Abstract
Introduction:
Vascular reconstruction requires technical expertise and is often time consuming. As a novel alternative to traditional hand-sewn vascular anastomoses, the VasoLock (VL), is a nonabsorbable, sutureless anastomosis device with traction anchors designed to hold free artery ends together. These anchors do not penetrate the vessel wall but adhere by leveraging the elasticity of the vessels to fasten blood vessels together. This pilot study assesses the performance and patency of this novel device in a porcine model of femoral artery injury.
Methods:
Female swine (n = 7) underwent femoral artery exposure for a total of 10 VL implanted. Study animals underwent hemodilution to a target hematocrit of 15% and ROTEM was used to assess coagulopathy, followed by an arterial injury via transection. The VL was inserted without any sutures. Flow-probe monitors were positioned proximal and distal to the device and flow rates were measured continuously for a total of 90 min. Flow was analyzed and presented as a ratio of distal to proximal flow with the slope of this ratio across time subsequently determined. Angiographic assessment was completed to evaluate for patency and technical complications after 90 min of implant.
Results:
The average animal weight was 44.1 ± 3.2 kg. The average mean arterial pressure at the time of implant was 51.2 ± 7.8 mmHg, median heart rate was 77.4 (IQR = 77.25–157.4) beats per minute, and average temperature was 36.1 ± 1.5°C. The baseline hematocrit was 13.5 ± 3.0%, average pH was 7.20 ± 0.1, average clotting time was 154.1 ± 58.7 s and average clot formation time was 103.4 ± 10.9 s all demonstrating the acidotic, hypothermic, and coagulopathic state of the swine at the time of insertion. During the 90-min observation period, the average flow gradient identified across the VL was 0.99 ± 0.24, indicating no significant change in flow across the VL. The average slope of the gradients was 0.0005 (P = 0.22), suggesting the ratio of proximal and distal flow did not change over the 90 min. Following 90 min of dwell time, all VL were patent without technical complication. Angiographic assessment at 90 min demonstrated no evidence of dissection, device migration, arterial extravasation, or thromboembolism with any of the 10 devices.
Conclusions:
This pilot study demonstrated technical feasibility of the novel VL device over a 90-min observation period. All VL were patent and no negative events or complications were identified. This technology demonstrated significant promise in a coagulopathic state: additional investigation, involving long-term survival, is warranted for further validation.
Keywords: Distal perfusion, Hemorrhage, Sutured anastomosis, Vascular anastomosis, Vascular injury
Introduction
Vascular injury is a common occurrence in trauma, both in the military and civilian populations.1–5 Frequently, vascular reconstruction requires the creation of an anastomosis to reestablish end-organ or limb perfusion. The technical ability to construct a reliable, high-fidelity anastomosis requires surgical expertise and procedural experience. Despite the prevalence of vascular injuries in trauma, the immediate availability of surgeons capable of these complex repairs is, at times, limited. Specifically, the technical specialization to repair vascular injuries may be limited in the military-specific, far-forward, resource-limited, austere environments and in the rural civilian setting that suffer from long transport time. Furthermore, the traditional skillset of the initial operating surgeon may lack the necessary experience for vascular repair regardless of the setting.
Delay in time to vascular injury repair leads directly to prolonged ischemia times. Early limb reperfusion has demonstrated superior outcomes with prompt restoration of distal perfusion resulted in improved limb salvage rates.6,7 The time to reperfusion is particularly important to limit ischemia reperfusion injuries and the subsequent associated complications.
The VasoLock (VL) (Fig. 1) device is a novel, sutureless anastomotic device that obviates the need for the typical, hand-sewn, sutured anastomosis. Instead, this device employs traction of anchors on either end of the device that secure it intraluminally. The VL device was designed to limit the need for vascular experts across all echelons of care while maintaining the ability to provide definitive repair to a traumatic vascular injury with minimum repair time. This study seeks to assess the short-term feasibility of this novel anastomotic device to address the time and technical complexity associated with a hand-sewn vascular anastomosis in a traumatic extremity vascular injury.
