Abstract
Objectives
Post-infarction ventricular septal defect (VSD) is one of the known complications after acute myocardial infarction. This study investigated the clinical results after surgical repair of VSD.
Methods
This retrospective study included all patients undergoing surgical repair of VSD from 1996 to 2020 in Oman.
Results
Out of a total of 75 patients, 62.5% were men, with a mean age of 59 years. The mean follow-up was 17.2 (7.5) years. Of the 75 patients, 34 (45.3%) patients died within 30 days. Total survival was 41.3% at 5 years, while the 10-year survival rate was 33.3%. Outcomes and predictors for 30 days mortality were the number of concomitant coronary involvement and anastomoses performed, residual postoperative shunt and postoperative dialysis.
Conclusion
Even with surgical repair, early mortality of post-infarction septal defect is still considerably high. Early repair and the anatomically posterior rupture are predictors of early mortality. In patients surviving the immediate postoperative period, long-term survival is limited by pre-existing coronary artery disease, postoperative renal failure and the presence of a residual postoperative shunt.
Keywords: Post Infarction Heart Rupture, Ventricular Septal Rupture, Coronary Artery Bypass Grafting, Mortality, Oman
Advances in Knowledge
- The timing for surgical intervention remains a critical factor in the overall prognosis of patients with post-myocardial infarction (MI) ventricular septal defect (VSD).
- The current approach still recommends that immediate intervention either surgical or device closure is vital to prevent irreversible haemodynamic collapse and multiorgan failure. However, the mortality is still very high using this approach.
- When the ventricular septal rupture (VSR) is large (>15mm), immediate surgical intervention is indicated over percutaneous closure as there are chances of device embolisation and residual shunting. In some cases or where the patient is contraindicated for surgery, these types of VSR can be closed by transcatheter PMI, AMVSD and Amplatzer septal ocluders.
Application to Patient Care
- To the best of the authors’ knowledge the surgical repair of post-infarction VSR is associated with a high risk of early mortality and late mortality; this risk has remained unchanged during the last 2–3 decades.
- This study found that delayed surgery was associated with better survival.
Post-infarction ventricular septal defect (vsd) is one of the outcomes as well as a known fatal complication following acute myocardial infarction (MI).1 The incidence is low and approximately 0.2% of all patients with an MI develop post-infarction-VSD.2,3 It is very well documented that the mortality with sole medical treatment without surgical intervention is extremely high (over 90%).2,4,5 There are a few studies on spontaneous closure of this type of MI complication, mainly small defects of less than 1 cm.6 In terms of interventional cardiology and anatomically accessible defect, transcatheter closure of VSD were performed in many centres.7,8 However, the fact remains that non-surgical treatment associated with its unacceptably high mortality is generally considered inadequate.9 On the other hand, with surgical intervention the mortality ranges from 19–60%.2,5,10 Cardiogenic shock is a profound circulatory failure, usually due to cumulative myocardial loss of approximately 40% or more and it is secondary to acute myocardial infarction that leads to rapid fall in the haemodynamic status of the patient which makes the surgical management often the only realistic option.8,11–13
There has always been a controversy in the literature regarding associated grafting of the involved coronaries (coronary artery bypass grafting) whether to be performed along with the closure of the VSD repair or not.14 Some researchers documented that the grafting of the involved coronaries (CABG) in these conditions is beneficial and helpful.15,16 Contrary to this, some studies recommend the opposite.15,17 The different complications and parameters, such as the patients’ preoperative haemodynamic state, the morpho-anatomical position of ventricular septal defect and types of operative strategy and techniques as well as the latest advances in assisted mechanical circulatory supports (MCSs), are very critical; therefore, important decisions need to be made in the pathways of the management of such cases and also whether to involve the concomitant grafting of the coronaries and its usefulness.4,10,18
The present study investigated the clinical results after surgical repair of VSD that developed post-MI in all patients in Oman during a 25-year period and to recognise the predictors, risk factors and outcomes for early and late mortality after surgery.
Methods
This retrospective study was conducted at the National Heart Centre (NHC) at the Royal Hospital, Muscat, Oman. The NHC and the Royal Hospital were the only place were major cardiothoracic surgeries and interventional cardiology procedures are carried for all patients in Oman; whereas now other centres also perform them from 2014. However, the majority of cases were operated on at the Royal Hospital and the NHC. Data were gathered regarding preoperative findings and postoperative results after surgical repair. Peri-operative variables were collected from each patient chart. All patients who underwent surgical repair from January 1996 to December 2020 were included. Mortality was calculated and the predictors for early and late mortality were identified.
After complete cardiological assessment, including echocardiography and coronary angiography, patients were operated on through a median sternotomy. All patients were operated using extra corporeal cardiopulmonary bypass (CPB) and moderate hypothermia (28°C). CPB was established after the insertion of aortic, superior and inferior vena cava cannula.
Descriptive statistics were used to summarise the data. For categorical variables, frequencies and percentages were reported. For continuous variables, the mean and standard deviation were used to summarize the data. Data were analysed using STATA, Version 13.1 (STATA Corporation, College Station, Texas, USA) Student’s t-test was used. The level of significance was set at a P value of 0.05.
Ethical Permission for the study was obtained from the Scientific Research Committee at the Royal Hospital.
