Abstract
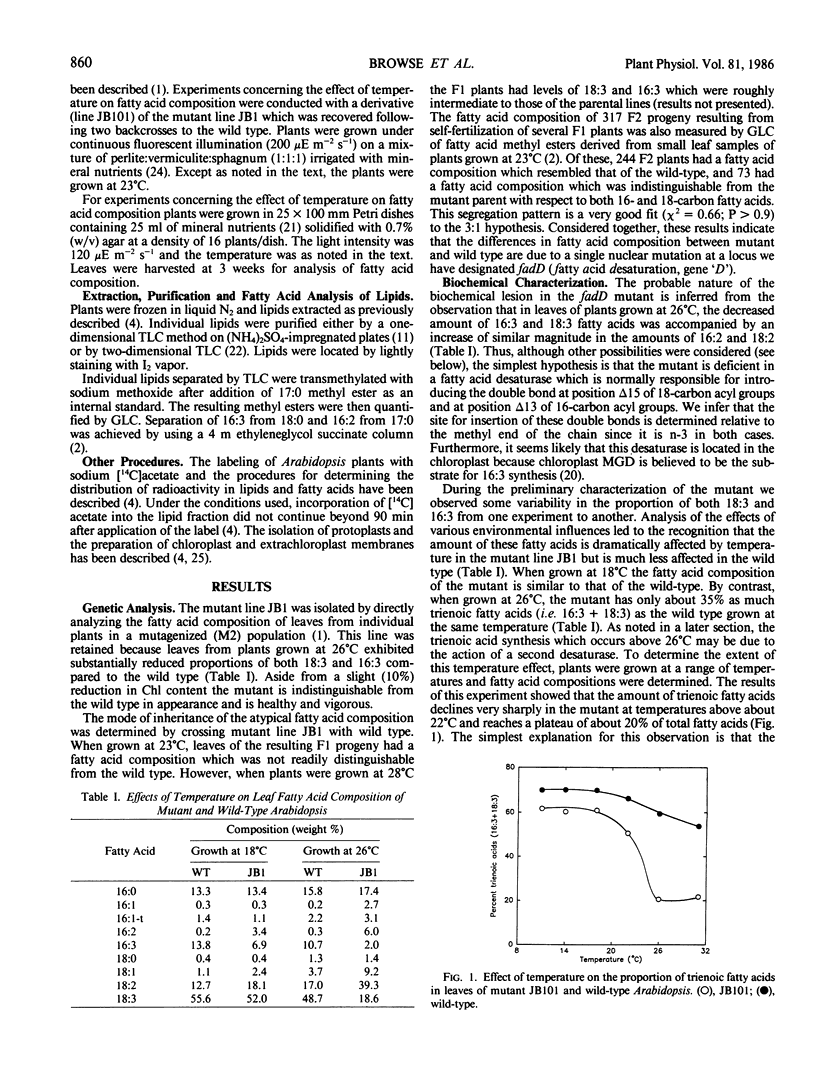
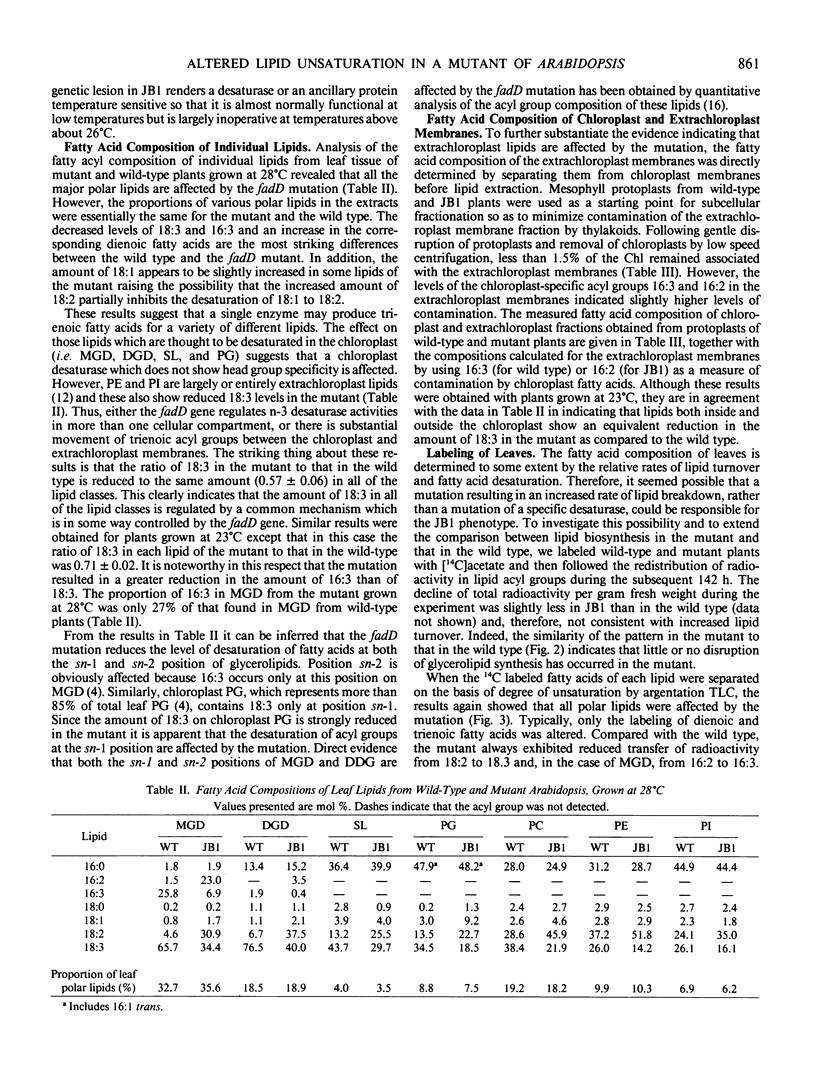
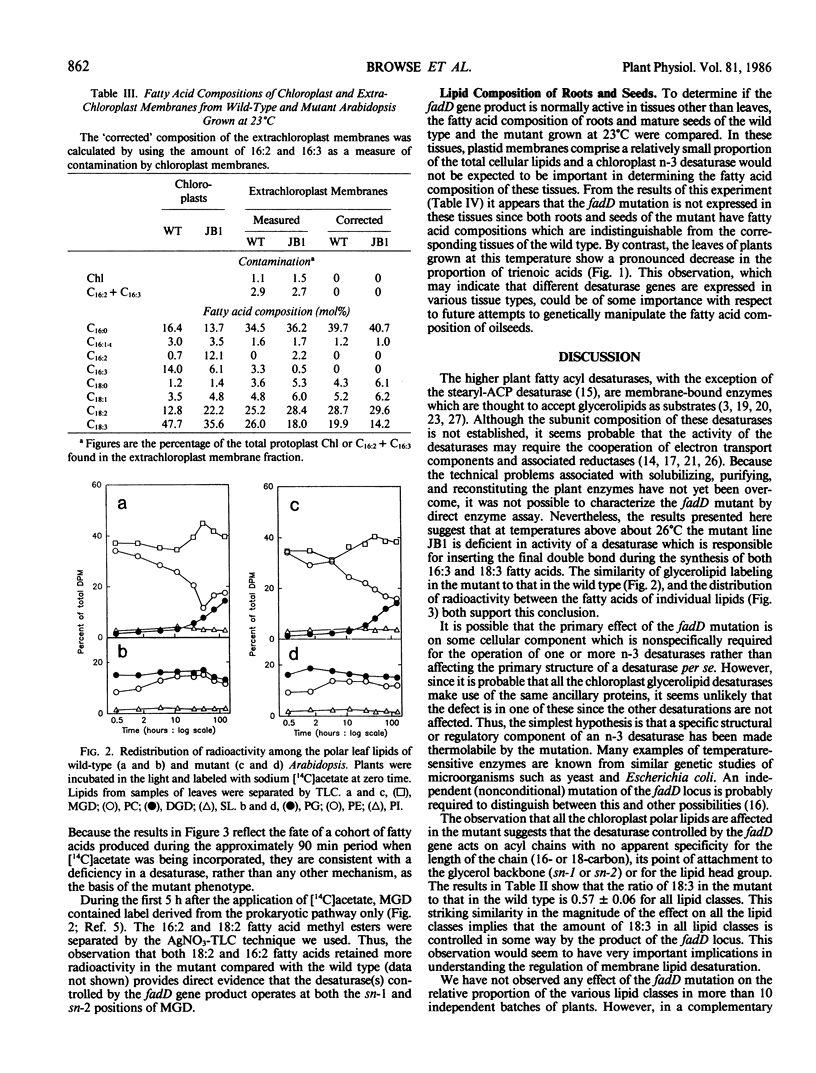
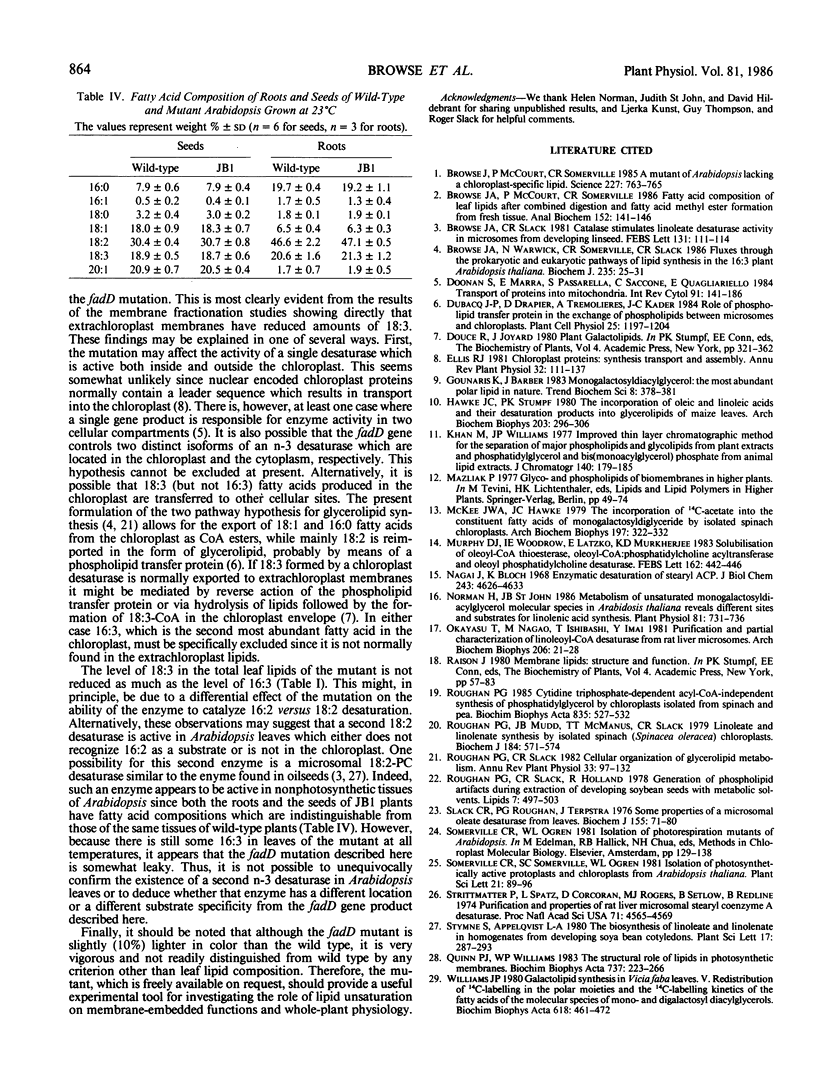
Leaf tissue of a mutant of Arabidopsis thaliana contains reduced levels of both 16:3 and 18:3 fatty acids and has correspondingly increased levels of the 16:2 and 18:2 precursors due to a single recessive nuclear mutation. The kinetics of in vivo labeling of lipids with [14C]acetate and quantitative analysis of the fatty acid compositions of individual lipids suggests that reduced activity of a glycerolipid n-3 desaturase is responsible for the altered lipid composition of the mutant. The effects of the mutation are most pronounced when plants are grown at temperatures above 26°C but are relatively minor below 18°C, suggesting a temperature-sensitive enzyme. Since the desaturation of both 16- and 18-carbon fatty acids is altered, it appears that the affected enzyme lacks specificity with respect to acyl group chain length and that it is located in the chloroplast where 16:3-monogalactosyldiglyceride is synthesized. Because the degree of unsaturation of all the major glycerolipids was similarly affected by the mutation, it is inferred that either the affected desaturase