Fig. 1 –
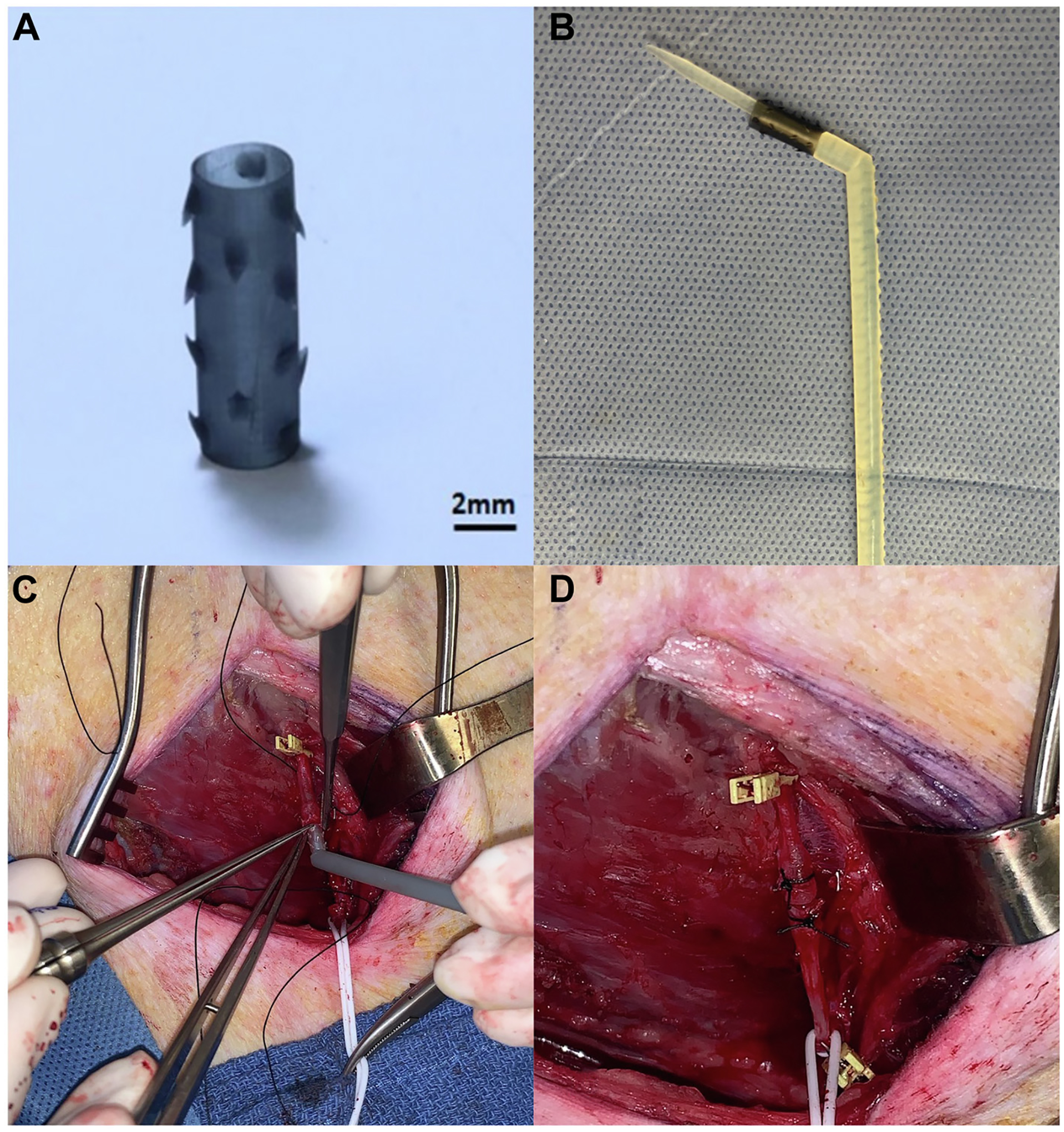
VasoLock Device ex vivo prior to implant (A) and with deployment device (B). Insertion of the VL into proximal arterial end (C) and completed anastomosis (D).
Methods
Overview
This study was approved by the Institutional Animal Care and Use Committee at the Uniformed Services University (USU), Bethesda, Maryland. This study was conducted by the Battlefield Shock and Organ Support Program at USU, Bethesda, Maryland. A Cooperative Research and Development Agreement was completed between USU, The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc, and Johns Hopkins University. All animal care and use were in strict compliance with the Guide for the Care and Use of Laboratory Animals, and the National Institutes of Health guide for the care and use of Laboratory Animals (NIH Publications No. 8023, revised 1978) in a facility accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International.
Study design
The study design was a prospective translational swine model with 1 pilot group comprised of 10 device insertions. All animals were subjected to the same preparation and instrumentation, baseline evaluation, hemodilution, vascular injury, device implantation, and critical care phases. Arterial lab samples were completed following preoperative set-up, after hemodilution, and at the completion of the 90 min dwell-time (ABL 800 FLEX; 201 Radiometer America, Brea, California).
Animal preparation
Anesthetic
Healthy adult, nonpregnant female Yorkshire-cross swine, (Sus scrofa) (44.1 kg ± 1.2 kg), were obtained from Animal Biotech Industries Inc (Dolyestown, Pennsylvania). They were allowed to acclimate for at least 3 d while housed at the USU animal vivarium. Animals were induced with 6 mg/kg of tiletamine/zolazepam (Telazol, Fort Dodge Animal Health, Fort Dodge, Iowa) intramuscularly, and subsequently intubated, and maintained with 1%−5% isoflurane. All animals were mechanically ventilated with tidal volumes of 7–10 mL per kilogram (mL/kg) and a respiratory rate of 10–15 breaths per minute sufficient to maintain end-tidal carbon dioxide (CO2) at 40 ± 5 mmHg. Swine were placed on a warming blanket to maintain temperature >36.7–39.2°C. Bilateral peripheral intravenous (IV) access was established on each ear, and IV fluids initiated at a rate of 5–10 mL/kg/h. Lidocaine infusion (20 mcg/kg/h) was initiated to prevent tachyarrhythmias.