Results
This study included a total of 75 patients, 62.7% (n = 44) were men. The mean age was 59 years and an age range was 47–73 years. Patient pathways of selection, surgical repair and technique were determined by local protocols. No patient was taken for percutaneous closure. Patient characteristics and associated risk factors are reported in Table 1.
Table 1.
Patient characteristics and associated risk factors
| Characteristic | n (%) |
|---|---|
| Patient number | 75 (100) |
| Mean age in years | 59 |
| Male/female ratio (% male) | 1.7:1 (62.7) |
| Previous CAD | 12 (16.0) |
| Hypertension | 31 (41.3) |
| Obesity | 37 (49.3) |
| Hypercholesterolemia | 34 (45.3) |
| Smoking | 15 (24.0) |
| Family history CAD | 20 (26.7) |
CAD = coronary artery disease.
Pre-operatively, 68 patients were in New York Heart Association (NYHA) class IV, while 7 were in class III. A preoperative transthoracic echocardiography was done in all patients. The anatomical region of the infarction and subsequent perforation of the septum was anterior in 53 (70.7%) patients and postero-inferior in 19 (25.3%) patients. A total of 3 (4%) patients had both anterior and inferior wall infarct. Medical history revealed the presence of obesity in 37 (49.3%), hypertension in 31 (41.3%), smoking in 15 (20%), diabetes mellitus in 28 (37.3%), previous myocardial infarct in 12 (16%) and family history of coronary artery disease in 15 (20%).
The median time from the onset of MI to VSD diagnosis was 4 days and the median time from diagnosis to surgical repair was 1 day. The percentage of patient operated within one week after diagnosis of MI were 79%, 14% between 1–3 weeks and 5% after 3 weeks. All the patients had preoperative coronary angiogram and 63% underwent associated coronary artery bypass grafting with the defect repair.
The preoperative haemodynamic and myocardial variables are shown in Table 2. A total of 68 (90.7%) patients presented with pulmonary oedema and cardiac failure. All patients needed dopamine, dobutamine and/or adrenaline 56 (75%) patients to support the failing myocardium. Thrombolytic therapy (streptokinase) was received by 40 (53.3%) patients prior to referral for cardiac surgery. A total of 44 (58.7%) patients needed counter pulsation with an intra-aortic balloon pump (IABP) preoperatively while 25 (33.3%) needed IABP intraoperatively; 6 (8%) patients did not need an IABP at all during the entire course of events. There were 6 (8%) patients who were ventilated preoperatively. All patients were taken for cardiac catheterisation after the initial MI or after VSD rupture and haemodynamic deterioration. Haemodynamic monitoring was done through indwelling arterial cannula, internal jugular cannula (all patients) and Swan Ganz catheter (last 37 patients). During cardiac catheterisation, oximetry and haemodynamic assessment were done. The target vessels were also identified for coronary artery revascularisation. Written consent was taken from the patients after explaining the high risk of the operation. A total of 59 (78.7%) patients were operated within the first week from the onset of ventricular septal rupture. Table 2 shows the preoperative haemodynamic and myocardial variables.
Table 2.
Preoperative haemodynamic and myocardial variables (N = 75)
| Variable | n (%) |
|---|---|
| Anterior wall MI | 53 (70.7) |
| Inferior wall MI | 19 (25.3) |
| Single vessel disease | 47 (62.7) |
| Double vessel disease | 12 (16) |
| Triple vessel disease | 15 (20) |
| LAD lesion | 71 (94.7) |
| RCA lesion | 25 (33.3) |
| Preop shock | 68 (90.7) |
| Preop IABP | 44 (58.6) |
| Preop PTCA | 3 (4) |
MI = myocardial infarction; LAD = left anterior descending; RCA = right coronary artery; IABP = intra-aortic balloon pump; PTCA = percutaneous transluminal coronary angioplasty.
After complete cardiological assessment, including echocardiography and coronary angiography, patients were operated on through a median sternotomy. All patients were operated using extra corporeal cardiopulmonary bypass (CPB) and moderate hypothermia (28°C). CPB was established after insertion of aortic, superior and inferior vena cava cannula. After cardiopulmonary bypass was established, cardioplegic arrest was achieved through antegrade cold blood cardioplegia. In the last 12 (16%) patients, retrograde cold blood cardioplegia was also given. Initially the distal anastomosis was performed and then the VSD was approached. Internal mammary artery was not used in any patient as the patients were very unstable. Hence, vein grafts were used as conduits in all patients.
The septal defect was managed and manipulated through the infarcted area of myocardium. All VSDs were approached through the left ventricle. The necrotic muscle around the VSD was carefully debrided back to healthy myocardium. The VSD was then patched using various materials such as Dacron 4 (5.3%), Teflon 4 (5.3%) or bovine 5 (20.8%) or native pericardium 11 (14.6%). The ventriculotomy was then closed using Teflon strips to support the suture line, beyond the infarcted myocardium. This was done using interrupted 3-0 prolene sutures reinforced with Teflon strips on each side. Concomitant CABG was done in 11 (14.6%) patients. CABG was not done in those patients when the LAD was too close to the ventriculotomy suture line. Apical amputation was not done in any patient. Inspection of the mitral valve apparatus was done in all patients. Also, the MV was tested for leak using saline flush. No patients needed any mitral valve repair or replacement.
