Abstract
Aminoacyl-tRNA synthetases (aaRSs) are ancient enzymes that serve a foundational role in the efficient and accurate translation of genetic information from messenger RNA to proteins. These proteins play critical, non-canonical functions in a multitude of cellular processes. Multiple viruses are known to hijack the functions of aaRSs for proviral outcomes, while cells modify antiviral responses through non-canonical functions of certain synthetases. Recent findings have revealed that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the etiological agent of coronaviral disease 19 (COVID-19), utilizes canonical and non-canonical functions of aaRSs, establishing a complex interplay of viral proteins, cellular factors and host aaRSs. In a striking example, an unconventional multi-aaRS complex consisting of glutamyl-prolyl-, lysyl-, arginyl- and methionyl-tRNA synthetases interact with a previously unknown RNA-element in the 3′-end of SARS-CoV-2 genomic and subgenomic RNAs. This review aims to highlight the aaRS-SARS-CoV-2 interactions identified to date, with possible implications for the biology of host aaRSs in SARS-CoV-2 infection.
Keywords: aminoacyl-tRNA synthetase, post-transcriptional region, RNA virus, SARS-COV-2, UTR
An introduction to aminoacyl-tRNA synthetases
Aminoacyl-tRNA synthetases (aaRSs) are essential cellular enzymes that provide aminoacylated tRNAs as substrates to ribosomes as genetic information is decoded from messenger RNA to proteins. AaRSs catalyze the aminoacylation reaction in two steps: first, activation of an amino acid (aa) by a condensation reaction with ATP accompanied by release of pyrophosphate followed by esterification of the amino acid with a tRNA, and release of AMP [1–3]. AaRS-catalyzed tRNA-charging serves two purposes — (i) the amino acid remains energetically activated, i.e. its subsequent condensation with another amino acid in a peptide chain does not require additional energy input, and (ii) more importantly, provides the information required to recognize the mRNA template that determines sequence specificity. Each aaRS specifically recognizes its corresponding amino acid and cognate tRNA. Specific structural features and nucleotide sequences in tRNA isoacceptor molecules (tRNAaa), the operational RNA code [4,5], as well as identity of the amino acids (aa) guide precise aminoacylation to generate the aa-tRNAaa. In addition to anticodon binding and catalysis, several aaRSs have cis-editing domains that recognize and correct mischarging on non-cognate tRNAs [6]. Proteins that lack a tRNA-charging function but otherwise resemble aaRS editing domains can proofread mischarged aminoacylated tRNAs by trans-editing [7]. There are 20 aaRSs for the 20 amino acids enshrined in the genetic code. With some degree of evolutionary mosaicism selenocysteine (Sec), the 21st proteinogenic amino acid, is present in the three domains of life (eubacteria, archaea, and eukarya), and is generated from serine on seryl-tRNASec, after tRNASec is charged with seryl-tRNA synthetase [8,9]. Certain methanogenic archaea possess an expanded genetic code that includes the 22nd proteinogenic amino acid pyrrolysine (Pyl) [10,11], that is charged to tRNAPyl by pyrrolysyl-tRNA synthetase [12].
The endosymbiotic origin of mitochondria in eukaryogenesis [13,14] resulted in two sets of operational RNA codes in the same cellular system: one from the host and another from the endosymbionts. Today, mitochondrial DNA encodes 22 tRNAs, while the cognate mitochondrial aaRSs (mt-aaRS) are nuclear DNA-encoded. To distinguish between human cytosolic and mt-aaRS, the standard nomenclature uses 1 or 2 as a suffix for any aaRS protein, e.g. seryl-tRNA synthetase 1 or SARS1 is cytosolic while SARS2 is mt-SARS. As exceptions: (i) human GARS1 gene encodes glycyl-tRNA synthetase that functions in both cytosol and mitochondria [15], (ii) human KARS2 protein is an isoform of KARS1 protein produced from the KARS1 gene [16], (iii) mt-QARS is absent from mammalian genomes; EARS2 misacylates mt-tRNAGln and glutamine is synthesized on Glu-mt-tRNAGln post-aminoacylation by the hGatCAB amidotransferase [17], and (iv) EPRS1 in complex eukaryotes is a unique, bifunctional aaRS that resides in the cytosol and consists of an N-terminus GluRS domain covalently joined by a linker domain to a C-terminus ProRS domain [18].
Aminoacyl-tRNA synthetases in virus infection
Viruses are obligate, intracellular parasites that carry minimal genetic information compared with its hosts, and thus depend on usurping host cellular resources. Most viruses do not encumber their genomes with aaRSs, instead they rely on host aaRSs and other host proteins and complexes that enable mRNA translation, e.g. ribosomes. Exceptions include several giant DNA viruses with protozoan hosts that encode a single aaRS or a small complement of aaRSs, e.g. Cafeteria roenbergensis virus (isoleucyl-tRNA synthetase) [19], Pandoraviruses (tyrosyl and tryptophanyl-tRNA synthetases) [20], Mimiviruses (tyrosyl, methionyl, arginyl and cysteinyl-tRNA synthetases) [21], Moumouviruses (tyrosyl, methionyl, arginyl, cysteinyl and isoleucyl-tRNA synthetases) [22] and Megavirus chilensis (tyrosyl, methionyl, arginyl, cysteinyl, isoleucyl, tryptophanyl and asparginyl-tRNA synthetases) [23], while Tupanviruses encode all 20 aaRSs [24]. Host aaRS aminoacylation activity can be hijacked by 3′-localized tRNA-like structures (TLS) that control replication of plant RNA viruses [25,26]. The 3′-TLS in ∼30 plant viruses are aminoacylated by YARS1 (e.g. brome mosaic virus, BMV), HARS1 (e.g. tobacco mosaic virus), or VARS1 (e.g. turnip yellow mosaic virus), and tRNA mimicry is essential for plant virus replication and gene expression [27]. The BMV tRNA mimic undergoes a conformational rearrangement, binding YARS1 in a structural form that differs dramatically from tRNA [28], exemplifying the dynamics of viral RNA structures in binding host machinery. In a second case of tRNA mimicry, domain V of the internal ribosome entry site (IRES) in poliovirus genome resembles tRNAGly, and binds GARS1 to activate translation–initiation [29].
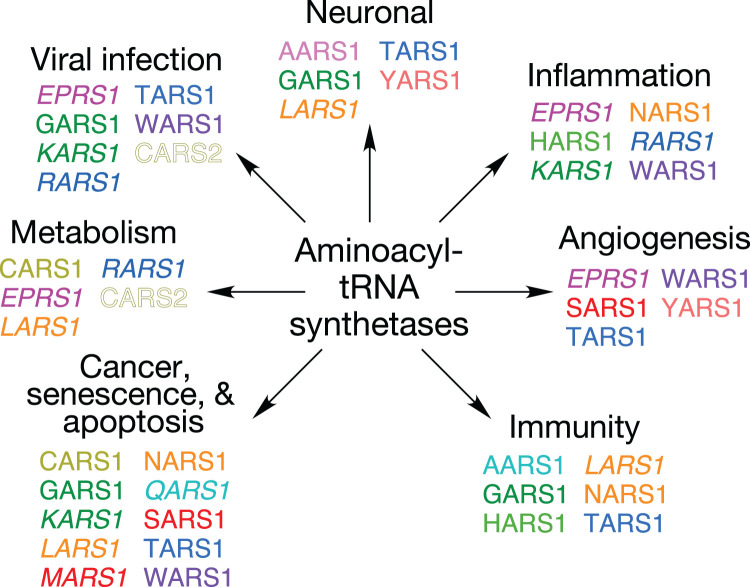
Many mammalian aaRSs exhibit non-canonical functions distinct from their ancient, primary function of tRNA aminoacylation (Figure 1). These moonlighting, or ‘ex-translational', activities generally depend on domains appended in evolution [30]. These functions are regulated by post-translational modifications [31–36] or by targeted cleavage [37–40]. These modified aaRS can act as cytokines, apoptosis and angiogenesis regulators, and non-enzymatic regulators of translation, with the potential to integrate genetic and environmental responses [41–43]. In mammalian cells, nine of the 20 cytoplasmic aaRS functions (in 8 proteins since EPRS1 is bifunctional) and 3 auxiliary proteins, AIMPs (aminoacyl-tRNA synthetase interacting multifunctional proteins) 1, 2, and 3, reside in a multi-tRNA synthetase complex (MSC), of uncertain function. The observation of MSC binding to ribosomes has led to the hypothesis that MSC ‘channeling' of charged tRNAs to ribosomes improves mRNA translation efficiency [44]. This concept is challenged by reports that global translation is not reduced when the majority of EPRS1, or all of RARS1 and QARS1, are excluded from the MSC [41,45]. Alternatively, the MSC might sequester aaRSs to reduce injurious cell activities of the free proteins, while permitting cue-dependent release of specific aaRSs for non-canonical functions [41,46].
Figure 1. Non-canonical functions of aminoacyl-tRNA synthetases.
Aminoacyl-tRNA synthetases are color-coded to highlight multiple functions of same protein. MSC-resident (italics), non-MSC cytoplasmic (plain font), and mitochondrial (outline font) synthetases are indicated.
Importantly, several aaRSs exhibit critical non-canonical, host-viral interactions, and consequent activities, as follows: infection-dependent release of EPRS1 from the MSC sequesters poly(rC)-binding protein 2 (PCBP2) and protects MAVS, an antiviral mitochondrial signaling molecule, from PCBP2-mediated ubiquitination in influenza A virus-infected cells [47]. EPRS1 and RARS1 bind an RNA element in porcine transmissible gastroenteritis coronavirus (TGEV), thereby facilitating innate immune evasion of viral RNA genome [48]. Human immunodeficiency virus type 1 (HIV-1) virions are packaged with tRNALys3 by KARS1, that is incorporated into the virus following regulated release from the MSC [49,50]. The HIV-1 genome contains a tRNA-like element (TLE) that acts as molecular mimic of tRNALys and aids tRNALys3 primer annealing by recruitment of KARS1 [51]. HIV-1 gag protein forms a stable complex with the MSC through a tRNA-dependent interaction with the EPRS1 linker domain, without specificity of the tRNA utilized [52].
