Abstract
Despite decades of effort, preservation of complex organs for transplantation remains a significant barrier that exacerbates the organ shortage crisis. Progress in organ preservation research is significantly hindered by suboptimal research tools that force investigators to sacrifice translatability over throughput. For instance, simple model systems, as single cell monolayers or co-cultures, lack native tissue structure and functional assessment, while mammalian whole organs are complex systems with confounding variables not compatible with high throughput experimentation. In response, diverse fields and industries have bridged this experimental gap through the development of rich and robust resources for the use of zebrafish as a model organism. Through this study, we aim to demonstrate the value zebrafish pose for the fields of solid organ preservation and transplantation, especially with respect to experimental transplantation efforts. A wide array of methods were customized and validated for preservation-specific experimentation utilizing zebrafish, including the development of assays at multiple developmental stages (larvae and adult), methods for loading and unloading preservation agents, and the development of viability scores to quantify functional outcomes. Using this platform, the largest and most comprehensive screen of cryoprotectant agents (CPAs) was performed to determine their toxicity and efficiency at preserving complex organ systems using a high subzero approach called partial freezing (i.e. storage in the frozen state at −10°C). As a result, adult zebrafish heart cardiac function was successfully preserved after 5 days of partial freezing storage. In combination, the methods and techniques developed have the potential to drive and accelerate research in the field of solid organ preservation and transplantation.
Graphical Abstract
Zebrafish as a high-throughput, relevant model organism for heart cryopreservation research.
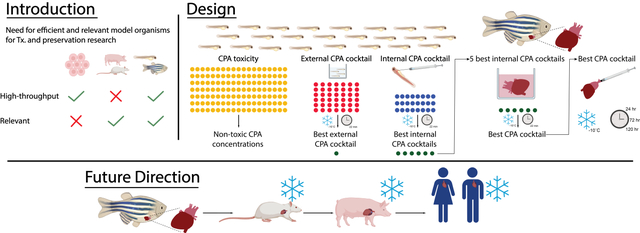
Introduction
Transplantation is the standard of care for end-stage organ failure as it increases quality of life and life expectancy of patients with chronic end-stage diseases [1, 2]. Although it is a successful treatment, its impact has been significantly hindered by a severe shortage of donor organs, resulting in over 100,000 people on the transplant waiting list in the United States alone, leading to major concerns about equitable access [3–5]. These difficulties arise in part from the inability to adequately preserve vascularized tissues over long periods of time and ensure their viability upon transplantation [3]. Furthermore, such constraints not only significantly limit the field of transplantation, but also heavily stymie a wide range of biomedical advances including research into more effective utilization of cadaveric donors, xenotransplantation, tissue engineering approaches, and laboratory-grown cell, tissue, and organs [6].
Cryopreservation aims to target the duration constraints of vascularized tissues by utilizing a wide array of below freezing temperatures techniques including vitrification, supercooling, isochoric sub-freezing, equilibrium non-frozen and partial freezing [4, 6–10]. Each of these techniques feature significantly different technical parameters (storage temperatures, cooling and thawing rates, applied pressures, etc.) with practically infinite number of protective molecules (cryoprotectant agents – CPAs) which can be chosen for their physicochemical properties or biological activity. This wide array of technical parameters results in a plethora of mechanistic effects, leading to different organ thermodynamic properties and a myriad of required optimizations indispensable for the success of organ preservation. Thus, the design of whole organ preservation protocols is a highly complex problem that would oblige screening of large numbers of combinations of candidate CPAs, supplements, and technical parameters.
The biggest hinderer of organ preservation advancements is the inaccessibility of high throughput, yet translationally relevant model organisms. Mammalian models, both large and small, provide the desired translation but lack high throughput capacity due to high costs (i.e. surgical facilities, anesthesia and post-transplant care) and experimental complexity (clinical, surgical and microsurgical skills). Alternatively, single cell monolayers or multiple cell co-cultures allow high throughput experimentation but are unable to interrogate functionality, as they lack important structural parameters present on tissues (cell-cell interaction, cell-scaffold interactions, etc.). Furthermore, the cellular diversity of tissues, and their accompanying variable responses to stimulus, can only be replicated in low throughput tissue engineering models, and even still they fail to fully recapitulate tissue function.
This work proposes the zebrafish (Danio rerio) as the ideal model organism to efficiently and effectively screen thousands of possible compounds/protocol parameters made possible by their perfect balance between translation potential and high throughput experimentation [11–13]. Zebrafish has been extensively used in other research fields due to its significant advantages, including low maintenance and breading costs, high percentage of gene orthology to humans (70% of total genes and 82% of genes associated with human disease), copious embryo production, fast developmental rate (major organs formed within 24 hours), easy genetic manipulation, etc. [11]. Furthermore, due to the popularity of this model, extensive and comprehensive assay development has been achieved using both adults and larvae, which provides a wide array of established scientific tools, surpassing even mouse models, that can be easily adapted for transplantation and preservation research. For instance, high throughput toxicity assays, using zebrafish larvae, have been developed to test the effects compounds pose at the whole organ level and can easily be adapted to test CPA toxicity, an ongoing major challenge in cryopreservation [12, 14, 15]. Additionally, experimental protocols have been developed to maintain adult zebrafish organs in culture with the ability of mimicking many aspects of ex vivo organ handling, common in transplantation research [16]. Therefore, for the fields of cryopreservation and organ transplantation, the zebrafish is an ideal model organism as their intact organs offer the desired tissue complexity while allowing for high throughput experimentation.
The purpose of this study is, therefore, to innovate in solid organ preservation research by investigating the usability of zebrafish as a high throughput model organism; which to our knowledge has not been done before. As the case study, this work focuses on heart preservation via partial freezing due to i) the urgency to improve heart preservation protocols, ii) the ability of zebrafish heart to recapitulate human cardiac function, and iii) the promising results seen previously in partial freezing of rodent and human liver, likely to translate into heart preservation [2, 10, 17, 18]. Partial freezing is a preservation method inspired by freeze-tolerant animals in nature and aims to achieve significant metabolic suppression by coupling high subzero storage temperatures (−10°C) with cellular dehydration, making it particularly difficult to test in classic in vitro models [10]. Therefore, by taking advantage of the versatility of zebrafish as a screening tool, both adult and larvae models were utilized for screening and viability assessments after partial freezing. In tandem, fifty combinations (CPA cocktails) of twelve different CPAs were tested for their ability to preserve heart viability and functionality after extended partial freezing in a scalable, high throughput format with the aim of identifying potential CPA cocktail candidates for the use in long-term storage of cardiac allografts. In addition, new viability assays capable of determining heart functionality, while being amenable to high throughput screening are introduced. In combination, the developed methods and techniques have the potential to drive and accelerate research in the field of cryobiology and solid organ transplantation.
Methods
All reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise stated. Zebrafish (Danio rerio) larvae, and adults were produced, grown, and maintained according to standard protocols approved by the Institutional Animal Care and Use Committees of Massachusetts General Hospital. The zebrafish embryos were produced by adult spawning. Healthy embryos were selected and developed to 3 day-post-fertilization (dpf) larvae for all experiments, unless otherwise stated. All experiments involving larvae were done in E3 water (300 mM NaCl, 10.2 mM KCl, 26.23 mM CaCl2, 20 mM MgSO47H2O) and 0.4% (w/v) tricaine in E3 water was used for anesthesia, unless otherwise stated. Fish lines used were: wild-type AB and Tg(cmlc2:nGFP, mKate-CAAX)fb18Tg. All zebrafish experiments were performed in accordance to the ethical guidelines and were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care under protocol #2018N000091.
Cryoprotectant screening in zebrafish larvae by immersion – CPAs Toxicity:
Individual cryoprotectants (CPAs), dimethyl sulfoxide (DMSO), glycerol and 1-Propanol (PROH) were tested at concentrations ranging from 5% to 50% (v/v). Ethylene glycol was tested from 1 to 30% (v/v). Trehalose, mannitol, lactobionic acid (lactobionate), 3-O-Methyl-d-glucose (3-OMG), dextrose, sorbitol, L-proline and 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) were tested at concentrations ranging from 5 to 500 mM. Raffinose was tested from 5 to 250 mM. Polyethylene glycol (PEG, 35 kDa), dextran (25–45 kDa), Pluronic F68, polyvinylpyrrolidone (PVP), Ficoll70, hydroxyethyl starch (HES) concentrations ranged from 1 to 12.5% (w/v). CPAs toxicity was assessed by incubating three day-post-fertilization larvae (n = 4 per well) in a total volume of 100μL of different concentrations of CPAs in E3 water, 0.4% Tricaine (1:10) and Snomax (1 mg/ml). Larvae were incubated in individual CPAs for 45 mins and their toxicity was determined by assessment of heart rate, analyzed from 10 sec video recordings (Axiocam 105 color, Zen 3.2 Blue Edition, Zeiss, Germany), circulation, and morphology (Pre-Freeze External Composite Viability Score - ).
Cryoprotectant screening in zebrafish larvae by immersion – CPAs Preservation Capability:
After working concentrations of CPAs were stablished, individual CPAs were combined into CPAs cocktails via a Taguchi orthogonal array design (Figure 1F). Zebrafish larvae were immersed (n = 4) in CPAs cocktails for 30 mins, followed by freezing at a rate of −1°C/min in a controlled rate freezer (Planer Kryo 560–16), until reaching −10°C, held for 22 min and thawed as fast as possible in a water bath set to 28°C. Cooling rate was determined by cryopreservation success in other organisms including zebrafish embryos [19]. To avoid edge effect during the thawing process, the outer edges of the 96 well-plate were not used for any of the larval experiments. The efficiency of CPAs cocktails on larvae preservation were determined by pre-freeze and post-thaw viability assessment (External Composite Viability Score - ).
