Abstract
Background
Most cases of stroke are caused by impairment of blood flow to the brain (ischaemia), which results in a reduction in available oxygen and subsequent cell death. It has been postulated that hyperbaric oxygen therapy (HBOT) may reduce the volume of brain that will die by greatly increasing available oxygen, and it may further improve outcomes by reducing brain swelling. Some centres are using HBOT routinely to treat people with stroke. This is an update of a Cochrane Review first published in 2005.
Objectives
To assess the effectiveness and safety of adjunctive HBOT in the treatment of people with acute ischaemic stroke.
Search methods
We searched the Cochrane Stroke Group Trials Register (last searched April 2014), the Cochrane Central Register of Controlled Trials (CENTRAL) (April 2014), MEDLINE (1966 to April 2014), EMBASE (1980 to April 2014), the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1982 to April 2014), the Database of Randomised Controlled Trials in Hyperbaric Medicine (DORCTIHM) (searched April 2014) and the reference lists of articles. We handsearched relevant publications and contacted researchers to identify additional published and unpublished studies.
Selection criteria
Randomised controlled trials (RCTs) that compared the effects of adjunctive HBOT versus those of no HBOT (no treatment or sham).
Data collection and analysis
Three review authors independently extracted data, assessed each trial for internal validity and resolved differences by discussion.
Main results
We included 11 RCTs involving 705 participants. The methodological quality of the trials varied. We could pool data only for case fatalities. No significant differences were noted in the case fatality rate at six months in those receiving HBOT compared with the control group (risk ratio (RR) 0.97, 95% confidence interval (CI) 0.34 to 2.75, P value 0.96). Four of 14 scale measures of disability and functional performance indicated improvement following HBOT, for example, the mean Trouillas Disability Scale score was lower with HBOT (mean difference (MD) 2.2 point reduction with HBOT, 95% CI 0.15 to 4.3, P value 0.04), and the mean Orgogozo Scale score was higher (MD 27.9 points, 95% CI 4.0 to 51.8, P value 0.02).
Authors' conclusions
We found no good evidence to show that HBOT improves clinical outcomes when applied during acute presentation of ischaemic stroke. Although evidence from the 11 RCTs is insufficient to provide clear guidelines for practice, the possibility of clinical benefit has not been excluded. Further research is required to better define the role of HBOT in this condition.
Plain language summary
Hyperbaric oxygen therapy for treating people with acute ischaemic stroke
Question
We wanted to compare the safety and effectiveness of hyperbaric oxygen therapy (HBOT) versus no HBOT (either no treatment or an ineffective intervention designed to mimic the true treatment) for treating people who had suffered an acute ischaemic stroke.
Background
Hyperbaric oxygen therapy (HBOT) is a treatment designed to increase the supply of oxygen to the part of the brain affected by stroke and to reduce the extent of irreversible damage. HBOT involves people breathing pure oxygen in a specially designed chamber (such as those used for deep sea divers with the bends) for a period of about an hour and a half each day for 10 to 20 days.
Study characteristics
We identified 11 studies, involving 705 participants, up to April 2014. All studies included adult participants (41% female) who had suffered an acute stroke within the past two weeks, although most studies enrolled participants within three days of the stroke. All trials evaluated the addition of HBOT to standard practice for study participants. Most trials reported the number of deaths and some measure of functional ability, although the actual measures used varied considerably, making comparisons between trials difficult. Follow‐up periods varied from 90 days to one year.
Key results
Too few patients have been studied to say whether HBOT decreases the chance of dying, and only three trials have suggested improvement in the ability to do everyday tasks. Overall, little evidence is currently available to support the use of HBOT for people with stroke.
Quality of the evidence
In general, the quality of the evidence was moderate, given the small numbers of participants and the many different instruments used to estimate the quality of life and the functional ability of participants. The methodology used in many of these trials was poorly described, making a good estimate of the reliability of the evidence difficult. We could pool data only for the chance of dying after the stroke, and although no evidence suggested that HBOT reduced the chance of death following a stroke, the confidence we have in this result is relatively low. We have not excluded the possibility that HBOT may be harmful or beneficial.
Summary of findings
Summary of findings for the main comparison. Should hyperbaric oxygen therapy be used as an adjuvant therapy to routine therapy and rehabilitation for acute ischaemic stroke?
| Should hyperbaric oxygen therapy be used as an adjuvant therapy to routine therapy and rehabilitation for acute ischaemic stroke? | ||||||
| Patient or population: people with acute ischaemic stroke Settings: hospital inpatients Intervention: hyperbaric oxygen therapy used as adjuvant therapy to Comparison: routine therapy and rehabilitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Routine therapy and rehabilitation | Hyperbaric oxygen therapy used as adjuvant therapy | |||||
| Death Follow‐up: 3 to 6 months | Low‐risk population | RR 0.97 (0.34 to 2.75) | 144 (4 studies) | ⊕⊕⊕⊝ moderatea | ||
| 20 per 1000 | 19 per 1000 (7 to 55) | |||||
| Medium‐risk population | ||||||
| 80 per 1000 | 78 per 1000 (27 to 220) | |||||
| High‐risk population | ||||||
| 120 per 1000 | 116 per 1000 (41 to 330) | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
a Small trials, with most not blinded to allocation.
Background
Description of the condition
Stroke may be defined as a sudden neurological deficit of presumed vascular origin (Bath 2000). It is both a leading cause of mortality worldwide, accounting for an estimated 4.4 million deaths in 1990 (Murray 1997), and a leading cause of disability. About one‐third of survivors require significant assistance in daily life at one year after the event (Bamford 1991; Bath 2000).
Stroke is divided into two broad subgroups: ischaemic (impairment of blood flow) and haemorrhagic (bleeding within the brain), with the former accounting for 73% to 86% of all cases (Sudlow 1997). On average, ischaemic stroke has a lower case fatality rate than haemorrhagic stroke (23% versus 62% at one year), and treatment differs for the two subgroups (Bamford 1991; Bath 2000). Therefore, early and accurate diagnosis is desirable. Because clinical assessment is unreliable in determining stroke type, neuroimaging (preferably using computerised tomography (CT) scan) is required for optimal management (Wardlaw 2004).
During a cerebral ischaemic event, neurological tissue suffers hypoxia. When hypoxia is prolonged, neurons lose their ability to maintain ionic homeostasis. Free oxygen radicals accumulate and degrade the cell membranes (Ikeda 1990; Siesjo 1989); irreversible changes result in unavoidable cell death. These changes may occur rapidly and before therapy can be instituted, but in some people the symptoms worsen gradually or in a step‐wise fashion over a matter of hours or days (Robertson 1989). This latter observation suggests that close management of haemodynamic, respiratory and metabolic factors designed to maintain oxygenation might be beneficial. Indeed, intensive stroke management protocols and antiplatelet therapy have been shown to positively influence the outcome (CASTCG 1997; ISTCG 1997; SUTC 2004).
Description of the intervention
Hyperbaric oxygen therapy (HBOT) is an adjunctive therapy that has been proposed for the treatment of people with ischaemic stroke (Hart 1971; Ingvar 1965). HBOT is the therapeutic administration of 100% oxygen at environmental pressures greater than 1.0 atmosphere absolute (ATA). Administration involves placing the patient in an airtight vessel, increasing pressure within that vessel and administering 100% oxygen for respiration. In this way, it is possible to deliver greatly increased partial pressure of oxygen to the tissues. Typically, treatments involve pressurisation to between 1.5 and 3.0 ATA for periods between 60 and 120 minutes, once or twice daily.
How the intervention might work
Potential benefits of HBOT include reversal of hypoxia through increased oxygen delivery and reduction of cerebral oedema (Hills 1999; Sukoff 1982), as well as several specific effects of hyperoxia, which include decreased lipid peroxidation, inhibition of leucocyte activation and restoration of the functional blood‐brain barrier (Mink 1995a; Mink 1995b; Thom 1993). It has been proposed that HBOT protects marginally viable brain (often termed 'the ischaemic penumbra') from further damage on reperfusion through these mechanisms, which act to regulate abnormal cellular metabolites (Badr 2001; Selman 2004). Conversely, oxygen in high doses may increase oxidative stress through the production of oxygen free‐radical species and is potentially toxic (Yusa 1987). Indeed, the brain is particularly at risk (Clark 1982). For this reason, it is appropriate to postulate that in some people with stroke, HBOT may do more harm than good.
Therefore, HBOT is associated with some risk of adverse effects, including damage to the ears, sinuses and lungs from the effects of pressure, temporary worsening of shortsightedness, claustrophobia and oxygen poisoning. Although serious adverse events are rare, HBOT cannot be regarded as an entirely benign intervention.
Why it is important to do this review
Despite 40 years of interest in the delivery of HBOT for people with stroke, little comparative evidence of effectiveness can be found. Most reports have described single or multiple cases, and the largest study includes a series of 122 cases reported in 1980 (Neubauer 1980). A review of these studies revealed that more than half of cases improved clinically or electrophysiologically with HBOT and concluded that a case could be made for setting up controlled studies (Nighoghossian 1997).
This is an update of a Cochrane review first published in 2005.
Objectives
To assess the effectiveness and safety of adjunctive HBOT in the treatment of people with acute ischaemic stroke.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs) that compared the effects of adjunctive HBOT versus those of no HBOT (no treatment or sham).
Types of participants
We focused on participants of any age or sex with acute ischaemic stroke. Acute ischaemic stroke was defined as a sudden neurological deficit of presumed vascular origin for which haemorrhage had been excluded by CT or magnetic resonance imaging (MRI) within 14 days of enrolment in the trial.
Types of interventions
Trials comparing standard treatment regimens designed to promote recovery after acute ischaemic stroke, including intensive combined therapies, with and without HBOT.