A slightly different technique was used in the last 11 (14.6%) patients. A large native pericardial patch was sutured to the ventricular septum far away from the margins of the VSD. Bio-glue was injected into the space between the pericardial patch and the septum; this was then reinforced with a second Dacron patch sutured over and over the previous pericardial patch finally. The ventriculotomy was closed along with the protruding pericardial patch with Teflon strips on either side of the suture line. On the other 40 patients the triple patch technique was used. In general, this technique uses 3 patching techniques (1 patch technique with exclusion of infarcted tissues, 2 patch technique reinforced with bio-glue and suturing, finally 3 patch technique). The various surgical approaches and techniques are illustrated in Figures 1–4.
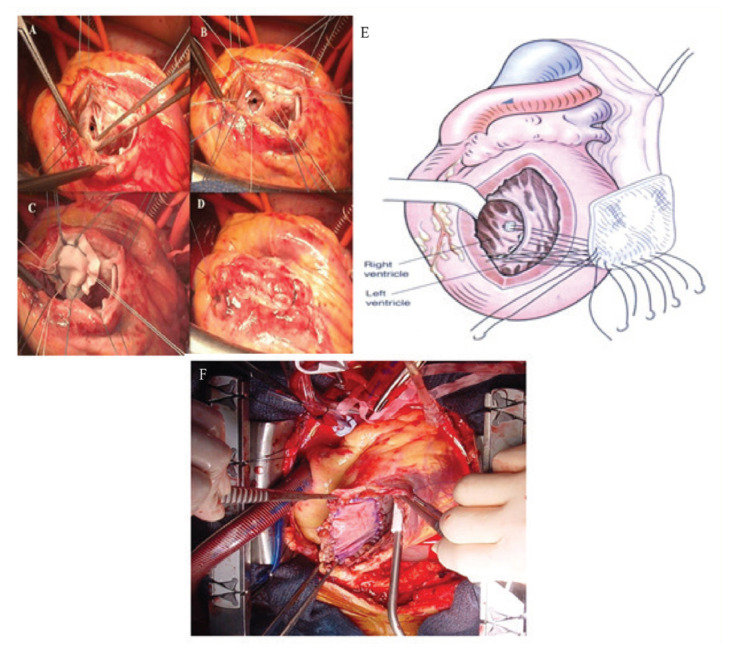
Figure 1.
A–D: Four panels showing the ventricular septum rupture repaired by trimming the necrotic and damaged myocardium and repaired by using a single patch for tension free repair. The ventriculotomy closed with Teflon felt strip and pledgeted suture. E: Drawing showing a single patch insertion with a pledgeted suture. F: Surgical view of the repaired of antero-apical post-infarction ventricular septal defect by pericardial patch.
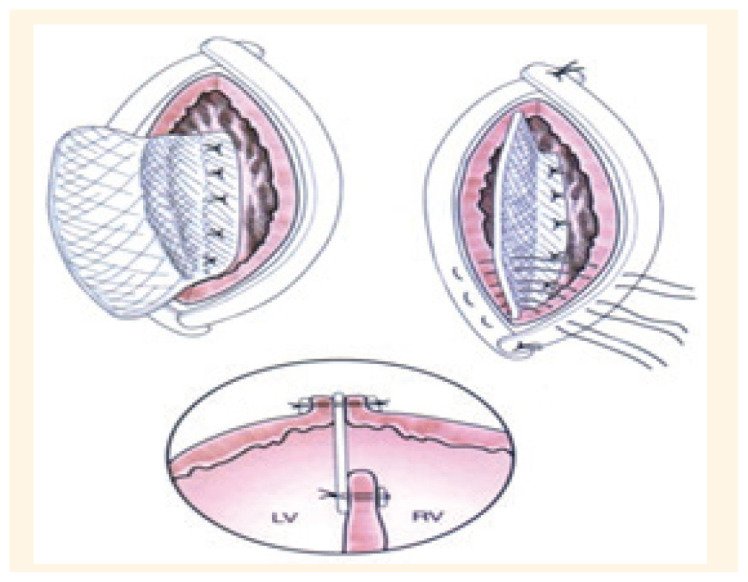
Figure 2.
Drawing of exclusion of infarction tissue with a single patch technique and the closure the edge of the ventricle with 2 Teflon felt including the single patch within them.
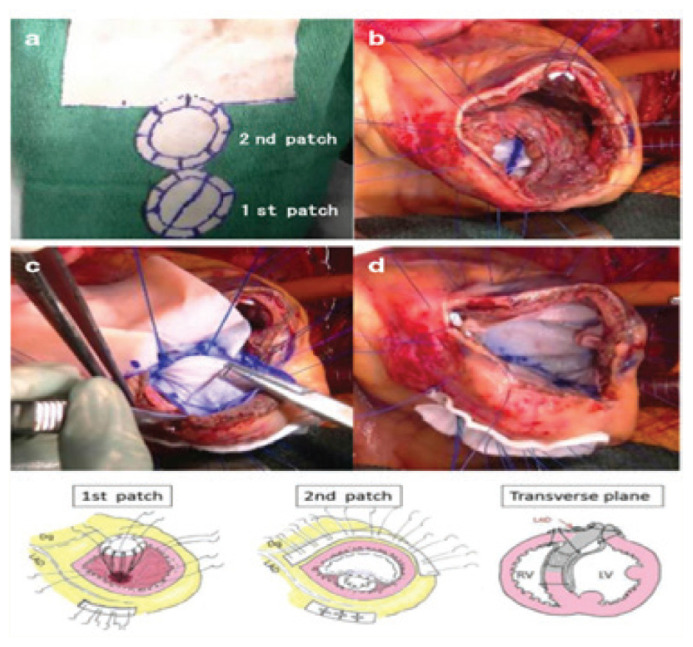
Figure 3.
