Homeopathy for COVID-19 in primary care: A randomized, double-blind, placebo-controlled trial (COVID-Simile study). J Integr Med 2022; 20(3): 221–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khorshiddoust RR, Khorshiddoust SR, Hosseinabadi T, et al. Efficacy of a multiple-indication antiviral herbal drug (Saliravira(R)) for COVID-19 outpatients: A pre-clinical and randomized clinical trial study. Biomed Pharmacother 2022; 149: 112729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hinks TSC, Cureton L, Knight R, et al. Azithromycin versus standard care in patients with mild-to-moderate COVID-19 (ATOMIC2): an open-label, randomised trial. Lancet Respir Med 2021; 9(10): 1130–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oldenburg CE, Pinsky BA, Brogdon J, et al. Effect of Oral Azithromycin vs Placebo on COVID-19 Symptoms in Outpatients With SARS-CoV-2 Infection: A Randomized Clinical Trial. JAMA 2021; 326(6): 490–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McCreary MR, Schnell PM, Rhoda DA. Randomized double-blind placebo-controlled proof-of-concept trial of resveratrol for outpatient treatment of mild coronavirus disease (COVID-19). Sci Rep 2022; 12(1): 10978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mitjà O, Corbacho-Monné M, Ubals M, et al. Hydroxychloroquine for Early Treatment of Adults With Mild Coronavirus Disease 2019: A Randomized, Controlled Trial. Clinical Infectious Diseases 2020; 73(11): e4073–e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schwartz I, Boesen ME, Cerchiaro G, et al. Assessing the efficacy and safety of hydroxychloroquine as outpatient treatment of COVID-19: a randomized controlled trial. 2021; 9(2): E693–E702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Johnston C, Brown ER, Stewart J, et al. Hydroxychloroquine with or without azithromycin for treatment of early SARS-CoV-2 infection among high-risk outpatient adults: A randomized clinical trial. Eclinicalmedicine 2021; 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Footnotes
Declaration of competing interest
DS, DFH, AC were investigators in the CSSC-004 study; D.F. and M.F. were investigators in the TSUNAMI RCT of CCP. DJS reports AliquantumRx Founder and Board member with stock options (macrolide for malaria), Hemex Health malaria diagnostics consulting and royalties for malaria diagnostic test control standards to Alere-all outside of submitted work. AC reports being part of the scientific advisory board of SabTherapeutics and has received personal fees from Ortho Diagnostics, outside of the submitted work. All other authors report no relevant disclosures.
Data availability/sharing
Datasets used for this systematic review are publicly available in PubMed, medRxiv and bioRxiv. Data files used to generate figures are available upon request.
References
- 1.Chen P, Nirula A, Heller B, et al. SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19. N Engl J Med. 2021;384(3):229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinreich DM, Sivapalasingam S, Norton T, et al. REGEN-COV Antibody Combination and Outcomes in Outpatients with Covid-19. N Engl J Med. 2021;385(23):e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson EJ, Rouphael NG, Widge AT, et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N Engl J Med. 2020;383(25):2427–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammond J, Leister-Tebbe H, Gardner A, et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. N Engl J Med. 2022;386(15):1397–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N Engl J Med. 2022;386(6):509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.FDA Takes Actions to Expand Use of Treatment for Outpatients with Mild-to-Moderate COVID-19 [press release]. 2022. [Google Scholar]
- 8.O’Shaughnessy JA. Convalescent Plasma EUA Letter of Authorization 12282021. In. Vol https://www.fda.gov/media/141477/download. FDA website; (https://www.fda.gov/media/141477/download)2021. [Google Scholar]