Studies were eligible for inclusion if HBOT was administered in a compression chamber at pressures between 1.5 ATA and 3.0 ATA for treatment times between 30 minutes and 120 minutes, at least once daily.
Types of outcome measures
Studies were eligible for inclusion in the meta‐analysis if they reported any of the following outcome measures. Because of variations in clinical practice, we divided timing and recording of outcomes into three stages for analysis: early (immediately after the treatment course), medium term (four to eight weeks after treatment) and longer term (at the end of scheduled follow‐up). Several assessment scales were used to quantify trial outcomes. We have taken information concerning these scales from the Internet Stroke Centre Stroke Trials Directory at http://www.strokecenter.org/trials/scales/index.htm.
Primary outcomes
Case fatality rate.
Severe functional disability rate (or death), defined as 'drowsy/stuporous/unconscious or unable to feed/dress independently.' Care was taken to ensure that death was included as a bad outcome when data were extracted.
Secondary outcomes
Functional status scale scores (e.g. National Institutes of Health Stroke Scale (NIHSS), Rankin Score, Glasgow Outcome Scale).
Deemed to have a good functional outcome assessed as a binary outcome using any of the above scales.
Activities of daily living (ADLs) (e.g. the Barthel Index).
CT or MRI estimate of infarct size or volume.
Adverse events following HBOT, such as proportion of participants with visual disturbance (short and long term), barotrauma (aural, sinus, pulmonary short and long term) and oxygen toxicity (short term); other recorded adverse effects were reported and discussed.
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module.
Electronic searches
We searched the Cochrane Stroke Group Trials Register, which was last searched by the Managing Editor on 3 April 2014. In addition, we searched MEDLINE (1966 to April 2014; Appendix 1), EMBASE (1980 to April 2014; Appendix 2) and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1982 to April 2014; Appendix 3), as well as the Cochrane Central Register of Controlled Trials (CENTRAL) (8 April 2014; Appendix 4). We adapted the MEDLINE search strategy for the other databases. We applied no language restrictions. We also searched the Database of Randomised Controlled Trials in Hyperbaric Medicine (DORCTIHM) (from inception to April 2014) (Bennett 2004). The DORCTIHM database was compiled through an unfocused search of PubMed using 'hyperbaric oxygenation' as a medical subject heading (MeSH) term, handsearching of primarily hyperbaric journals (see below) since first publication and checking of references in identified RCTs. This site is now interactive and receives citations for formal review from healthcare professionals in the field.
Searching other resources
We searched the reference lists of articles and contacted researchers in an effort to identify additional published and unpublished studies.
-
We handsearched the following relevant publications.
Hyperbaric textbooks (Jain 1999; Jain 2009; Kindwall 1999; Mathieu 2006; Oriani 1996).
Journals (Undersea and Hyperbaric Medicine 1992 to 2014, Hyperbaric Medicine Review 1986 to 1992, South Pacific Underwater Medicine Society (SPUMS) Journal 1973 to 2007, European Journal of Hyperbaric Medicine 1998 to 2007, Diving and Hyperbaric Medicine 2007 to 2014 and Aviation, Space and Environmental Medicine Journal 1980 to 2014).
Conference proceedings (Undersea and Hyperbaric Medical Society, SPUMS, European Undersea and Baromedical Society, International Congress of Hyperbaric Medicine) published from 1980 to 2014.
Data collection and analysis
Trial identification
Three review authors (MB, JW and PK) scanned records identified through the electronic searches and excluded obviously irrelevant studies. Two review authors (MB and AS) assessed the remaining records and retrieved the full‐text articles of all potentially relevant studies. From these, three review authors (MB, AS and PK) selected studies for inclusion based on the criteria described previously. In all instances, review authors resolved differences of opinion by discussion.
Data extraction
Three review authors (MB, AS and CF) independently extracted data from the studies using standardised forms developed for this review. We contacted the authors of primary studies to request further information when data were missing or incomplete. Review authors resolved all differences by discussion.
Quality assessment
We assessed study quality using the 'Risk of bias' tables for each individual study, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011); the results are presented both descriptively and graphically.
Analyses
We used a fixed‐effect model when no evidence of significant heterogeneity between studies was found and planned to use a random‐effects model when such heterogeneity was likely (DerSimonian 1986). We considered the appropriateness of meta‐analysis in the presence of significant clinical or statistical heterogeneity. We assessed statistical heterogeneity using the I2 statistic. We planned to explore heterogeneity and to perform subgroup analyses if appropriate.
For proportions (dichotomous outcomes), we used risk ratio (RR). We converted continuous data to mean differences (MDs) using the inverse variance method and calculated an overall MD. We planned to test publication bias using funnel plots; however, this was not appropriate given the small number of studies found.
We planned to perform subgroup analysis by calculating the RR or MD in each subgroup and examining 95% confidence intervals (CIs). Non‐overlap in intervals would have been taken to indicate a statistically significant difference between subgroups; however, subgroup analysis was not appropriate given the available data.
All analyses were performed on an intention‐to‐treat basis, if possible, and if not possible, this was clearly stated.
We intended to perform sensitivity analyses for missing data and study quality, if appropriate.
Missing data
We had planned to employ sensitivity analyses by using different approaches to imputing missing data. However, no binary outcomes involved incomplete data, and this analysis was not required.
Study quality
If appropriate, we had planned to conduct a sensitivity analysis by study quality based on the presence or absence of a reliable random allocation method, concealment of allocation and blinding of participants or outcome assessors.
If appropriate data were found, we planned to consider subgroup analysis based on the following.
Time elapsed between stroke and institution of HBOT.
Dose of oxygen received (pressure less than 2.0 ATA versus greater than or equal to 2.0 ATA), time of treatment (less than 60 minutes versus 60 minutes or longer) and length of treatment course (fewer than five sessions versus five or more sessions).
Nature of comparative treatment modalities.
Volume of cerebral infarction as measured by baseline presentation of CT imaging.
Results
Description of studies
We identified a total of 962 references in 2004, a further 104 by September 2008 and a further 396 by April 2014, yielding a total of 1462 citations. Following elimination of duplicates, independent scrutiny of the titles and abstracts revealed 40 potentially relevant articles. One was an ongoing trial first announced in 2008 (Michalski 2008), but our attempts to contact the study authors were unsuccessful, and this trial may have been abandoned. Following review of the full text of the remaining 39 articles, we excluded 18 further trials because they were not RCTs, five because they gave inappropriate treatment, four because they included inappropriate participants (with long‐standing or haemorrhagic stroke) and one review article (see Characteristics of excluded studies and Figure 1). The remaining 11 randomised trials formed the basis of this review and are described below. Seven trials are now included in the quantitative review.
1.
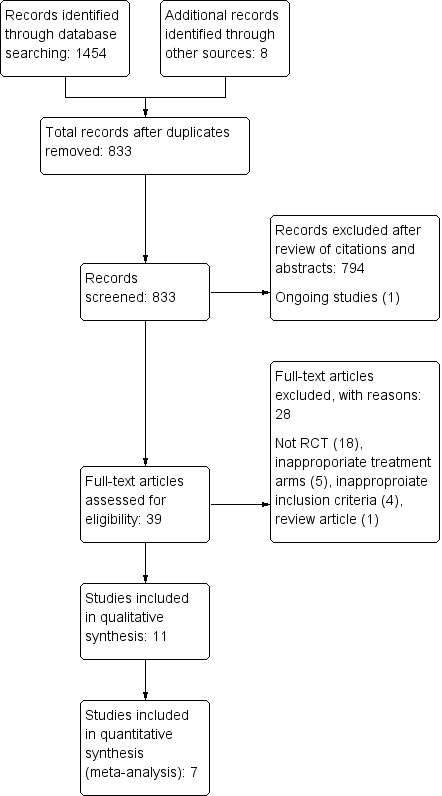
Study flow diagram.
Between the previous search in September 2008 and the most recent search in April 2014, we found 396 new citations, of which only one was a new trial, which contributed 38 further participants to the quantitative analysis (Imai 2006). In this review, we also included four relevant trials that did not report on our outcomes of interest and so did not contribute to the quantitative review. Together these trials enrolled a total of 334 participants.
In total, the 11 included trials enrolled 705 participants (361 into trials contributing to the quantitative review and 344 into trials contributing to the qualitative review only).
Anderson 1991 enrolled 39 non‐pregnant participants (sex distribution was not given) between 20 and 90 years of age, presenting with a neurological deficit and score greater than 20 on a scale devised for this trial (0 = no deficit, 100 = completely unresponsive). The deficit was presumed to be due to ischaemic cerebral infarction in the brain region perfused by one carotid artery and to have occurred during the preceding two weeks. Once enrolled, all participants received standard care in a neurological intensive care unit and 400 mg of vitamin A daily. Those randomly assigned to the HBOT arm received 100% oxygen (for 60 minutes at 1.5 ATA) every eight hours to a total of 15 sessions, and those in the control arm received a sham treatment of breathing air (at 1.5 ATA) on the same schedule. Changes in the graded neurological examination performed on admission were used to assess neurological outcomes at day five, week six and year one. Infarct volume was estimated by CT at four months, and deaths were recorded.
Nighoghossian 1995 enrolled 34 participants (21 men), between 20 and 75 years of age, presenting with a neurological deficit highly suggestive of middle cerebral artery (MCA) occlusion. All participants presented within 24 hours of the cerebral event and with a score less than 80 on the Orgogozo Functional Scale (0 = completely unresponsive, 100 = no deficit). Once enrolled, all participants received supportive care including low‐dose heparin (10,000 units divided into two doses daily), nursing care, rehabilitation, speech therapy and occupational therapy. Those allocated to HBOT received 100% oxygen (in a monoplace chamber at 1.5 ATA daily for 40 minutes for 10 days), while controls received sham therapy (at 1.2 ATA) of breathing air on the same schedule. Outcomes included case fatality rate and changes on three healthcare scales (acute assessment scale: Orgogozo (100 to 0), Trouillas (0 to 10) and functional assessment scale (Rankin Scale)) that were used to assess neurological outcomes at six months and at one year.