Non-MSC, cytoplasmic aaRSs and mitochondrial KARS2 contribute to viral life cycles [53]. Similarly, WARS1 is an interferon (IFN)-γ-inducible mediator of enterovirus, e.g. EV-A71, cell entry, and a cell type-specific restriction factor [54]. In contrast, immune cells infected with vesicular stomatitis virus (VSV) or herpes simplex virus (HSV) secrete WARS1 that functions as an antiviral cytokine, promoting the production of inflammatory cytokines and type-I IFNs to suppress virus replication [55]. Likewise, TARS1 is secreted from vascular endothelial cells in response to tumor necrosis factor (TNF)-α and sculpts a T-helper-1 response for clearing H1N1 influenza A virus infection [56].
SARS-CoV-2 and the COVID-19 pandemic
During the last 4 years, coronaviral disease-19 (COVID-19) has upended lives with levels of mortality and morbidity unprecedented in recent history [57–60]. COVID-19 is the first documented coronavirus pandemic in human history [61], instigating widespread public discourse on pharmaceutical and non-pharmaceutical interventions [62–64]. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative agent of COVID-19, and the seventh coronavirus known to cause a human disease [65,66]. SARS-CoV-2 is an enveloped betacoronavirus with positive, single-stranded genomic RNA, closely related to SARS-CoV-1 and to bat sarbecoviruses [67]. Serious cases of COVID-19 exhibit acute respiratory distress syndrome (ARDS) [68] and can be lethal, particularly if exacerbated by co-morbidities [69]. Extended ramifications of the disease manifest as ‘long COVID' that remains incompletely understood or even phenotyped [70–73]. Possibly, waves of SARS-CoV-2 infection in the COVID-19 pandemic has reduced genetic diversity of other respiratory viruses, such as respiratory syncytial virus (RSV) and influenza A virus, through bottleneck effects, thereby re-shaping future disease outbreaks [74]. Based on adaptive functions of other viruses, it is not surprising that SARS-CoV-2 took advantage of host systems, including piggybacking on cellular aaRSs.
Mitochondrial aaRSs as mediators of SARS-CoV-2 infection
To evaluate the roles of aaRSs on SARS-CoV-2 infection and pathogenesis, a meta-analysis summarized findings from multiple datasets [75]. A graded cell survival analysis was done by genome-wide CRISPR screening of Vero-E6 cells following infection with SARS-CoV-2 and other coronaviruses (CoVs) [76]. Disruption of 14 mitochondrial aaRSs (AARS2, DARS2, EARS2, FARS2, HARS2, LARS2, MARS2, NARS2, PARS2, RARS2, SARS2, TARS2, VARS2, and YARS2) sensitized cells to cell death following infection by SARS-CoV-2, suggesting antiviral activity of the aaRSs. PARS2 and EARS2 appear to exert pan-coronavirus antiviral activity as the screen showed sensitization to cell death upon infections with SARS-CoV-2, HKU5-SARSCoV-1-S, and MERS-CoV. EARS2 was further implicated in cellular entry of SARS-CoV-2. Mitochondrial aaRSs appeared to be more effective than cytosolic aaRSs as antiviral effectors/modulators following SARS-CoV-2 infection [75].
SARS-CoV-2 initiates a global shutdown of splicing, mRNA nuclear export, and translation [77]. Consistent with this repression, down-regulation of mRNAs encoding cytosolic and mitochondrial aaRSs was observed in transcriptomic studies of bronchoalveolar lavage fluid (BALF) [78] and post-mortem lung tissues [79] from COVID-19 patients, and from SARS-CoV-2-infected A549-hACE2 (human angiotensin-converting enzyme) [79] and human bronchial epithelial (NHBE) cells [80]. Likewise, low levels of many cytosolic aaRS proteins were observed in proteomic analyses of BALF [81], peripheral blood mononuclear cells (PBMCs) [82], and liver cells [83] from COVID-19 patients, and from SARS-CoV-2-infected human Caco-2 cells [84]. Many mt-aaRS proteins are expressed at high levels in liver cells from COVID-19 patients, but the levels of mt-aaRS mRNAs were not reported. The phenomenon seems to be generalizable as multiple mitochondrial ribosomal proteins are also expressed at high levels in liver cells from COVID-19 patients, likely reflecting increased mitochondrial translation. Phosphoproteomic analysis of Vero-E6 cells infected with SARS-CoV-2 revealed phosphorylation of CARS1, TARS2, and HARS2 [85].
Meta-analysis of proteomic studies sub-divided coronaviral protein-interacting aaRSs into three classes, namely, down-regulated mt-aaRSs, down-regulated cyto-aaRSs, and up-regulated mt-aaRSs [75]. Network analysis of aaRSs implicated EARS2, IARS1, IARS2, and TARS1 as mediators between first responders, i.e. SARS-CoV-2-interacting proteins and late-stage effectors. SARS-CoV-2 membrane protein (M) interacts with TARS2 in HEK293T cells [75], but the physiological significance is unknown. While the analysis implicates mt-aaRSs, particularly HARS2, EARS2, and TARS2 in SARS-COV-2 infection severity, the consequences of differential aaRS expression remain unclear. Whether such changes benefit the virus or the host cell is unclear, but would differentially implicate either proviral or antiviral outcomes. Multiple recent studies [86–108] have expanded the aaRS-interaction datasets beyond those in this meta-analysis; interactions of cellular aaRSs and SARS-CoV-2 proteins based on BioGrid repository are enumerated as in Table 1.
Table 1. Putative interactions between host aminoacyl-tRNA synthetases and SARS-CoV-2 proteins curated from the BioGrid database [143].
| AaRS | SARS-CoV-2 non-structural proteins | SARS-CoV-2 structural proteins | References | |
|---|---|---|---|---|
| AARS1 | NSP10, NSP11, NSP16 | S, ORF9B, ORF10 | [82, 87–89, 106] | |
| CARS1 | - | - | ||
| DARS1 | NSP4, NSP5, NSP6, NSP12, NSP13, NSP14 | S, ORF3A, ORF3B, E, M, ORF6, ORF7A, ORF7B, ORF8 | [90, 104, 106] | |
| EPRS1 | NSP5, NSP12 | ORF8, ORF14 (ORF9C) | [90, 102–104] | |
| FARSA | NSP2 | ORF10 | [88, 92] | |
| FARSB | - | ORF8, N | [103] | |
| GARS | - | - | ||
| HARS1 | - | - | ||
| IARS1 | NSP5 | - | [90] | |
| KARS1/ KARS2 | NSP5, NSP7, NSP15 | ORF3A, ORF3B, E, M, ORF6, ORF7A, ORF7B, ORF8, N, ORF9B, ORF10 | [87, 90, 91, 105, 106] | |
| LARS1 | - | - | ||
| MARS1 | NSP1, NSP5 | ORF14 (ORF9C) | [90, 94, 102] | |
| NARS1 | NSP2, NSP4, NSP6 | ORF3A, M, ORF7B, ORF10 | [87, 106] | |
| QARS1 | NSP4 | S, ORF6, ORF7B, ORF8, ORF9B, ORF10 | [87, 106] | |
| RARS1 | - | N | [91] | |
| SARS1 | NSP15 | N | [87, 105] | |
| TARS1 | NSP2, NSP4 | S, ORF7B, ORF8, N, ORF10 | [87, 93] | |
| VARS1 | NSP2 | S, ORF8, ORF10 | [88, 103] | |
| WARS1 | NSP9, NSP15 | N | [93, 103] | |
| YARS1 | NSP4, NSP5 | S, E, M, ORF6, ORF8, N, ORF9B, ORF10 | [87, 90, 91, 106] | |
| AARS2 | NSP5, NSP9 | ORF7B, ORF9B | [90, 95, 105, 106] | |
| CARS2 | - | ORF9B | [106] | |
| DARS2 | NSP4, NSP5 | M, ORF9B | [90, 106] | |
| EARS2 | NSP4 | S, ORF7A, ORF7B, ORF9B, ORF14 (ORF9C) | [105, 106] | |
| FARS2 | - | - | ||
| HARS2 | - | M, ORF9B | [86, 106] | |
| IARS2 | NSP2, NSP3, NSP5, NSP6, NSP9, NSP13, NSP14, NSP16 | S, ORF3A, ORF14 (ORF9C), ORF10 | [87–89, 102] | |
| LARS2 | - | - | ||
| MARS2 | NSP1, NSP14 | - | [93] | |
| NARS2 | NSP8 | M | [86, 96, 97] | |
| PARS2 | - | - | ||
| RARS2 | NSP6, NSP8 | ORF9B | [82, 100, 106, 107] | |
| SARS2 | NSP8 | - | [87] | |
| TARS2 | NSP7, NSP10, NSP12, NSP13 | M, ORF9B | [82, 85, 96, 97, 100, 104, 105, 108] | |
| VARS2 | - | ORF9B | [106] | |
| WARS2 | - | - | ||
| YARS2 | NSP1, NSP4 | - | [87] | |
| Non-AARS, MSC-resident proteins | ||||
| AIMP1 | NSP5 | ORF3B, ORF6, N | [87, 90, 98, 103] | |
| AIMP2 | - | ORF8, N | [103] | |
| AIMP3 | NSP5, NSP13 | ORF3A, ORF9B, ORF10 | [87, 99] | |
All references listed in text.