Surgical photographs showing (A) the two patch (double) technique being made to sandwich the septum evenly with an 8– needle mattress, (B) the first patch covering the ventricular septal defect, (C) a set of needle threads penetrating the ventricular septum passing through the second patch and (D) the patch was trimmed to exclude the infarcted muscle from the left ventricular cavity. The outcome is shown in the drawing below and in the transverse plan.
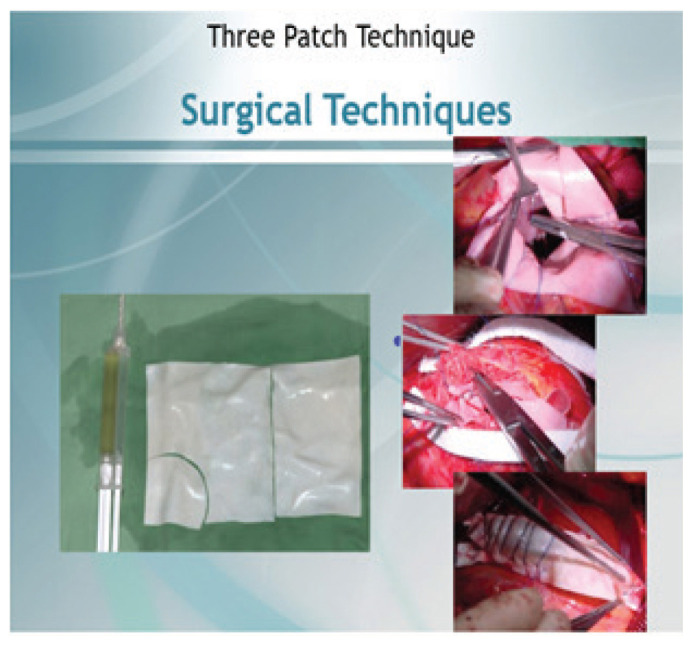
Figure 4.
Images of the triple patch technique.
Once CPB was terminated, inotropic support and IABP were used as indicated to stabilise the patient in the immediate postoperative period. Atrial and ventricular epicardial pacing wires were sutured in place in all patients. As 2 patients were stable pre-operatively, IABP was not inserted then. They also came off CPB effortlessly and remained very stable. Hence, IABP was not inserted in these 2 patients at all.
The 30-day hospital mortality post-MI-VSD was 45.3%; 2 patients could not be weaned off cardiopulmonary bypass and died on the operating table. These 2 patients were in cardiogenic shock, on ventilator and IABP and had altered renal function preoperatively. All the patients that died had old MI, poor ejection fraction, were in NYHA Class IV, needed IABP and were on high inotropic supports.
All these patients had multiorgan failure in the postoperative period; 28 (37.3%) patients needed continuous veno-venous haemodialysis to maintain optimal renal parameters. A total of 6 (8%) patients had gastrointestinal bleeding which was controlled medically and 24 (32.0%) patients developed ventricular tachy-arrhythmias that were controlled pharmacologically.
Overall, 18 (24%) patients had small residual defect postoperatively of which 3 died. Percutaneous closure of these defects could have improved their outcome. However, this could not be done as the facility was unavailable at our centre at that time. A total of 6 (8%) patients had mild mitral valve regurgitation in the postoperative echocardiography which was treated with vasodilators.
When analysing the death of the 3 patients with residual VSD in postoperative bedside echocardiography its associated with poor haemodynamics, low cardiac output, deranged coagulation profile and multiorgan failure; these patients died in the first week. These patients were too critical to undergo surgical closure; if a percutaneous device closure was available, the prognosis could have been different.
Of the total 75 patients, 15 were operated on more than a week after diagnosis, details of which are shown in Table 3. In this group of patients, 3 patients that expired had old inferior wall myocardial infarction, 3 were in pulmonary oedema preoperatively and 3 underwent concomitant CABG for one vessel.
Table 3.
Details of the 15 patients, who were operated more than a week after diagnosis with post-myocardial infarction ventricular septal defect
| No. | Time between diagnosis and surgery in days | Outcome | Coronary artery bypass surgery | Reason for delay | Associated risk factors |
|---|---|---|---|---|---|
| 3 | 10 | Survived | No | Delay in diagnosis | Smoking |
| 3 | 10 | Survived | CABGX1 | Delay in diagnosis | Hypertension |
| 3 | 20 | Survived | No | No consent | Old IWMI |
| 3 | 26 | Survived | No | Delay in diagnosis | Smoking |
| 3 | 9 | Died | CABGX1 | No consent | Old IWMI |
CABG = coronary artery bypass surgery; IWMI = inferior wall myocardial infarction.
