Abstract
The possible role of photosynthesis in the mechanism of action of the herbicide acifluorfen (2-chloro-4-(trifluoromethyl)phenoxy-2-nitrobenzoate; AF) was examined. The sensitivity to AF of cotyledons of cucumber (Cucumis sativus L.) which had been grown under far red light (FR) and white light were compared. FR grown tissues which were photosynthetically imcompetent were hypersensitive to AF under white light and had approximately the same relative response to AF under blue and red light as green, white-light-grown tissues. Ultrastructural damage was apparent in FR-grown, AF-treated tissues within an hour after exposure to white light, with cytoplasmic and plastidic disorganization occurring simultaneously. In cucumber cotyledon tissue which had been greening for various time periods, there was no correlation between photosynthetic capacity and herbicidal efficacy of AF. PSII inhibitors (atrazine and DCMU) and the photophosphorylation inhibitor, tentoxin, had no effect on AF activity. Atrazine did not reduce AF activity at any concentration or light intensity tested, indicating that there is no second, photosynthetic-dependent mechanism of action operating at low AF concentrations or low fluence rates. Carbon dioxide-dependent O2 evolution of intact chloroplasts of spinach (Spinacia oleracea L.) had an AF I50 of 125 micromolar compared to 1000 micromolar for cucumber, whereas AF was much more herbicidally active in tissues of cucumber than of spinach. Differences in activity could not be accounted for by differences in uptake of AF. Our results indicate that there is no photosynthetic involvement in the mechanism of action of AF in cucumber.
Full text
PDF
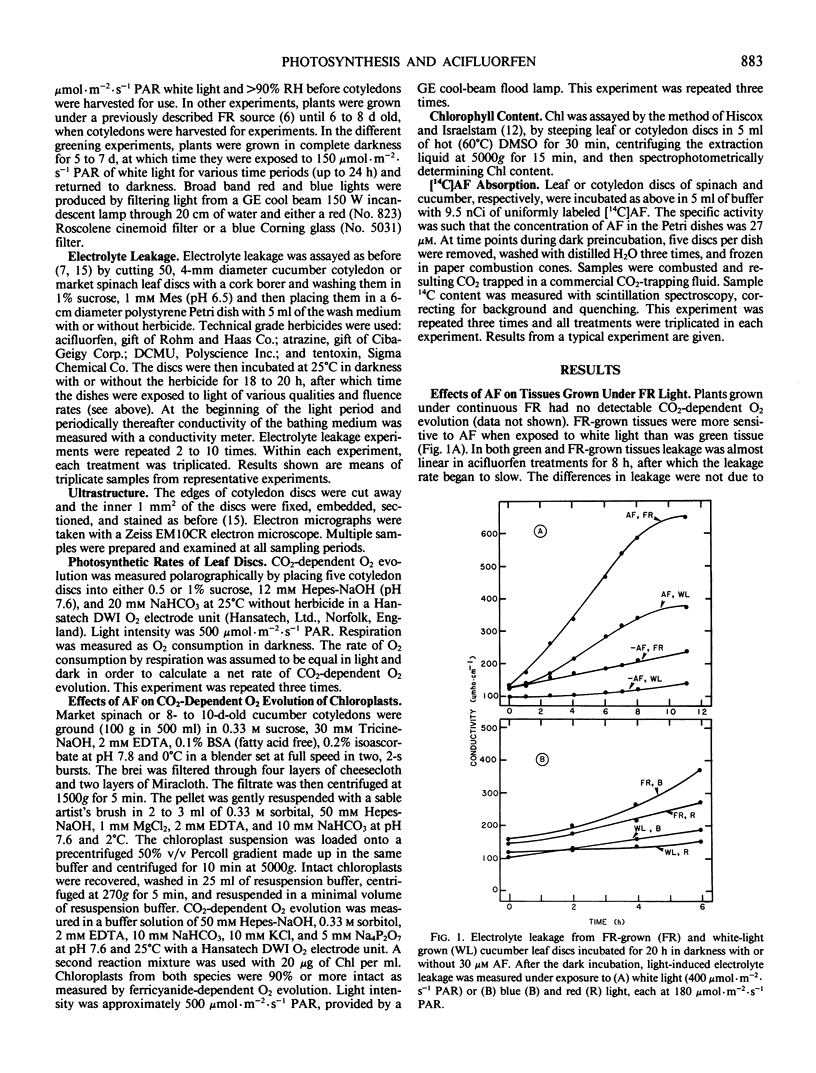

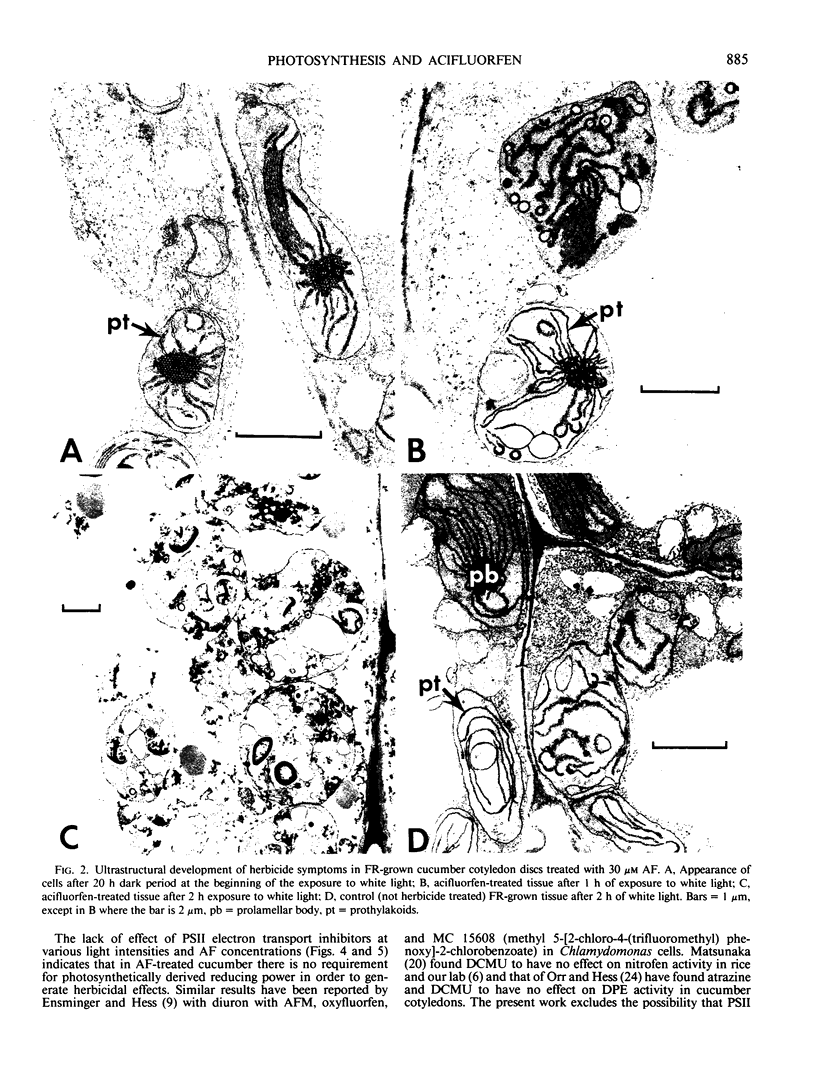
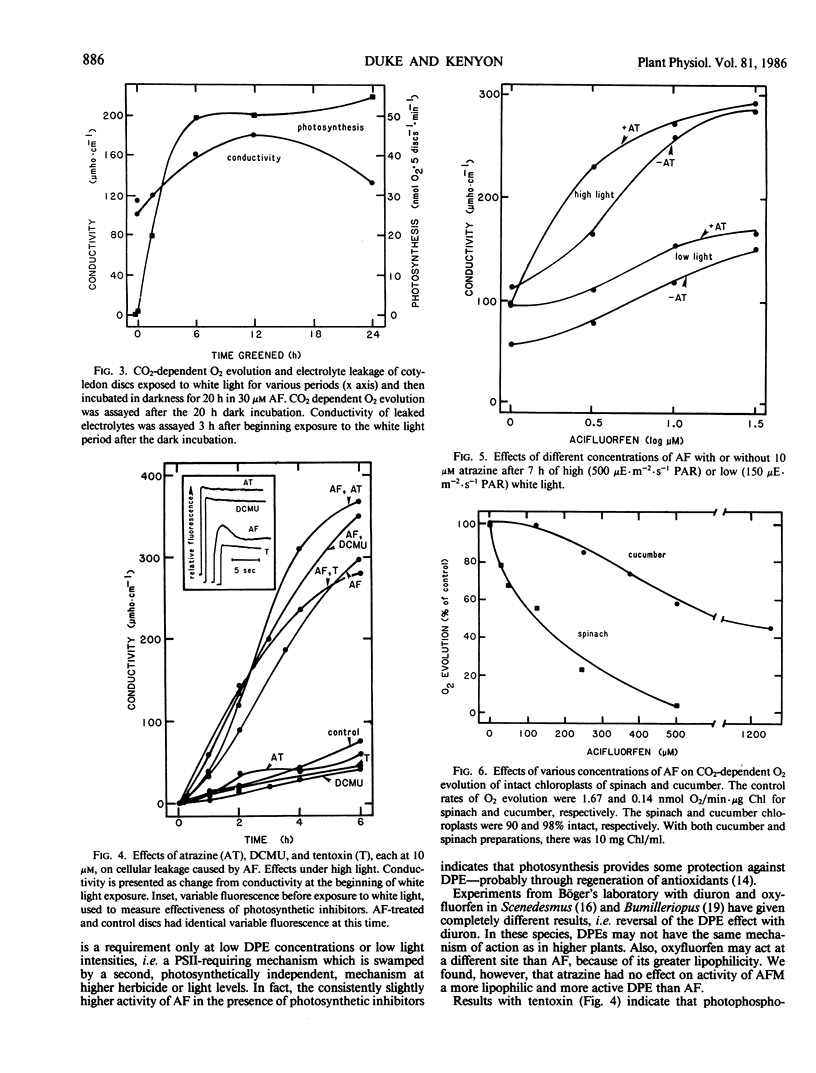

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arntzen C. J. Inhibition of photophosphorylation by tentoxin, a cyclic tetrapeptide. Biochim Biophys Acta. 1972 Dec 14;283(3):539–542. doi: 10.1016/0005-2728(72)90273-3. [DOI] [PubMed] [Google Scholar]
- Bugg M. W., Whitmarsh J., Rieck C. E., Cohen W. S. Inhibition of photosynthetic electron transport by diphenyl ether herbicides. Plant Physiol. 1980 Jan;65(1):47–50. doi: 10.1104/pp.65.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensminger M. P., Hess F. D. Action Spectrum of the Activity of Acifluorfen-methyl, a Diphenyl Ether Herbicide, in Chlamydomonas eugametos. Plant Physiol. 1985 Feb;77(2):503–505. doi: 10.1104/pp.77.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensminger M. P., Hess F. D. Photosynthesis involvement in the mechanism of action of diphenyl ether herbicides. Plant Physiol. 1985 May;78(1):46–50. doi: 10.1104/pp.78.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon W. H., Duke S. O. Effects of Acifluorfen on Endogenous Antioxidants and Protective Enzymes in Cucumber (Cucumis sativus L.) Cotyledons. Plant Physiol. 1985 Nov;79(3):862–866. doi: 10.1104/pp.79.3.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele J. A., Uchytil T. F., Durbin R. D., Bhatnagar P., Rich D. H. Chloroplast coupling factor 1: A species-specific receptor for tentoxin. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2245–2248. doi: 10.1073/pnas.73.7.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettlaufer S. H., Alscher R., Strick C. Chloroplast-Diphenyl Ether Interactions II. Plant Physiol. 1985 Jun;78(2):215–220. doi: 10.1104/pp.78.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]