A ‘post-canonical' protective activity of CARS2
Recently, protective functions of cysteine hydropersulfides and cysteine supersulfides were identified in lung diseases, including viral airway infections such as influenza and COVID-19 [109]. Cysteine hydropersulfides (CysSSH) have been implicated in cellular redox protection. Mitochondrial cysteinyl-tRNA synthetase (CARS2) exhibits cysteine persulfide synthase (CPERS) activity in vivo [110]. CARS2 generates CysS–(S)n–H from cysteine post-aminoacylation through CPERS activity, forming CysS–(S)n–tRNACys from Cys-tRNACys, and catalyzing co-translational protein polysulfidation. Importantly, CPERS-mediated polysulfidation occurs on aminoacylated tRNA, contingent on and following the canonical function of CARS2, thereby revealing a ‘post-canonical' aaRS function. CARS2-derived cysteine hydropersulfides and cysteine supersulfides sustain mitochondrial biogenesis and activity of the electron transport chain [110]. Also, CysSSH is released from the mitochondria into the cytoplasm for production of CysS–(S)n–H and polysulfidation in extra-mitochondrial compartments. Increased SARS-CoV-2 yield was observed in CARS2 knockdown VeroE6/TMPRSS2 cells. Cars2AINK mutant mice possess undiminished cysteinyl-tRNA synthetase activity, but exhibit reduced CPERS activity and low supersulfide production [109]. Homozygous Cars2AINK/AINK mice are embryonic lethal indicating a developmental role of supersulfides. SARS-CoV-2 infection was assessed in Cars2AINK mice crossed with ACE2-transgenic mice, and lethality was significantly exacerbated. Thus, CARS2 has a central role in innate defense functions of supersulfiides, protecting the lung and airways, as well as associated vasculature, against SARS-CoV-2 [109].
ProRS and proline-rich proteins are potential therapeutic targets
Febrifugine, isolated from Dichroa febrifuga in the family Hydrangeaceae, is an alkaloid and active ingredient in a traditional Chinese medicinal herb, chángshān [111,112]. Recognized for its antiprotozoal activity, chángshān extract historically has been used as an anti-malarial treatment [113]. Halofuginone is a halogenated derivative of febrifugine that potently inhibits the differentiation of pro-inflammatory Th17 cells [114]. It binds the ProRS domain of human glutamyl-prolyl-tRNA synthetase (EPRS1), acting as a competitive inhibitor of prolyl-tRNA synthetase activity [115]. Halofuginone has been used in preclinical and clinical studies to treat fibrotic disease and to reduce hyperinflammation [116]. Screening a library of small-molecule antagonists of SARS-CoV-2 spike receptor-binding domain (RBD) interaction with extracellular heparan sulfate in Hep3B human hepatoma cells, halofuginone was identified as a potent hit [117]. The drug reduced RBD binding to Hep3B, Calu-3, and Caco-2 cells, and at low nanomolar amounts inhibited SARS-CoV-2 infection of Hep3B and air–liquid interface cultures of primary human bronchial epithelial cells. Spike binding to cells is heparan sulfate (HS)-dependent [118]. Halofuginone decreased cellular HS synthesis without altering HS-specific sulfation, and reduced expression of core heparan sulfate proteoglycans (HSPGs) [117]. These results established that pre-infection treatment with halofuginone inhibits SARS-CoV-2 entry in these models. Further, post-infection treatment inhibited subsequent viral genome replication in Huh7.5 cell line, chosen for high viral replication due to a RIG-I mutation suppressing innate antiviral signaling [119]), suggesting potential functions beyond cell entry. Extracellular HSPGs are relatively proline-rich and viral polyproteins ORF1a and ORF1ab, as well as spike protein, are likewise proline-rich even compared with proline-rich cellular collagen. The inhibition of ProRS activity by halofuginone suggests that infection-directed synthesis of proline-rich viral and cellular proteins might be an attractive target for design of novel antiviral therapeutics. Consistent with this rationale, two other ProRS inhibitors, i.e. ProSA (a non-hydrolyzable prolyl-AMP analog) and halofuginol, also inhibited infection by SARS-CoV-2 [117].
An unconventional multi-aaRS complex directed by COVID-19 cues
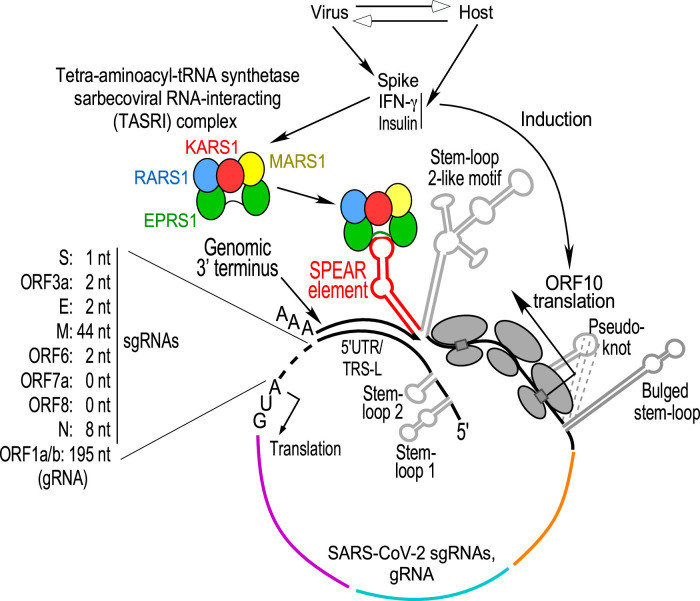
mRNA termini are critical for agonist-driven, post-transcriptional regulons, in which functionally related mRNAs are co-regulated by specific RNA-binding proteins targeting similar sequence or structural elements [120]. The Gamma-interferon-Activated Inhibitor of Translation (GAIT) RNA element in a family of inflammation-related human mRNAs [48,121–124] is targeted by an inducible, heterotetrameric GAIT complex comprised of EPRS1, the direct RNA-interacting constituent, as well as ribosomal protein L13a, heterogenous ribonucleoprotein Q or NSAP1, and GAPDH; the system is a classical archetype of a post-transcriptional regulon. SARS-CoV-2 transcription generates an ensemble of nested 3′-co-terminal subgenomic RNAs (sgRNAs) that contain 5′-leader and 3′-end sequences identical with each other, and to the genomic RNA (gRNA). Upon interrogation of the 3′-end of SARS-CoV-2 for GAIT element-like RNA elements, a novel 39 nt element present in all viral RNAs was described [125]. This cis-element is structurally homologous to the pig alphacoronaviral TGEV (transmissible gastroenteritis virus) GAIT-like element [48]. The RNA sequence is conserved in SARS-CoV-1 and other viruses of the subgenus Sarbecovirus suggesting an invariant function. Insulin and IFN-γ, agents associated with COVID-19 severity and outcome, increase SARS-CoV-2 sgRNA expression and translation contingent upon an intact cis-element; disruption of the proposed secondary structure led to loss of agonist-mediated induction. Maximal reporter activation was observed following co-treatment with spike subunit S1 (the RBD) and IFN-γ in lung and colon cell lines expressing the ACE2 receptor. The newly discovered cis-element was termed Sarbecoviral Pan-End Activating RNA (SPEAR) element.
Beyond its influence on sgRNA reporter expression, an intact SPEAR element was required for maximal −1 programmed ribosomal frameshifting (−1 PRF) efficiency in frameshift-assay reporters bearing SARS-CoV-2 genomic termini, thereby expanding the role of SPEAR. The SPEAR element is rooted in the structurally ‘fluid' 3′-end hypervariable region (HVR) in subgenus Sarbecovirus — a region of weak sequence conservation among different Betacoronavirus subgenera, and therefore this sequence is absent from the subgenera Embecovirus, Nobecovirus and Merbecovirus, while in the monotypic subgenus Hibecovirus, only the sequence corresponding to the SPEAR proximal stem is conserved [125].
GAIT and GAIT-like TGEV RNA elements bind EPRS1, an aaRS that resides in the multi-tRNA synthetase complex (MSC) [41,42,126]. The SPEAR interacts with EPRS1 and NSAP1, but not with RPL13a or GAPDH, in IFN-γ-programmed U937 monocytic cell extracts [125]. Disruption of the SPEAR element by mutation prevents binding of EPRS1, but not NSAP1, further distinguishing the SPEAR-binding and GAIT complexes. More importantly, SPEAR disruption inhibits sgRNA expression and further distinguishes SPEAR function from GAIT function as the former activates and the latter inhibits expression. EPRS1 requirement for SPEAR activation was demonstrated by genetic perturbation in adipocytes and knockdown in a lung cell line. Unexpectedly, a subset of MSC constituents in addition to EPRS1, namely MARS1, KARS1, and RARS1, interacts with SPEAR in extracts from an IFN-γ- or insulin-treated lung cell line. UV cross-linking revealed both EPRS1 and KARS1 directly interacted with the SPEAR element; EPRS1 binding was mediated by the linker domain — the region that also binds the GAIT element. Interaction of EPRS1 with SARS-CoV-2 sgRNAs as well as gRNA was confirmed in IFN-γ stimulated SARS-CoV-2 replicon. Following cell induction with IFN-γ or spike S1, or both, the four SPEAR-binding aaRSs mobilize to form an extra-MSC, ∼500 kDa tetra-aminoacyl-tRNA synthetase sarbecoviral RNA-interacting (TASRI) complex — the second largest known human aaRS complex, after the MSC. The agonists induce relocalization of the TASRI complex to the endoplasmic reticulum (ER), a primary source of the double-membrane organelles supporting SARS-CoV-2 genome replication [127]. Importantly, cell-penetrating peptide-phosphorodiamidate morpholine oligonucleotide (PPMO) conjugates antisense to the SPEAR element block EPRS1 (and TASRI complex) binding, exhibit nearly 1.5-log reduction in SARS-CoV-2 titers, reduce viral protein levels in infected cells, attenuated growth kinetics in a SARS-CoV-2 EGFP reporter virus and effectively reduce viral genomic and subgenomic RNAs, indicating an important role of the interaction of the TASRI complex with SPEAR-element in SARS-CoV-2, and its potential as a therapeutic target [125].