- 9.Sullivan DJ, Gebo KA, Shoham S, et al. Early Outpatient Treatment for Covid-19 with Convalescent Plasma. N Engl J Med. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Libster R, Perez Marc G, Wappner D, et al. Early High-Titer Plasma Therapy to Prevent Severe Covid-19 in Older Adults. N Engl J Med. 2021;384(7):610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Brien MP, Forleo-Neto E, Musser BJ, et al. Subcutaneous REGEN-COV Antibody Combination to Prevent Covid-19. New England Journal of Medicine. 2021;385(13):1184–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen TV, Ferrand G, Cohen-Boulakia S, et al. RCT studies on preventive measures and treatments for COVID-19. Zenodo. 2020. [Google Scholar]
- 13.Boutron I, Chaimani A, Meerpohl JJ, et al. The COVID-NMA Project: Building an Evidence Ecosystem for the COVID-19 Pandemic. Ann Intern Med. 2020;173(12):1015–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.JPT H, S G, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. www.handbook.cochrane.org; 2011. The Cochrane Collaboration, 2011. [Google Scholar]
- 15.Gottlieb RL, Vaca CE, Paredes R, et al. Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients. N Engl J Med. 2022;386(4):305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bramante CT, Huling JD, Tignanelli CJ, et al. Randomized Trial of Metformin, Ivermectin, and Fluvoxamine for Covid-19. N Engl J Med. 2022;387(7):599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dougan M, Azizad M, Chen P, et al. Bebtelovimab, alone or together with bamlanivimab and etesevimab, as a broadly neutralizing monoclonal antibody treatment for mild to moderate, ambulatory COVID-19. medRxiv. 2022:2022.2003.2010.22272100. [Google Scholar]
- 18.Reis G, Moreira Silva E, Medeiros Silva DC, et al. Effect of Early Treatment With Hydroxychloroquine or Lopinavir and Ritonavir on Risk of Hospitalization Among Patients With COVID-19: The TOGETHER Randomized Clinical Trial. JAMA Netw Open. 2021;4(4):e216468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reis G, Silva E, Silva DCM, et al. Effect of Early Treatment with Ivermectin among Patients with Covid-19. N Engl J Med. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tardif JC, Bouabdallaoui N, L’Allier PL, et al. Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial. Lancet Respir Med. 2021;9(8):924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reis G, Dos Santos Moreira-Silva EA, Silva DCM, et al. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob Health. 2022;10(1):e42–e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossignol JF, Bardin MC, Fulgencio J, Mogelnicki D, Brechot C. A randomized double-blind placebo-controlled clinical trial of nitazoxanide for treatment of mild or moderate COVID-19. EClinicalMedicine. 2022;45:101310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Millat-Martinez P, Gharbharan A, Alemany A, et al. Prospective individual patient data meta-analysis of two randomized trials on convalescent plasma for COVID-19 outpatients. Nature Communications. 2022;13(1):2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alemany A, Millat-Martinez P, Corbacho-Monne M, et al. High-titre methylene blue-treated convalescent plasma as an early treatment for outpatients with COVID-19: a randomised, placebo-controlled trial. Lancet Respir Med. 2022;10(3):278–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dougan M, Nirula A, Azizad M, et al. Bamlanivimab plus Etesevimab in Mild or Moderate Covid-19. N Engl J Med. 2021;385(15):1382–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Streinu-Cercel A, Sandulescu O, Preotescu LL, et al. Efficacy and Safety of Regdanvimab (CT-P59): A Phase 2/3 Randomized, Double-Blind, Placebo-Controlled Trial in Outpatients With Mild-to-Moderate Coronavirus Disease 2019. Open Forum Infect Dis. 2022;9(4):ofac053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim JY, Sandulescu O, Preotescu LL, et al. A Randomized Clinical Trial of Regdanvimab in High-Risk Patients With Mild-to-Moderate Coronavirus Disease 2019. Open Forum Infect Dis. 2022;9(8):ofac406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oldenburg CE, Pinsky BA, Brogdon J, et al. Effect of Oral Azithromycin vs Placebo on COVID-19 Symptoms in Outpatients With SARS-CoV-2 Infection: A Randomized Clinical Trial. JAMA. 2021;326(6):490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou S, Hill CS, Sarkar S, et al. beta-d-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells. J Infect Dis. 2021;224(3):415–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta K, Strymish J, Stack G, Charness M. Rapid Relapse of Symptomatic SARS-CoV-2 Infection Following Early Suppression with Nirmatrelvir/Ritonavir. Research Square 2022. [Google Scholar]