Sansone 1997 has been presented only in abstract format. This trial enrolled 17 participants with an average age of 62 years who had presented for therapy within 24 hours of CT scanning, showing a cerebral ischaemic event in the middle or anterior cerebral arterial territory. Study participants included 11 males. The degree of neurological deficit was not clear, although scores were provided in a table. Once enrolled, participants were randomly assigned by a method not stated to receive HBOT (1.5 to 1.8 ATA for 60 minutes daily for 8 to 10 days) or sham treatment of breathing air. A neurological recovery score (0 to 10) was used to assess outcomes at six and 12 months.
Rusyniak 2003 enrolled 33 participants (including 22 men) presenting to the emergency department within 24 hours of stroke onset with a deficit on an acute impairment scale—the National Institutes of Health Stroke Scale (NIHSS)—of less than 23 (30 is maximum disability) and without evidence of haemorrhage on CT. Those randomly assigned to the HBOT arm received a single session (breathing 100% oxygen for 60 minutes in a monoplace chamber at 2.5 ATA), while those in the control arm received sham treatment (breathing oxygen at 1.14 ATA). Primary outcome measures included percentages of participants with improvement at 24 hours and at 90 days. Secondary measurements included complications of treatment and mortality at 90 days. Outcomes included case fatality rate; adverse effects of treatment and changes in the following: NIHSS score (at 24 hours and at 90 days); a functional assessment scale—the modified Rankin Scale (90 days); and two outcome scales—the Barthel Index of ADL (90 days) and the Glasgow Outcome Scale (90 days).
Yang 2003 enrolled 80 participants (43 men) with radiological or MRI evidence of ischaemic stroke within 72 hours of the event. These strokes occurred in a range of vascular territories. Participants were randomly assigned to receive HBOT at 2.0 ATA for 80 minutes daily, probably for 20 days, or a standard therapy that may have included a vasodilator, an anticoagulant or a 'nerve cell protectant.' The only outcome measure was a four‐point scale from 'cure' to 'no effect' that was measured at 21 days.
Yang 2004 enrolled 120 participants with radiological or MRI evidence of ischaemic stroke within 72 hours of the event; however 40 of these were randomly assigned to an arm that was not relevant to this review. The strokes were all 'first events,' associated with neurological deficits and during which the patient was conscious and co‐operative. Eighty participants were randomly assigned to HBOT at 2.0 ATA for 60 minutes daily for 20 days or to a standard treatment regimen that was not described in detail. Outcomes measured include degree of neurological dysfunction (scale not described), Fugl‐Meyer motor assessment score and Barthel Index of activities of daily living. The timing of these assessments was not stated.
Imai 2006 enrolled 38 adult participants with acute embolic stroke in the MCA territory within 48 hours of the event. Initial diagnosis was determined by neurological signs and was confirmed by MRI. Patients were excluded if they had received fibrinolytic therapy. All participants received standard care plus a free‐radical scavenger—edaravone 30 mg intravenously twice daily. In addition, the 19 participants in the HBOT group received 100% oxygen at 2.0 ATA for 60 minutes daily for 10 days. Reported outcomes included death, unfavourable outcomes by the Modified Rankin Scale (mRS), adverse events and median NIHSS scores—all at 90 days.
Trials included but not contributing to the quantitative review
Li 1998 enrolled 86 participants with cerebral infarction (time not stated but described as 'acute') and provided 'routine' therapy with 'Salvia Milirrhiza Co.' to both groups. The 43 participants assigned to the HBOT group received 10 daily treatments at 2 ATA breathing 100% oxygen for 120 minutes. The study authors reported cerebral blood flow, electroencephalography (EEG), haemorrheology and serum superoxide dismutase (SOD) before and after the intervention period but included no outcomes of interest for this review. EEG, cerebral blood flow and serum SOD were reported as improved in the HBOT group versus the control group (P value < 0.01). Haemorrheology was not significantly different.
Peng 2003 enrolled 60 participants with cerebral infarction within the previous seven days, and 30 were allocated to each arm of the study. All participants received 'standard pharmacological care,' while the HBOT group received 10 daily treatments at 2.3 ATA breathing 100% oxygen for 80 minutes. The study authors reported within‐group differences for several markers of free‐radical activity, including serum nitric oxide (NO), SOD and monoaldehyde (MDA) but did not directly compare groups. Levels of NO and SOD were significantly increased following HBOT, but not in the control group. Participants in the HBOT group had significantly improved scores on an undefined neurological deficit scale (HBOT 9 ± 8 versus 15 ± 9, t = ‐2.176, P value < 0.05). Researchers also reported improved 'clinical efficacy' in the HBOT group (HBOT 87% versus control 63%, x2 = 4.356, P value < 0.05).
Hong 2008 is an abstract only and has not been published in full. This trial enrolled 86 young inpatients with ischaemic stroke, but there are no further details. The cohort was randomised into two groups (method unknown), but the number of participants in each group is not given. One group received 'routine therapy' while the other received 'routine therapy and hyperbaric therapy'. Neither treatment arm was described. The authors reported the 'Nerve Function Deficit Score' (undefined) was improved in 70% in the HBOT group and 42.6% in the control group, that the effectiveness of treatment in the HBOT group was 81.4% compared with 35.9% in the control group, and that the total progress rate was 93% versus 60.5% in favour of the HBOT group. None of these outcomes were defined.
Zhao 2008 enrolled 112 adults admitted with an acute stroke involving the internal carotid artery territory and confirmed with CT or MRI within 24 hours. They were randomised by a method not described. Sixty‐two participants received routine therapy including anticoagulation, antiplatelet aggregation agents, cerebral vasodilators, reduction of intracranial pressure by dehydration, as well as supplementary rehabilitation exercise. Fifty participants received the same routine treatment plus HBOT for 60 minutes daily for 10 days at 2.0 ATA (plus a 30‐minute decompression). Serum levels of soluble intercellular adhesion molecule, soluble vascular cell adhesion molecule, soluble E‐selectin, and matrix metalloproteinase‐9 were significantly increased on admission and decreased over the study period in both groups. The decrease was significantly greater in the HBO group, compared with the routine treatment group (P value < 0.05 or P value < 0.01).
Risk of bias in included studies
See the Characteristics of included studies table for further details. These trials varied in methodological quality, and only six provided full reports of completed trials in a peer‐reviewed publication. Risk of bias dimensions are presented for each included study in Figure 2 and Figure 3.
2.
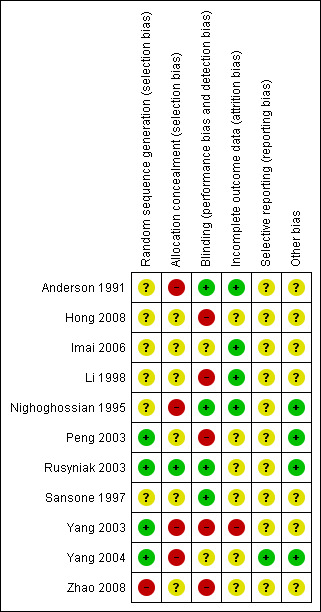
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
3.
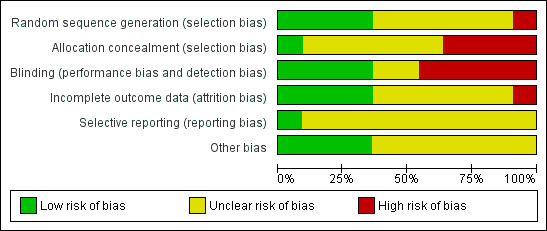
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Randomisation
Randomisation procedures were described in two trials ‐ Rusyniak 2003 (sealed envelopes) and Peng 2003 (coin toss) ‐ but not in the other nine trials. Allocation concealment was adequately described in Rusyniak 2003, but in the other 10 reports, it was not clear whether investigators were unable to predict the prospective group to which a participant would be allocated.
Participant baseline characteristics
All trials enrolled participants with clinical evidence of neurological deficit attributable to ischaemic stroke and lasting longer than 24 hours. Five trials specifically excluded haemorrhagic stroke with CT or MRI evidence before the time of enrolment (Imai 2006; Rusyniak 2003; Yang 2003; Yang 2004; Zhao 2008). Three trials enrolled participants within 24 hours of the cerebral event (Rusyniak 2003; Nighoghossian 1995; Sansone 1997), one trial within 48 hours (Imai 2006), two trials within 72 hours (Yang 2003; Yang 2004) and one trial within seven days (Peng 2003), and one trial accepted participants up to two weeks following the event (Anderson 1991). The extent and severity of deficit on enrolment were poorly described and difficult to compare across trials, given the different neurological and health status scales used to establish baseline status. Three trials specified the clinical appearance of deficits in an area supplied by one internal carotid artery (Anderson 1991; Nighoghossian 1995; Sansone 1997): Two trials specified the anterior cerebral artery territory (Anderson 1991; Imai 2006) and two the anterior or middle cerebral territory (Nighoghossian 1995; Sansone 1997); three studies did not specify the area (Rusyniak 2003; Yang 2003; Yang 2004). All trials enrolled both male and female adults. In total, 118 participants (41%) were female.