Surgical preparation and instrumentation
The bilateral carotid arteries were accessed either via percutaneous or open cut down with a 7 French (Fr) arterial sheath placed on the right and a 5 Fr arterial sheath on the left. The 7 Fr sheath facilitated placement of a Solid-State Pressure Catheter (Transonic Systems Inc, Ithaca, New York) for measuring proximal arterial blood pressure and for laboratory draws throughout the experiment. A limited, lower midline laparotomy was performed for open cystostomy, and abdominal domain was then restored using clamps. Common femoral arterial exposure was completed through a standard groin incision.8 The common femoral artery was circumferentially isolated for several centimeters to facilitate creation of a femoral artery transection injury. Lastly, two 4 mm (mm) Basic Vascular Flowprobes (Transonic Systems Inc) were placed around the proximal and distal common femoral artery to allow for continuous flow monitoring. The lidocaine infusion was discontinued prior to hemodilution.
The animals underwent hemodilution via a 50%−60% exchange transfusion of 6% hetastarch in lactated electrolyte solution to induce acidosis and coagulopathy. The target hematocrit was 15% and rotational thromboelastometry (ROTEM, intrinsic thromboelastometry [INTEM]) was obtained to assess and ensure adequate coagulopathy. Baseline physiologic parameters including mean arterial pressure (MAP), heart rate, and temperature were recorded prior to and following hemodilution.
Common femoral artery transection and VasoLock device placement
Following hemodilution and baseline measurements, proximal and distal control of the femoral artery was obtained and the vessel was transected. A 3.5 mm outer diameter VL device was inserted in a nonbeveled artery to restore arterial continuity allowing distal perfusion without hand-sewn sutured anastomosis. As the primary goal of this pilot study was to assess patency, the device was secured after arterial insertion with an externally positioned silk suture. Following implant, blood flow was confirmed using continuous wave Doppler signal evaluation.
Critical care phase
Upon initiating the critical care phase, all animals were administered 500 mL (mL) hetastarch bolus then continued on 5–10 mL/kg/h of normal saline for maintenance fluids. Physiologic and hemodynamic parameters were continuously measured using PowerLab data acquisition system (AD Instruments, Colorado Springs, Colorado). Phenylephrine boluses were used to supplement blood pressure to maintain a MAP greater than 50 mmHg. Of note, no blood products were administered throughout the protocol. A 90-min critical care phase was observed. Predevice and postdevice arterial flow rates were measured continuously for a total of 90 min after VL implantation.
Arterial flow assessment
Device flow assessment was completed utilizing two different modalitiese–flow probe assessment and angiography. The flow probe data was analyzed and presented as a ratio of distal to proximal flow with the slope of this ratio across the 90 min. Angiographic assessment was completed at the conclusion of the 90 min via the carotid artery sheath with catheter selection of the ipsilateral external iliac artery to evaluate for patency and technical complications. The contrast median used for angiography was Iodixanol (320 mg Iodine per mL).
Euthanasia
At the completion of the critical care phase, animals were humanely euthanized using an overdose of pentobarbital-based euthanasia solution (100 mg/kg) consistent with the 2020 American Veterinary Medical Association (AVMA) Guidelines for the Euthanasia of Animals.9
Data analysis
The primary outcome of the study was VL device patency over the observed period. We also assessed technical complications associated with implant including device migration/dislodgement, dissection, arterial extravasation, or thromboembolism. The gradient of flow was calculated as a ratio of proximal to distal and plotted using GraphPad Prism, version 9.2 (San Diego). Normality was assessed with Shapiroe–Wilk testing, and data are presented as mean ± standard deviation, or (where noted) median and interquartile range, as appropriate.
Results
Baseline characteristics
A total of 7 animals were utilized and 10 VL were implanted in the femoral arteries. The baseline characteristics of the animals following hemodilution is shown in Table and demonstrated no difference between animals. Following hemodilution, shock was confirmed with an average reduced MAP (51.2 ± 7.8 mmHg) and acidosis (pH 7.20 ± 0.1). They were also shown to be coagulopathic with a decreased alpha angle, prolonged clot formation time and decreased maximum clot firmness more than two standard deviations out of the accepted references.10
Table –
Initial hemodynamic measurements at T0 of implant and INTEM values following hemodilution.
| Specimen number | Weight (kg) | Hematocrit (%) | Mean arterial pressure (MAP) | Heart rate | Temperature (Celsius) | pH | Clotting time (CT, s) | Alpha angle | Clot formation time (CFT, s) | A10 (mm) | Maximum clot firmness (MCF, mm) | Maximum lysis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 44 | 17.8 | 52.0 | 77.2 | 34.1 | 7.10 | 235 | 70 | 101 | 52 | 55 | 1 |
| 2 | 40 | 15.4 | 62.3 | 77.3 | 35.7 | 7.21 | 211 | 69 | 109 | 43 | 46 | 0 |
| 3 | 44 | 8.2 | 50.9 | 77.4 | 35.7 | 7.23 | 125 | 72 | 88 | 54 | 56 | 5 |
| 4 | 40 | 12.7 | 39.0 | 105.7 | 36.7 | 7.17 | 167 | 66 | 121 | 42 | 46 | 1 |
| 5 | 48 | 14.6 | 55.4 | 77.3 | 34.7 | 7.22 | 157 | 71 | 104 | 45 | 48 | 1 |
| 6 | 48 | 13.0 | 54.9 | 157.4 | 37.3 | 7.22 | 59 | 72 | 93 | 60 | 66 | 0 |
| 7 | 45 | 12.9 | 43.7 | 102.0 | 38.2 | 7.26 | 125 | 70 | 108 | 57 | 61 | 0 |
| Overall | 44.1 ± 3.2 | 13.5 ± 3.0 | 51.2 ± 7.8 | 77.4 (77.3–157.4)* | 36.1 ± 1.5 | 7.20 ± 0.1 | 154.1 ± 58.7 | 70 ± 2.1 | 103.4 ± 10.9 | 50.4 ± 7.1 | 54.0 ± 7.8 | 1 (0–1)* |
INTEM = intrinsic thromboelastometry; IQR = interquartile range; T0 = time 0.
