TO THE EDITOR: On May 11, 2023, the World Health Organization announced that mpox (formerly known as monkeypox) was no longer a public health emergency of international concern. However, at approximately the same time, a cluster of cases that included vaccine break-throughs1 may have forecasted a potential for resurgence.
The multicountry outbreak in 2022 disproportionately affected the LGBTQ+ community and persons with human immunodeficiency virus (HIV) infection.2 On August 9, 2022, Emergency Use Authorization was approved for a one-fifth dose of an intradermal regimen of the JYNNEOS vaccine (modified vaccinia Ankara, Bavarian Nordic [MVA-BN]) as a dose-sparing alternative to the two-dose subcutaneous regimen licensed in 2019. However, the intradermal route had not been studied in persons with HIV infection, and the durability of the antibody against mpox virus (MPXV) after MVA-BN vaccination was unknown.
In the New York City Observational Study of Mpox Immunity (NYC OSMI), we recruited study participants among persons who were receiving the MVA-BN vaccine (with either intradermal or subcutaneous administration or a combination of the two) at multiple city clinics (ClinicalTrials.gov number, NCT05654883) (Fig. S1 in the Supplementary Appendix, available with the full text of this letter at NEJM.org). As of May 15, 2023, a total of 145 participants without previous mpox had been enrolled. Of these participants, 24% were persons with HIV infection, 20% had received previous smallpox vaccination, 89% were LGBTQ+ (of whom 85% were men), and 37% were from underrepresented communities (Table S1).
Previous studies have shown that IgG titers against vaccinia virus correlate with vaccinia neutralization.3–5 Here, we similarly report IgG titers against MPXV H3L protein as a measure of MPXV neutralization (Fig. S2). The study was approved by the institutional review board at NYU Grossman School of Medicine. All the participants provided written informed consent. Details regarding the conduct of the study are provided in the protocol, available at NEJM.org.
In participants without a history of smallpox vaccination, IgG titers against MPXV H3L decreased after the peak following the second dose of vaccine (antibody half-life, 107.9 days with wide confidence intervals) (Fig. 1A). Participants who had received previous smallpox vaccination had a higher level of durability of IgG through 3 months after the second dose of vaccine (Fig. 1B). There was no indication of decreased titers with longer intervals between the recommended two doses (Fig. 1C [left]). Geometric mean titers were four times as high after two doses as compared with one dose (199.4 vs. 49.6) among participants without previous smallpox vaccination (Fig. S3).
Figure 1. Longevity of Anti–MPXV H3L IgG Titers after MVA-BN Vaccination.
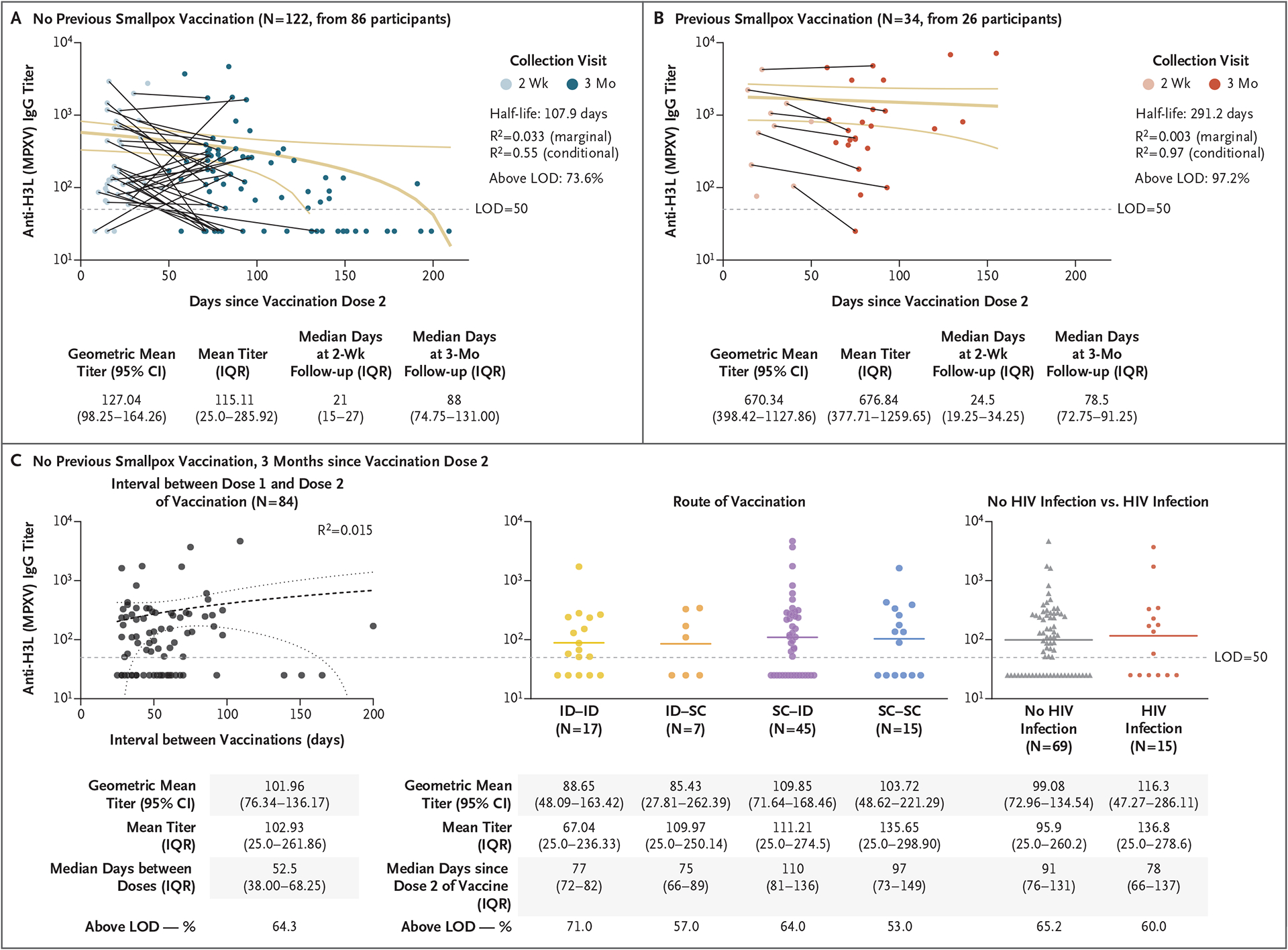
Shown are antibody titers among study participants who had received two doses of the MVA-BN vaccine among those without a history of smallpox vaccination (Panel A) and among those with a history of smallpox vaccination (Panel B). The marginal R2 is the proportion of variance explained by the fixed effects relative to the overall variance, and the conditional R2 is the proportion of variance explained by both fixed and random effects relative to the overall variance. Three months after the second dose of vaccine in participants who had not received previous smallpox vaccination, there was no indication of decreased titers with longer intervals between the recommended two doses (Panel C, left); IgG titers were similar regardless of the route of administration (Panel C, center) or HIV status (Panel C, right). For each of the two doses, the route of administration was intradermal (ID), subcutaneous (SC), or a combination of the two routes. Confidence intervals (CI) have not been adjusted for multiplicity and should not be used for hypothesis testing. IQR denotes interquartile range, and LOD limit of detection.