Blinding
Four trials utilised sham therapy to mask participants to HBOT (Anderson 1991; Nighoghossian 1995; Rusyniak 2003, Sansone 1997). Two trials blinded the investigators (Anderson 1991; Rusyniak 2003); one trial also specified outcome assessor blinding (Anderson 1991). No study author formally tested the success of the blinding strategy applied.
Participants lost to follow‐up
Nighoghossian 1995 reported a total of seven participants who were withdrawn from therapy (control group: four ‐ all because of worsening neurological status, with one dead at six‐month follow‐up; HBOT group: three ‐ one with worsening of neurological state, one with myocardial infarction and one with claustrophobia). Anderson 1991 lost a total of 12 participants to follow‐up at six months (control group: five ‐ two deaths, two unavailable and one refusal; HBOT group: seven ‐ two deaths, one unavailable, three refusals and one following a second stroke); Rusyniak 2003 lost seven participants to final follow‐up (control group: six ‐ two dead, four not explained; HBOT group: one dead). Yang 2003 reported a participant group of 80 but reported demographic and outcome data for only 70 participants—it is not clear whether this was an error or if 10 participants were lost to follow‐up. No comparisons of baseline characteristics were made between those lost to follow‐up and those remaining in the study.
Intention‐to‐treat analysis
Two trials performed intention‐to‐treat analyses as they reported inclusion of those lost to follow‐up or not completing the allocated therapy (Nighoghossian 1995; Rusyniak 2003). For mortality, one trial also used intention‐to‐treat; however, it is not clear if this approach was used for other outcomes (Anderson 1991).
Effects of interventions
See: Table 1
We could pool data from only seven studies enrolling a total of 361 participants and for a limited number of clinically important outcomes. For most outcomes, we have been limited to a description of outcomes reported individually for each study.
Primary outcomes
Comparison 1. Death at three to six months
Data were available for four trials involving 144 participants (45% of the total participants in this review) (Anderson 1991; Imai 2006; Nighoghossian 1995; Rusyniak 2003; ). No significant differences in case fatality rates were noted (six deaths (8%) in those receiving HBOT versus six (8.5%) among those given sham therapy). The risk ratio (RR) of dying after receiving HBOT was 0.97 (95% CI 0.34 to 2.75, P value 0.96). Moderate heterogeneity between trials was indicated (I2 = 34.7%), which was explained by removal of the single trial testing the combination of HBOT and a free‐radical scavenger (edaravone) versus edaravone only. No participants were lost to follow‐up for this outcome (Analysis 1.1).
1.1. Analysis.
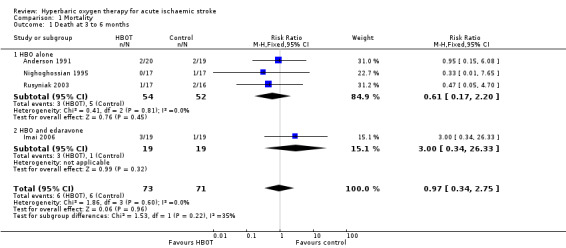
Comparison 1 Mortality, Outcome 1 Death at 3 to 6 months.
Comparison 2. No effect of therapy
Only Yang 2003 presented data on this outcome, involving 70 participants (25% of the total). In the HBOT group, three of 38 participants (8%) were judged to have received 'no effect' from therapy and were unable to care for themselves, compared with seven of 32 cases (22%) in the control group. The risk ratio for a poor outcome with HBOT was 0.36 but was not statistically significant (95% CI 0.1 to 1.28, P value = 0.12) (Analysis 2.1).
2.1. Analysis.
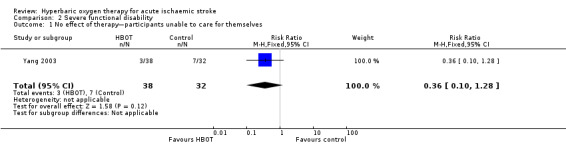
Comparison 2 Severe functional disability, Outcome 1 No effect of therapy—participants unable to care for themselves.
Secondary outcomes
Comparison 3. Functional scales
Mean neurological assessment score at day five
Only Anderson 1991 reported this outcome. The mean score was lower (better outcome) in the control group (38.5 versus 43.8: MD 5.3 points, 95% CI ‐7.5 to 18.1, P value 0.42) (Analysis 3.1).
3.1. Analysis.
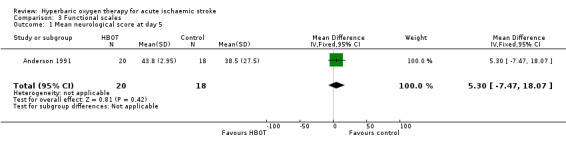
Comparison 3 Functional scales, Outcome 1 Mean neurological score at day 5.
Mean neurological assessment score at week six
Only Anderson 1991 reported this outcome. The mean score was lower (better outcome) in the control group (28.3 versus 38.5: MD 10.2 points, 95% CI ‐8.5 to 28.9, P value 0.28) (Analysis 3.2).
3.2. Analysis.
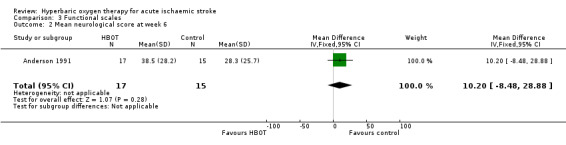
Comparison 3 Functional scales, Outcome 2 Mean neurological score at week 6.
Mean neurological assessment score at one year
Only Anderson 1991 reported this outcome. The mean score was lower (better outcome) in the control group (25.8 versus 31.4: MD 5.6 points, 95% CI ‐15.0 to 26.2, P value 0.59) (Analysis 3.3).
3.3. Analysis.
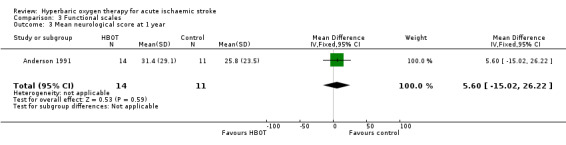
Comparison 3 Functional scales, Outcome 3 Mean neurological score at 1 year.
Mean Orgogozo Scale score at six months
Only Nighoghossian 1995 reported this outcome. The mean score was higher (better outcome) in the HBOT group (72.9 versus 54.7: MD 18.2 points, 95% CI ‐5.2 to 41.6, P value 0.13) (Analysis 3.4).
3.4. Analysis.
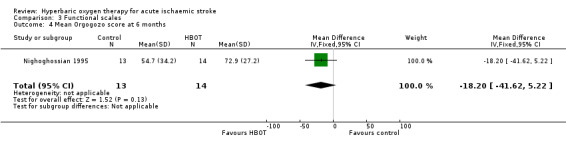
Comparison 3 Functional scales, Outcome 4 Mean Orgogozo score at 6 months.
Mean Orgogozo Scale score at one year
Only Nighoghossian 1995 reported this outcome. The mean score was higher (better outcome) in the HBOT group (78.2 versus 50.3: MD 27.9 points, 95% CI 4.0 to 51.8, P value 0.02) (Analysis 3.5).
3.5. Analysis.
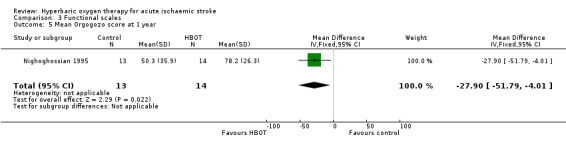
Comparison 3 Functional scales, Outcome 5 Mean Orgogozo score at 1 year.
Mean Trouillas Disability Scale score at six months
Only Nighoghossian 1995 reported this outcome. The mean score was lower (better outcome) in the HBOT group (4.6 versus 6.1: MD 1.5 points, 95% CI ‐1.2 to 4.2, P value 0.27) (Analysis 3.6).
3.6. Analysis.
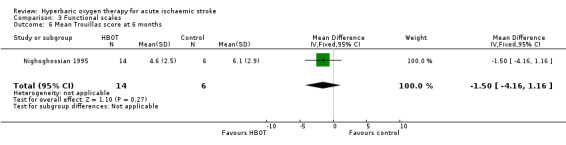
Comparison 3 Functional scales, Outcome 6 Mean Trouillas score at 6 months.
Mean Trouillas Disability Scale score at one year
Only Nighoghossian 1995 reported this outcome. The mean score was lower (better outcome) in the HBOT group (4.1 versus 6.3: MD 2.2 points, 95% CI 0.15 to 4.3, P value 0.04) (Analysis 3.7).
3.7. Analysis.
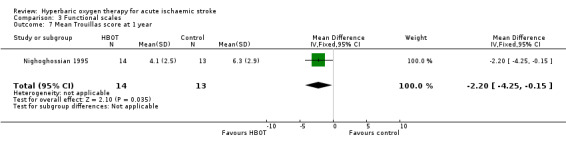
Comparison 3 Functional scales, Outcome 7 Mean Trouillas score at 1 year.
Mean Modified Rankin Functional Assessment Scale score at six months
Only Nighoghossian 1995 reported this outcome. The mean score was lower (better outcome) in the HBOT group (2.6 versus 3.2: MD 0.6 points, 95% CI ‐0.18 to 1.4, P value 0.13) (Analysis 3.8).
3.8. Analysis.
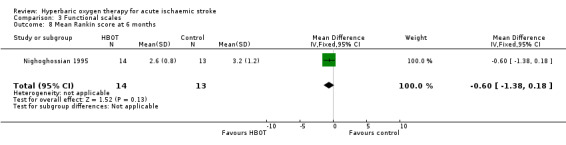
Comparison 3 Functional scales, Outcome 8 Mean Rankin score at 6 months.