Table 4 shows the data of patients with NYHA Classes and survival at 30 days and up to 25 years of follow-up after surgery. At 1 year follow-up 41 (54.6%) patients had survived; 9 (12%) were in NYHA class III while 32 (42.6%) were in NYHA class II. At 5 years follow-up, 31 (41.3%) patients had survived, all were in NYHA Class II. These patients were sent back to their respective peripheral hospitals for follow-up. At 10–25 years follow-up, 25 (33.3%) patients had survived and are all in NYHA Class II. These patients are clinically well and have not been prescribed digitalis or diuretics. All these patients receive aspirin, simvastatin and drugs for hypertension; 2 patients needed intermittent haemodialysis for altered renal function and 2 patients were lost to follow-up. Since these patients are in remote territories, it has not been possible to contact them. The causes of mortality of the expired patients mentioned earlier included pre-existing coronary artery disease, postoperative renal failure and dialysis, the presence of a residual postoperative shunt with other pre-existing preoperative and postoperative associated complications explained previously.
In summary, the total mortality within 30 days were 34 (45.3%) patients and the remaining 41 patients survived more than 30 days. At the 1-year follow-up 41 (54.6%) patients survived, at 5-year follow-up 31 (41.3%) patients had survived and at 10–25 years follow-up 25 (33.3%) patients had survived. Most cases of death that occurred within 30 days were because of poor haemodynamics, low cardiac output, deranged coagulation profile and multiorgan failure.
Discussion
This retrospective longitudinal cohort analysis study described the experience at our centre with post-infarction VSD and its known lethal complication after acute MI. The primary objective of the study was to recognise the predictors, risk factors and outcomes for early and late mortality after surgery and the effect of associated grafting of the coronaries.
Post infarction VSD occurs in 1–2% cases of MI and is the cause of 5% mortality after MI.8,19 With the advent of aggressive pharmaceutical treatment, prompt control of hypertension, thrombolytic interventional therapy, the early detection and treatment of acute MI has reduced the incidence of post-MI VSD to less than 1% during the past decade.3,20 In the present study, there were only 75 cases in a time span of 25 years.
Various studies and researchers have revealed that 25% of patients with post-infarction septal defects who do not receive surgical intervention died within the first 24 hours, and approximately 50% of the patients with post-infarction VSD died within 1 week, 65% of these patients died within 2 weeks if left without surgical intervention and 80% within 4 weeks; 7% lived longer than 1 year, most probably these patients had small defects less than 1 cm.4,21 On the other hand, researchers have reported that almost up to 75% of patients with post-infarction VSDs died within 1 month.10,19,21 All these findings and reports indicate that the risk of death after post-infarction VSD peaks immediately after infarction and septal rupture and then gradually declines.22 Also, emergent surgical intervention is indicated despite the associated mortality and morbidity.23
In the current study, 10 (52.6%) of the 19 patients who were operated on in less than 7 days, died. In comparison, only 1 (20%) of the 5 patients operated within 7 days after septal perforation died. This shows that improvement in the preoperative haemodynamic status does aid the overall prognosis positively.24 Researchers found that a short waiting time after the diagnosis of post-MI VSD helps development of a better and stronger tissue scar, improving the outcomes of the repair and decreasing the risk of recurrent and/or residual VSD.4,22 However, it is not possible to wait in every single case of post-MI VSD until the haemodynamic condition improves.8,22,25 An unstable patient with low cardiac output, on ionotropic supports and IABP is the usual scenario when a patient gets a post-MI VSD, where emergent surgery is indicated.23
The hospital mortality in the current study is comparable to other studies (20–50%).2,8,22 It has been stated that a hospital mortality rate of 33% for surgical and 42% mortality for percutaneous closure of post-MI VSD.5,24 In the current study the hospital mortality rate was 45%. Other studies have found that approximately 60% of patients with post-MI VSD had severe congestive heart failure and low cardiac output which required intensive care unit stay and therapy.5,24 In the current study, 91.6% patients presented in cardiogenic shock and all needed inotropic support while 54.1 % were on IABP support preoperatively and 33% needed IABP insertion intra-operatively.
Concomitant CABG during closure of post-MI VSD has been a subject of controversy.3,20 Patients who had undergone coronary angiography underwent repair of a post-infarction ventricular septal defect had significantly increased long-term survival compared with patients with unbypassed coronary artery disease.10,18,26 Other groups of researchers in the literature found that concomitant CABG results in additional cross-clamp and CPB time for a patient with compromised LV function following a post-MI VSD which may lead to further complications and affect the outcome.10,18,21,26
Dalrymple-Hay et al. stated that the outcome of CABG, in the same setting of the post-infarction VSD repair, remains questionable and controversial.14 A total of 179 patients have undergone repair of a post-MI VSD, of which 40 had CABG concomitant with VSD repair.10,18,19 They reported that the 30-day mortality was 32% and found that concomitant CABG did not decrease operative death or mortality. It was concluded that coronary bypass grafting with post-infarction VSD repair does not affect early mortality nor the survival benefits.10,14,19,21 These findings demonstrated no benefit in revascularisation at the time of repair and, therefore, accordingly it may be unnecessary to perform CABG or coronary angiography in these patients.14,17,27
In the current study, it was found to be difficult to graft the LAD artery in presence of a left ventriculotomy. Despite leaving an adequate margin, prior to ventriculotomy, the pledgeted sutures taken to close the ventriculotomy were near the LAD. Also, the anterior surface of the left ventricle looked too unhealthy to benefit from a graft. In such a situation, the LAD was not grafted. A total of 4 of the 11 patients who died had undergone CABG for a single vessel. However, 7 of the 11 patients who underwent CABG did survive. This anastomosis takes only a few minutes, thus not affecting the cross-clamp time significantly. The current study also found that associated CABG may not affect the immediate postoperative outcome significantly.