- 31.Hasan Ali O, Bomze D, Risch L, et al. Severe COVID-19 is associated with elevated serum IgA and antiphospholipid IgA-antibodies. Clinical Infectious Diseases. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levine AC, Fukuta Y, Huaman MA, et al. COVID-19 Convalescent Plasma Outpatient Therapy to Prevent Outpatient Hospitalization: A Meta-analysis of Individual Participant Data From Five Randomized Trials. Clin Infect Dis. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Focosi D, Quiroga R, McConnell S, Johnson MC, Casadevall A. Convergent Evolution in SARS-CoV-2 Spike Creates a Variant Soup from Which New COVID-19 Waves Emerge. International Journal of Molecular Sciences. 2023;24(3):2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Focosi D, Franchini M, Joyner MJ, Casadevall A. Comparative analysis of antibody responses from COVID-19 convalescents receiving various vaccines reveals consistent high neutralizing activity for SARS-CoV-2 variant of concern Omicron. 2021:2021.2012.2024.21268317. [Google Scholar]
- 35.Gharbharan A, Jordans C, Zwaginga L, et al. Outpatient convalescent plasma therapy for high-risk patients with early COVID-19. A randomized placebo-controlled trial. Clin Microbiol Infect. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korley FK, Durkalski-Mauldin V, Yeatts SD, et al. Early Convalescent Plasma for High-Risk Outpatients with Covid-19. N Engl J Med. 2021;385(21):1951–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab. N Engl J Med. 2021. [DOI] [PubMed] [Google Scholar]
- 38.Norton T, Ali S, Sivapalasingam S, et al. REGEN-COV Antibody Combination in Outpatients With COVID-19 – Phase 1/2 Results. medRxiv. 2022:2021.2006.2009.21257915. [Google Scholar]
- 39.Montgomery H, Hobbs FDR, Padilla F, et al. Efficacy and safety of intramuscular administration of tixagevimab-cilgavimab for early outpatient treatment of COVID-19 (TACKLE): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2022;10(10):985–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Butler CC, Hobbs FDR, Gbinigie OA, et al. Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial. Lancet. 2023;401(10373):281–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tippabhotla SK, Lahiri S, Rama Raju D, Kandi C, Prasad VN. Efficacy and Safety of Molnupiravir for the Treatment of Non-Hospitalized Adults With Mild COVID-19: A Randomized, Open-Label, Parallel-Group Phase 3 Trial. SSRN. 2022;4042673. [Google Scholar]
- 42.Reis G, Moreira Silva EAS, Medeiros Silva DC, et al. Early Treatment with Pegylated Interferon Lambda for Covid-19. N Engl J Med. 2023;388(6):518–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feld JJ, Kandel C, Biondi MJ, et al. Peginterferon lambda for the treatment of outpatients with COVID-19: a phase 2, placebo-controlled randomised trial. Lancet Respir Med. 2021;9(5):498–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jagannathan P, Andrews JR, Bonilla H, et al. Peginterferon Lambda-1a for treatment of outpatients with uncomplicated COVID-19: a randomized placebo-controlled trial. Nature Communications. 2021;12(1):1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roozbeh F, Saeedi M, Alizadeh-Navaei R, et al. Sofosbuvir and daclatasvir for the treatment of COVID-19 outpatients: a double-blind, randomized controlled trial. Journal of Antimicrobial Chemotherapy. 2020;76(3):753–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bosaeed M, Alharbi A, Mahmoud E, et al. Efficacy of favipiravir in adults with mild COVID-19: a randomized, double-blind, multicentre, placebo-controlled clinical trial. Clin Microbiol Infect. 