Mean Modified Rankin Functional Assessment Scale score at one year
Only Nighoghossian 1995 reported this outcome. The mean score was lower (better outcome) in the HBOT group (2.4 versus 3.0: MD 0.6 points, 95% CI ‐0.18 to 1.4, P value 0.13) (Analysis 3.9).
3.9. Analysis.
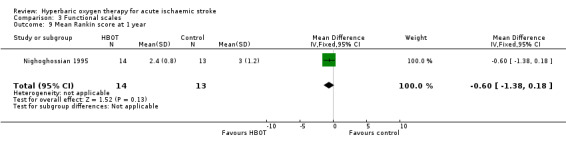
Comparison 3 Functional scales, Outcome 9 Mean Rankin score at 1 year.
Mean Neurological Recovery Score at 12 months
Only Sansone 1997 reported this outcome. The mean score was better in the HBOT group (HBOT score 4.2 versus control 6.4, MD 2.2 points better with HBOT, 95% CI 1.6 to 2.8, P value < 0.0001) (Analysis 3.10).
3.10. Analysis.
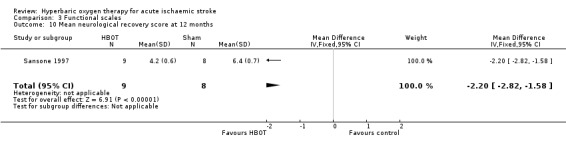
Comparison 3 Functional scales, Outcome 10 Mean neurological recovery score at 12 months.
Mean Neurological Functional Deficit Score at three weeks
Only Yang 2004 reported this outcome and did not define this scale. The mean score after therapy was better with HBOT than with sham (HBOT 17 versus control 20, MD 3 points better with HBOT, 95% CI 0.4 to 5.6, P value 0.03) (Analysis 3.11).
3.11. Analysis.
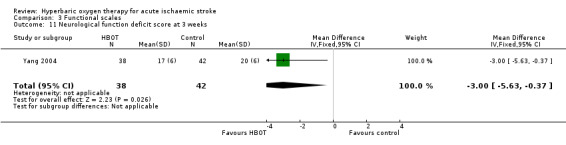
Comparison 3 Functional scales, Outcome 11 Neurological function deficit score at 3 weeks.
Comparison 4. Deemed to have a good outcome
Two trials reported the numbers of participants achieving a predefined 'good outcome' (Imai 2006; Rusyniak 2003). Rusyniak 2003 used four different outcome scales, and Imai 2006 used the Modified Rankin Scale. Differences are reported below.
NIHSS zero or improved by four points at 24 hours
Three participants in the HBOT group achieved this outcome versus five in the control group (RR 0.56, 95% CI 0.2 to 2.0, P value 0.37, for a good outcome with HBOT) (Analysis 4.1).
4.1. Analysis.
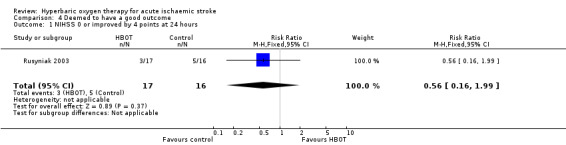
Comparison 4 Deemed to have a good outcome, Outcome 1 NIHSS 0 or improved by 4 points at 24 hours.
Rankin Scale score less than two at 90 days
In Rusyniak 2003, five participants in the HBOT group achieved this outcome versus nine in the control group (RR 0.52, 95% CI 0.2 to 1.2, P value 0.14 for a good outcome with HBOT), while in Imai 2006, six participants achieved a good outcome with HBOT versus only one who received control (RR 6.0, 95% CI 0.8 to 45.2, P value 0.08). Pooled analysis was subject to high heterogeneity (I2 = 82%) and has not been reported (Analysis 4.2).
4.2. Analysis.
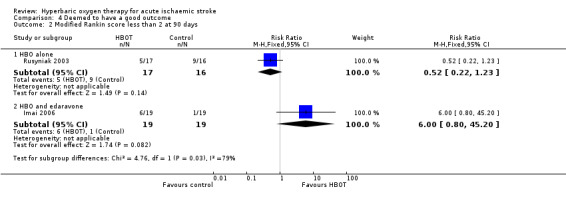
Comparison 4 Deemed to have a good outcome, Outcome 2 Modified Rankin score less than 2 at 90 days.
Glasgow Outcome score of five at 90 days
Six participants in the HBOT group achieved this outcome versus 10 in the control group (RR 0.6, 95% CI 0.3 to 1.2, P value 0.13 for a good outcome with HBOT) (Analysis 4.3).
4.3. Analysis.
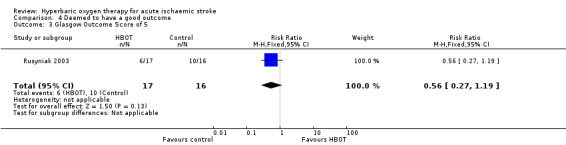
Comparison 4 Deemed to have a good outcome, Outcome 3 Glasgow Outcome Score of 5.
NIHSS score less than two at 90 days
Five participants in the HBOT group achieved this outcome versus eight in the control group (RR 0.59, 95% CI 0.2 to 1.4, P value 0.24 for a good outcome with HBOT) (Analysis 4.4).
4.4. Analysis.
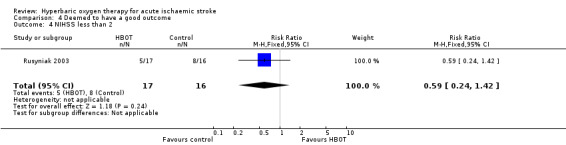
Comparison 4 Deemed to have a good outcome, Outcome 4 NIHSS less than 2.
Deemed cured and self‐caring at 21 days
Yang 2003 reported on the proportion of participants achieving 'cure' and able to provide self‐care. Nine participants in the HBOT were 'cured' versus four in the control group (RR 1.9, 95% CI 0.6 to 5.6, P value 0.25 for a good outcome with HBOT) (Analysis 4.5).
4.5. Analysis.
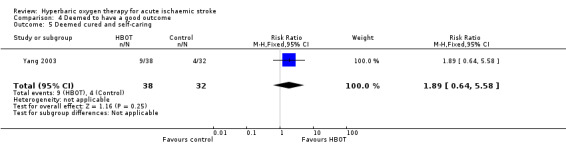
Comparison 4 Deemed to have a good outcome, Outcome 5 Deemed cured and self‐caring.
Comparison 5. Activities of daily living
Barthel Index of 95 or 100 at 90 days
Only Rusyniak 2003 reported the number of participants with this outcome. Eight participants in the HBOT group achieved this score versus nine in the control group (RR 1.2, 95% CI 0.6 to 2.3, P value 0.6 for a good outcome with HBOT) (Analysis 5.1).
5.1. Analysis.
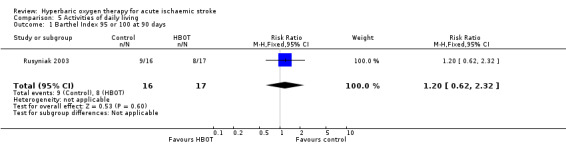
Comparison 5 Activities of daily living, Outcome 1 Barthel Index 95 or 100 at 90 days.
Mean Barthel Index at three weeks
Only Yang 2004 reported this outcome. The mean Barthel Index was higher (better) in the HBOT group (74 versus 54). The MD of 20 points was statistically significant (95% CI 13.7 to 26.3, P value < 0.0001) (Analysis 5.2).
5.2. Analysis.
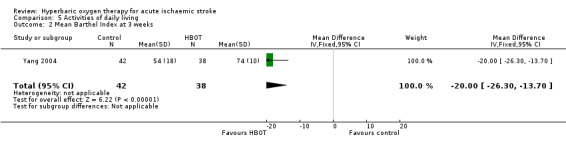
Comparison 5 Activities of daily living, Outcome 2 Mean Barthel Index at 3 weeks.
Comparison 6. Mean volume of infarct on CT
Mean infarct volume at four months
Only Anderson 1991 reported this outcome. The mean infarct volume was smaller in the control group (29.0 cm3 versus 49.2 cm3) but not significantly so (MD 20.2 cm3, 95% CI ‐13.4 to 53.8, P value 0.24) (Analysis 6.1).
6.1. Analysis.
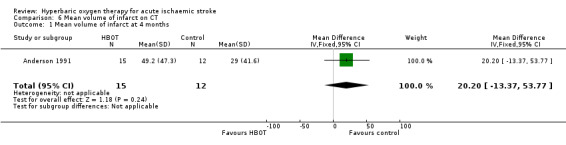
Comparison 6 Mean volume of infarct on CT, Outcome 1 Mean volume of infarct at 4 months.
Comparison 7. Adverse effects of therapy
Ear pain
Two trials reported on this outcome, enrolling a total of 71 participants (22% of the total) (Imai 2006; Rusyniak 2003). Four participants in the HBOT group suffered significant ear pain versus none in the control group (RR 4.9, 95% CI 0.6 to 39.7, P value 0.14) (Analysis 7.1).
7.1. Analysis.
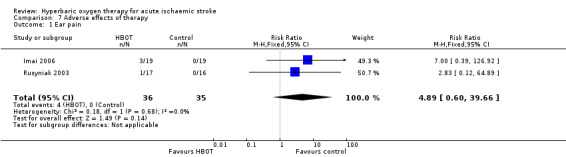
Comparison 7 Adverse effects of therapy, Outcome 1 Ear pain.
Claustrophobia with related discomfiture was a significant problem in the monoplace vessels used in all trials for both arms. The severity of these problems varied across studies and was differentially reported. Anderson 1991 reported 15 participants (39%) who could not complete scheduled therapy, and Rusyniak 2003 did not report withdrawal of any participants from therapy. This is likely to reflect the single‐treatment design of Rusyniak 2003 on the one hand and the very intensive schedule planned for Anderson 1991 on the other.