Figure 1:
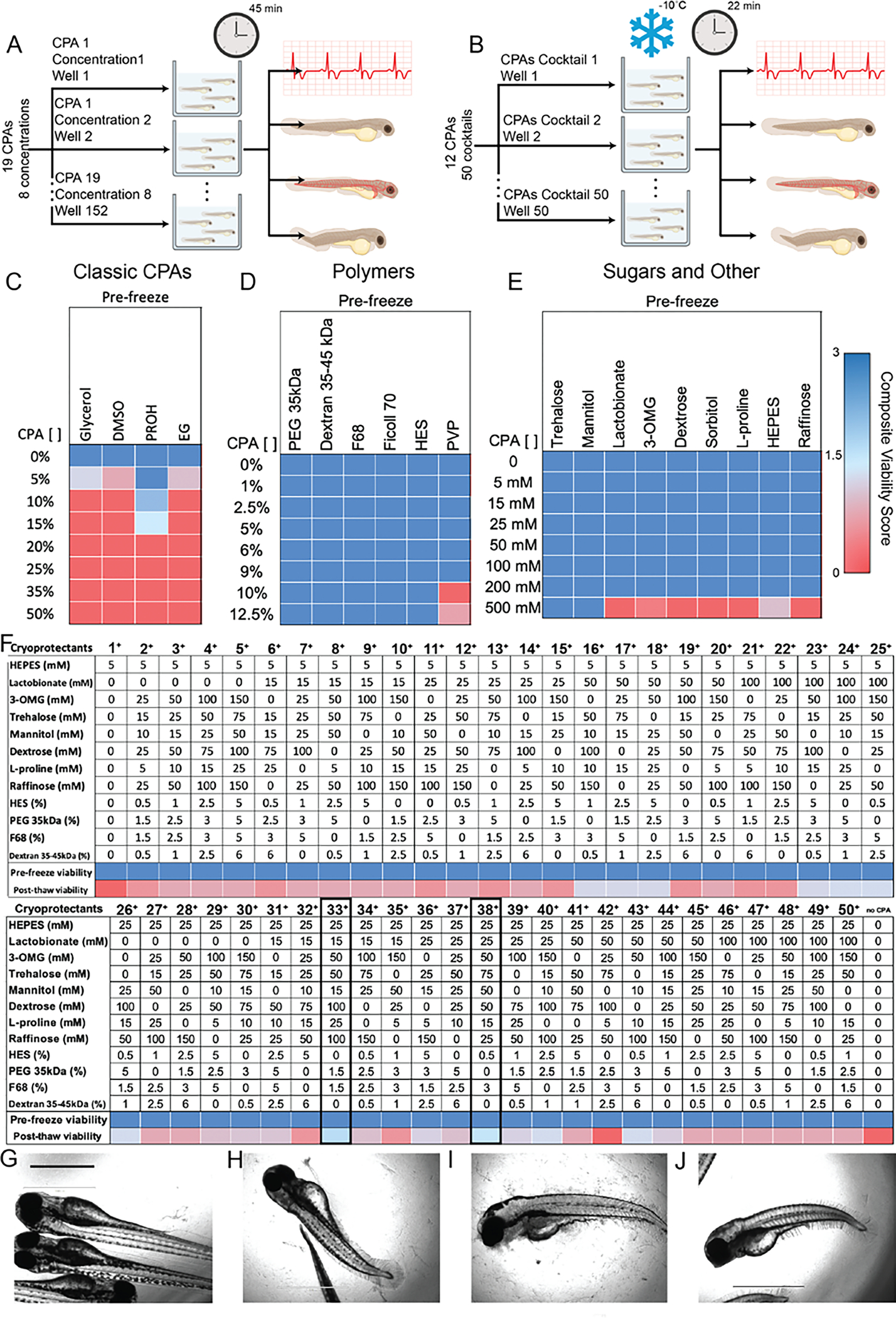
Individual CPAs toxicity Assay (A) and identification of best performing external CPAs cocktail capable of retaining larvae viability after 22 mins of storage at −10°C when immersed in CPAs (B). Larvae viability was determined by quantifying heart rate, dehydration, blood circulation and fish morphology and reported as the External Composite Viability Score (). (C-E) Four classic CPAs, six polymers, seven sugars and two other components (total of 19) were tested to determine their individual toxicity pre-freeze at eight different concentrations by incubating larvae for 45 mins (n = 4 animals per CPAs concentration). (F) Twelve CPAs, from the previously tested nineteen, were arranged in a L50 Taguchi experimental design and tested at different concentrations (7) yielding 50 CPAs cocktails (n = 4 animals per CPAs cocktail). Pre-freeze toxicity was determined after 30 mins of incubation, with CPAs cocktails 33+ and 38+ resulting in the best larvae outcomes as determined by above threshold (>1.5) post-thaw (1.75 and 1.625 respectively). Storage with above the-threshold CPAs cocktails resulted in post-thaw retention of larva morphology (G) compared to untreated/unfrozen larvae (H), whereas storage without CPAs (I) or below the-threshold CPAs (J) resulted in significant loss of morphology and overall embryo integrity. Scale bar 1000 um.
Viability Scoring system – External Composite Viability Score - :
Toxicity and freezing efficiency of CPAs during immersion experiments were assessed by assigning numerical values to qualitative metrics including heart rate, circulation, fish morphology, and dehydration (Table 1). A was calculated for each individual larva by adding the score for each applicable viability parameter and dividing them by the number of viability parameters assessed. Individual for the larvae within each treatment were averaged to provide a single score per CPA/CPAs cocktail.
Table 1: External Composite Viability Score () – CPAs immersion.
CPAs toxicity and their effects in external morphology were determined via the assessment of Pre – Freeze viability parameters. Similarly, CPAs cocktail preservation efficiency of external morphology was determined by the assessment of Pre – Freeze and Post – Thaw viability parameters.
| Viability Parameter |
Morphology • Necrosis in yolk area • Dorsal curvature of the tail • Loss of the caudal fin |
Heartbeat • Beats per minute |
Dehydration • Visible swim bladder • Size of head and eyes |
Circulation • Visible blood movement through the yolk, duct of Cuvier and caudal artery |
||||
|---|---|---|---|---|---|---|---|---|
| Pre – Freeze (CVSpF) | Not preserved – Complete necrosis and/or significant dorsal curvature and/or loss of the caudal fin | 0 | No heartbeat no preserved morphology | 0 | N/A | No circulation and no preserved morphology – Complete lack of visible blood movement | 0 | |
| Somewhat preserved – Some necrosis and/or loss of the caudal fin | 1 | No heartbeat, preserved morphology | 1 | No circulation preserved morphology – Complete lack of blood movement | 1 | |||
| Completely preserved structures | 2 | Slowed heartbeat (< 75 bpm) | 2 | Some circulation – Slow blood movement | 2 | |||
| Normal heartbeat (> 75 bpm) | 3 | Normal circulation – Normal blood movement | 3 | |||||
| Post – Thaw (CVSpT) | Not preserved – Complete necrosis and/or significant dorsal curvature and/or loss of the caudal fin | 0 | Absence | 0 | Severe dehydration – No visible swim bladder and/or significant reduction in head and eye size | 0 | No circulation – Complete lack of visible blood movement | 0 |
| Somewhat preserved – Some necrosis and/or loss of the caudal fin | 1 | Presence | 3 | Some dehydration – Small swim bladder and/or slightly reduce head and eye size | 1 | Some circulation – Slow blood movement | 1 | |
| Completely preserved structures | 2 | No dehydration – Normal swim bladder, normal head and eye size | 2 | Normal circulation – Normal blood movement | 2 | |||
Viability Scoring system – Internal Composite Viability Score – CVSInt:
Toxicity and freezing efficiency of CPAs loaded via microinjection were assessed, as mentioned previously, by assigning numerical values to similar qualitative metrics (Table 2). For the CVSInt, however, morphology was eliminated from the scoring system due to the efficiency of the External CPAs cocktail to retain morphology. Similar to the , an CVSInt was calculated for each individual larva by adding the score for each applicable viability parameters and dividing them by the number of viability parameters (Pre – Freeze 3, Post – Thaw 4) assessed.
Table 2: Internal Composite Viability Score () – CPAs Injection.