2022;28(4):602–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vaezi A, Salmasi M, Soltaninejad F, Salahi M, Javanmard SH, Amra B. Favipiravir in the Treatment of Outpatient COVID-19: A Multicenter, Randomized, Triple-Blind, Placebo-Controlled Clinical Trial. Adv Respir Med. 2023;91(1):18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lowe DM, Brown LK, Chowdhury K, et al. Favipiravir, lopinavir-ritonavir, or combination therapy (FLARE): A randomised, double-blind, 2 × 2 factorial placebo-controlled trial of early antiviral therapy in COVID-19. PLoS Med. 2022;19(10):e1004120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaizer AM, Shapiro NI, Wild J, et al. Lopinavir/ritonavir for treatment of non-hospitalized patients with COVID-19: a randomized clinical trial. Int J Infect Dis. 2023;128:223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parienti JJ, Prazuck T, Peyro-Saint-Paul L, et al. Effect of Tenofovir Disoproxil Fumarate and Emtricitabine on nasopharyngeal SARS-CoV-2 viral load burden amongst outpatients with COVID-19: A pilot, randomized, open-label phase 2 trial. EClinicalMedicine. 2021;38:100993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reis G, Dos Santos Moreira Silva EA, Medeiros Silva DC, et al. Effect of early treatment with metformin on risk of emergency care and hospitalization among patients with COVID-19: The TOGETHER randomized platform clinical trial. Lancet Reg Health Am. 2022;6:100142. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52.Lenze EJ, Mattar C, Zorumski CF, et al. Fluvoxamine vs Placebo and Clinical Deterioration in Outpatients With Symptomatic COVID-19: A Randomized Clinical Trial. JAMA. 2020;324(22):2292–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McCarthy MW, Naggie S, Boulware DR, et al. Effect of Fluvoxamine vs Placebo on Time to Sustained Recovery in Outpatients With Mild to Moderate COVID-19: A Randomized Clinical Trial. JAMA. 2023;329(4):296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reis G, Dos Santos Moreira Silva EA, Medeiros Silva DC, et al. Oral Fluvoxamine With Inhaled Budesonide for Treatment of Early-Onset COVID-19 : A Randomized Platform Trial. Ann Intern Med. 2023;176(5):667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reis G, Silva E, Silva DCM, et al. Effect of Early Treatment with Ivermectin among Patients with Covid-19. N Engl J Med. 2022;386(18):1721–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rezai MS, Ahangarkani F, Hill A, et al. Non-effectiveness of Ivermectin on Inpatients and Outpatients With COVID-19; Results of Two Randomized, Double-Blinded, Placebo-Controlled Clinical Trials. Front Med (Lausanne). 2022;9:919708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Naggie S, Boulware DR, Lindsell CJ, et al. Effect of Ivermectin vs Placebo on Time to Sustained Recovery in Outpatients With Mild to Moderate COVID-19: A Randomized Clinical Trial. JAMA. 2022;328(16):1595–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Naggie S, Boulware DR, Lindsell CJ, et al. Effect of Higher-Dose Ivermectin for 6 Days vs Placebo on Time to Sustained Recovery in Outpatients With COVID-19: A Randomized Clinical Trial. JAMA. 2023;329(11):888–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Skipper CP, Pastick KA, Engen NW, et al. Hydroxychloroquine in Nonhospitalized Adults With Early COVID-19 : A Randomized Trial. Ann Intern Med. 2020;173(8):623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schwartz I, Boesen ME, Cerchiaro G, et al. Assessing the efficacy and safety of hydroxychloroquine as outpatient treatment of COVID-19: a randomized controlled trial. CMAJ Open. 2021;9(2):E693–E702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mitja O, Reis G, Boulware DR, et al. Hydroxychloroquine for treatment of non-hospitalized adults with COVID-19: A meta-analysis of individual participant data of randomized trials. Clin Transl Sci. 2023;16(3):524–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johnston C, Brown ER, Stewart J, et al. Hydroxychloroquine with or without azithromycin for treatment of early SARS-CoV-2 infection among high-risk outpatient adults: A randomized clinical trial. EClinicalMedicine. 2021;33:100773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spivak AM, Barney BJ, Greene T, et al. A Randomized Clinical Trial Testing Hydroxychloroquine for Reduction of SARS-CoV-2 Viral Shedding and Hospitalization in Early Outpatient COVID-19 Infection. Microbiol Spectr. 