Discussion
Summary of main results
Pooled data analysis for clinical outcomes of interest could be performed with respect to only one primary analysis: death at three to six months. We found no convincing evidence that HBOT improves outcomes when applied during the acute presentation of ischaemic stroke. Pooled data from four trials did not suggest significant benefit for the case fatality rate in the six months following presentation (RR 0.97) (Anderson 1991; Imai 2006; Nighoghossian 1995; Rusyniak 2003). Although no deaths were reported in the other trials, it is not clearly stated whether all participants enrolled reached final follow‐up. It is of interest that the only trial to administer the combination of a free‐radical scavenger and HBOT reported three deaths in the HBOT arm and one in the control group (Imai 2006). Use of antioxidants during HBOT remains a controversial practice.
Some indications showed improvement on some functional and clinical scales, but this finding was not consistent. Given the heterogeneity of participants enrolled and the poor descriptors used for some of these scales, we believe that pooling of results using a standardised mean difference was not justified. In all, of the 15 comparisons made, three studies reported a total of four comparisons with a statistically significant difference in favour of HBOT, although the clinical importance of these differences is not clear (Nighoghossian 1995 (two scores); Sansone 1997 (one score); Yang 2004 (one score)). When estimated at longer follow‐up times by Nighoghossian 1995, these improvements were present at one year but not at six months after therapy was completed.
Overall completeness and applicability of evidence
This review has included data from 11 trials. We believe these represent all randomised trials in this area, both published and unpublished, at the time of our database search. Only seven trials with 361 participants were available for evaluation using our planned comparisons, and meta‐analysis was not appropriate or possible for most outcomes. Four randomised trials enrolling a further 344 participants reported no outcomes of interest for our meta‐analysis.
Although we have made every effort to locate further unpublished data, it remains possible that this review is subject to a positive publication bias, with generally favourable trials more likely to provide reporting. With regard to long‐term outcomes following HBOT and any effect on the quality of life of these study participants, we have located no relevant data.
These trials were published over a 17‐year period, up to 2008. We had planned to perform subgroup analyses with respect to time elapsed between stroke and institution of HBOT, dose of oxygen received and duration of treatment course. None of these analyses were appropriate given the small number of pooled analyses and the relatively uniform lack of effect shown in individual studies. In one pooled analysis that was possible, statistical heterogeneity was moderate (I2 = 34.7%), and the two included trials were clinically heterogenous in that one trial included only participants with embolic stroke, while using combination therapy of HBOT and a free‐radical scavenger (edaravone) (Imai 2006).
A wide range of oxygen doses was reported across these studies: Rusyniak 2003 gave one session only, Anderson 1991 provided an intensive regimen of eight‐hourly exposures for three days, Imai 2006 held daily sessions for seven days, five trials reported daily therapy for 10 days (Li 1998; Nighoghossian 1995; Sansone 1997; Yang 2003; Zhao 2008) and Yang 2004 provided therapy for 20 days. Anderson 1991, Nighoghossian 1995 and Sansone 1997 applied modest hyperbaric pressure of 1.5 ATA versus the 2.5 ATA applied by Rusyniak 2003 in the single therapy session. Five trials used 2.0 ATA (Imai 2006; Li 1998; Yang 2003; Yang 2004; Zhao 2008). This review does not permit conclusions with regard to oxygen dosing schedule.
HBOT is regarded as a relatively benign intervention. Few possible major adverse effects have been described (pulmonary barotrauma, drug reactions, injury or death related to chamber fire), and none of the included studies reported any such events. Various more minor complications may occur commonly but were similarly not reported in the included studies. Visual disturbance, usually reduction in visual acuity secondary to conformational changes in the lens, is very commonly reported in perhaps as many as 50% of those given a course of 30 treatments (Khan 2003). Although a great majority of people experiencing visual disturbances recover spontaneously over days to weeks, a small proportion of people continue to require correction to restore sight to pretreatment levels. The second most common adverse effect associated with HBOT is barotrauma. Barotrauma can affect any air‐filled cavity in the body (including middle ear, lungs and respiratory sinuses) and occurs as a direct result of compression. Aural barotrauma is by far the most common as the middle ear air space is small and is largely surrounded by bone and the sensitive tympanic membrane. Active effort by the individual is usually required to inflate the middle ear through the Eustachian tube on each side. Barotrauma is a consequence not of HBOT directly but rather of the physical conditions required to administer it. Most episodes of barotrauma are mild, easily treated or recover spontaneously and do not require the therapy to be abandoned. Less commonly, HBOT may be associated with acute neurological toxicity manifesting as seizure.
Quality of the evidence
A major problem for this review was the multiple outcome scales used to assess functional ability and clinical severity across trials. No scale was used and reported by more than one of these trials, making pooling of data for analysis impossible. Further, analysis of these ordinal scales to produce mean scores for group comparisons may not be appropriate. This is particularly true of Rankin, Trouillas and other unnamed recovery scales (Sansone 1997; Yang 2003; Yang 2004), given their limited range of scores from zero to 10, but none of the reports provided plots of experimental data for any of these scales to justify the use of such parametric tests. The usefulness of such scales for estimating outcomes at all has been questioned (Van Gijn 1992). Many of them offer distinct advantages, including the tendency to equate multiple small deficits with a few major deficits and a poor ability to estimate stroke deficit at the extremes of severity (Orgogozo 1998). One review concluded that of nine stroke scales tested, the NIHSS was one of the three most reliable and the Barthel Index was the most reliable disability scale (D'Olhaberriague 1996).
The problem of multiple alternative outcome measures when potential stroke therapies are investigated is not confined to the area of HBOT. Considerable efforts are being made to develop a standard suite of outcomes to improve this situation, most notably the 'Core Outcome Measures in Effectiveness Trials (COMET) initiative.' More information is available at http://www.cometinitiative.org/.
Other problems associated with assessing data include failure to report on primary functional outcomes, variability in time intervals between stroke event and enrolment, and variable doses of HBOT. These problems demand cautious interpretation of the results.
Potential biases in the review process
We have made an exhaustive search of the literature, including handsearching of multiple proceedings and relevant journals in all languages. All apparently relevant study reports have been translated, and we believe we have not missed any significant studies in the area. This review has been conducted in accordance with the Cochrane methodology and is explicit. We have reported potential biases noted in contributing studies.
Agreements and disagreements with other studies or reviews
A number of reviews have examined the use of HBOT in neurological conditions, including acute stroke. Despite this, the question of the efficacy and effectiveness of HBOT for this purpose remains controversial. The conclusions reached by individual authors are largely dependent on the relative weight given to animal experimentation and non‐randomised clinical trials on the one hand versus randomised clinical trials on the other. Al‐Waili 2005 reviewed both clinical and preclinical evidence and concluded that "previous studies have demonstrated promising results—strong enough to warrant further clinical study of HBO therapy in patients with stroke," implying agreement with our analysis that the clinical case is not yet sufficiently strong to support routine use of HBOT.
Similarly, in a systematic review of the clinical data, Carson 2005 suggested that the best evidence shows no benefit of hyperbaric oxygen therapy in people with stroke. However, review authors agree that because of considerable clinical heterogeneity, particularly with respect to timing and dose of HBOT, firm conclusions are difficult to make.
Authors' conclusions
Implications for practice.
We found very few clinical data on which to base recommendations. In the 11 small trials published in full or as abstracts, evidence is insufficient to confirm that HBOT significantly affects outcomes following acute ischaemic stroke. Use of HBOT as routine therapy for people with stroke cannot be justified by this review.
Implications for research.
Given the small number of participants in the included trials, we cannot be certain that benefit from HBOT can be excluded. Although a case can be made for further trials, such investigations would have to be carefully planned. More information may be useful on a subset of disease severity, particularly on the timing of therapy. The effects of differing oxygen dosage and of other therapies administered simultaneously are not known. Future trials will need to consider, in particular:
appropriate sample sizes with power to detect clinically important differences;
careful definition and selection of target patients;
appropriate range of oxygen doses per treatment session (pressure and time);
appropriate and carefully defined comparator therapy;
use of an effective sham therapy;
effective and explicit blinding of outcome assessors;
appropriate outcome measures, including those listed in this review;
careful elucidation of adverse effects; and
the cost‐effectiveness of the treatment.
What's new
| Date | Event | Description |
|---|---|---|
| 9 July 2014 | New citation required but conclusions have not changed | Conclusions have not changed |
| 9 July 2014 | New search has been performed | We have included in the qualitative review one trial that was previously excluded and two new citations, all of which are randomised controlled trials (RCTs) but do not report any outcomes of interest. The review now includes 11 RCTs involving 705 participants. One new citation was added to the quantitative synthesis, but conclusions have not changed |
History
Protocol first published: Issue 4, 2004 Review first published: Issue 3, 2005
| Date | Event | Description |
|---|---|---|
| 31 December 2008 | New search has been performed | This review has been updated to 31 December 2008. We have included three new studies, but no substantial changes have been made to the conclusions. Six further studies previously awaiting classification have now been excluded |
| 20 July 2008 | Amended | This review has been converted to the new review format |
Acknowledgements
The review authors acknowledge the support and suggestions of Hazel Fraser and the editors of the Cochrane Stroke Group for assistance provided in preparation of this review. In particular, we acknowledge the help of Brenda Thomas in developing the search strategy used and of Daniel Rusyniak, Peter Langhorne, Ale Algra, Anne Rowat and Steff Lewis for extensive editorial assistance provided.