CPAs toxicity and preservation toxicity of CPAs cocktails loaded via microinjection were determined via the assessment of Pre – Freeze and Post – Thaw viability parameters.
| Viability Parameter |
Heartbeat • Beats per minute |
Dehydration • Visible swim bladder • Size of head and eyes |
Circulation • Visible blood movement through the yolk, duct of Cuvier and caudal artery |
|||
|---|---|---|---|---|---|---|
| Pre – Freeze (CVSpF) | No heartbeat no preserved morphology | 0 | N/A | No circulation and no preserved morphology – Complete lack of visible blood movement | 0 | |
| No heartbeat, preserved morphology | 1 | No circulation preserved morphology – Complete lack of blood movement | 1 | |||
| Slowed heartbeat (< 75 bpm) | 2 | Some circulation – Slow blood movement | 2 | |||
| Normal heartbeat (> 75 bpm) | 3 | Normal circulation – Normal blood movement | 3 | |||
| Post – Thaw (CVSpT) | Absence | 0 | Severe dehydration – No visible swim bladder and/or significant reduction in head and eye size | 0 | No circulation – Complete lack of visible blood movement | 0 |
| Presence | 3 | Some dehydration – Small swim bladder and/or slightly reduce head and eye size | 1 | Some circulation – Slow blood movement | 1 | |
| No dehydration – Normal swim bladder, normal head and eye size | 2 | Normal circulation – Normal blood movement | 2 | |||
Cryoprotectant screening in zebrafish larvae by microinjection:
Larvae were anesthetized and placed in polydimethylsiloxane (PDMS) devices for microinjection. Approximately 1.8 nl of CPA solutions (~ 3% of total blood volume) combined with FITC salt (1.25 μg/mL final concentration) was injected at the Duct of Cuvier. Larvae were screened using a fluorescence microscope (EVOS FL) and FITC positive larvae were further frozen. Following CPAs injection, larvae (n = 4 per CPAs cocktail) were immersed in 100μL of the CPA cocktail designed to preserve external morphology (external CPAs) for 10 mins and pre-freeze assessment was performed via the Internal Composite Viability Score (CVSInt). Following assessment, larvae were frozen at −10°C for 22 mins and thawed as fast as possible in a 28°C water bath. Immediately after thawing, larvae were recovered by immersion in decreasing osmolarities of E3 water (Supplemental Table 1) for 5 mins each, following by regular osmolarity E3 water (Supplemental Figure 1). The three best performing internal CPAs cocktails (CPAs 6, 7 and 8) where replicated in higher replicate number (n = 8) to confirm the findings.
Cryoprotectant screening in adult zebrafish hearts:
Wild-type AB zebrafish ranging in age between 3 and 24 months and approximately equal sex ratios were used for experiments. Animals were euthanized by immersion in 0.7% (w/v) tricaine. Once no reflexes were present, they were transferred to a petri dish containing excision buffer (1x phosphate-buffered saline (PBS), 150 U/ml of heparin, 1% antibiotic/antimycotic: Penicillin, Streptomycin, Amphotericin B) and placed ventral side up in a foam holder under the dissecting microscope. The hearts were carefully excised and placed in 24-well plates containing heart culture medium (L-15 Leibovitz medium, Gibco; 10% fetal bovine serum (FBS); 1% antibiotic/antimycotic; 1.25 mM CaCl2; 800 mg/l glucose and 2 U/ml of heparin). A maximum of four hearts were excised each time. Three controls were done including a time-matched heart (dissected at the same time and kept in culture, TimeCON), a heart frozen without any cryoprotectants (FrozenCON) and a freshly excised heart (FreshCON). The six CPAs cocktails that yielded best results in previously screened larvae were tested in the adult hearts. After excision, hearts were placed in a 28 °C incubator and given a 1-hour recovery time. Following the recovery time, 10 sec videos of the hearts were acquired under the microscope (Axiocam 105 color, Zen 3.2 Blue Edition, Zeiss, Germany) and beats-per-minute was acquired by multiplying the number of beats times six. After video acquisition, CPA loading was done in an orbital shaker set at 100 rpm (Jeio Tech, Inc., Billerica, MA) on ice via a 4-step loading procedure. Hearts were placed in 100 μL each solution for 5 mins, starting with 4°C culture media containing Snomax (0 CPA), followed by a 1:4 dilution of the full CPA cocktail, a 1:2 dilution, and finally the full concentration cocktail. After CPAs loading, hearts were frozen in a controlled rate freezer (Planer Kryo 560–16) by decreasing temperature by 1°C/min until reaching −10°C and held for 22 minutes. They were thawed as fast as possible in a water bath set to 28 °C and CPAs cocktails were unloaded at room temperature in the same step-wise procedure as the loading. Post-thaw heart rate was recorded via 10 sec video acquisition (representative videos Supplemental Video 4 and 5) and multiplied by six to obtain beats-per-minute (bpm). Hearts were transferred to a 96-well plate containing 180 ul of media and 20 ul of PrestoBlue™ reagent (Life Technologies) and incubated at 28 °C under agitation, and fluorescence was measure according to the manufacturers’ instructions. Cocktail formulations were as follows: Cocktail 1: 5% DMSO, 10% Glycerol; Cocktail 2: 5% DMSO, 10% Glycerol, 25 mM Raffinose, 200 mM 3-OMG, 15 mM Mannitol; Cocktail 6: 5% DMSO, 5% Glycerol, 25 mM Raffinose, 15 mM Mannitol, 170 U/ml Heparin; Cocktail 7: 10% Glycerol, 200 mM 3-OMG, 15 mM Mannitol, 170 U/ml Heparin; Cocktail 8: 10% Glycerol, 25 mM Raffinose, 5% PEG, 170 U/ml Heparin. Once the best CPA cocktail was identified, a refined protocol was applied for the subsequent replicates that involved less handling, with recovery time lowered to 15-min incubation. CPA loading was performed by cannulating the atrioventricular canal with a 33G SteriJect needle (TKS, Industrial Company, Michigan) and injecting 100 μL of CPA cocktail. Four controls were done including a time-matched heart (dissected at the same time and kept in culture medium, TimeCON), a heart frozen without any cryoprotectants (FrozenCON), a freshly excised heart (FreshCON) and a heart stored at 4°C (HypothermicCON). Hearts were held at −10 °C for 24 h, 72 h or 120 h. After thawing, 0.5 mM norepinephrine was added to the hearts to initiate beating.
Cell death analysis:
Hearts were fixed at 4°C in 4% paraformaldehyde (PFA) overnight, followed by several washes in PBS-Tween, ethanol, xylene and lastly paraffin embedding and sectioning. Cell death stain was performed using TUNEL labeled fluorescein-labeled dUTP (Roche, Basel, Switzerland). Briefly, paraffin embedded scaffold sections were deparaffinized, antigen retrieved via incubation in proteinase K (Dako, Denmark) for 15 mins, followed by incubation in TUNEL stain with for 1 hr at 37°C. All fluorescent images were acquired via a Nikon Eclipse Ni-E microscopy and quantification was performed on six different heart slices per sample and analyzed using MATLAB (Natick, MA).
Statistical Analysis
All graphing and statistical analyses were performed with Prism 7.03 (GraphPad Software Inc., La Jolla, CA, USA). All data were analyzed for outliers using the ROUT method with Q = 1%, followed by ordinary one-way ANOVA with Tukey’s multiple comparison test. Extended preservation Heart Rate and Cardiac Viability analyses were conducted using two-way ANOVA and Tukey-Kramer HSD post-hoc analyses on means. All data are expressed as mean ± standard deviation. Statistical significance is defined at p < 0.05.
Results
Zebrafish larvae screening for CPAs toxicity and preservation performance via immersion:
Zebrafish larvae were the ideal model organism for the initial screen of a large pool of candidate CPAs for toxicity and effectiveness due to their ability to provide valuable information about a wide array of physiological functions in a high through-put manner. For this set of results, the physiological functions quantified to determine larvae viability pre-freeze included heart rate, blood circulation, and fish morphology (Table1). Post-thaw larvae viability assessment included all of the pre-freeze metrics, plus dehydration. The viability metrics were combined in a single viability metric labeled External Composite Viability Score (), as described further in the Materials and Methods. To best preserve the multiple organ systems in zebrafish larvae, two different CPAs cocktails were devised to separately target the preservation of external and internal organs. To determine the CPAs cocktail that would best preserve external tissues and structures, an initial toxicity screen was performed on a large list of candidate components (19) at varying concentrations (8) to establish non-toxic concentrations (Fig. 1A).
Zebrafish larvae were exposed to the individual components via immersion, their effects monitored and reported via the (Fig. 1 C–E). The four classic CPAs tested demonstrated high toxicity, which resulted in zero for most components at most concentrations pre-freeze. Among the non-zero pre-freeze were dimethyl sulfoxide (DSMO) and ethylene glycol (EG) with CVS of 0.75 and 1, respectively, at a concentration of 5%. Glycerol resulted in pre-freeze non-zero at concentrations of 5% (1.25), while the best performing classic CPAs, Isopropyl (PROH), maintained a pre-freeze above the-threshold (≥1.5) at concentrations of 5% (3), 10% (2.25) and 15% (1.5, Fig. 1C).
Alternatively, all the polymers tested in this screen proved less toxic for larvae pre-freeze with all polymers achieving the maximum (3) for all concentrations, with the exception of polyvinylpyrrolidone (PVP) which resulted in low larvae viability at concentrations of 10% and 12.5% (0 and 0.67, respectively, Fig, 1D). Similar to the polymer results, the sugars and other components tested in this screen demonstrated low toxicity pre-freeze. All components retained complete larvae function with of 3 for all concentrations with the exception of the highest concentration (500 mM), where only the sugars, trehalose and mannitol, remained non-toxic for larvae with a of 3, for both. Other non-zero but below the threshold at the 500 mM concentration include 3-O-Methyl-d-glucose (3-OMG, 0.25) and 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES, 1, Fig. 1E).
Based on the CPAs toxicity on zebrafish larvae and elimination due to redundancy, seven CPAs from the toxicity assay were discarded with the remaining twelve arranged in a L50 Taguchi experimental design and tested at seven concentrations yielding a total of 50 CPAs cocktails (Fig. 1F). Zebrafish larvae were immersed in the CPAs cocktails, stored at −10°C for 22 mins and their viability assessed via the post-thaw (Fig. 1B). All 50 CPAs cocktails tested resulted non-toxic for the larvae pre-freeze with of 3. Forty-eight cocktails resulted in post-thaw below the threshold with only cocktails 33+ and 38+ resulting in above the-threshold post-thaw (1.75 and 1.625, respectively). Cocktail 33+ (Fig. 1F) was determined to be the best performing CPAs cocktail and utilized further as the external CPAs cocktail in other larvae experiments in this manuscript. Interestingly, most of the components within the best performing cocktails are non-permeating CPAs, suggesting lack of CPAs permeability was not a factor in external CPAs cocktail preservation capabilities.