Many publications have implicated that the cases who survived the surgical interventions had a good long-term prognosis.10,18,28,29 They reported, among the patients who survived the first 30 days postoperatively, a long-term mortality of only 6%. Researchers have also reported a late survival for patients who survived the surgical repair (69% at 5 years, 50% at 10 years and 37% at 14 years).10,30 In the current study, at 1-year follow-up 41 (54.6%) patients, at 5-year follow-up 31 (41.3%) patients and at 10–25-year follow-up 25 (33.3%) patients had survived and are were in NYHA Class II. These patients are clinically well and have not been prescribed digitalis or diuretics.
After an acute post-MI VSD has developed, it is known by evidence and that the edges of the VSD are extremely friable.20,28 This results in the stitches cutting through and leaving behind a residual VSD resulting in low cardiac output postoperatively. If possible, waiting for a short period after the diagnosis will improve the tissue quality and allow the margins of the infracted muscle to develop a firmer scar, which can facilitate successful surgical VSD closure, thus preventing residual VSD.4,22,31 However, some studies have reported a better survival after immediate surgical intervention.2,22,28,29
Current guidelines by both the American College of Cardiology-American Heart Association and European Society of Cardiology recommend immediate intervention in patients with post-MI VSD, irrespective of the clinical condition.23,32 In the current study, surgical closure of VSD was done a week after VSD diagnosis in 20%. Of the 5 cases that were operated on more than a week after diagnosis of post-infarction VSD, only 1 died. In contrast, 10 of the 19 patients who were operated in the first week died. Though, waiting for the parameters to improve or the VSD edges to heal is of help, this may not be the best strategy for all patients.8 At the same time, the complications of operating on extremely sick patient can outweigh the benefits promised by surgery.8,10,27 The decision for timing of operative intervention can vary slightly from case to case.23
The time from MI to detection VSD and the postoperative residual VSD have been reported to be significant predictors of death.4,19,22 In the current study 18 patients (24%) had residual shunt of which 3 died of multiorgan failure. It’s clearly documented in the literature that the preoperative haemodynamic status plays a more important role than the shunt size or even the ejection fraction in the overall postoperative outcome.22,25 However, trials efforts to stabilise the patient’s condition with medical therapy result in deterioration and even death if no surgical intervention is done.8,22,25 Hence, early surgical intervention is necessary.
Post-infarction catheter-based devices closure of the VSD percutaneously has been reported as an optional treatment modality instead of surgery if possible depending on the site and the size of the defect.11,24,33 Complications of percutaneous techniques include migration of device into pulmonary artery, rupture of right ventricle, tamponade and low cardiac output.28 Friable margins and large size of a post-MI VSD cause technical difficulties while using the device.22,29 The in-hospital mortality after percutaneous technique has been reported at 42%.5,24,28
Percutaneous technique has also been used for closure of residual VSD after surgical repair.28,33 Researchers suggested the use of device closure to improve and stabilise the haemodynamic state of the patient and to allow time for the myocardium to fibrose and allow for better surgical closure.10,20,22
A second open heart surgery for closure of residual defect or revascularisation of an under-perfused area of the heart in an already compromised patient can be avoided.20,27,28 A residual VSD can be closed with device and an angioplasty can help restore myocardial oxygenation.20,24 Hybrid procedures in a post-MI VSD situation can be used to improve the overall outcome of the patient.8,10,20,24,28
In recent times, when device closure is offered as a treatment option for post-MI VSD, hybrid procedures can help to improve patient prognosis. Device closure of a residual VSD after emergent VSD closure can be indicated in cases with critical haemodynamic parameters. This needs a dedicated team of surgeons and interventionists working in close co-ordination. Modern assisting devices and surgical techniques may improve the surgical results and outcomes of post-MI VSD.
In the future, aggressive control of risk factors for prevention of MI, early detection of post-MI VSD, early surgical intervention for deteriorating patient and hybrid procedures can help reduce the mortality and morbidity associated with this potentially lethal situation.
This study was subject to certain limitations. This study collected data from a single centre, but it is the only centre for the whole country. Also, there was no patient taken for device closure. In addition, no comparisons were made between device closure and surgical closure.
Conclusion
The perioperative period of post-MI VSD is usually stormy, needing close monitoring and timely intervention. These patients are usually in cardiac failure, on significant pharmacological supports, IABP and mechanical ventilation. The poor cardiac output puts the patient in an impending or even fully developed multiorgan failure situation. The risk of operating on such a compromised patient is high. Once the patient has been diagnosed, management includes any combination of aggressive medical management, mechanical circulatory support (MCS), surgical repair, transcatheter closure, novel surgical/percutaneous hybrid procedures and palliative care. There remain controversies regarding timing of repair; the optimal timing of treatment remains unclear, with guidelines recommending early intervention whereas published data suggest better outcomes with a delayed approach. The current literature is limited by the rarity of the complication and patient selection bias. Further work is required in this field. Use of early MCS in patients with haemodynamic instability appears to be a promising modality to bridge patients to a decision of delayed repair and chance for the tissues fibrosis to take place for better surgical outcomes and less residual defects.