Appendices
Appendix 1. MEDLINE search strategy
MEDLINE (Ovid)
1. cerebrovascular disorders/ or basal ganglia cerebrovascular disease/ or brain ischemia/ or exp brain infarction/ or hypoxia‐ischemia, brain/ or carotid artery diseases/ or carotid artery thrombosis/ or carotid artery, internal, dissection/ or intracranial arterial diseases/ or cerebral arterial diseases/ or infarction, anterior cerebral artery/ or infarction, middle cerebral artery/ or infarction, posterior cerebral artery/ or exp "intracranial embolism and thrombosis"/ or exp stroke/ or vertebral artery dissection/ 2. (isch?emi$ adj6 (stroke$ or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva or attack$)).tw. 3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation or basilar artery or vertebral artery) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw. 4. 1 or 2 or 3 5. Hyperbaric Oxygenation/ 6. Oxygen Inhalation Therapy/ 7. Oxygen/ae, tu 8. atmospheric pressure/ 9. Atmosphere Exposure Chambers/ 10. (hyperbar$ or HBO$).tw. 11. (high pressure oxygen or 100% oxygen).tw. 12. ((monoplace or multiplace) adj5 chamber$).tw. 13. 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 14. 4 and 13 15. exp animals/ not humans.sh. 16. 14 not 15
The following line used to update the search: 17. limit 16 to ed=20080701‐20140404
Appendix 2. EMBASE search strategy
EMBASE (Ovid)
1. stroke/ or cerebrovascular disease/ or brain infarction/ or brain stem infarction/ or cerebellum infarction/ or exp brain ischemia/ or carotid artery disease/ or exp carotid artery obstruction/ or cerebral artery disease/ or exp cerebrovascular accident/ or exp occlusive cerebrovascular disease/ or stroke patient/ or stroke unit/ 2. (isch?emi$ adj6 (stroke$ or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva or attack$)).tw. 3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation or basilar artery or vertebral artery) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw. 4. 1 or 2 or 3 5. oxygen therapy/ or hyperbaric oxygen/ 6. *oxygen/ 7. atmospheric pressure/ 8. pressure chamber/ 9. (hyperbar$ or HBO$).tw. 10. (high pressure oxygen or 100% oxygen).tw. 11. ((monoplace or multiplace) adj5 chamber$).tw. 12. or/5‐11 13. 4 and 12 14. (exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/) not (human/ or normal human/ or human cell/) 15. 13 not 14
The following line used to update the search: 16. limit 15 to dd=20080701‐20140404
Appendix 3. CINAHL search strategy
CINAHL (EBSCO)
S1 .(MH "Cerebrovascular Disorders") OR (MH "Basal Ganglia Cerebrovascular Disease") OR (MH "Carotid Artery Diseases") OR (MH "Carotid Artery Thrombosis") OR (MH "Cerebral Ischemia+") OR (MH "Intracranial Arterial Diseases") OR (MH "Cerebral Arterial Diseases") OR (MH "Intracranial Embolism and Thrombosis+") OR (MH "Stroke") or (MH "Stroke Patients") S2 .TI ( isch#emi* N6 (stroke* or apoplex* or cerebral vasc* or cerebrovasc* or cva or attack*) ) OR AB ( isch#emi* N6 (stroke* or apoplex* or cerebral vasc* or cerebrovasc* or cva or attack*) ) S3 .TI ( (brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebr* or mca* or anterior circulation or basilar artery or vertebral artery) N5 (isch?emi* or infarct* or thrombo* or emboli* or occlus* or hypoxi*) ) S4 .AB ( (brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebr* or mca* or anterior circulation or basilar artery or vertebral artery) N5 (isch?emi* or infarct* or thrombo* or emboli* or occlus* or hypoxi*) ) S5 .S1 OR S2 OR S3 OR S4 S6 .(MH "Oxygen Therapy") OR (MH "Hyperbaric Oxygenation") S7 .(MH "Oxygen/AE/TU") OR (MH "Oxygenation") S8 .(MH "Atmospheric Pressure") S9 .TI ( hyperbar* or HBO* ) OR AB ( hyperbar* or HBO* ) S10 .TI (high pressure oxygen or 100% oxygen) or AB (high pressure oxygen or 100% oxygen) S11 .TI ( ((monoplace or multiplace) N5 chamber*) ) OR AB ( ((monoplace or multiplace) N5 chamber*) ) S12 .S6 OR S7 OR S8 OR S9 OR S10 OR S11 S13 .S5 AND S12
The following lines to update the search: S14 .EM 200807‐ S15 .S13 AND S14
Appendix 4. CENTRAL search strategy
#1 [mh ^"cerebrovascular disorders"] or [mh ^"basal ganglia cerebrovascular disease"] or [mh ^"brain ischemia"] or [mh "brain infarction"] or [mh ^"hypoxia‐ischemia, brain"] or [mh ^"carotid artery diseases"] or [mh ^"carotid artery thrombosis"] or [mh ^"carotid artery, internal, dissection"] or [mh ^"intracranial arterial diseases"] or [mh ^"cerebral arterial diseases"] or [mh ^"infarction, anterior cerebral artery"] or [mh ^"infarction, middle cerebral artery"] or [mh ^"infarction, posterior cerebral artery"] or [mh "intracranial embolism and thrombosis"] or [mh stroke] or [mh ^"vertebral artery dissection"] #2 isch*mi* near/6 (stroke* or apoplex* or cerebral next vasc* or cerebrovasc* or cva or attack*):ti,ab #3 (brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle next cerebr* or mca* or "anterior circulation" or "basilar artery" or "vertebral artery") near/5 (isch*mi* or infarct* or thrombo* or emboli* or occlus* or hypoxi*):ti,ab #4 #1 or #2 or #3 #5 [mh ^"Hyperbaric Oxygenation"] #6 [mh ^"Oxygen Inhalation Therapy"] #7 [mh ^Oxygen/AE,TU] #8 [mh ^"atmospheric pressure"] #9 [mh ^"Atmosphere Exposure Chambers"] #10 (hyperbar* or HBO*):ti,ab #11 ("high pressure oxygen" or "100% oxygen"):ti,ab #12 ((monoplace or multiplace) near/5 chamber*):ti,ab #13 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 #14 #4 and #13
Data and analyses
Comparison 1. Mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death at 3 to 6 months | 4 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.34, 2.75] |
| 1.1 HBO alone | 3 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.17, 2.20] |
| 1.2 HBO and edaravone | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.34, 26.33] |
Comparison 2. Severe functional disability.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No effect of therapy—participants unable to care for themselves | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.10, 1.28] |
Comparison 3. Functional scales.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean neurological score at day 5 | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 5.30 [‐7.47, 18.07] |
| 2 Mean neurological score at week 6 | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 10.2 [‐8.48, 28.88] |
| 3 Mean neurological score at 1 year | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | 5.60 [‐15.02, 26.22] |
| 4 Mean Orgogozo score at 6 months | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | ‐18.20 [‐41.62, 5.22] |
| 5 Mean Orgogozo score at 1 year | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | ‐27.90 [‐51.79, ‐4.01] |
| 6 Mean Trouillas score at 6 months | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐1.5 [‐4.16, 1.16] |
| 7 Mean Trouillas score at 1 year | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | ‐2.2 [‐4.25, ‐0.15] |
| 8 Mean Rankin score at 6 months | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.38, 0.18] |
| 9 Mean Rankin score at 1 year | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.38, 0.18] |
| 10 Mean neurological recovery score at 12 months | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | ‐2.2 [‐2.82, ‐1.58] |
| 11 Neurological function deficit score at 3 weeks | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐3.0 [‐5.63, ‐0.37] |
Comparison 4. Deemed to have a good outcome.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NIHSS 0 or improved by 4 points at 24 hours | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.16, 1.99] |
| 2 Modified Rankin score less than 2 at 90 days | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 HBO alone | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.22, 1.23] |
| 2.2 HBO and edaravone | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.0 [0.80, 45.20] |
| 3 Glasgow Outcome Score of 5 | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.27, 1.19] |
| 4 NIHSS less than 2 | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.24, 1.42] |
| 5 Deemed cured and self‐caring | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [0.64, 5.58] |
Comparison 5. Activities of daily living.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Barthel Index 95 or 100 at 90 days | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.62, 2.32] |
| 2 Mean Barthel Index at 3 weeks | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐20.0 [‐26.30, ‐13.70] |
Comparison 6. Mean volume of infarct on CT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean volume of infarct at 4 months | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 20.20 [‐13.37, 53.77] |
Comparison 7. Adverse effects of therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Ear pain | 2 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.89 [0.60, 39.66] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anderson 1991.
| Methods | RCT stratified for disease severity Powered to find a 30% relative improvement in HBOT group with 45 participants in each group (stopped early, no clear stopping rule applied) | |
| Participants | 39 adults with onset of ischaemic cerebral infarction within 2 weeks and greater than 20 severity score out of 100 on a graded neurological scale designed to test for deficits in the areas supplied by the internal carotid artery No contraindications to HBOT or unstable medical conditions | |
| Interventions | Both groups received standard measures in neurological intensive care and vitamin E 400 mg 8‐hourly during compression period Control: sham therapy on air at 1.5 ATA for 60 minutes within 6 hours of enrolment, then every 8 hours to a total of 15 exposures (over 5 days) HBOT: 100% oxygen at 1.5 ATA on the same schedule as above | |
| Outcomes | Graded neurological examination on a 0 to 100 scale, as at admission Repeated at day 5, week 6, year 1 Infarct volume on CT scan at 4 months | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but method not clear |
| Allocation concealment (selection bias) | High risk | Not mentioned in the text and may not have been done |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participant and outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12 participants lost to final follow‐up and all accounted for in the text |
| Selective reporting (reporting bias) | Unclear risk | No protocol report available for checking |
| Other bias | Unclear risk | Mortality reported as ITT, but not clear for other outcomes Recruited some participants relatively late—up to 2 weeks |
Hong 2008.