Storage with above the threshold CPAs cocktails resulted in the retention of larva morphology and overall integrity post-thaw (Fig. 1G) when compared to untreated/unfrozen larvae (Fig. 1H), whereas storage without CPAs (Fig. 1I) or below the-threshold CPAs (Fig. 1J) resulted in significant loss of morphology.
Zebrafish larvae screening for CPAs toxicity and preservation performance via microinjection:
Once a working external CPAs cocktail was determined, a CPAs cocktail focused on preserving internal organ function was devised. Zebrafish larvae were injected through the duct of Cuvier with 24 new CPAs cocktails, immersed in the previously determined external CPAs cocktail and stored at −10°C for 22 mins (Fig. 2A). Due to zebrafish larvae versatility, larvae viability pre-freeze and post-thaw was determined via the evaluation of physiological functions such as heart rate, blood circulation and dehydration, heart rate quantification and immunofluorescence imaging. Morphology assessment was removed from the internal composite viability score (CVSInt) due to the capability of external CPAs cocktail (cocktail 33+) to retain larvae morphology through the freezing and thawing process (i.e. always a score of 3). Fourteen of the twenty-four CPAs cocktails achieved a CVSInt of 3 pre-freeze. From the twenty-four CPAs cocktails tested, five (CPAs cocktails 1, 2, 6, 7 and 8) were able to achieve an above the-threshold (>1.5) CVSInt (CPAs 1: 1.78, CPAs2: 1.75, CPAs6: 1.93, CPAs7 & CPAs8: 2.3) denoting retention of circulation, heartbeat and normal hydration post-thaw (Fig. 2B). Heartbeat analysis of larvae treated with above-the-threshold CPAs cocktails (CPAs 1, 2, 6, 7 and 8) demonstrated post-thaw heart beat presence in three out of the four (3/4) larvae treated with CPAs 1 and 2, and four out of the four (4/4) larvae treated with CPAs 6, 7 and 8, while no heart beat was retained by any of the larvae (0/4) frozen without CPAs (FrozenCON, Fig. 2C). Heart rate measurements were made for larvae treated (n = 8 per cocktail) with CPAs 6 (74.3 bpm ± 15.7, p < 0.0001), CPAs 7 (72.8 bpm ± 13.4, p < 0.0001) and CPAs 8 (59.3 bpm ± 15.2, p < 0.0001) showing the retention of approximately half the heart rate for all groups when compared with their respective pre-freeze rates (CPAs 6: 134 bpm ± 10, CPAs 7: 128 bpm ± 8, CPAs 8: 144 bpm ± 8, Fig. 2D and Supplemental Videos 1–3). Cardiomyocyte viability was assessed post-thaw from microscopic images of larvae heart for FrozenCON, CPAs 6, 7 and 8, suggesting cardiomyocyte viability was retained in hearts of larvae treated with all CPAs cocktails, while reduced in FrozenCON hearts determined by decreased GFP signal brightness (Fig. 2E).
Figure 2:
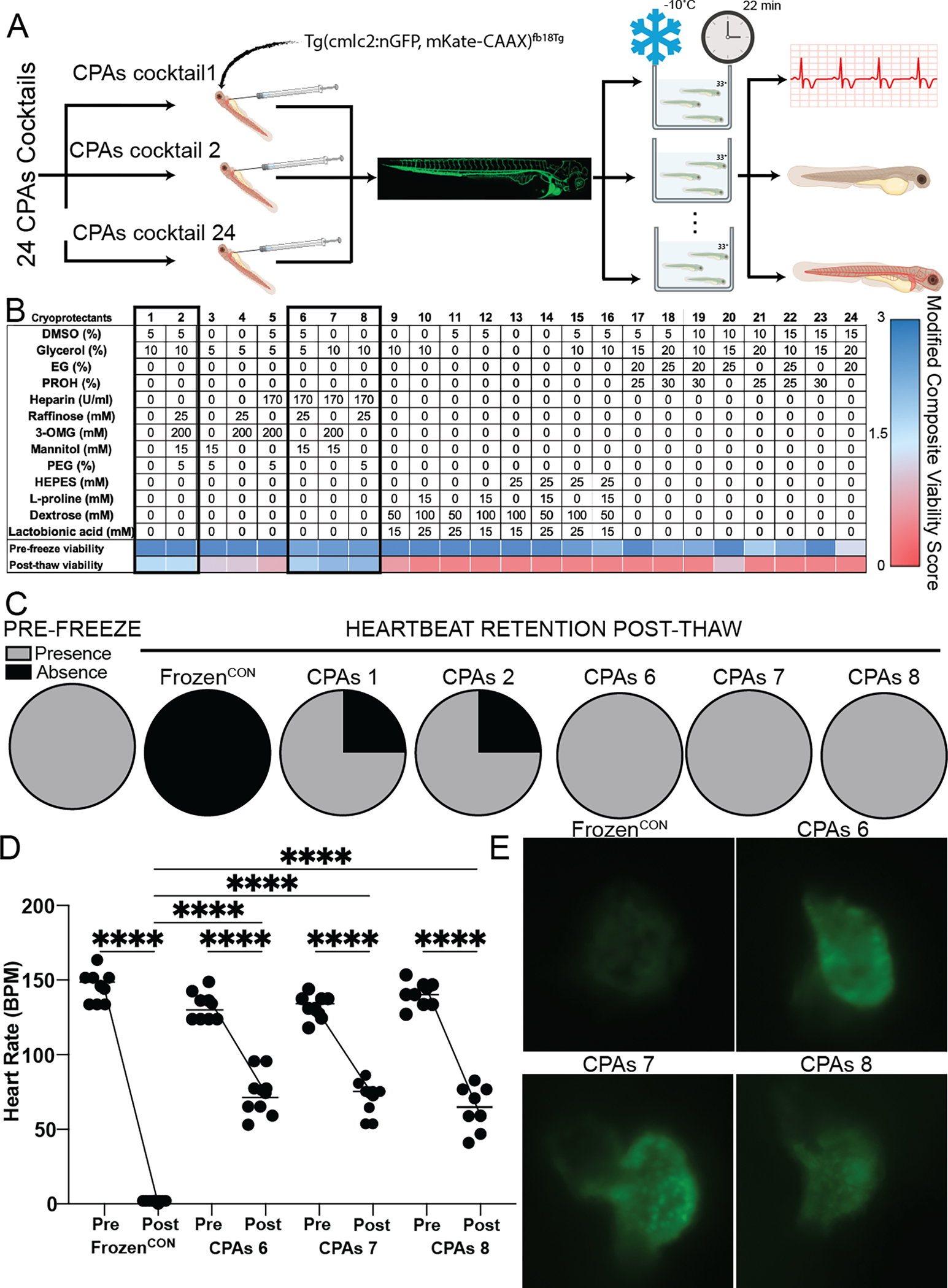
Identification of best performing internal CPAs cocktail capable of retaining larvae (Tg(cmlc2:nGFP, mKate-CAAX)fb18Tg) viability after 22 mins of storage at −10°C when injected through the duct of Cuvier and immersed in CPAs cocktail 33+ (A). Larvae viability was determined by quantifying heart rate, dehydration and blood circulation and reported as the internal composite viability score (CVSInt, n = 4 animals per treatment). (B) Thirteen CPAs were arranged in a L25 Taguchi experimental design and tested at different concentrations (6) yielding 24 CPAs cocktails. Pre-freeze toxicity was determined after 10 mins of incubation with CPAs cocktails 1, 2, 6, 7 and 8 resulted in the best larvae outcomes as determined by above threshold (>1.5) CVSInt (1.78, 1.75, 1.93, 2.3 and 2.3 respectively) post-thaw. (C) Heartbeat retention was seen in all larvae (4 per group) treated with CPAs cocktails 6, 7 and 8, while heartbeat retention was only seen 3 out of the 4 larvae treated with CPAs cocktails 1 and 2. (D) The best performing cocktails were re-tested in larger sample sizes (n = 8 per cocktail). Heart rate analysis of larvae treated with CPAs 6, 7 and 8 demonstrated the presence of approximately half the heart rate post-thaw when compared to pre-freeze rates. (E) Representative microscopic images of larvae GFP-labeled heart. High GFP signal intensity revealed the presence of viable cardiomyocytes in the hearts of larvae treated with CPAs 6, 7 and 8, while decreased signal intensity in hearts frozen without CPAs (FrozenCON) indicate a reduction of cardiomyocyte viability. All statistical data is expressed as mean ± standard deviation and analyzed via ordinary one-way ANOVA with Tukey’s multiple comparison test. **** = p < 0.001.