Median (IQR).
Flow data
The flow rates for proximal and distal flow were plotted for each animal with the average above and below flow for all 10 implanted VL devices shown in Figure 2. The flow gradient, or ratio of proximal to distal flow, was then calculated for each animal for each time point and plotted for the duration of the experiment (Fig. 3). The overall flow gradient across all 10 devices was determined was 0.99 ± 0.24. The combined gradients of all implanted VL were analyzed with the mean gradient for the 90 min shown in Figure 4. A simple linear regression was performed and the slope of this line was 0.0005 (P = 0.22).
Fig. 2 –
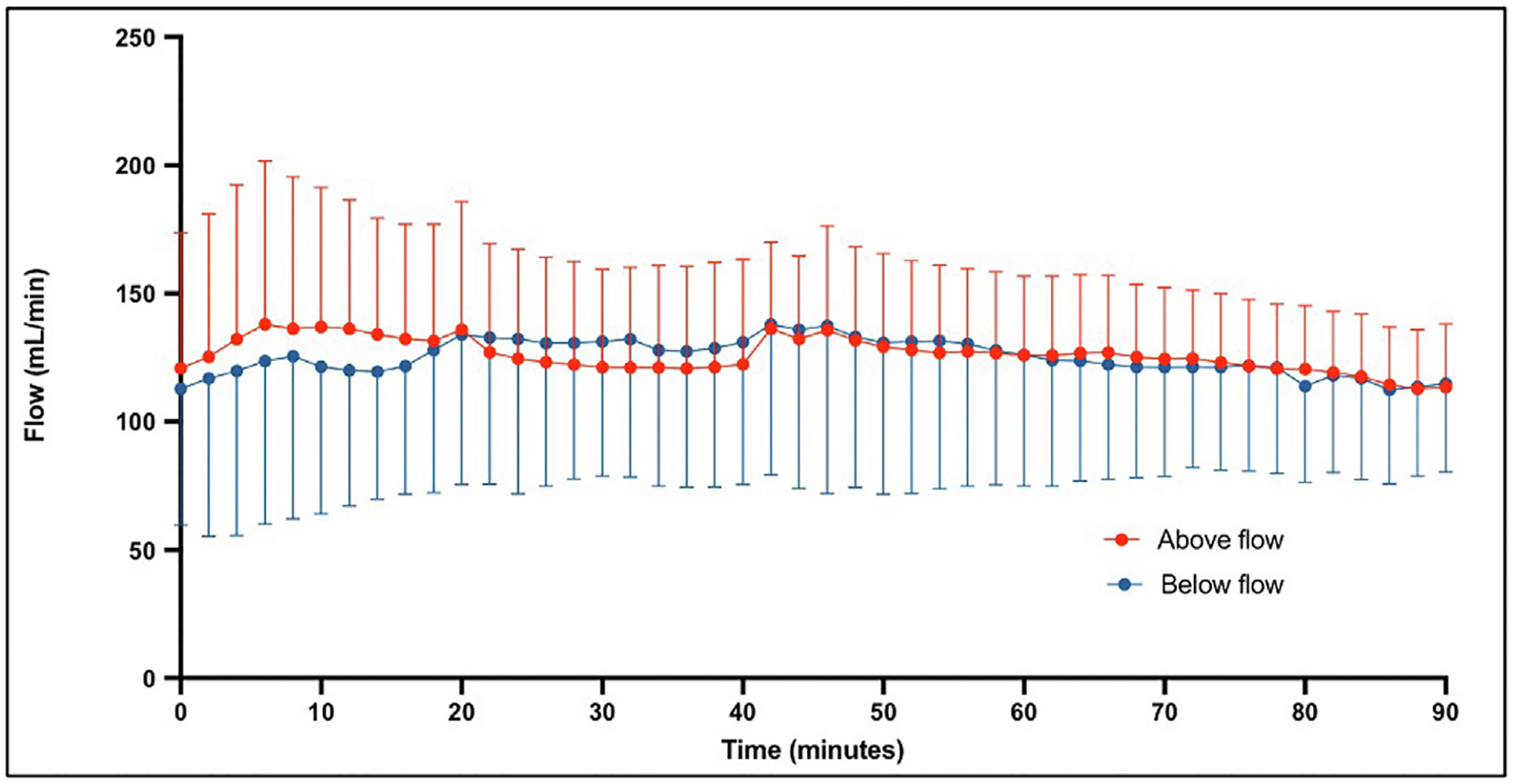
Average above flow (red) and below flow (blue) for all 10 implanted VasoLock.