After the two-dose series of vaccines, IgG titers were similar regardless of the route of administration (Fig. 1C [center] and Fig. S4) or HIV status (Fig. 1C [right] and Fig. S5). In persons with HIV infection, CD4+ counts were positively associated with IgG titers (R2 = 0.41), with a median CD4+ count of 682 cells per cubic millimeter (interquartile range, 591 to 876) (Fig. S5).
These findings provide data for policymakers in case of mpox resurgence and the need for reinvigorated education and vaccination campaigns. We observed similar MPXV immunogenicity regardless of the vaccination route or HIV status. The IgG data through 3 months suggest a need for studies to determine whether booster vaccination may be needed for longer-term immunity and the correlation of the antibody titer with vaccine protection.
Supplementary Material
Acknowledgments
Supported by the Blavatnik Family Foundation, by grants (AI148574 and 75N93021C00014, to Dr. Mulligan) from the National Institutes of Health, by the New York City Department of Health and Mental Hygiene, and by the NYU Grossman School of Medicine.
Footnotes
The findings and conclusions in this letter are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
Contributor Information
Angelica C. Kottkamp, NYU Grossman School of Medicine, New York, NY
Marie I. Samanovic, NYU Grossman School of Medicine, New York, NY
Ralf Duerr, NYU Grossman School of Medicine, New York, NY
Aaron L. Oom, NYU Grossman School of Medicine, New York, NY
Hayley M. Belli, NYU Grossman School of Medicine, New York, NY
Jane R. Zucker, New York City Department of Health and Mental Hygiene, New York, NY
Jennifer B. Rosen, New York City Department of Health and Mental Hygiene, New York, NY
Mark J. Mulligan, NYU Grossman School of Medicine, New York, NY
References
- 1.City of Chicago. Resurgence of mpox — provider update: May 9, 2023. CHI Health Alert Network, May 9, 2023. (https://www.chicagohan.org/alert-detail/-/alert-details/46678186). [Google Scholar]
- 2.World Health Organization. Multi-country outbreak of mpox, external situation report #15. February 2, 2023. (https://www.who.int/publications/m/item/multi-country-outbreak-of-mpox--external-situation-report-15--2-february-2023).
- 3.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol 2003; 171: 4969–73. [DOI] [PubMed] [Google Scholar]
- 4.Overton ET, Stapleton J, Frank I, et al. Safety and immunogenicity of modified vaccinia Ankara-Bavarian Nordic smallpox vaccine in vaccinia-naive and experienced human immunodeficiency virus-infected individuals: an open-label, controlled clinical phase II trial. Open Forum Infect Dis 2015; 2: ofv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frey SE, Wald A, Edupuganti S, et al. Comparison of lyophilized versus liquid modified vaccinia Ankara (MVA) formulations and subcutaneous versus intradermal routes of administration in healthy vaccinia-naïve subjects. Vaccine 2015; 33: 5225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