Excised adult zebrafish hearts are a suitable model organism to determine CPAs capacity to preserve cardiac tissue and function after short-term partial freezing:
The best performing internal CPAs cocktails (1, 2, 6, 7 and 8) were tested for their ability to preserve adult cardiac tissue via freezing adult zebrafish hearts immersed in the CPAs cocktails. CPAs cocktail performance was determined based on retention of heart rate (beats per minute - bpm) and cardiac metabolism post-thaw. Excised hearts were immersed in CPAs cocktails and stored for 25 mins at −10 °C. Heart rate was retained by most of the CPAs cocktails (CPAs cocktail 2 - 77 bpm ± 7, CPAs cocktail 6 - 50 bpm ± 21, CPAs cocktail 7 - 64 ± 20 and CPAs cocktail 8 - 70 bpm ± 21, Fig. 3B), with only CPAs cocktail 1 recovering statistically lower heart rate (7 bpm ± 6) when compared to Fresh (88 bpm ± 21, CPAs cocktail 1 – p = 0.938 CPAs cocktail 2 – p < 0.0001, CPAs cocktail 6 – p = 0.056, CPAs cocktail 7 – p 0.502 and CPAs cocktail 8 -p = 0.808) and Time controls (74 bpm ± 41, CPAs cocktail 1 – p = 0.999, CPAs cocktail 2 – p < 0.0001, CPAs cocktail 6 – p = 0.004, CPAs cocktail 7 – p = 0.0001 and CPAs cocktail 8 – p < 0.0001). Although, most of the CPAs cocktails were able to retain heart beat post-freeze, only CPAs 6 (1.27 AU ± 0.13,) retained cardiac metabolism comparable to Fresh controls (1.14 ± 0.31, p = 0.896) and statistically higher than Frozen controls (0.44 AU ± 0.08, p < 0.0001, Fig. 3C). Hearts stored in all other cocktails resulted in statistically lower cardiac viability (CPAs cocktail 1 – 0.39 AU ± 0.11, CPAs cocktail 2 – 0.63 AU ± 0.19, CPAs cocktail 7 – 0.56 ± 0.14 and CPAs cocktail 8 – 0.71 AU ± 0.13) when compared with Fresh controls (CPAs cocktail 1 – p < 0.0001, CPAs cocktail 2 – p = 0.0001, CPAs cocktail 7 – p < 0.0001 and CPAs cocktail 8 – p = 0.0014) and non-statistical different when compared to Frozen controls (0 bpm ± 0, CPAs cocktail 1 – p = 0.99, CPAs cocktail 2 – p = 0.504, CPAs cocktail 7 – p = 0.914 and CPAs cocktail 8 – p = 0.119).
Figure 3:
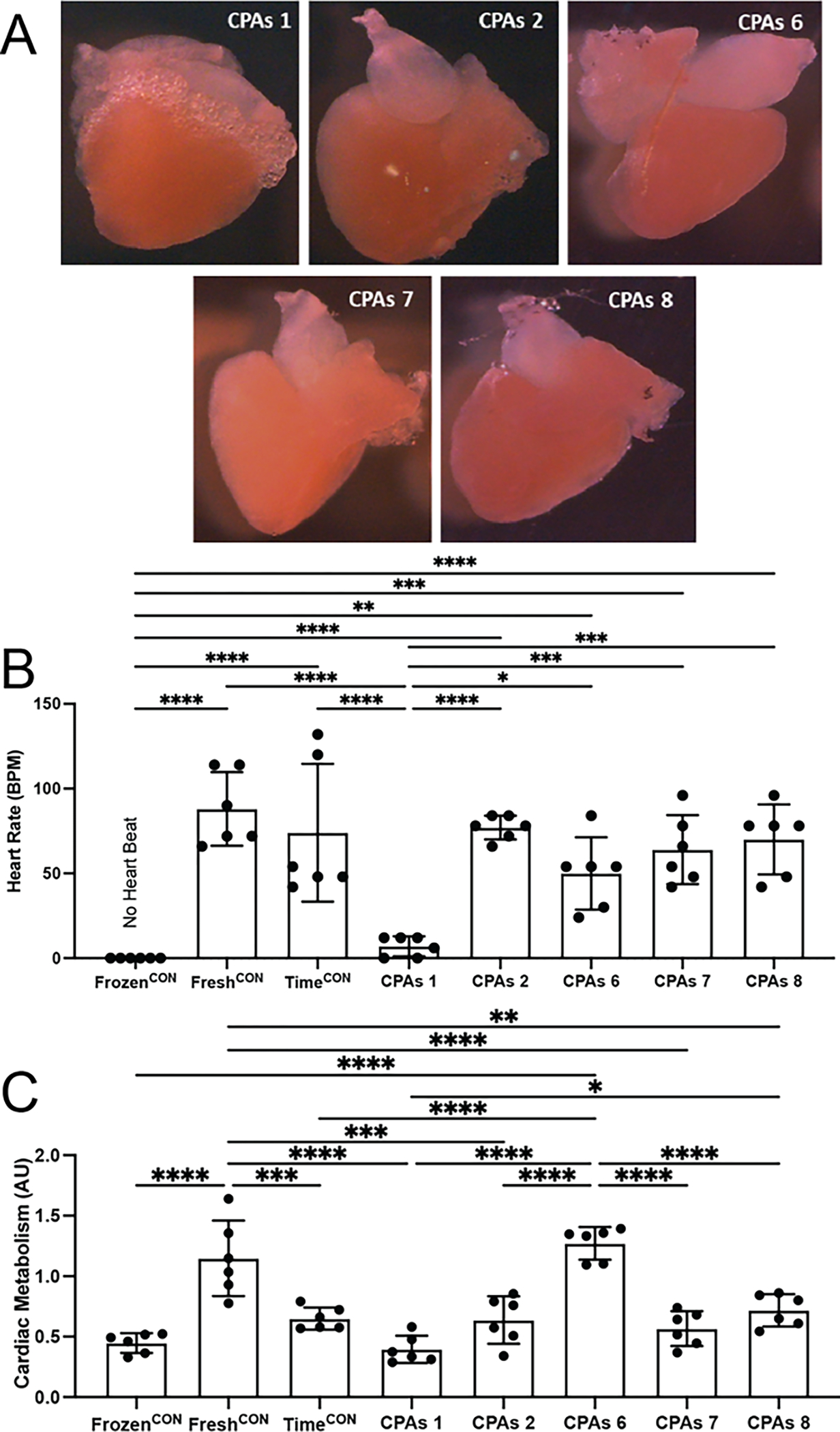
CPAs cocktails 2, 6, 7 and 8 partially retain cardiac function of adult zebrafish hearts after 25 mins of storage at −10 °C when loaded via immersion (n = 6 animals per treatment). (A) Representative microscopic images of adult zebrafish hearts treated with different CPAs cocktails. (B) Heart rate (beats per minute – bpm) was best retained in hearts treated with CPAs 2, 6, 7 and 8 with no statistical difference seen between fresh and the time control hearts. (C) Cardiac metabolism was best retained by CPAs 6 having no statistical difference with Fresh controls. All other CPAs cocktails resulted in statistically lower cell metabolism when compared to Fresh controls and similar metabolism of hearts frozen without CPAs. All statistical data is expressed as mean ± standard deviation and analyzed via ordinary one-way ANOVA with Tukey’s multiple comparison test. * = p < 0.05, ** = p < 0.01, *** = p < 0.001, **** = p <0.0001.
Excised adult zebrafish hearts are a suitable model organism to determine CPAs capacity to preserve cardiac tissue and function after long-term partial freezing:
CPAs cocktail 6 was discerned to be the best performing cocktail for short-term storage due to its capability to retain heart rate and cardiac viability post-thaw of adult zebrafish hearts (Fig. 3), plus its ability to retain heart rate in all treated larvae when loaded via injection (Fig. 2B). Therefore, CPAs cocktail 6 was further tested to determine its efficiency at preserving adult cardiac tissue viability and function under longer-term storage times. Adult zebrafish hearts were perfused and immersed in CPAs cocktail 6 and stored for 24 h, 72 h and 120 h at −10°C and evaluated for heart rate, atrial and ventricular contractions and cardiac viability (as per TUNEL assay). Hearts stored in CPAs cocktail 6 were compared to freshly excised hearts (FreshCON) and hearts stored via the current standard for preservation (UW at 4°C - HypothermicCON). Heart rate in both storage conditions ( 24 h: CPAs cocktail 6 – 77 bpm ± 26 and HypothermicCON – 36 bpm ± 19, 72 h: CPAs cocktail 6 – 66 bpm ± 26 and HypothermicCON - 49 bpm ± 16, 120 h: CPAs cocktail 6 – 61 bpm ± 21 and HypothermicCON - 75 bpm ± 18) were statistically similar to the heart rate seen in the FreshCON hearts (48 bpm ± 10, 24 h: CPAs cocktail 6 – p = 0.729, HypothermicCON – p < 0.999, 72 h: CPAs cocktail 6 – p = 0.998, HypothermicCON – p > 0.999, 120 h: 24 h: CPAs cocktail 6 – p > 0.998, HypothermicCON – p = 0.802, Fig. 4A) at all time points. Unlike hearts stored for short-term preservation times (Figure 3B), long-term storage hearts did not recover synchronous contractions. Therefore a difference between the rate of atrial contraction and ventricular contraction was observed, indicating the atrium ( 24 h: CPAs cocktail 6 – 77 cpm ± 26 and HypothermicCON – 41 cpm ± 25, 72 h: CPAs cocktail 6 – 64 cpm ± 11 and HypothermicCON - 49 cpm ± 16, 120 h: CPAs cocktail 6 – 66 cpm ± 24 and HypothermicCON - 75 cpm ± 18) was able to contract over twice as often as the ventricle (24 h: CPAs cocktail 6 – 18 cpm ± 8 and HypothermicCON – 10 cpm ± 4, 72 h: CPAs cocktail 6 – 33 cpm ± 9 and HypothermicCON - 30 cpm ± 25, 120 h: CPAs cocktail 6 – 28 cpm ± 17 and HypothermicCON - 75 cpm ± 18) in both conditions at all storage times (Fig. 4C) with ventricular contractions being statistically lower for CPAs 6 at 24 hr (p < 0.0001 ) and, for both Hypothermic control and CPAs 6 hearts at 120 hr (p < 0.0001 and p = 0.028, respectively) when compared to their respective atrial contraction rate. Assessment of cardiomyocyte viability, via the quantification of DAPI and TUNEL immunofluorescent stains of heart sections, demonstrated a trend in temperature vs. time dependent damage of hearts (Fig. 4B–D). Cardiac viability decreased initially in both experimental groups with no further changes seen in cell viability of CPAs cocktail 6 hearts over time (24 h: 36.3% ± 10.8, 72 h: 28% ± 11.5, 120 h: 39.4 ±8.4), and a slight decrease, although not statically significant, in cell viability of hearts stored under clinical conditions (24 h: 50% ± 20.7, 72 h: 53.9% ± 15.2, 120 h: 38.8 ± 10.8, Fig. 4B). Although, a decrease in cell viability was seen in both storage conditions, both experimental groups achieved statistically higher number of viable cardiomyocytes (CPAs cocktail 6 - 24 h: p = 0.006, 72 h: p < 0.0001, 120 h: p = 0.0013 and HypothermicCON hearts - 24 h: p < 0.0001, 72 h: p < 0.0001, 120 h: p < 0.0) when compared to FrozenCON hearts (9.8% ± 4.3). Comparison between cardiac viability at individual storage times indicates no significant difference between CPAs cocktail hearts and HypothermicCON hearts at 24 h (p = 0.588) and 120 h (p > 0.999) of storage time, but statistical difference at 72 h (, p = 0.007) of storage time.