does not exhibit head group specificity or there is substantial transfer of trienoic acyl groups between different lipid classes. Both chloroplast and extrachloroplast lipids are equally affected by the mutation. Thus, either the desaturase is located both outside and inside the chloroplast, or 18:3 formed inside the chloroplast is reexported to other cellular sites.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Browse J., McCourt P. J., Somerville C. R. Fatty acid composition of leaf lipids determined after combined digestion and fatty acid methyl ester formation from fresh tissue. Anal Biochem. 1986 Jan;152(1):141–145. doi: 10.1016/0003-2697(86)90132-6. [DOI] [PubMed] [Google Scholar]
- Browse J., McCourt P., Somerville C. R. A mutant of Arabidopsis lacking a chloroplast-specific lipid. Science. 1985 Feb 15;227(4688):763–765. doi: 10.1126/science.227.4688.763. [DOI] [PubMed] [Google Scholar]
- Browse J., Warwick N., Somerville C. R., Slack C. R. Fluxes through the prokaryotic and eukaryotic pathways of lipid synthesis in the '16:3' plant Arabidopsis thaliana. Biochem J. 1986 Apr 1;235(1):25–31. doi: 10.1042/bj2350025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doonan S., Marra E., Passarella S., Saccone C., Quagliariello E. Transport of proteins into mitochondria. Int Rev Cytol. 1984;91:141–186. doi: 10.1016/s0074-7696(08)61316-9. [DOI] [PubMed] [Google Scholar]