2023;11(2):e0467422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rodrigues C, Freitas-Santos RS, Levi JE, et al. Hydroxychloroquine plus azithromycin early treatment of mild COVID-19 in an outpatient setting: a randomized, double-blinded, placebo-controlled clinical trial evaluating viral clearance. Int J Antimicrob Agents. 2021;58(5):106428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Puskarich MA, Cummins NW, Ingraham NE, et al. A multi-center phase II randomized clinical trial of losartan on symptomatic outpatients with COVID-19. EClinicalMedicine. 2021;37:100957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cairns DM, Dulko D, Griffiths JK, et al. Efficacy of Niclosamide vs Placebo in SARS-CoV-2 Respiratory Viral Clearance, Viral Shedding, and Duration of Symptoms Among Patients With Mild to Moderate COVID-19: A Phase 2 Randomized Clinical Trial. JAMA Network Open. 2022;5(2):e2144942–e2144942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Connors JM, Brooks MM, Sciurba FC, et al. Effect of Antithrombotic Therapy on Clinical Outcomes in Outpatients With Clinically Stable Symptomatic COVID-19: The ACTIV-4B Randomized Clinical Trial. JAMA. 2021;326(17):1703–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gonzalez-Ochoa AJ, Raffetto JD, Hernandez AG, et al. Sulodexide in the Treatment of Patients with Early Stages of COVID-19: A Randomized Controlled Trial. Thromb Haemost. 2021;121(7):944–954. [DOI] [PubMed] [Google Scholar]
- 69.Cools F, Virdone S, Sawhney J, et al. Thromboprophylactic low-molecular-weight heparin versus standard of care in unvaccinated, at-risk outpatients with COVID-19 (ETHIC): an open-label, multicentre, randomised, controlled, phase 3b trial. Lancet Haematol. 2022;9(8):e594–e604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barco S, Voci D, Held U, et al. Enoxaparin for primary thromboprophylaxis in symptomatic outpatients with COVID-19 (OVID): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet Haematol. 2022;9(8):e585–e593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Clemency BM, Varughese R, Gonzalez-Rojas Y, et al. Efficacy of Inhaled Ciclesonide for Outpatient Treatment of Adolescents and Adults With Symptomatic COVID-19: A Randomized Clinical Trial. JAMA Intern Med. 2022;182(1):42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Duvignaud A, Lhomme E, Onaisi R, et al. Inhaled ciclesonide for outpatient treatment of COVID-19 in adults at risk of adverse outcomes: a randomised controlled trial (COVERAGE). Clin Microbiol Infect. 2022;28(7):1010–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thomas S, Patel D, Bittel B, et al. Effect of High-Dose Zinc and Ascorbic Acid Supplementation vs Usual Care on Symptom Length and Reduction Among Ambulatory Patients With SARS-CoV-2 Infection: The COVID A to Z Randomized Clinical Trial. JAMA Netw Open. 2021;4(2):e210369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Adler UC, Adler MS, Padula AEM, et al. Homeopathy for COVID-19 in primary care: A randomized, double-blind, placebo-controlled trial (COVID-Simile study). J Integr Med. 2022;20(3):221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Khorshiddoust RR, Khorshiddoust SR, Hosseinabadi T, et al. Efficacy of a multiple-indication antiviral herbal drug (Saliravira(R)) for COVID-19 outpatients: A pre-clinical and randomized clinical trial study. Biomed Pharmacother. 2022;149:112729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hinks TSC, Cureton L, Knight R, et al. Azithromycin versus standard care in patients with mild-to-moderate COVID-19 (ATOMIC2): an open-label, randomised trial. Lancet Respir Med. 2021;9(10):1130–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McCreary MR, Schnell PM, Rhoda DA. Randomized double-blind placebo-controlled proof-of-concept trial of resveratrol for outpatient treatment of mild coronavirus disease (COVID-19). Sci Rep. 2022;12(1):10978. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets used for this systematic review are publicly available in PubMed, medRxiv and bioRxiv. Data files used to generate figures are available upon request.