Figure 4:
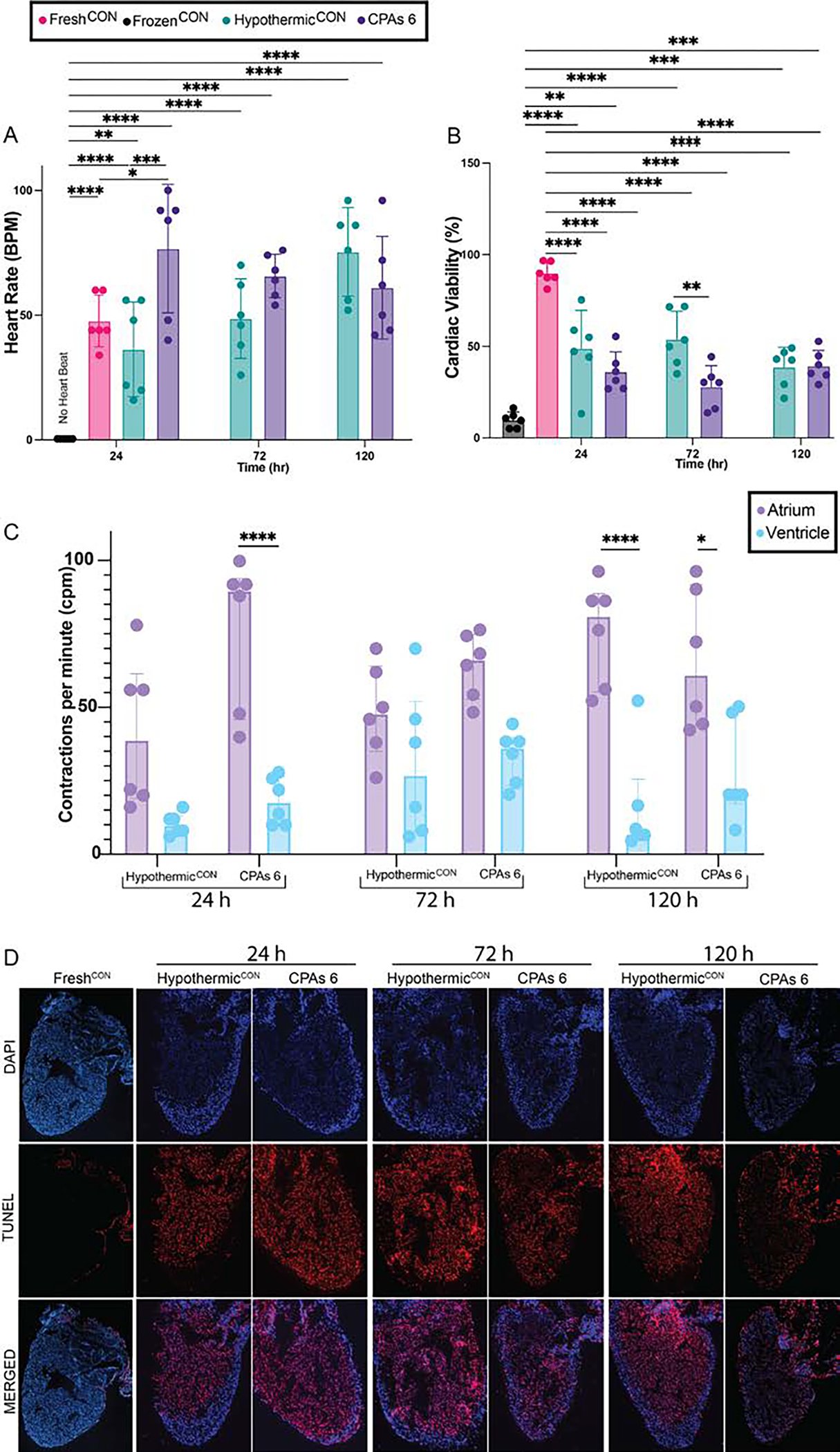
CPAs cocktail 6 partially retained cardiac function of adult zebrafish hearts after 24 h, 72 h and 120 h of storage at −10 °C when loaded via perfusion (n = 6 animals per treatment). (A) Heart beat analysis for hearts stored in CPAs 6 resulted in similar heart rate recovery at all storage times. No statistical difference was seen between heart rate of CPAs cocktail 6 hearts over time or when compared to the beat ratio of hearts stored in clinical standard conditions (HypothermicCON) at each individual storage times. (B) Similarly, storage in CPAs cocktail 6 resulted in ~40% of cardiac tissue to remain viable post-thaw, irrespective of freezing time, suggesting temperature dependent injury but no time dependent injury. (C) Separate analysis of ventricular and atrial beat for hearts stored in CPAs 6 resulted in similar atrial and ventricular contraction (contraction per minute – cpm) recovery at all storage times, with the atrium contracting over twice as often as the ventricle. No statistical difference was seen between beat ratio of CPAs cocktail 6 hearts over time or when compared to the beat ratio of hearts stored in clinical standard conditions (HypothermicCON) at each individual storage times (D) Representative histological images of TUNEL and DAPI staining. Scale bar 250 μm. All statistical data is expressed as mean ± standard deviation. Heart rate and Cardiac Viability were analyzed via a two-way ANOVA and Tukey-Kramer HSD post-hoc analyses. Contraction rate was analyzed via an ordinary one-way ANOVA with Tukey’s multiple comparison test. **** = p < 0.000
Discussion
Despite a record-breaking 41,354 transplants performed in the United States in 2021, the severe donor organ shortage continues to prohibit the field from being a broad solution for patients with end-stage organ failure. While significant effort has been devoted to solve the donor shortage crisis, less effort has been dedicated to preservation techniques that hold the promise of overcoming several challenges in organ transplantation [3]. Among the difficulties associated with the advancement of preservation techniques is the under-utilization of high throughput model organisms that provide insightful information regarding complex organ systems response to preservation variables. The zebrafish (Danio rerio) is an excellent model organism for high-throughput experimentation, which with few exceptions, have same tissue homeostasis as humans [20]. These results demonstrate the advantages of zebrafish as model organism in heart transplantation research and the easiness in which a wide array of scientific tools can be re-purposed to advance, simplify and maximize research in organ preservation.
Cryoprotective agents (CPAs) are molecules that play a pivotal role in subzero preservation, due to their capability to eliminate ice formation within the organ’s cells during storage. Although, absolutely necessary for proper organ cryopreservation, the inherent toxicity of these molecules at higher concentrations makes them the major challenge for cryopreservation progress [21]. Although, some effort has been dedicated to understanding the mechanisms of CPAs toxicity, all of these efforts have been focused on resolving preservation challenges for specific, homogeneous cell lines by testing the individual effects of just a couple CPAs [22–26]. Similarly, the sparse effort attempts to advance preservation techniques for cellular heterogenous composites or complex organ systems have focused on testing the effects/efficiency of a very small pool of cryoprotectants [27, 28].
To fill this gap in knowledge zebrafish larvae were utilized to test, in a high throughput manner, CPAs toxicity and efficiency to preserve solid organ systems, such as the heart. Over 500 tests by immersion were performed to test the individual toxicity of CPAs at different concentrations and their effects in 3 day-post-fertilization (dpf) larvae survivability (Fig. 1C–D). Although immersion experiments with 3 dpf larvae are extremely efficient, they pose challenges in correlating whole organism survivability to the internal organ system of interest, such as the heart. As a result, this study required the formulation of a highly specific external CPAs cocktail to maintain external larvae morphology, in addition to screening heart-specific CPAs cocktails through microinjection. Regardless of these challenges, the development of a successful external CPAs cocktail can now be used routinely in future studies that may target other organs relevant to the field of experimental transplantation, such as the liver. Further, while microinjection of CPAs does technically lower throughput as compared to immersion experiments, the development of a heart-specific CPA cocktail was significantly streamlined and ultimately reduced reliance on mammalian model systems in research.