Fig. 3 –
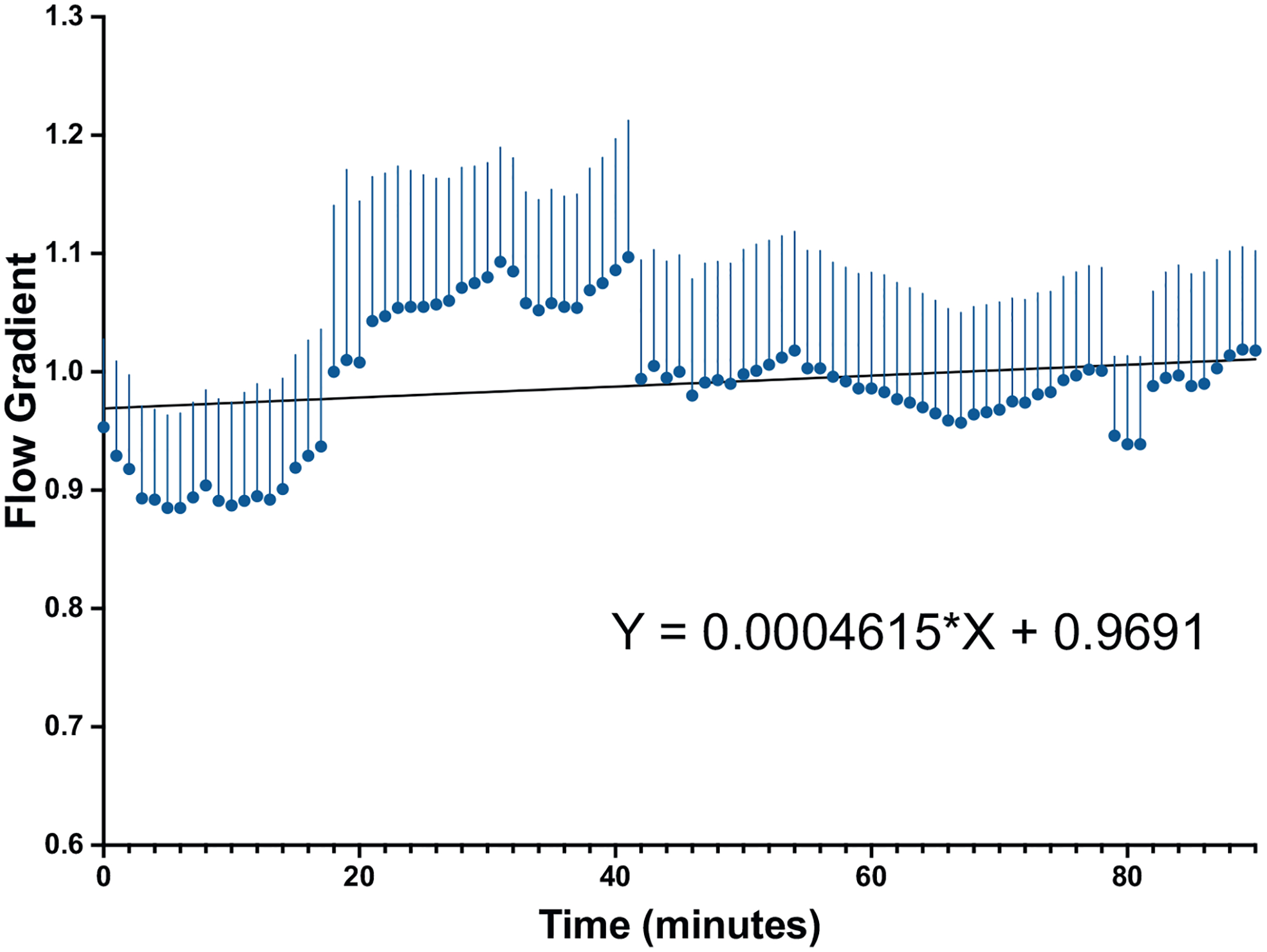
Mean proximal to distal gradient across the VasoLock device over a 90-min time observation period with linear regression.