Within the MSC, EPRS1 and MARS1 reside in a subcomplex joined by interacting GST-like domains; according to a current model RARS1 and KARS1 reside in a disconnected MSC region, complexed with AIMP2 and QARS1 [126]. Agonist-induced release of EPRS1 and KARS1 from the cytoplasmic MSC has been reported [45,49]. Formation of the TASRI complex is not understood and might not be a one-step dissociation from the cytoplasmic MSC, but rather an orchestrated, multi-step dissociation-association process. Moreover, parts of the TASRI complex may be derived from newly generated, free cytoplasmic pools. Translational control of SARS-CoV-2 sgRNAs that is SPEAR-dependent and regulated by a host aaRS complex is a unique example of a cellular complex-stimulated regulatory function of an RNA element in the SARS-CoV-2 3′-end.
The SPEAR element resides in the structurally ambiguous HVR. Internal initiation at ORF10, a 3′-end co-terminal feature newly acquired in SARS-CoV-2, plays a critical role in SPEAR-mediated induction of sgRNA expression [125], Mechanistically, this finding suggests a potential role of translating ribosomes in activating the SPEAR element by TASRI complex-assisted RNA refolding. We propose a framework to decode structure–function relationships of SARS-CoV-2 3′-end regulatory regions, where (i) functional translation of ORF10 forms a SPEAR-permissive 3′ end, (ii) an agonist-inducible TASRI complex directs SPEAR element formation in the structurally fluid HVR, (iii) the SPEAR-adjacent genomic terminus base-pairs with the 5′UTR in gRNA/5′TRS-L (transcription regulatory sequence of leader) in sgRNAs [128], and (iv) sgRNA or gRNA circularization places the SPEAR element proximate to the start codon to regulate translation and PRF [125] (Figure 2). Additionally, the SPEAR element is near the virus 3′-end triple-helix junction necessary for recognition by the replication–transcription complex (RTC), possibly bringing the TASRI complex in proximity to the viral replication machinery as well.
Figure 2. An integrated model of SARS-CoV-2 ORF10, SPEAR element, and virus RNA cyclization [125].
RNAs are cyclized by base-pairing of the 3′-end genomic terminus immediately downstream of the SPEAR element, with 16 complementary nucleotides of the TRS-L (transcription regulatory sequence-L) or 21 nt of the 5′UTR [128]. The start codon lies 0–2 nt downstream of this base-paired region in most sgRNAs, except in N (8 nt) and M (44 nt); in gRNA, the ORF1a/b start codon lies further downstream, with intervening 5′UTR stem–loop structures (not shown). Cell activation by SARS-CoV-2 spike, IFN-γ, or insulin induces ORF10 translation and formation of the SPEAR element-binding TASRI (tetra-aminoacyl-tRNA synthetase sarbecoviral RNA-interacting) complex.
SPEAR element activation by IFN-γ, insulin, and spike in multiple cell types suggests pathophysiologic significance. EPRS1 binding to the SPEAR element was markedly increased in lysates from epididymal white adipose tissue from fat-fed obese mice compared with normal mice. Adipose tissues are virus reservoirs, as well as sources of inflammatory adipokines, contributing to COVID-19 severity in obese patients [129,130]. The relative risk of death in severely obese COVID-19 patients is ∼4.2-fold higher compared with non-obese patients [131]. Likewise, visceral fat area is associated with an increased need for treatment in intensive care units and for mechanical ventilation [132]. Elevated EPRS1 binding to the SPEAR element in adipose tissue from obese mice suggests a mechanism underlying risk of severe COVID-19 in obese patients [125]. Similarly, insulin stimulates EPRS1 binding to SPEAR in differentiated adipocytes, possibly illuminating the clinical observation that insulin treatment, like obesity, is associated with increased mortality in COVID-19 patients [133]. Importantly, substantial weight loss following bariatric surgery in obese patients is associated with improved outcomes of COVID-19 infection, suggesting a causal relationship, and that obesity is a modifiable risk factor for COVID-19 severity [134]. IFN-γ, like other inflammatory cytokines, exhibits potent antiviral activity. However, uncontrolled levels of circulating cytokines and immune cell hyperactivation are characteristic of the ‘cytokine storm', a life-threatening, systemic inflammatory syndrome, and a principal contributor to COVID-19 pathogenesis [135]. The circulating level of IFN-γ, unique among cytokines measured, is higher in COVID-19 patients that succumbed, compared with survivors [136]. Treatment of mice with IFN-γ, in combination with TNF-α, induces a cytokine shock that mirrors the tissue damage of COVID-19, and neutralizing antibodies targeting these cytokines protect mice from mortality following SARS-CoV-2 infection [137]. IFN-γ induces expression of the ACE2 receptor of SARS-CoV-2 in enterocytes and promotes virus production in infected cells [138]. IFN-γ-induced activation of the SPEAR-binding complex might provide an additional pathogenic mechanism [125]. In addition to host agonists, SARS-CoV-2 spike also activates the SPEAR-binding complex. Spike engages the cellular ACE2 receptor for virus entry and elicits cell signal transduction pathways that promote pulmonary and cardiovascular complications [139,140]. Additionally, spike can drive viral infection and pathogenesis via non-ACE2 interactions in cells with low ACE2 receptor levels [141,142]. The S1 subunit of spike, containing N-terminal and receptor-binding domains, induces EPRS1 incorporation into a complex with three other MSC-constituent aaRSs, and binding to the SPEAR element via the EPRS1 non-catalytic linker domain [125]. Together, the results show that SPEAR is a novel, pan-sgRNA translation–activation element that, along with a newly elucidated host-derived TASRI complex, defines a SARS-CoV-2 post-transcriptional regulon.
Future directions
Accelerating disruptive activities of mankind have led to habitat destruction of many organisms, increasing future likelihood of zoonotic spillover of viruses. Lessons learned from COVID-19 will be important for navigating a future pandemic from known or as-yet unknown viruses, including sarbecoviruses [60,143,144]. Near-term, elucidation of the role of aaRSs in translation, replication, infection, and pathogenesis of SARS-CoV-2 variants might explain differences in their pathogenesis. The knowledge gathered in aaRS biology as mediators of virus infection will facilitate design of new antiviral therapeutic strategies.
Perspectives
Aminoacyl-tRNA synthetases have canonical, post-canonical, and non-canonical functions in cellular homeostasis and in response to environmental stimuli.
The repertoire of non-canonical functions of aminoacyl-tRNA synthetases extend to being host modulators of virus infection.
Recent elucidation of molecular aspects in SARS-CoV-2 infection have expanded the galaxy of interactions exhibited by these proteins, as also exemplified by a previously unrecognized TASRI complex comprised of four aminoacyl-tRNA synthetases.
Acknowledgements
We would like to thank Prof. Ivan Robert Nabi for inviting this manuscript and all members of the Fox laboratory for discussions.
Abbreviations
- aa
amino acid
- aaRS
aminoacyl-tRNA synthetase
- ACE2
angiotensin converting enzyme 2
- AIMP
aminoacyl-tRNA synthetase complex-interacting multifunctional protein
- AMP
adenosine monophosphate
- ARDS
acute respiratory distress syndrome
- ATP
adenosine triphosphate
- BALF
bronchoalveolar lavage fluid
- BMV
brome mosaic virus
- COVID-19
coronaviral disease-19
- CRISPR
clustered regularly interspaced short palindromic repeats
- CysSSH
cysteine hydropersulfide
- cyto-aaRS
cytosolic aminoacyl-tRNA synthetase
- GAIT
gamma-interferon activated inhibitor of translation
- gRNA
genomic RNA
- GST
glutathione S transferase
- H1N1 influenza A virus subtype-hemagglutinin 1
neuraminidase 1
- hGatCAB
human glutamyl-tRNA(Gln) amidotransferase subunits C-A-B
- HIV
human immunodeficiency virus
- HS
heparan sulfate
- HSPG
heparan sulfate proteoglycan
- HSV
herpes simplex virus
- HVR
hypervariable region
- IFN
interferon
- MAVS
mitochondrial antiviral-signaling protein
- MERS-CoV
middle east respiratory syndrome coronavirus
- mRNA
messenger RNA
- MSC
multi-aminoacyl tRNA synthetase complex
- mt-aaRS
mitochondrial aminoacyl-tRNA synthetase
- NSP
non-structural protein
- ORF
open reading frame
- PCBP2
poly(rC)-binding protein 2
- RBD
receptor-binding domain
- RSV
respiratory syncytial virus
- RTC
replication–transcription complex
- S1
spike subunit 1
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- sgRNA
subgenomic RNA
- SPEAR
sarbecoviral pan-end activating RNA
- TASRI complex
tetra-aminoacyl-tRNA synthetase sarbecoviral RNA-interacting complex
- TGEV
transmissible gastroenteritis coronavirus
- TLE
tRNA-like element
- TLS
tRNA-like structures
- TMPRSS2
transmembrane serine protease 2
- TNF
tumor necrosis factor
- tRNA
transfer RNA
- TRS-L
transcription regulatory sequence of leader
- UTR
untranslated region
- VSV
vesicular stomatitis virus
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by NIH grants R01 DK124203, R01 DK130377, R01 AG067146, R01 NS124547, and R01 NS124581 to PLF. D.K. was partly supported by American Heart Association Grant #19POST34380687/DebjitKhan/2019.
Open Access
Open access for this article was enabled by the participation of Cleveland Clinic in an all-inclusive Read & Publish agreement with Portland Press and the Biochemical Society under a transformative agreement with Individual.
Author Contributions
D.K. and P.L.F. wrote and edited the manuscript.