Therefore, based on this toxicity profile, and the fact that combination of multiple CPAs (vs. single CPAs) achieve improved preservation, combination of CPAs were screened, using over 270 tests, to determine their capability to preserve larvae internal (24 cocktails, Fig. 2B) and external (50 cocktails, Fig. 1F) structure and function. The utilization of cocktails conformed by multiple CPAs aimed to address the multi-cellulated nature of the zebrafish larvae and the different organismal demands during subzero storage. By targeting internal and external larvae structures individually, preservation efficiency was improved, resulting in an increase in composite viability score (CVS) from 1.75 to 2.3. The use of combination CPAs, additionally of targeting multi-cellular demands, has been demonstrated to lower CPAs toxicity when compared to single CPAs at the same total concentrations [29, 30]. This synergistic effect on toxicity is seen in these results where the CPAs cocktails that best preserve internal larvae functionality (Fig. 2B) contained concentrations of Glycerol (5% or 10%) and DMSO (5%) that were deemed too toxic by the individual toxicity profile (Fig. 1C). However, it should be noted that toxicity profiles of immersion versus injection screens may not be directly comparable, particularly for freshwater fish that may have different sensitivities to external osmotic effects. Nonetheless, due to the lack of understanding on CPAs toxicity mechanism and the potential benefits of their combined effects, thorough CPAs screenings in relevant model organisms are further needed [31].
The lack of understanding of CPAs action mechanisms, albeit a major issue in preservation advancement, is not the only problem for the field. The assessment of preservation success, intuitively important, is extremely difficult in complex organ preservation. Due to lack of translatability from simpler systems, complex organ preservation relies on extremely cumbersome experiments (i.e. machine perfusion, long-term survival studies post-transplant in mammals) to assess organ functionality after experimentation [10, 32, 33]. The inherent difficulty of these experiments requires extensive training and development of very specialized skills that further hinder the advancement of complex organ preservation and transplantation research.
Alternatively, the transparent nature of zebrafish larvae allows unprecedented levels of direct observation into many of the physiological processes that would be affected by subzero preservation and subsequent transplantation. This permitted the development and implementation of a robust assessment system capable of determining success/failure of preservation by evaluating circulatory/cardiac function, dehydration/edema and larvae morphology (the composite viability score - CVS). The physiological functions quantified for the current CVS were specifically selected to determine the degree of cardiac function retention, but can be replaced for other parameters when the focus is a different organ system [34–36]. Additional to function retention, the transparency of zebrafish larvae provides a platform where the even and proper distribution of CPAs can be tracked visually via the use of fluorescently tagged CPAs, a commodity that requires cumbersome imaging techniques in bulky organs [37–39].
Similar to larvae, the use of adult zebrafish organs ex vivo for preservation techniques has extreme potential to simplify preservation research. The ability to maintain adult zebrafish hearts in static culture, while retaining significant function even after 3 days, is an excellent surrogate for ex vivo whole organs handling systems [16]. This survivability and function retention of hearts in significantly less complicated experimental setups facilitates immensely the assessment of cardiac function retention, even facilitating high-throughput experimentation. For instance, atrial and ventricular function can easily be quantified via simple observation (Fig. 3B and Fig. 4A), myocardial cells metabolism can be assessed in their native structure via mainstream metabolic assays (Fig. 3D), and even the electrical signals of the heart can be recorded via ECG measurements while on static culture [40].
It is important to point out, that despite the capability of zebrafish to significantly simplify many aspects of preservation research, there are other biological and physiological aspects that make it a very robust model organism. For heart preservation specifically, despite the simplicity of zebrafish heart compared to mammals, the conservation of most cardiac cell types and structures leads to significant physiological similarities [41]. The loss of ventricular function, and retention of atrial function, as seen in long-term storage times (Fig. 4C), may be due to the higher sensitivity of the atrioventricular (AV) node to ischemia (an unavoidable consequence of any preservation technique), making it more susceptible to irreversible damage [42, 43]. This difference in ischemia sensitivity between the AV and the sinoatrial (SA) node could be explained by the difference in their cellular composition and the resultative proneness to ischemia/reperfusion injury. The SA node is mainly composed by P cells, void looking cells that resemble embryonal primitive myocardial cells and count with very few or no mitochondria [44]. In comparison, the AV node is composed by transitional cells which contain more myofibrils, more sarcomeres and more mitochondria [45]. Ischemia/reperfusion injury is mediated by the release of cytochrome c, a small protein bound in the inner membrane of the mitochondria, which activates apoptotic signaling [46]. Therefore, higher number of mitochondria within the AV node likely results in increased damage and reduced function. This higher sensitivity of pacemaker cells to damage is, also, a very likely explanation for the significant difference in heartbeat retention post-thaw between the different CPAs/control solution (Fig. 3B and Fig. 4A), while maintaining similar cardiac viability (Fig. 4B–D) and metabolism (Fig. 3D).
Fortunately, mitigating the adverse effects of ischemia/reperfusion injury on the pacemaker cells of the AV node, even after extended storage (120 hr), might be possible as demonstrated by the retention of functional electrical impulses that lead to quantifiable heart contractions in adult zebrafish hearts (Fig. 4A). Due to high conservation of specific molecular components that comprise pacemaker cells within a wide array of species (fish, rodents and humans), the function retention achieved in zebrafish hearts by CPAs cocktails 1, 2 and 6 could potentially translate into the retention of functional electrical impulses in mammalian hearts after sub-zero freezing [47].
It is important to point out, however, these cross-species similarities are not present in other aspects of the cardiac physiology between zebrafish and mammals, complicating the extrapolation of preservation capabilities. Zebrafish cardiomyocytes more closely resemble neonatal mammalian cells, rather than adult, potentially resulting in a higher tolerance to ischemia than mammalian adult cardiomyocytes [48–50]. Additionally, zebrafish are capable of retaining normal cardiac function in temperatures as low as 6°C, whereas mammalian hearts function at a very narrow temperature range, potentially impacting the fish cardiac graft sensitivity to cold damage [51]. As a result, lower cardiac cell survival could occur during the translation of these CPAs cocktails and protocol into mammalian hearts and species-tailored optimization will be required.
The purpose of these series of experiments is to contribute towards the elimination of barriers in organ transplantation by targeting pressing issues in preservation research at different levels. First, the introduction of a novel model organism and research platform capable of substituting complicated experimental setups, while maintaining organismal complexity aims to simplify and accelerate research in organ preservation. Aided by this simplicity, the easy protocol development and plethora of adaptable assays, zebrafish results as the ideal model organism to tackle research challenges in the fields of organ preservation and transplantation. Second, these results help narrow the large gap in knowledge on individual CPAs toxicity and combined CPAs preservation efficiency on solid organ systems via the insights generated by the high-throughput screening of CPAs effects in zebrafish larvae. Although, the preservation efficiency of these CPAs was only tested in one (Partial Freezing) of the many cryopreservation techniques (i.e. vitrification, supercooling, isochoric storage), this comprehensive CPAs screening provides a base knowledge of the ability of different CPAs to complement their effects and achieve improved preservation. This knowledge, along with the techniques and tools developed herein can be used as the basis for other cryopreservation research. Lastly, via heart subzero storage experiments, these results demonstrated the high susceptibility of pacemaker cells to cold temperature storage, uncovering a key focus area for future heart preservation research.
Supplementary Material
Significance Statement.
Research tools are the most fundamental element for scientific advancements. Unfortunately, the fields of organ preservation and solid organ transplantation lack the appropriate research tools to facilitate large scale experimentation in relevant model organisms, leading to a significant stagnation of scientific progress. The lack of progress in both of these fields plays a direct role in the shortage of organs available for transplant and creates enormous logistical challenges to many areas of biomedical research. Zebrafish is introduced as a platform, along with a wide array of research tools to fill the gap in efficient and relevant assays in the fields of solid organ preservation and transplantation, capable of accelerating progress in both of these fields.
Acknowledgments
This research was funded through SNT from the US National Institutes of Health (NIH), including K99/R00 HL1431149 and R01HL157803, American Heart Association (AHA; 18CDA34110049), Harvard Medical School Eleanor and Miles Shore Fellowship, and the Claflin Distinguished Scholar Award on behalf of the Massachusetts General Hospital (MGH) Executive Committee on Research. We also thankfully acknowledge support to MT (National Science Foundation under Grant No. EEC 1941543), DML (NIH R24OD031955; MGH Research Scholars), and JMGR (NIH R01HL164749–01; AHA 19CDA34660207; Hassenfeld Scholar Award; Corrigan-Minehan Heart Center of MGH SPARK Award).
Footnotes
Competing Interests
Dr. Tessier, Dr. Toner, Dr. Uygun, Dr. de Vries, Mrs. Cronin, and Ms. Pendexter have patent applications relevant to this study. Dr. Uygun has a financial interest in Organ Solutions, a company focused on developing organ preservation technology. Drs. Toner, Uygun, and Tessier serve on the Scientific Advisory Board for Sylvatica Biotech Inc., a company focused on developing high subzero organ preservation technology. All competing interests are managed by the MGH and Partners HealthCare in accordance with their conflict-of-interest policies.