Fig. 4 –
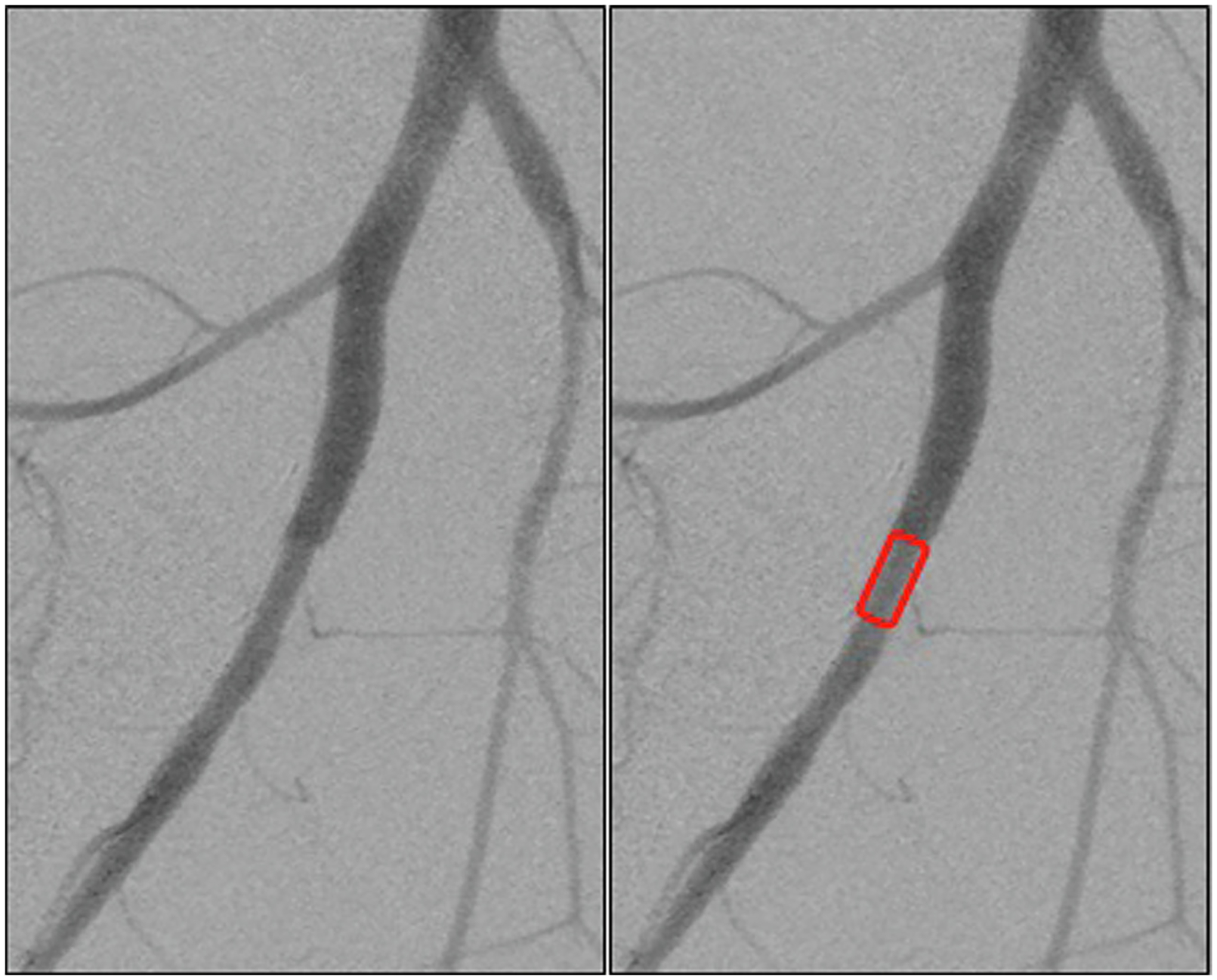
Completion angiogram demonstrating normal flow through the VasoLock device without evidence of complication from implantation. Device location outlined in red box.
Patency and angiographic confirmation
At the conclusion of the 90-min dwell time, angiographic assessment was completed to evaluate for device patency and technical complications (Fig. 4). All 10 of the implanted VL devices were confirmed to have distal flow to the extremity. There was no evidence of device migration or dislodgment, extravasation of contrast, dissection of the native vessel, or thromboembolism for any of the devices. Additionally, the limbs all appeared to be clinically well-perfused with no evidence of temperature changes or mottling.
Discussion
This pilot study aimed to assess the technical feasibility of the novel VL device for establishing distal perfusion following a femoral arterial injury in a swine model of coagulopathic, hemorrhagic shock. The VL device demonstrated and delivered technical success and feasibility and allowed for prompt restoration of flow. Importantly, all devices implanted in this pilot remained patent and without significant complications, including arterial extravasation, arterial dissection, arterial thrombosis or embolization, device thrombosis, or device migration/dislodgement throughout a 90-min postinsertion observation period. Furthermore, the materials and geometric design of the VL device offers surgeons with a potential alternative to the traditional hand-sewn anastomosis, which could potentially be utilized across several specialties of surgery. Lastly, this unique device design demonstrated early promise at a relatively small device diameter and represents significant potential for early reconstruction in the setting of arterial trauma.