References
- 1.Schmelz, S. and Naismith, J.H. (2009) Adenylate-forming enzymes. Curr. Opin. Struct. Biol. 19, 666–671 10.1016/j.sbi.2009.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagel, G.M. and Doolittle, R.F. (1995) Phylogenetic analysis of the aminoacyl-tRNA synthetases. J. Mol. Evol. 40, 487–498 10.1007/BF00166617 [DOI] [PubMed] [Google Scholar]
- 3.Brown, J.R. and Doolittle, W.F. (1995) Root of the universal tree of life based on ancient aminoacyl-tRNA synthetase gene duplications. Proc. Natl Acad. Sci. U.S.A. 92, 2441–2445 10.1073/pnas.92.7.2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schimmel, P., Giege, R., Moras, D. and Yokoyama, S. (1993) An operational RNA code for amino acids and possible relationship to genetic code. Proc. Natl Acad. Sci. U.S.A. 90, 8763–8768 10.1073/pnas.90.19.8763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carter, Jr, C.W. and Wills, P.R. (2019) Class I and II aminoacyl-tRNA synthetase tRNA groove discrimination created the first synthetase-tRNA cognate pairs and was therefore essential to the origin of genetic coding. IUBMB Life 71, 1088–1098 10.1002/iub.2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perona, J.J. and Gruic-Sovulj, I. (2014) Synthetic and editing mechanisms of aminoacyl-tRNA synthetases. Top. Curr. Chem. 344, 1–41 10.1007/128_2013_456 [DOI] [PubMed] [Google Scholar]
- 7.Kuzmishin Nagy, A.B., Bakhtina, M. and Musier-Forsyth, K. (2020) Trans-editing by aminoacyl-tRNA synthetase-like editing domains. Enzymes 48, 69–115 10.1016/bs.enz.2020.07.002 [DOI] [PubMed] [Google Scholar]
- 8.Labunskyy, V.M., Hatfield, D.L. and Gladyshev, V.N. (2014) Selenoproteins: molecular pathways and physiological roles. Physiol. Rev. 94, 739–777 10.1152/physrev.00039.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Copeland, P.R. (2005) Making sense of nonsense: the evolution of selenocysteine usage in proteins. Genome Biol. 6, 221 10.1186/gb-2005-6-6-221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srinivasan, G., James, C.M. and Krzycki, J.A. (2002) Pyrrolysine encoded by UAG in Archaea: charging of a UAG-decoding specialized tRNA. Science 296, 1459–1462 10.1126/science.1069588 [DOI] [PubMed] [Google Scholar]
- 11.Polycarpo, C., Ambrogelly, A., Ruan, B., Tumbula-Hansen, D., Ataide, S.F., Ishitani, R.et al. (2003) Activation of the pyrrolysine suppressor tRNA requires formation of a ternary complex with class I and class II lysyl-tRNA synthetases. Mol. Cell 12, 287–294 10.1016/s1097-2765(03)00280-6 [DOI] [PubMed] [Google Scholar]
- 12.Polycarpo, C., Ambrogelly, A., Berube, A., Winbush, S.M., McCloskey, J.A., Crain, P.F.et al. (2004) An aminoacyl-tRNA synthetase that specifically activates pyrrolysine. Proc. Natl Acad. Sci. U.S.A. 101, 12450–12454 10.1073/pnas.0405362101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sagan, L. (1967) On the origin of mitosing cells. J. Theor. Biol. 14, 255–274 10.1016/0022-5193(67)90079-3 [DOI] [PubMed] [Google Scholar]
- 14.Gray, M.W. (2012) Mitochondrial evolution. Cold Spring Harb. Perspect. Biol. 4, a011403 10.1101/cshperspect.a011403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie, W., Nangle, L.A., Zhang, W., Schimmel, P. and Yang, X.L. (2007) Long-range structural effects of a charcot-Marie-Tooth disease-causing mutation in human glycyl-tRNA synthetase. Proc. Natl Acad. Sci. U.S.A. 104, 9976–9981 10.1073/pnas.0703908104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tolkunova, E., Park, H., Xia, J., King, M.P. and Davidson, E. (2000) The human lysyl-tRNA synthetase gene encodes both the cytoplasmic and mitochondrial enzymes by means of an unusual alternative splicing of the primary transcript. J. Biol. Chem. 275, 35063–35069 10.1074/jbc.M006265200 [DOI] [PubMed] [Google Scholar]
- 17.Hotchkiss, R.D. (1948) The quantitative separation of purines, pyrimidines, and nucleosides by paper chromatography. J. Biol. Chem. 175, 315–332 10.1016/S0021-9258(18)57261-6 [DOI] [PubMed] [Google Scholar]
- 18.Ray, P.S. and Fox, P.L. (2014) Origin and evolution of glutamyl-prolyl tRNA synthetase WHEP domains reveal evolutionary relationships within holozoa. PLoS ONE 9, e98493 10.1371/journal.pone.0098493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer, M.G., Allen, M.J., Wilson, W.H. and Suttle, C.A. (2010) Giant virus with a remarkable complement of genes infects marine zooplankton. Proc. Natl Acad. Sci. U.S.A. 107, 19508–19513 10.1073/pnas.1007615107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Philippe, N., Legendre, M., Doutre, G., Coute, Y., Poirot, O., Lescot, M.et al. (2013) Pandoraviruses: amoeba viruses with genomes up to 2.5 Mb reaching that of parasitic eukaryotes. Science 341, 281–286 10.1126/science.1239181 [DOI] [PubMed] [Google Scholar]
- 21.Abergel, C., Rudinger-Thirion, J., Giege, R. and Claverie, J.M. (2007) Virus-encoded aminoacyl-tRNA synthetases: structural and functional characterization of mimivirus TyrRS and MetRS. J. Virol. 81, 12406–12417 10.1128/JVI.01107-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoosuf, N., Yutin, N., Colson, P., Shabalina, S.A., Pagnier, I., Robert, C.et al. (2012) Related giant viruses in distant locations and different habitats: Acanthamoeba polyphaga moumouvirus represents a third lineage of the Mimiviridae that is close to the megavirus lineage. Genome Biol. Evol. 4, 1324–1330 10.1093/gbe/evs109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arslan, D., Legendre, M., Seltzer, V., Abergel, C. and Claverie, J.M. (2011) Distant Mimivirus relative with a larger genome highlights the fundamental features of Megaviridae. Proc. Natl Acad. Sci. U.S.A. 108, 17486–17491 10.1073/pnas.1110889108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abrahao, J., Silva, L., Silva, L.S., Khalil, J.Y., Rodrigues, R., Arantes, T.et al. (2018) Tailed giant Tupanvirus possesses the most complete translational apparatus of the known virosphere. Nat. Commun. 9, 749 10.1038/s41467-018-03168-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yot, P., Pinck, M., Haenni, A.L., Duranton, H.M. and Chapeville, F. (1970) Valine-specific tRNA-like structure in turnip yellow mosaic virus RNA. Proc. Natl Acad. Sci. U.S.A. 67, 1345–1352 10.1073/pnas.67.3.1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sherlock, M.E., Hartwick, E.W., MacFadden, A. and Kieft, J.S. (2021) Structural diversity and phylogenetic distribution of valyl tRNA-like structures in viruses. RNA 27, 27–39 10.1261/rna.076968.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dreher, T.W. (2009) Role of tRNA-like structures in controlling plant virus replication. Virus Res. 139, 217–229 10.1016/j.virusres.2008.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonilla, S.L., Sherlock, M.E., MacFadden, A. and Kieft, J.S. (2021) A viral RNA hijacks host machinery using dynamic conformational changes of a tRNA-like structure. Science 374, 955–960 10.1126/science.abe8526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andreev, D.E., Hirnet, J., Terenin, I.M., Dmitriev, S.E., Niepmann, M. and Shatsky, I.N. (2012) Glycyl-tRNA synthetase specifically binds to the poliovirus IRES to activate translation initiation. Nucleic Acids Res. 40, 5602–5614 10.1093/nar/gks182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo, M., Yang, X.L. and Schimmel, P. (2010) New functions of aminoacyl-tRNA synthetases beyond translation. Nat. Rev. Mol. Cell Biol. 11, 668–674 10.1038/nrm2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arif, A., Jia, J., Mukhopadhyay, R., Willard, B., Kinter, M. and Fox, P.L. (2009) Two-site phosphorylation of EPRS coordinates multimodal regulation of noncanonical translational control activity. Mol. Cell 35, 164–180 10.1016/j.molcel.2009.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee, E.Y., Hwang, J. and Kim, M.H. (2023) Phosphocode-dependent glutamyl-prolyl-tRNA synthetase 1 signaling in immunity, metabolism, and disease. Exp. Mol. Med. 55, 2116–2126 10.1038/s12276-023-01094-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim, D.G., Lee, J.Y., Kwon, N.H., Fang, P., Zhang, Q., Wang, J.et al. (2014) Chemical inhibition of prometastatic lysyl-tRNA synthetase-laminin receptor interaction. Nat. Chem. Biol. 10, 29–34 10.1038/nchembio.1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yannay-Cohen, N., Carmi-Levy, I., Kay, G., Yang, C.M., Han, J.M., Kemeny, D.M.et al. (2009) LysRS serves as a key signaling molecule in the immune response by regulating gene expression. Mol. Cell 34, 603–611 10.1016/j.molcel.2009.05.019 [DOI] [PubMed] [Google Scholar]
- 35.Yoon, I., Nam, M., Kim, H.K., Moon, H.S., Kim, S., Jang, J.et al. (2020) Glucose-dependent control of leucine metabolism by leucyl-tRNA synthetase 1. Science 367, 205–210 10.1126/science.aau2753 [DOI] [PubMed] [Google Scholar]
- 36.Sung, Y., Yoon, I., Han, J.M. and Kim, S. (2022) Functional and pathologic association of aminoacyl-tRNA synthetases with cancer. Exp. Mol. Med. 54, 553–566 10.1038/s12276-022-00765-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim, S.B., Kim, H.R., Park, M.C., Cho, S., Goughnour, P.C., Han, D.et al. (2017) Caspase-8 controls the secretion of inflammatory lysyl-tRNA synthetase in exosomes from cancer cells. J. Cell Biol. 216, 2201–2216 10.1083/jcb.201605118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wakasugi, K. and Schimmel, P. (1999) Two distinct cytokines released from a human aminoacyl-tRNA synthetase. Science 284, 147–151 10.1126/science.284.5411.147 [DOI] [PubMed] [Google Scholar]