- Hawke J. C., Stumpf P. K. The incorporation of oleic and linoleic acids and their desaturation products into the glycerolipids of maize leaves. Arch Biochem Biophys. 1980 Aug;203(1):296–306. doi: 10.1016/0003-9861(80)90180-0. [DOI] [PubMed] [Google Scholar]
- Khan M. U., Williams J. P. Improved thin-layer chromatographic method for the separation of major phospholipids and glycolipids from plant lipid extracts and phosphatidyl glycerol and bis(monoacylglyceryl) phosphate from animal lipid extracts. J Chromatogr. 1977 Oct 11;140(2):179–185. doi: 10.1016/s0021-9673(00)88412-5. [DOI] [PubMed] [Google Scholar]
- McKee J. W., Hawke J. C. The incorporation of [14C]acetate into the constituent fatty acids of monogalactosyldiglyceride by isolated spinach chloroplasts. Arch Biochem Biophys. 1979 Oct 1;197(1):322–332. doi: 10.1016/0003-9861(79)90252-2. [DOI] [PubMed] [Google Scholar]
- Nagai J., Bloch K. Enzymatic desaturation of stearyl acyl carrier protein. J Biol Chem. 1968 Sep 10;243(17):4626–4633. [PubMed] [Google Scholar]
- Norman H. A., John J. B. Metabolism of Unsaturated Monogalactosyldiacylglycerol Molecular Species in Arabidopsis thaliana Reveals Different Sites and Substrates for Linolenic Acid Synthesis. Plant Physiol. 1986 Jul;81(3):731–736. doi: 10.1104/pp.81.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayasu T., Nagao M., Ishibashi T., Imai Y. Purification and partial characterization of linoleoyl-CoA desaturase from rat liver microsomes. Arch Biochem Biophys. 1981 Jan;206(1):21–28. doi: 10.1016/0003-9861(81)90061-8. [DOI] [PubMed] [Google Scholar]
- Roughan P. G., Mudd J. B., McManus T. T., Slack C. R. Linoleate and alpha-linolenate synthesis by isolated spinach (Spinacia oleracea) chloroplasts. Biochem J. 1979 Dec 15;184(3):571–574. doi: 10.1042/bj1840571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C. R., Roughan P. G., Terpstra J. Some properties of a microsomal oleate desaturase from leaves. Biochem J. 1976 Apr 1;155(1):71–80. doi: 10.1042/bj1550071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter P., Spatz L., Corcoran D., Rogers M. J., Setlow B., Redline R. Purification and properties of rat liver microsomal stearyl coenzyme A desaturase. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4565–4569. doi: 10.1073/pnas.71.11.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]