The VL device is a new alternative to a traditional hand-sewn vascular anastomosis. While a number of other vascular anastomosis devices have been previously proposed, none of them include entirely sutureless reconstruction using an intraluminal design that can be applied to arterial applications.11 VL was developed using an iterative design process to combine clinical and bioengineering considerations to optimize anchor geometry and orientation, and device anastomosis performance with end-user usability feedback. A custom accompanying deployment device, which both dilates and inserts the VL into the vessel intraluminally (Fig. 1) was developed in parallel to ensure a consistent and timely anastomosis using the VL. The VL is comprised of synthetic thermoplastic polymer polyetheretherketone (Fig. 1) and exerts mechanical frictional forces onto surrounding elastic tissues. Polyetheretherketone is a moldable, nonabsorbable implantgrade material with antithrombotic properties. A durable, nonabsorbable material was chosen given the potential risk for distal embolization during material absorption and breakdown for absorbable products which would introduce ambiguity with long-term patency outcomes. The integral traction anchor design of the VL device generations frictional forces and the anchors take advantage of the elasticity of the vessels to approximate vessels together with a tight seal, while minimizing tissue trauma or penetration. Given the less compliant nature of arteries, extraluminal devices have difficulty with seating and obtaining seal which is why an intraluminal design was chosen. The VL was not studied in veins and as a result, is currently indicated for arterial use only as the device exploits the elasticity of the arterial wall to establish device fixation. Patent protection on the VL device is currently pending as PCT/US2021/040749.
With respect to technical feasibility, all 10 VL devices were inserted into the femoral artery without procedural difficulty and remained patent for the 90-min dwell time. There was no technical complication following device implant given the normal postdeployment angiography (Fig. 4). The angiograms demonstrated no evidence of thrombosis, occlusion, or dissection. The expected flow gradient, or ratio of proximal to distal flow, across an artery without an injury or hemodynamically significant stenosis should be 1. The VL device demonstrated an average flow gradient of 1 indicating no significant change in flow across the device. Additionally, the expected slope of the mean gradient for a noninjured artery should be 0, representing the absence of change in flow. The slope of the mean gradient across the VL device was 0.0005, indicating that there was no change in flow during the 90-min device dwell time. These promising results further suggest that distal perfusion was maintained at near normal flow while the device was implanted. Furthermore, there was no loss of flow inherent to the device or due to complications. The device also demonstrated patency during considerable variability in blood pressure related to the dynamic nature and physiology of the testing model used. Even in this dynamic setting, the VL device maintained patency without thrombosis during low-flow states.
The VL device attempts to overcome potential limitations of inadequate resources or surgeon experience by minimizing time to definitive restoration of blood flow to the injured extremity, as well as making implant easy to use with minimal technical expertise required. Specifically, new users can place the device with minimal training and still avoid major adverse problems in the acute setting. While we are not able to present data on time required for implantation, in general this process took less than 3 min. Future studies will quantify how skill level effect length of time required for implant. Temporary intravascular shunts (TIVS) have been described as a bridge to definitive repair of vascular injuries.12–15 Studies from both military and civilian cohorts have shown the benefit of early restoration of arterial flow and its impact on the opportunity for successful limb salvage.4,6,16 In the trauma patient, TIVS also allow for concomitant management of other injuries or physiologic abnormalities without requiring time-consuming, definitive vascular repair. While there are several commercially available shunts in the market including, ones specifically designed for trauma patients,17 TIVS have shown poor patency in the distal arterial beds with patency rates as low as 12% in the tibial vessels.1 While the VL device showed promise in vessels of a similar size to adult human tibial arteries, a direct comparison to TIVS at a smaller vessel size range would be required in order to establish a more definitive characterization. The implanted VL device was 3.5 mm and demonstrated reliable patency at this size during the pilot investigation. A reliable device that allows surgeons an alternative to the technically demanding hand-sewn anastomosis has specific viability and benefit to the military in the austere, far-forward deployed setting where early establishment of arterial perfusion following traumatic injury is imperative.
Given that this is the first study utilizing the VL device, there are inherent limitations. While 100% of the VL was patent during the study period, a 90 min dwell time is relatively short. The data we present here support the feasibility for performing arterial injury anastomoses in a trauma model. Long-term performance studies are ongoing to simulate permanent placement before the human applications can be considered. These include histologic evaluation of the artery and distal muscle beds to assess for ischemic changes as well as any potential intimal injury from the traction anchors. Additionally, this experiment was completed in a trauma-specific model with acidotic and coagulopathic animals. While coagulopathy in trauma patients is common, these conditions are not universal and thus the application of the VL device in other patient populations is unclear. Further testing under normal hematologic conditions is needed to assess patency for other uses. Additional investigation would be required to demonstrate the need for antiplatelet or anticoagulation to maintain patency as well as infectious outcomes particularly in traumatic wounds. As with any vascular anastomosis, size match is imperative in order to maximize device patency and limit turbulence. Currently the device is only sized 3 mm to 6 mm which may limit its arterial targets and the investigators are planning future designs suitable for end-to-side reconstruction. Despite these limitations, the VL device demonstrated early technical promise and offers a unique alternative to the traditional end-to-end, handsewn anastomosis.
Conclusions
This study demonstrates the technical feasibility of the VL device for repair of extremity vascular injury and restoration of distal perfusion over a 90 min period. There was no significant change in flow across the device and flows remained constant for the duration of the experiment. This early evaluation of the VL shows that it can be used to quickly reestablish arterial flow with encouraging patency rates and no observed complications. The results warrant additional study over a longer duration.
Funding
The research was partially supported by the Johns Hopkins University Cohen Translational Engineering Fund and the Bisciotti Foundation Translational Fund and the Maryland Innovation Initiative.