- 39.Jobin, P.G., Solis, N., Machado, Y., Bell, P.A., Kwon, N.H., Kim, S.et al. (2019) Matrix metalloproteinases inactivate the proinflammatory functions of secreted moonlighting tryptophanyl-tRNA synthetase. J. Biol. Chem. 294, 12866–12879 10.1074/jbc.RA119.009584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jobin, P.G., Solis, N., Machado, Y., Bell, P.A., Rai, S.K., Kwon, N.H.et al. (2020) Moonlighting matrix metalloproteinase substrates: enhancement of proinflammatory functions of extracellular tyrosyl-tRNA synthetase upon cleavage. J. Biol. Chem. 295, 2186–2202 10.1074/jbc.RA119.010486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cui, H., Kapur, M., Diedrich, J.K., Yates, J.R., Ackerman, S.L. and Schimmel, P. (2021) Regulation of ex-translational activities is the primary function of the multi-tRNA synthetase complex. Nucleic Acids Res. 49, 3603–3616 10.1093/nar/gkaa1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo, M. and Schimmel, P. (2013) Essential nontranslational functions of tRNA synthetases. Nat. Chem. Biol. 9, 145–153 10.1038/nchembio.1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yao, P. and Fox, P.L. (2020) Aminoacyl-tRNA synthetases in cell signaling. Enzymes 48, 243–275 10.1016/bs.enz.2020.04.002 [DOI] [PubMed] [Google Scholar]
- 44.Kyriacou, S.V. and Deutscher, M.P. (2008) An important role for the multienzyme aminoacyl-tRNA synthetase complex in mammalian translation and cell growth. Mol. Cell 29, 419–427 10.1016/j.molcel.2007.11.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sampath, P., Mazumder, B., Seshadri, V., Gerber, C.A., Chavatte, L., Kinter, M.et al. (2004) Noncanonical function of glutamyl-prolyl-tRNA synthetase: gene-specific silencing of translation. Cell 119, 195–208 10.1016/j.cell.2004.09.030 [DOI] [PubMed] [Google Scholar]
- 46.Ray, P.S., Arif, A. and Fox, P.L. (2007) Macromolecular complexes as depots for releasable regulatory proteins. Trends Biochem. Sci. 32, 158–164 10.1016/j.tibs.2007.02.003 [DOI] [PubMed] [Google Scholar]
- 47.Lee, E.Y., Lee, H.C., Kim, H.K., Jang, S.Y., Park, S.J., Kim, Y.H.et al. (2016) Infection-specific phosphorylation of glutamyl-prolyl tRNA synthetase induces antiviral immunity. Nat. Immunol. 17, 1252–1262 10.1038/ni.3542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marquez-Jurado, S., Nogales, A., Zuniga, S., Enjuanes, L. and Almazan, F. (2015) Identification of a gamma interferon-activated inhibitor of translation-like RNA motif at the 3’ end of the transmissible gastroenteritis coronavirus genome modulating innate immune response. mBio 6, e00105 10.1128/mBio.00105-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duchon, A.A., St Gelais, C., Titkemeier, N., Hatterschide, J., Wu, L. and Musier-Forsyth, K. (2017) HIV-1 exploits a dynamic multi-aminoacyl-tRNA synthetase complex to enhance viral replication. J. Virol. 91, e01240-17 10.1128/JVI.01240-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dewan, V., Wei, M., Kleiman, L. and Musier-Forsyth, K. (2012) Dual role for motif 1 residues of human lysyl-tRNA synthetase in dimerization and packaging into HIV-1. J. Biol. Chem. 287, 41955–41962 10.1074/jbc.M112.421842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu, S., Comandur, R., Jones, C.P., Tsang, P. and Musier-Forsyth, K. (2016) Anticodon-like binding of the HIV-1 tRNA-like element to human lysyl-tRNA synthetase. RNA 22, 1828–1835 10.1261/rna.058081.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jin, D., Zhu, Y., Schubert, H.L., Goff, S.P. and Musier-Forsyth, K. (2023) HIV-1 Gag binds the multi-aminoacyl-tRNA synthetase complex via the EPRS subunit. Viruses 15, 474 10.3390/v15020474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaminska, M., Shalak, V., Francin, M. and Mirande, M. (2007) Viral hijacking of mitochondrial lysyl-tRNA synthetase. J. Virol. 81, 68–73 10.1128/JVI.01267-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yeung, M.L., Jia, L., Yip, C.C., Chan, J.F., Teng, J.L., Chan, K.H.et al. (2018) Human tryptophanyl-tRNA synthetase is an IFN-gamma-inducible entry factor for enterovirus. J. Clin. Invest. 128, 5163–5177 10.1172/JCI99411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee, H.C., Lee, E.S., Uddin, M.B., Kim, T.H., Kim, J.H., Chathuranga, K.et al. (2019) Released tryptophanyl-tRNA synthetase stimulates innate immune responses against viral infection. J. Virol. 93, e01291-18 10.1128/JVI.01291-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jung, H.J., Park, S.H., Cho, K.M., Jung, K.I., Cho, D. and Kim, T.S. (2020) Threonyl-tRNA synthetase promotes T helper type 1 cell responses by inducing dendritic cell maturation and IL-12 production via an NF-kappaB pathway. Front. Immunol. 11, 571959 10.3389/fimmu.2020.571959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morens, D.M. and Fauci, A.S. (2020) Emerging pandemic diseases: how we got to COVID-19. Cell 182, 1077–1092 10.1016/j.cell.2020.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.COVID-19 Excess Mortality Collaborators. (2022) Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020-21. Lancet 399, 1513–1536 10.1016/S0140-6736(21)02796-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Long, C.C., Wulf Hanson, S., Abbafati, C., Aerts, J.G., Al-Aly, Z., Ashbaugh, C.et al. (2022) Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA 328, 1604–1615 10.1001/jama.2022.18931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sachs, J.D., Karim, S.S., Aknin, L., Allen, J., Brosbol, K., Colombo, F.et al. (2022) The Lancet Commission on lessons for the future from the COVID-19 pandemic. Lancet 400, 1224–1280 10.1016/S0140-6736(22)01585-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu, Y.C., Kuo, R.L. and Shih, S.R. (2020) COVID-19: the first documented coronavirus pandemic in history. Biomed. J. 43, 328–333 10.1016/j.bj.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kickbusch, I. and Liu, A. (2022) Global health diplomacy-reconstructing power and governance. Lancet 399, 2156–2166 10.1016/S0140-6736(22)00583-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prada, E., Langbecker, A. and Catalan-Matamoros, D. (2023) Public discourse and debate about vaccines in the midst of the COVID-19 pandemic: a qualitative content analysis of Twitter. Vaccine 41, 3196–3203 10.1016/j.vaccine.2023.03.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xue, J., Chen, J., Chen, C., Zheng, C., Li, S. and Zhu, T. (2020) Public discourse and sentiment during the COVID 19 pandemic: using Latent Dirichlet Allocation for topic modeling on Twitter. PLoS ONE 15, e0239441 10.1371/journal.pone.0239441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu, R., Zhao, X., Li, J., Niu, P., Yang, B., Wu, H.et al. (2020) Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 395, 565–574 10.1016/S0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Andersen, K.G., Rambaut, A., Lipkin, W.I., Holmes, E.C. and Garry, R.F. (2020) The proximal origin of SARS-CoV-2. Nat. Med. 26, 450–452 10.1038/s41591-020-0820-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. (2020) The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 5, 536–544 10.1038/s41564-020-0695-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marini, J.J. and Gattinoni, L. (2020) Management of COVID-19 respiratory distress. JAMA 323, 2329–2330 10.1001/jama.2020.6825 [DOI] [PubMed] [Google Scholar]
- 69.Russell, C.D., Lone, N.I. and Baillie, J.K. (2023) Comorbidities, multimorbidity and COVID-19. Nat. Med. 29, 334–343 10.1038/s41591-022-02156-9 [DOI] [PubMed] [Google Scholar]
- 70.Soriano, J.B., Murthy, S., Marshall, J.C., Relan, P., Diaz, J.V. and WHO Clinical Case Definition Working Group on Post-COVID-19 Condition (2022) A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 22, e102–e107 10.1016/S1473-3099(21)00703-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Munblit, D., O'Hara, M.E., Akrami, A., Perego, E., Olliaro, P. and Needham, D.M. (2022) Long COVID: aiming for a consensus. Lancet Respir. Med. 10, 632–634 10.1016/S2213-2600(22)00135-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rubin, E.J., Baden, L.R., Rosen, C.J. and Morrissey, S. (2022) Audio interview: studying long COVID. N. Engl. J. Med. 386, e20 10.1056/NEJMe2201619 [DOI] [PubMed] [Google Scholar]