Footnotes
AUTHORS’ CONTRIBUTION: All authors have contributed equally and approved the final version of the manuscript.
CONFLICTS OF INTEREST: The authors declare no conflict of interests.
FUNDING: No funding was received for this study.
References
- 1.Elkattawy S, Alyacoub R, Noori MAM, Talpur A, Khimani K. A Rare Complication of Myocardial Infarction: Ventricular Septal Defect. Cureus. 2020;12:e9725. doi: 10.7759/cureus.9725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeppsson A, Liden H, Johnsson P, Hartford M, Rådegran K. Surgical repair of post infarction ventricular septal defects: a national experience. Eur J Cardiothorac Surg. 2005;27:216–21. doi: 10.1016/j.ejcts.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 3.Moreyra AE, Huang MS, Wilson AC, Deng Y, Cosgrove NM, Kostis JB, et al. Trends in incidence and mortality rates of ventricular septal rupture during acute myocardial infarction. Am J Cardiol. 2010;106:1095–100. doi: 10.1016/j.amjcard.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Park SJ, Kim JB, Jung SH, Choo SJ, Chung CH, Lee JW. Surgical Repair of Ventricular Septal Defect after Myocardial Infarction: A Single Center Experience during 22 Years. Korean J Thorac Cardiovasc Surg. 2013;46:433–8. doi: 10.5090/kjtcs.2013.46.6.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abbasnejad M, Vand MT, Khamnian Z, Separham A. In-hospital outcome of patients with post-MI VSD: a single-center study. Kardiochir Torakochirurgia Pol. 2018;15:227–32. doi: 10.5114/kitp.2018.80918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mittal CM, Aslam N, Mohan B, Tandon R, Sood N, Wander GS. Spontaneous closure of post-myocardial infarction ventricular septal rupture. Tex Heart Inst J. 2011;38:596–7. [PMC free article] [PubMed] [Google Scholar]
- 7.Shabestari MM, Ghaderi F, Hamedanchi A. Transcatheter Closure of Postinfarction Ventricular Septal Defect: A Case Report and Review of Literature. J Cardiovasc Thorac Res. 2015;7:75–7. doi: 10.15171/jcvtr.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suder B, Janik Ł, Wasilewski G, Konstanty-Kalandyk J, Sadowski J, Kapelak B, et al. Post-myocardial infarction ventricular septal defect. Is it better to operate on a fresh infarction or to wait? A case study. Kardiochir Torakochirurgia Pol. 2016;13:39–41. doi: 10.5114/kitp.2016.58963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof E, Fleischmann KE, et al. ACC/AHA 2007 Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery. Circulation. 2007;116:e418–500. [Google Scholar]
- 10.Arnaoutakis GJ, Zhao Y, George TJ, Sciortino CM, McCarthy PM, Conte JV. Surgical repair of ventricular septal defect after myocardial infarction: outcomes from the Society of Thoracic Surgeons National Database. Ann Thorac Surg. 2012;94:436–44. doi: 10.1016/j.athoracsur.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schlotter F, de Waha S, Eitel I, Desch S, Fuernau G, Thiele H. Interventional post-myocardial infarction ventricular septal defect closure: a systematic review of current evidence. EuroIntervention. 2016;12:94–102. doi: 10.4244/EIJV12I1A17. [DOI] [PubMed] [Google Scholar]
- 12.Van de Werf F, Ardissino D, Betriu A, Cokkinos DV, Falk E, Fox KA, et al. Management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2003;24:28–66. doi: 10.1016/s0195-668x(02)00618-8. [DOI] [PubMed] [Google Scholar]
- 13.Van de Werf F, Bax J, Betriu A, Blomstrom-Lundqvist C, Crea F, Falk V, et al. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: The Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology. Eur Heart J. 2008;29:2909–45. doi: 10.1093/eurheartj/ehn416. [DOI] [PubMed] [Google Scholar]
- 14.Dalrymple-Hay MJ, Langley SM, Sami SA, Haw M, Allen SM, Livesey SA, et al. Should coronary artery bypass grafting be performed at the same time as repair of a post-infarct ventricular septal defect? Eur J Cardiothorac Surg. 1998;13:286–92. doi: 10.1016/s1010-7940(98)00010-4. [DOI] [PubMed] [Google Scholar]
- 15.Hawkes AL, Nowak M, Bidstrup B, Speare R. Outcomes of coronary artery bypass graft surgery. Vasc Health Risk Manag. 2006;2:477–84. doi: 10.2147/vhrm.2006.2.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomson P, Niven CA, Peck DF, Eaves J. Patients’ and partners’ health-related quality of life before and 4 months after coronary artery bypass grafting surgery. BMC Nurs. 2013;12:16. doi: 10.1186/1472-6955-12-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eagle KA, Guyton RA, Davidoff R, Ewy GA, Fonger J, Gardner TJ, et al. ACC/AHA Guidelines for Coronary Artery Bypass Graft Surgery: Executive Summary and Recommendations. Circulation. 1999;100:1464–80. doi: 10.1161/01.cir.100.13.1464. [DOI] [PubMed] [Google Scholar]