Footnotes
Disclosure
The opinions and assertions expressed herein are those of the author(s) and do not necessarily reflect the official policy or position of the USU or the Department of Defense or The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. Mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. Government. David Stonko is an SEC registered investment advisor at Catalio Capital Management, LP, which is a biotechnology focused investment firm. This firm is invested in several medical device companies but none are related to this work. The authors have no financial interest related to the conduct of this research or the products tested or discussed during the project. In conducting research using animals, the investigators adhere to the laws of the United States and regulations of the Department of Agriculture.
Meeting Presentation
This abstract was presented at the 17th Annual Academic Surgical Congress (ASC) in Orlando, Florida, February 1–3, 2022.
REFERENCES
- 1.Rasmussen TE, Clouse WD, Jenkins DH, Peck MA, Eliason JL, Smith DL. The use of temporary vascular shunts as a damage control adjunct in the management of wartime vascular injury. J Trauma. 2006;61:8–12. discussion 12–5. [DOI] [PubMed] [Google Scholar]
- 2.Rasmussen TE, Clouse WD, Jenkins DH, Peck MA, Eliason JL, Smith DL. Echelons of care and the management of wartime vascular injury: a report from the 332nd EMDG/Air Force Theater Hospital, Balad Air Base, Iraq. Perspect Vasc Surg Endovasc Ther. 2006;18:91–99. [DOI] [PubMed] [Google Scholar]
- 3.White JM, Stannard A, Burkhardt GE, Eastridge BJ, Blackbourne LH, Rasmussen TE. The epidemiology of vascular injury in the wars in Iraq and Afghanistan. Ann Surg. 2011;253:1184–1189. [DOI] [PubMed] [Google Scholar]
- 4.Inaba K, Aksoy H, Seamon MJ, et al. Multicenter evaluation of temporary intravascular shunt use in vascular trauma. J Trauma Acute Care Surg. 2016;80:359–364. discussion 364–5. [DOI] [PubMed] [Google Scholar]
- 5.Patel JA, White JM, White PW, Rich NM, Rasmussen TE. A contemporary, 7-year analysis of vascular injury from the war in Afghanistan. J Vasc Surg. 2018;68:1872–1879. [DOI] [PubMed] [Google Scholar]
- 6.Polcz JE, White JM, Ronaldi AE, et al. Temporary intravascular shunt use improves early limb salvage after extremity vascular injury. J Vasc Surg. 2021;73:1304–1313. [DOI] [PubMed] [Google Scholar]
- 7.Alarhayem AQ, Cohn SM, Cantu-Nunez O, Eastridge BJ, Rasmussen TE. Impact of time to repair on outcomes in patients with lower extremity arterial injuries. J Vasc Surg. 2019;69:1519–1523. [DOI] [PubMed] [Google Scholar]
- 8.Edwards J, Abdou H, Patel N, et al. The functional vascular anatomy of the swine for research. Vascular. 2022;30:392–402. [DOI] [PubMed] [Google Scholar]
- 9.Underwood W, Anthony R. AVMA guidelines for the euthanasia of animals: 2020 edition. Retrieved March. 2020;2013:2020–2021. [Google Scholar]
- 10.Hoareau GL, Barthélemy A, Goy-Thollot I, et al. Reference intervals for and the effects of sample handling and sex on rotational thromboelastometry in healthy adult pigs. J Am Assoc Lab Anim Sci. 2020;59:322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mallela DP, Bose S, Shallal CC, et al. A systematic review of sutureless vascular anastomosis technologies. Semin Vasc Surg. 2021;34:247–259. [DOI] [PubMed] [Google Scholar]
- 12.Hancock H, Rasmussen TE, Walker AJ, Rich NM. History of temporary intravascular shunts in the management of vascular injury. J Vasc Surg. 2010;52:1405–1409. [DOI] [PubMed] [Google Scholar]
- 13.Eger M, Golcman L, Goldstein A, Hirsch M. The use of a temporary shunt in the management of arterial vascular injuries. Surg Gynecol Obstet. 1971;132:67–70. [PubMed] [Google Scholar]
- 14.Feliciano DV, Subramanian A. Temporary vascular shunts. Eur J Trauma Emerg Surg. 2013;39:553–560. [DOI] [PubMed] [Google Scholar]
- 15.Chambers LW, Green DJ, Sample K, et al. Tactical surgical intervention with temporary shunting of peripheral vascular trauma sustained during Operation Iraqi Freedom: one unit’s experience. J Trauma. 2006;61:824–830. [DOI] [PubMed] [Google Scholar]
- 16.Subramanian A, Vercruysse G, Dente C, Wyrzykowski A, King E, Feliciano DV. A decade’s experience with temporary intravascular shunts at a civilian level I trauma center. J Trauma. 2008;65:316–324. discussion 324–6. [DOI] [PubMed] [Google Scholar]
- 17.Stigall KS, Sleeter JJ, Thomas SB, et al. Performance of a novel temporary arterial shunt in a military-relevant controlled hemorrhage swine model. J Trauma Acute Care Surg. 2021;91:S74–S80. [DOI] [PubMed] [Google Scholar]