- 73.Davis, H.E., McCorkell, L., Vogel, J.M. and Topol, E.J. (2023) Long COVID: major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 21, 133–146 10.1038/s41579-022-00846-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chow, E.J., Uyeki, T.M. and Chu, H.Y. (2023) The effects of the COVID-19 pandemic on community respiratory virus activity. Nat. Rev. Microbiol. 21, 195–210 10.1038/s41579-022-00807-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Feng, Y., Tang, K., Lai, Q., Liang, J., Feng, M., Zhou, Z.W.et al. (2021) The landscape of aminoacyl-tRNA synthetases involved in severe acute respiratory syndrome coronavirus 2 infection. Front. Physiol. 12, 818297 10.3389/fphys.2021.818297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wei, J., Alfajaro, M.M., DeWeirdt, P.C., Hanna, R.E., Lu-Culligan, W.J., Cai, W.L.et al. (2021) Genome-wide CRISPR screens reveal host factors critical for SARS-CoV-2 infection. Cell 184, 76–91 10.1016/j.cell.2020.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Banerjee, A.K., Blanco, M.R., Bruce, E.A., Honson, D.D., Chen, L.M., Chow, A.et al. (2020) SARS-CoV-2 disrupts splicing, translation, and protein trafficking to suppress host defenses. Cell 183, 1325–1339 10.1016/j.cell.2020.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xiong, Y., Liu, Y., Cao, L., Wang, D., Guo, M., Jiang, A.et al. (2020) Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg. Microbes Infect. 9, 761–770 10.1080/22221751.2020.1747363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Blanco-Melo, D., Nilsson-Payant, B.E., Liu, W.C., Uhl, S., Hoagland, D., Moller, R.et al. (2020) Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 181, 1036–1045.e1039 10.1016/j.cell.2020.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vishnubalaji, R., Shaath, H. and Alajez, N.M. (2020) Protein coding and long noncoding RNA (lncRNA) transcriptional landscape in SARS-CoV-2 infected bronchial epithelial cells highlight a role for interferon and inflammatory response. Genes (Basel) 11, 760 10.3390/genes11070760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zeng, H.-L., Chen, D., Yan, J., Yang, Q., Han, Q.-Q., Li, S.-S.et al. (2021) Proteomic characteristics of bronchoalveolar lavage fluid in critical COVID-19 patients. FEBS J. 288, 5190–5200 10.1111/febs.15609 [DOI] [PubMed] [Google Scholar]
- 82.Li, J., Guo, M., Tian, X., Wang, X., Yang, X., Wu, P.et al. (2021) Virus-host interactome and proteomic survey reveal potential virulence factors influencing SARS-CoV-2 pathogenesis. Med 2, 99–112.e117 10.1016/j.medj.2020.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Leng, L., Cao, R., Ma, J., Lv, L., Li, W., Zhu, Y.et al. (2021) Pathological features of COVID-19-associated liver injury-a preliminary proteomics report based on clinical samples. Signal Transduct. Target. Ther. 6, 9 10.1038/s41392-020-00406-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Klann, K., Bojkova, D., Tascher, G., Ciesek, S., Munch, C. and Cinatl, J. (2020) Growth factor receptor signaling inhibition prevents SARS-CoV-2 replication. Mol. Cell 80, 164–174.e164 10.1016/j.molcel.2020.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bouhaddou, M., Memon, D., Meyer, B., White, K.M., Rezelj, V.V., Correa Marrero, M.et al. (2020) The global phosphorylation landscape of SARS-CoV-2 infection. Cell 182, 685–712.e619 10.1016/j.cell.2020.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stukalov, A., Girault, V., Grass, V., Karayel, O., Bergant, V., Urban, C.et al. (2021) Multilevel proteomics reveals host perturbations by SARS-CoV-2 and SARS-CoV. Nature 594, 246–252 10.1038/s41586-021-03493-4 [DOI] [PubMed] [Google Scholar]
- 87.Liu, X., Huuskonen, S., Laitinen, T., Redchuk, T., Bogacheva, M., Salokas, K.et al. (2021) SARS-CoV-2-host proteome interactions for antiviral drug discovery. Mol. Syst. Biol. 17, e10396 10.15252/msb.202110396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang, Y., Shang, L., Zhang, J., Liu, Y., Jin, C., Zhao, Y.et al. (2022) An antibody-based proximity labeling map reveals mechanisms of SARS-CoV-2 inhibition of antiviral immunity. Cell Chem. Biol. 29, 5–18.e16 10.1016/j.chembiol.2021.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Iacobucci, I., Monaco, V., Cane, L., Bibbo, F., Cioffi, V., Cozzolino, F.et al. (2022) Spike S1 domain interactome in non-pulmonary systems: a role beyond the receptor recognition. Front. Mol. Biosci. 9, 975570 10.3389/fmolb.2022.975570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Koudelka, T., Boger, J., Henkel, A., Schonherr, R., Krantz, S., Fuchs, S.et al. (2021) N-terminomics for the identification of in vitro substrates and cleavage site specificity of the SARS-CoV-2 main protease. Proteomics 21, e2000246 10.1002/pmic.202000246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu, H., Bai, Y., Zhang, X., Gao, T., Liu, Y., Li, E.et al. (2022) SARS-CoV-2 N protein antagonizes stress granule assembly and ifn production by interacting with G3BPs to facilitate viral replication. J. Virol. 96, e0041222 10.1128/jvi.00412-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zheng, Y.X., Wang, L., Kong, W.S., Chen, H., Wang, X.N., Meng, Q.et al. (2021) Nsp2 has the potential to be a drug target revealed by global identification of SARS-CoV-2 Nsp2-interacting proteins. Acta Biochim. Biophys. Sin. (Shanghai) 53, 1134–1141 10.1093/abbs/gmab088 [DOI] [PubMed] [Google Scholar]
- 93.Meyers, J.M., Ramanathan, M., Shanderson, R.L., Beck, A., Donohue, L., Ferguson, I.et al. (2021) The proximal proteome of 17 SARS-CoV-2 proteins links to disrupted antiviral signaling and host translation. PLoS Pathog. 17, e1009412 10.1371/journal.ppat.1009412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim, D.K., Weller, B., Lin, C.W., Sheykhkarimli, D., Knapp, J.J., Dugied, G.et al. (2023) A proteome-scale map of the SARS-CoV-2-human contactome. Nat. Biotechnol. 41, 140–149 10.1038/s41587-022-01475-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mihalic, F., Benz, C., Kassa, E., Lindqvist, R., Simonetti, L., Inturi, R.et al. (2023) Identification of motif-based interactions between SARS-CoV-2 protein domains and human peptide ligands pinpoint antiviral targets. Nat. Commun. 14, 5636 10.1038/s41467-023-41312-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gordon, D.E., Jang, G.M., Bouhaddou, M., Xu, J., Obernier, K., White, K.M.et al. (2020) A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 583, 459–468 10.1038/s41586-020-2286-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gordon, D.E., Hiatt, J., Bouhaddou, M., Rezelj, V.V., Ulferts, S., Braberg, H.et al. (2020) Comparative host-coronavirus protein interaction networks reveal pan-viral disease mechanisms. Science 370, eabe9403 10.1126/science.abe9403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee, J.G., Huang, W., Lee, H., van de Leemput, J., Kane, M.A. and Han, Z. (2021) Characterization of SARS-CoV-2 proteins reveals Orf6 pathogenicity, subcellular localization, host interactions and attenuation by Selinexor. Cell Biosci. 11, 58 10.1186/s13578-021-00568-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou, Y., Liu, Y., Gupta, S., Paramo, M.I., Hou, Y., Mao, C.et al. (2023) A comprehensive SARS-CoV-2-human protein-protein interactome reveals COVID-19 pathobiology and potential host therapeutic targets. Nat. Biotechnol. 41, 128–139 10.1038/s41587-022-01474-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.St-Germain, J.R., Astori, A., Samavarchi-Tehrani, P., Abdouni, H., Macwan, V., Kim, D.-K.et al. (2020) A SARS-CoV-2 BioID-based virus-host membrane protein interactome and virus peptide compendium: new proteomics resources for COVID-19 research. bioRxiv 10.1101/2020.08.28.269175 [DOI] [Google Scholar]
- 101.Bouhaddou, M., Reuschl, A.-K., Polacco, B.J., Thorne, L.G., Ummadi, M.R., Ye, C.et al. (2022) Global landscape of the host response to SARS-CoV-2 variants reveals viral evolutionary trajectories. bioRxiv 10.1101/2022.10.19.512927 [DOI] [Google Scholar]
- 102.Dominguez Andres, A., Feng, Y., Rosa Campos, A., Yin, J., Yang, C.-C., James, B.et al. (2020) SARS-CoV-2 ORF9c is a membrane-associated protein that suppresses antiviral responses in cells. bioRxiv 10.1101/2020.08.18.256776 [DOI] [Google Scholar]
- 103.Whitworth, I.T., Knoener, R.A., Puray-Chavez, M., Halfmann, P., Romero, S., Baddouh, M.et al. (2023) Defining distinct RNA-protein interactomes of SARS-CoV-2 genomic and subgenomic RNAs. bioRxiv 10.1101/2023.05.15.540806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Alruwaili, M., Armstrong, S., Prince, T., Erdmann, M., Matthews, D.A., Davidson, A.W.et al. (2023) SARS-CoV-2 NSP12 associates with the TRiC complex and the P323L substitution is a host adaption. bioRxiv 10.1101/2023.03.18.533280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Laurent, E.M., Sofianatos, Y., Komarova, A., Gimeno, J.-P., Samarchi-Tehrani, P., Kim, D.-K.et al. (2020) Global BioID-based SARS-CoV-2 proteins proximal interactome unveils novel ties between viral polypeptides and host factors involved in multiple COVID19-associated mechanisms. bioRxiv 10.1101/2020.08.28.272955 [DOI] [Google Scholar]
- 106.Samavarchi-Tehrani, P., Abdouni, H., Knight, J.D., Astori, A., Samson, R., Lin, Z.-Y.et al. (2020) A SARS-CoV-2 – host proximity interactome. bioRxiv 10.1101/2020.09.03.282103 [DOI] [Google Scholar]
- 107.May, D.G., Martin-Sancho, L., Anschau, V., Liu, S., Chrisopulos, R.J., Scott, K.L.et al. (2022) A BioID-derived proximity interactome for SARS-CoV-2 proteins. Viruses 14 10.3390/v14030611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Feng, K., Min, Y.Q., Sun, X., Deng, F., Li, P., Wang, H.et al. (2021) Interactome profiling reveals interaction of SARS-CoV-2 NSP13 with host factor STAT1 to suppress interferon signaling. J. Mol. Cell Biol. 13, 760–762 10.1093/jmcb/mjab068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Matsunaga, T., Sano, H., Takita, K., Morita, M., Yamanaka, S., Ichikawa, T.et al. (2023) Supersulphides provide airway protection in viral and chronic lung diseases. Nat. Commun. 14, 4476 10.1038/s41467-023-40182-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Akaike, T., Ida, T., Wei, F.Y., Nishida, M., Kumagai, Y., Alam, M.M.et al. (2017) Cysteinyl-tRNA synthetase governs cysteine polysulfidation and mitochondrial bioenergetics. Nat. Commun. 8, 1177 10.1038/s41467-017-01311-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Koepfli, J.B., Mead, J.F. and Brockman, J.A. (1947) An alkaloid with high antimalarial activity from Dichroa febrifuga. J. Am. Chem. Soc. 69, 1837 10.1021/ja01199a513 [DOI] [PubMed] [Google Scholar]