- 18.Barker TA, Ramnarine IR, Woo EB, Grayson AD, Au J, Fabri BM, et al. Repair of post-infarct ventricular septal defect with or without coronary artery bypass grafting in the northwest of England: A 5-year multi-institutional experience. Eur J Cardiothorac Surg. 2003;24:940–6. doi: 10.1016/s1010-7940(03)00465-2. [DOI] [PubMed] [Google Scholar]
- 19.Deja MA, Szostek J, Widenka K, Szafron B, Spyt TJ, Hickey MS, et al. Post infarction ventricular septal defect – can we do better? Eur J Cardiothorac Surg. 2000;18:194–201. doi: 10.1016/s1010-7940(00)00482-6. [DOI] [PubMed] [Google Scholar]
- 20.Jones BM, Kapadia SR, Smedira NG, Robich M, Tuzcu EM, Menon V, et al. Ventricular septal rupture complicating acute myocardial infarction: a contemporary review. Eur Heart J. 2014;35:2060–8. doi: 10.1093/eurheartj/ehu248. [DOI] [PubMed] [Google Scholar]
- 21.Khan MY, Waqar T, Qaisrani PG, Khan AZ, Khan MS, Zaman H, et al. Surgical Repair of post-infarction ventricular septal rupture: Determinants of operative mortality and survival outcome analysis. Pak J Med Sci. 2018;34:20–6. doi: 10.12669/pjms.341.13906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malhotra A, Patel K, Sharma P, Wadhawa V, Madan T, Khandeparkar J, et al. Techniques, Timing & Prognosis of Post Infarct Ventricular Septal Repair: a Re-look at Old Dogmas. Braz J Cardiovasc Surg. 2017;32:147–55. doi: 10.21470/1678-9741-2016-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2017;39:119–77. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 24.Assenza GE, McElhinney DB, Valente AM, Pearson DD, Volpe M, Martucci G, et al. Transcatheter Closure of Post-myocardial Infarction Ventricular Septal Rupture. Circ Cardiovasc Interv. 2013;6:59–67. doi: 10.1161/CIRCINTERVENTIONS.112.972711. [DOI] [PubMed] [Google Scholar]
- 25.Labrousse L, Choukroun E, Chevalier JM, Madonna F, Robertie F, Merlico F, et al. Surgery for post infarction ventricular septal defect (VSD): Risk factors for hospital death and long term results. Eur J Cardiothorac Surg. 2002;21:725–32. doi: 10.1016/s1010-7940(02)00054-4. [DOI] [PubMed] [Google Scholar]
- 26.Muehrcke DD, Daggett WM, Jr, Buckley MJ, Akins CW, Hilgenberg AD, Austen WG. Postinfarct ventricular septal defect repair: effect of coronary artery bypass grafting. Ann Thorac Surg. 1992;54:876–82. doi: 10.1016/0003-4975(92)90640-p. [DOI] [PubMed] [Google Scholar]
- 27.Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction) Circulation. 2004;110:588–636. doi: 10.1161/01.CIR.0000134791.68010.FA. [DOI] [PubMed] [Google Scholar]
- 28.Baldasare MD, Polyakov M, Laub GW, Costic JT, McCormick DJ, Goldberg S. Percutaneous repair of post-myocardial infarction ventricular septal defect: current approaches and future perspectives. Tex Heart Inst J. 2014;41:613–19. doi: 10.14503/THIJ-13-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coskun KO, Coskun ST, Popov AF, Hinz J, Schmitto JD, Bockhorst K, et al. Experiences with surgical treatment of ventricle septal defect as a post infarction complication. J Cardiothorac Surg. 2009;4:3. doi: 10.1186/1749-8090-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menon V, Webb JG, Hillis LD, Sleeper LA, Abboud R, Dzavik V, et al. Outcome and profile of ventricular septal rupture with cardiogenic shock after myocardial infarction: a report from the SHOCK Trial Registry. J Am Coll Cardiol. 2000;36:1110–16. doi: 10.1016/s0735-1097(00)00878-0. [DOI] [PubMed] [Google Scholar]
- 31.Yang C, Sun Y, Zou D, Sun Z, Zhang X, Su G, et al. Transcatheter closure of ventricular septal rupture with prolonged support of intra-aortic balloon pump after primary PCI: a case report. BMC Cardiovasc Disord. 2021;21:605. doi: 10.1186/s12872-021-02392-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Damluji AA, van Diepen S, Katz JN, Menon V, Tamis-Holland JE, Bakitas M, et al. Mechanical Complications of Acute Myocardial Infarction: A Scientific Statement From the American Heart Association. Circulation. 2021;144:e16–35. doi: 10.1161/CIR.0000000000000985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abuelatta R, Alrashidy T, Taha F, Naeim HA. Concomitant transcatheter closure of post-myocardial infarction ventricular septal defect and inferior wall aneurysm: Case report. Eur Heart J Case Rep. 2020;4:1–7. doi: 10.1093/ehjcr/ytaa408. [DOI] [PMC free article] [PubMed] [Google Scholar]