Data Availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1.Pinezich M and Vunjak-Novakovic G, Bioengineering approaches to organ preservation ex vivo. Exp Biol Med (Maywood), 2019. 244(8): p. 630–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kilic A, et al. , Donor selection in heart transplantation. J Thorac Dis, 2014. 6(8): p. 1097–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giwa S, et al. , The promise of organ and tissue preservation to transform medicine. Nat Biotechnol, 2017. 35(6): p. 530–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiu-Lam A, et al. , Perfusion, cryopreservation, and nanowarming of whole hearts using colloidally stable magnetic cryopreservation agent solutions. Sci Adv, 2021. 7(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.in Realizing the Promise of Equity in the Organ Transplantation System, Hackmann M, English RA, and Kizer KW, Editors. 2022: Washington (DC). [PubMed] [Google Scholar]
- 6.Taylor MJ, et al. , New Approaches to Cryopreservation of Cells, Tissues, and Organs. Transfus Med Hemother, 2019. 46(3): p. 197–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finger EB and Bischof JC, Cryopreservation by vitrification: a promising approach for transplant organ banking. Curr Opin Organ Transplant, 2018. 23(3): p. 353–360. [DOI] [PubMed] [Google Scholar]
- 8.de Vries RJ, et al. , Subzero non-frozen preservation of human livers in the supercooled state. Nat Protoc, 2020. 15(6): p. 2024–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powell-Palm MJ, et al. , Isochoric supercooled preservation and revival of human cardiac microtissues. Commun Biol, 2021. 4(1): p. 1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tessier SN, et al. , Partial freezing of rat livers extends preservation time by 5-fold. Nat Commun, 2022. 13(1): p. 4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howe K, et al. , The zebrafish reference genome sequence and its relationship to the human genome. Nature, 2013. 496(7446): p. 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kokel D, et al. , Rapid behavior-based identification of neuroactive small molecules in the zebrafish. Nat Chem Biol, 2010. 6(3): p. 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez-Rosa JM, Zebrafish Models of Cardiac Disease: From Fortuitous Mutants to Precision Medicine. Circ Res, 2022. 130(12): p. 1803–1826. [DOI] [PubMed] [Google Scholar]
- 14.Cornet C, et al. , ZeGlobalTox: An Innovative Approach to Address Organ Drug Toxicity Using Zebrafish. Int J Mol Sci, 2017. 18(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dyballa S, et al. , Comparison of zebrafish larvae and hiPSC cardiomyocytes for predicting drug induced cardiotoxicity in humans. Toxicol Sci, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pieperhoff S, et al. , Heart on a plate: histological and functional assessment of isolated adult zebrafish hearts maintained in culture. PLoS One, 2014. 9(5): p. e96771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Genge CE, et al. , The Zebrafish Heart as a Model of Mammalian Cardiac Function. Rev Physiol Biochem Pharmacol, 2016. 171: p. 99–136. [DOI] [PubMed] [Google Scholar]
- 18.Cronin S, et al. Partial Freezing: Subzero Whole Liver Preservation In The Presence Of Ice. in AMERICAN JOURNAL OF TRANSPLANTATION. 2019. WILEY 111 RIVER ST, HOBOKEN 07030–5774, NJ USA. [Google Scholar]
- 19.Liu XH, Zhang T, and Rawson DM, Effect of cooling rate and partial removal of yolk on the chilling injury in zebrafish (Danio rerio) embryos. Theriogenology, 2001. 55(8): p. 1719–31. [DOI] [PubMed] [Google Scholar]
- 20.Patton EE, Zon LI, and Langenau DM, Zebrafish disease models in drug discovery: from preclinical modelling to clinical trials. Nat Rev Drug Discov, 2021. 20(8): p. 611–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pegg DE, Principles of cryopreservation. Methods Mol Biol, 2007. 368: p. 39–57. [DOI] [PubMed] [Google Scholar]
- 22.Tessier SN, et al. , Effect of Ice Nucleation and Cryoprotectants during High Subzero-Preservation in Endothelialized Microchannels. ACS Biomater Sci Eng, 2018. 4(8): p. 3006–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norris MM, et al. , 3-O-methyl-D-glucose improves desiccation tolerance of keratinocytes. Tissue Eng, 2006. 12(7): p. 1873–9. [DOI] [PubMed] [Google Scholar]
- 24.Usta OB, et al. , Supercooling as a viable non-freezing cell preservation method of rat hepatocytes. PLoS One, 2013. 8(7): p. e69334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warner RM, et al. , Rapid quantification of multi-cryoprotectant toxicity using an automated liquid handling method. Cryobiology, 2021. 98: p. 219–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin C, Zhang T, and Rawson DM, Cryopreservation of zebrafish (Danio rerio) blastomeres by controlled slow cooling. Cryo Letters, 2009. 30(2): p. 132–41. [PubMed] [Google Scholar]
- 27.Zhan L, et al. , Cryopreservation method for Drosophila melanogaster embryos. Nat Commun, 2021. 12(1): p. 2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khosla K, et al. , Cryopreservation and Laser Nanowarming of Zebrafish Embryos Followed by Hatching and Spawning. Adv Biosyst, 2020. 4(11): p. e2000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campbell LH and Brockbank KG, Cryopreservation of porcine aortic heart valve leaflet-derived myofibroblasts. Biopreserv Biobank, 2010. 8(4): p. 211–7. [DOI] [PubMed] [Google Scholar]
- 30.Fahy GM, Cryoprotectant toxicity neutralization. Cryobiology, 2010. 60(3 Suppl): p. S45–53. [DOI] [PubMed] [Google Scholar]
- 31.Best BP, Cryoprotectant Toxicity: Facts, Issues, and Questions. Rejuvenation Res, 2015. 18(5): p. 422–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Que W, et al. , Prolonged Cold Ischemia Time in Mouse Heart Transplantation Using Supercooling Preservation. Transplantation, 2020. 104(9): p. 1879–1889. [DOI] [PubMed] [Google Scholar]
- 33.Bruinsma BG, et al. , Supercooling preservation and transplantation of the rat liver. Nat Protoc, 2015. 10(3): p. 484–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He JH, et al. , A zebrafish phenotypic assay for assessing drug-induced hepatotoxicity. J Pharmacol Toxicol Methods, 2013. 67(1): p. 25–32. [DOI] [PubMed] [Google Scholar]
- 35.Guo S, Using zebrafish to assess the impact of drugs on neural development and function. Expert Opin Drug Discov, 2009. 4(7): p. 715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Field HA, et al. , Analysis of gastrointestinal physiology using a novel intestinal transit assay in zebrafish. Neurogastroenterol Motil, 2009. 21(3): p. 304–12. [DOI] [PubMed] [Google Scholar]
- 37.Pegg DE, Wang L, and Vaughan D, Cryopreservation of articular cartilage. Part 3: the liquidus-tracking method. Cryobiology, 2006. 52(3): p. 360–8. [DOI] [PubMed] [Google Scholar]
- 38.Corral A, et al. , Assessment of the cryoprotectant concentration inside a bulky organ for cryopreservation using X-ray computed tomography. Cryobiology, 2015. 71(3): p. 419–31. [DOI] [PubMed] [Google Scholar]
- 39.Isbell SA, et al. , Measurement of cryoprotective solvent penetration into intact organ tissues using high-field NMR microimaging. Cryobiology, 1997. 35(2): p. 165–72. [DOI] [PubMed] [Google Scholar]
- 40.Stoyek MR, et al. , Zebrafish heart as a model to study the integrative autonomic control of pacemaker function. Am J Physiol Heart Circ Physiol, 2016. 311(3): p. H676–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bowley G, et al. , Zebrafish as a tractable model of human cardiovascular disease. Br J Pharmacol, 2022. 179(5): p. 900–917. [DOI] [PubMed] [Google Scholar]
- 42.Bagdonas AA, et al. , Effects of ischemia and hypoxia on the specialized conducting system of the canine heart. American heart journal, 1961. 61(2): p. 206–218. [DOI] [PubMed] [Google Scholar]
- 43.Senges J, et al. , Effect of hypoxia on the sinoatrial node, atrium, and atrioventricular node in the rabbit heart. Circulation Research, 1979. 44(6): p. 856–863. [DOI] [PubMed] [Google Scholar]
- 44.James TN, et al. , Comparative ultrastructure of the sinus node in man and dog. Circulation, 1966. 34(1): p. 139–63. [DOI] [PubMed] [Google Scholar]
- 45.Sherf L, James TN, and Woods WT, Function of the atrioventricular node considered on the basis of observed histology and fine structure. J Am Coll Cardiol, 1985. 5(3): p. 770–80. [DOI] [PubMed] [Google Scholar]
- 46.Lutz J, Thurmel K, and Heemann U, Anti-inflammatory treatment strategies for ischemia/reperfusion injury in transplantation. J Inflamm (Lond), 2010. 7: p. 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mandla R, Jung C, and Vedantham V, Transcriptional and Epigenetic Landscape of Cardiac Pacemaker Cells: Insights Into Cellular Specialization in the Sinoatrial Node. Front Physiol, 2021. 12: p. 712666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iorga B, et al. , Micromechanical function of myofibrils isolated from skeletal and cardiac muscles of the zebrafish. J Gen Physiol, 2011. 137(3): p. 255–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ostadalova I, et al. , Tolerance to ischaemia and ischaemic preconditioning in neonatal rat heart. J Mol Cell Cardiol, 1998. 30(4): p. 857–65. [DOI] [PubMed] [Google Scholar]
- 50.Riva E and Hearse DJ, Age-dependent changes in myocardial susceptibility to ischemic injury. Cardioscience, 1993. 4(2): p. 85–92. [PubMed] [Google Scholar]
- 51.Spence R, et al. , The behaviour and ecology of the zebrafish, Danio rerio. Biol Rev Camb Philos Soc, 2008. 83(1): p. 13–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.