- 112.Kuehl, F.A., Spencer, C.F. and Folkers, K. (1948) Alkaloids of Dichroa febrifuga Lour. J. Am. Chem. Soc. 70, 2091–2093 10.1021/ja01186a031 [DOI] [PubMed] [Google Scholar]
- 113.Wiesner, J., Ortmann, R., Jomaa, H. and Schlitzer, M. (2003) New antimalarial drugs. Angew. Chem. Int. Ed. Engl. 42, 5274–5293 10.1002/anie.200200569 [DOI] [PubMed] [Google Scholar]
- 114.Sundrud, M.S., Koralov, S.B., Feuerer, M., Calado, D.P., Kozhaya, A.E., Rhule-Smith, A.et al. (2009) Halofuginone inhibits TH17 cell differentiation by activating the amino acid starvation response. Science 324, 1334–1338 10.1126/science.1172638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Keller, T.L., Zocco, D., Sundrud, M.S., Hendrick, M., Edenius, M., Yum, J.et al. (2012) Halofuginone and other febrifugine derivatives inhibit prolyl-tRNA synthetase. Nat. Chem. Biol. 8, 311–317 10.1038/nchembio.790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pines, M. and Spector, I. (2015) Halofuginone - the multifaceted molecule. Molecules 20, 573–594 10.3390/molecules20010573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sandoval, D.R., Clausen, T.M., Nora, C., Cribbs, A.P., Denardo, A., Clark, A.E.et al. (2021) The prolyl-tRNA synthetase inhibitor halofuginone inhibits SARS-CoV-2 infection. bioRxiv 10.1101/2021.03.22.436522 [DOI] [Google Scholar]
- 118.Clausen, T.M., Sandoval, D.R., Spliid, C.B., Pihl, J., Perrett, H.R., Painter, C.D.et al. (2020) SARS-CoV-2 infection depends on cellular heparan sulfate and ACE2. Cell 183, 1043–1057.e1015 10.1016/j.cell.2020.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sumpter, R., Loo, Y.M., Foy, E., Li, K., Yoneyama, M., Fujita, T.et al. (2005) Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J. Virol. 79, 2689–2699 10.1128/JVI.79.5.2689-2699.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Keene, J.D. (2007) RNA regulons: coordination of post-transcriptional events. Nat. Rev. Genet. 8, 533–543 10.1038/nrg2111 [DOI] [PubMed] [Google Scholar]
- 121.Mukhopadhyay, R., Ray, P.S., Arif, A., Brady, A.K., Kinter, M. and Fox, P.L. (2008) DAPK-ZIPK-L13a axis constitutes a negative-feedback module regulating inflammatory gene expression. Mol. Cell 32, 371–382 10.1016/j.molcel.2008.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ray, P.S. and Fox, P.L. (2007) A post-transcriptional pathway represses monocyte VEGF-A expression and angiogenic activity. EMBO J. 26, 3360–3372 10.1038/sj.emboj.7601774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sampath, P., Mazumder, B., Seshadri, V. and Fox, P.L. (2003) Transcript-selective translational silencing by gamma interferon is directed by a novel structural element in the ceruloplasmin mRNA 3′ untranslated region. Mol. Cell. Biol. 23, 1509–1519 10.1128/MCB.23.5.1509-1519.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vyas, K., Chaudhuri, S., Leaman, D.W., Komar, A.A., Musiyenko, A., Barik, S.et al. (2009) Genome-wide polysome profiling reveals an inflammation-responsive post-transcriptional operon in IFN-gamma-activated monocytes. Mol. Cell. Biol. 29, 458–470 10.1128/MCB.00824-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Khan, D., Terenzi, F., Liu, G., Ghosh, P.K., Ye, F., Nguyen, K.et al. (2023) A viral pan-end RNA element and host complex define a SARS-CoV-2 regulon. Nat. Commun. 14, 3385 10.1038/s41467-023-39091-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Khan, K., Baleanu-Gogonea, C., Willard, B., Gogonea, V. and Fox, P.L. (2020) 3-Dimensional architecture of the human multi-tRNA synthetase complex. Nucleic Acids Res. 48, 8740–8754 10.1093/nar/gkaa569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Snijder, E.J., Limpens, R., de Wilde, A.H., de Jong, A.W.M., Zevenhoven-Dobbe, J.C., Maier, H.J.et al. (2020) A unifying structural and functional model of the coronavirus replication organelle: tracking down RNA synthesis. PLoS Biol. 18, e3000715 10.1371/journal.pbio.3000715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ziv, O., Price, J., Shalamova, L., Kamenova, T., Goodfellow, I., Weber, F.et al. (2020) The short- and long-range RNA-RNA interactome of SARS-CoV-2. Mol. Cell 80, 1067–1077 10.1016/j.molcel.2020.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Reiterer, M., Rajan, M., Gomez-Banoy, N., Lau, J.D., Gomez-Escobar, L.G., Ma, L.et al. (2021) Hyperglycemia in acute COVID-19 is characterized by insulin resistance and adipose tissue infectivity by SARS-CoV-2. Cell Metab. 33, 2484 10.1016/j.cmet.2021.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Malavazos, A.E., Corsi Romanelli, M.M., Bandera, F. and Iacobellis, G. (2020) Targeting the adipose tissue in COVID-19. Obesity (Silver Spring) 28, 1178–1179 10.1002/oby.22844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tartof, S.Y., Qian, L., Hong, V., Wei, R., Nadjafi, R.F., Fischer, H.et al. (2020) Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization. Ann. Intern. Med. 173, 773–781 10.7326/M20-3742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Petersen, A., Bressem, K., Albrecht, J., Thiess, H.M., Vahldiek, J., Hamm, B.et al. (2020) The role of visceral adiposity in the severity of COVID-19: highlights from a unicenter cross-sectional pilot study in Germany. Metabolism 110, 154317 10.1016/j.metabol.2020.154317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yu, B., Li, C., Sun, Y. and Wang, D.W. (2021) Insulin treatment is associated with increased mortality in patients with COVID-19 and type 2 diabetes. Cell Metab. 33, 65–77 10.1016/j.cmet.2020.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Aminian, A., Tu, C., Milinovich, A., Wolski, K.E., Kattan, M.W. and Nissen, S.E. (2022) Association of weight loss achieved through metabolic surgery with risk and severity of COVID-19 infection. JAMA Surg. 157, 221–230 10.1001/jamasurg.2021.6496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fajgenbaum, D.C. and June, C.H. (2020) Cytokine storm. N. Engl. J. Med. 383, 2255–2273 10.1056/NEJMra2026131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gadotti, A.C., de Castro Deus, M., Telles, J.P., Wind, R., Goes, M., Garcia Charello Ossoski, R.et al. (2020) IFN-gamma is an independent risk factor associated with mortality in patients with moderate and severe COVID-19 infection. Virus Res. 289, 198171 10.1016/j.virusres.2020.198171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Karki, R., Sharma, B.R., Tuladhar, S., Williams, E.P., Zalduondo, L., Samir, P.et al. (2021) Synergism of TNF-alpha and IFN-gamma triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell 184, 149–168 10.1016/j.cell.2020.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Heuberger, J., Trimpert, J., Vladimirova, D., Goosmann, C., Lin, M., Schmuck, R.et al. (2021) Epithelial response to IFN-gamma promotes SARS-CoV-2 infection. EMBO Mol. Med. 13, e13191 10.15252/emmm.202013191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Suzuki, Y.J., Nikolaienko, S.I., Dibrova, V.A., Dibrova, Y.V., Vasylyk, V.M., Novikov, M.Y.et al. (2021) SARS-CoV-2 spike protein-mediated cell signaling in lung vascular cells. Vascul. Pharmacol. 137, 106823 10.1016/j.vph.2020.106823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Patra, T., Meyer, K., Geerling, L., Isbell, T.S., Hoft, D.F., Brien, J.et al. (2020) SARS-CoV-2 spike protein promotes IL-6 trans-signaling by activation of angiotensin II receptor signaling in epithelial cells. PLoS Pathog. 16, e1009128 10.1371/journal.ppat.1009128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cantuti-Castelvetri, L., Ojha, R., Pedro, L.D., Djannatian, M., Franz, J., Kuivanen, S.et al. (2020) Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 370, 856–860 10.1126/science.abd2985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wei, C., Wan, L., Yan, Q., Wang, X., Zhang, J., Yang, X.et al. (2020) HDL-scavenger receptor B type 1 facilitates SARS-CoV-2 entry. Nat. Metab. 2, 1391–1400 10.1038/s42255-020-00324-0 [DOI] [PubMed] [Google Scholar]
- 143.Vetter, T.R. (2022) The next viral pandemic: not a question of if, but when and how. Anesth. Analg. 135, 900–902 10.1213/ANE.0000000000006198 [DOI] [PubMed] [Google Scholar]
- 144.Tung, A., Dalton, A., Hastie, J., Jabaley, C.S., Mittel, A.M., Nunnally, M.E.et al. (2022) The next next wave: how critical care might learn from COVID in responding to the next pandemic. Anesth. Analg. 135, 903–910 10.1213/ANE.0000000000006204 [DOI] [PubMed] [Google Scholar]
- 145.Oughtred, R., Rust, J., Chang, C., Breitkreutz, B.J., Stark, C., Willems, A.et al. (2021) The BioGRID database: a comprehensive biomedical resource of curated protein, genetic, and chemical interactions. Protein Sci. 30, 187–200 10.1002/pro.3978 [DOI] [PMC free article] [PubMed] [Google Scholar]