| Methods | RCT | |
| Participants | 86 young inpatients with ischaemic stroke | |
| Interventions | Routine treatment versus routine treatment plus HBOT | |
| Outcomes | Nerve function deficit score, effectiveness of HBOT and routine therapy | |
| Notes | Abstract only with very little detail and no clear explanation of the results given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given; "randomly divided into two groups' |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding appears unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information in the paper |
| Selective reporting (reporting bias) | Unclear risk | No information in the paper and no protocol available for checking |
| Other bias | Unclear risk | Too little information available |
Imai 2006.
| Methods | RCT | |
| Participants | Adults with acute embolic stroke (within 48 hours) in the MCA territory. Diagnosis with neurological signs, MRI findings; excluded if they received fibrinolytic treatment or had a seizure | |
| Interventions | Both groups received standard care. Intervention group received HBOT at 2.0 ATA for 60 minutes daily for 7 days and a free‐radical scavenger, edaravone 30 mg iv, before and after each HBOT session | |
| Outcomes | Death, unfavourable outcomes by mRS, adverse events of otalgia and cerebral haemorrhage, median NIHSS scores | |
| Notes | Methodology not well elucidated. Used combination of HBOT and free‐radical scavenger | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details of methods; "patients were randomised" |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No mention of any attempt at blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up to 90 days |
| Selective reporting (reporting bias) | Unclear risk | No protocol available for checking |
| Other bias | Unclear risk | No indication of other bias |
Li 1998.
| Methods | RCT | |
| Participants | 86 patients with cerebral infarction divided into 2 groups of 43 each | |
| Interventions | Control group received 'Salvia Miltirrhiza Co,' and HBOT group received the same therapy plus daily treatment of breathing 100% oxygen at 2.0 ATA for 120 minutes for 10 days | |
| Outcomes | Cerebral blood flow, EEG, haemorrheology and SOD | |
| Notes | No outcomes relevant to this review reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Were randomly divided' |
| Allocation concealment (selection bias) | Unclear risk | No mention of this |
| Blinding (performance bias and detection bias) All outcomes | High risk | No mention but unlikely in this trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Results for all participants reported |
| Selective reporting (reporting bias) | Unclear risk | No protocol available for checking |
| Other bias | Unclear risk | No evidence of other bias |
Nighoghossian 1995.
| Methods | RCT | |
| Participants | 34 adults (21 men) with ischaemic cerebral infarction confirmed by CT and presenting within 24 hours of onset of neurological deficit suggestive of MCA occlusion and scoring less than 80 on the Orgogozo scale (100 is normal) No contraindications to HBOT, old infarct, congestive heart failure or uncontrolled hypertension 17 participants allocated to each arm | |
| Interventions | Both groups received standard measures including low‐dose heparin and supportive care Control: sham therapy on air at 1.2 ATA daily for 40 minutes for 10 days HBOT: 100% oxygen at 1.5 ATA on the same schedule as above Participants were withdrawn from protocol if they deteriorated to coma or did not tolerate therapy (7 in total: 4 control, 3 HBOT) | |
| Outcomes | Graded neurological examination on 3 scales: Orgogozo (100 to 0), Trouillas (0 to 10) and Rankin Disability Scale Orgogozo was calculated at baseline; all were calculated at 6 months and at 1 year Adverse effects of HBOT | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, probably true |
| Allocation concealment (selection bias) | High risk | No mention in the text—probably not done |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No indication of blinding but sham therapy employed, suggesting that at least participants were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 participants did not reach final follow‐up and were accounted for |
| Selective reporting (reporting bias) | Unclear risk | No protocol available for checking |
| Other bias | Low risk | No indication of other bias |
Peng 2003.
| Methods | RCT using coin toss | |
| Participants | Adults 30 years of age and older with cerebral infarction within the past 7 days, diagnosed using a 1995 conference consensus guideline. Excluded if contraindication to HBOT or already on medication that affected free‐radical production | |
| Interventions | Both groups received standard pharmacological treatment (no details); HBOT group received 10 daily treatments at 2.3 ATA breathing 100% oxygen for 80 minutes | |
| Outcomes | Serum nitric oxide, SOD, MDA and a clinical neurological deficit score. Clinical efficacy also estimated but meaning of this measure not clear | |
| Notes | Text translated by Dr Ting Liu | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation described as by coin toss, so probably reliably random |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information available |
| Selective reporting (reporting bias) | Unclear risk | No protocol available for checking |
| Other bias | Low risk | No evidence of other bias |
Rusyniak 2003.
| Methods | RCT stratified by time from onset to enrolment (0 to 12 hours and 12 to 24 hours) | |
| Participants | 33 adults with presumed ischaemic cerebral infarction with no evidence of cerebral bleed on CT and presenting within 24 hours of onset of neurological deficit All participants scored less than 23 points on the NIHSS No contraindications to HBOT, previous infarct within 3 months or evidence of potentially serious cardiac arrhythmia | |
| Interventions | Control: sham therapy on oxygen at 1.14 ATA for 60 minutes HBOT: 100% oxygen at 2.5 ATA on the same schedule as above | |
| Outcomes | NIHSS measured at 24 hours and 90 days Barthel Index, mRS and Glasgow Outcome Scale calculated at 90 days Mortality Adverse effects of HBOT | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Described in the text |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Both participants and outcome assessors unaware of allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 7 participants did not reach final follow‐up; 4 of these were not accounted for |
| Selective reporting (reporting bias) | Unclear risk | No protocol available for checking |
| Other bias | Low risk | — |
Sansone 1997.
| Methods | RCT reported in abstract | |
| Participants | 17 participants (9 allocated to HBOT, 8 allocated to control) with a mean age of 61 years and presenting within 24 hours of CT‐demonstrated infarction in middle or anterior cerebral arterial territory | |
| Interventions | Control: sham treatment of breathing air without pressurisation HBOT: 100% oxygen at 1.5 to 1.8 ATA for 60 minutes once daily for 8 to 10 days | |
| Outcomes | Neurological recovery score (10 = no disability, 0 = dead) at 6 months and at 12 months | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study authors state that participants were randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment—probably did not happen |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Sham therapy arm suggests blinding for participants and outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Mean and SD were given without number of participants |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available for checking |
| Other bias | Unclear risk | This small trial may not have completed recruitment at reporting |
Yang 2003.
| Methods | RCT of HBOT versus 'standard therapy' | |
| Participants | 80 participants with ischaemic stroke proven by CT or MRI within 72 hours of the event | |
| Interventions | All participants received standard medical therapy including vasodilators, anticoagulants and 'nerve cell protectant' HBOT: 38 participants received 100% oxygen for 80 minutes daily at 2 ATA for 10 days Control: 32 participants received standard therapy only | |
| Outcomes | Neurological recovery was measured on a scale.
|
|
| Notes | Unusual outcome measure | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were described as 'divided randomly' |
| Allocation concealment (selection bias) | High risk | No mention of allocation concealment—probably did not happen |
| Blinding (performance bias and detection bias) All outcomes | High risk | No evidence of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 10 missing participants unaccounted for |
| Selective reporting (reporting bias) | Unclear risk | No protocol available for checking |
| Other bias | Unclear risk | Short report with relatively little detail |
Yang 2004.
| Methods | RCT with 3 treatment arms | |
| Participants | 120 participants with ischaemic stroke proven by CT or MRI within 72 hours of admission Had to be first event CVA, conscious and co‐operative and accompanied by neurological deficits | |
| Interventions | All participants received standard medical therapy and were enrolled in rehabilitation programmes as appropriate for this institution
HBOT: 60 minutes treatment at 2.0 ATA breathing 100% oxygen once daily for a total of 20 treatments
Control: standard therapy only 1 arm received a direct intravascular oxygen injection of 250 mL of oxygen twice daily: these participants were not included in this meta‐analysis |
|
| Outcomes | Neurological deficit Fugl‐Meyer motor assessment Barthel Index of activities of daily living | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study authors state that participants were randomly assigned |
| Allocation concealment (selection bias) | High risk | No mention of allocation concealment, so probably not done |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Single blinding (participant), but not clear whether this applied to HBOT/non‐HBOT comparators—a sham therapy is not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No protocol available for checking |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No obvious biases |
Zhao 2008.
| Methods | RCT with a third control group of normal individuals | |
| Participants | 112 participants with acute cerebral infarction | |
| Interventions | Routine treatment group received routine drug treatment and rehabilitation exercise. HBO group received the same routine therapy plus HBO treatment at 2.0 ATA for 60 minutes plus 30 minutes of slow depressurisation to 1.0 ATA, once each day for a total of 10 days | |
| Outcomes | Serum levels of soluble intercellular adhesion molecule, soluble vascular cell adhesion molecule, soluble E‐selectin and matrix metalloproteinase‐9 were detected by enzyme‐linked immunosorbent assay | |
| Notes | No outcomes of interest were reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No details. Participants "were recruited and randomized to a HBO group (n = 50) and a routine treatment group (n = 62)." Large difference in the size of groups reduces our confidence in this process |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | No mention, but unlikely in this study unless stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers contributing to final outcome not given |
| Selective reporting (reporting bias) | Unclear risk | No protocol available for checking |
| Other bias | Unclear risk | No clear indication of other biases |
ADL: activities of daily living ATA: atmosphere absolute CT: computerised tomography CVA: cerebrovascular accident EEG: electroencephalography HBOT: hyperbaric oxygen therapy ITT: intention‐to‐treat iv: intravenous MCA: middle cerebral artery MDA: malondialdehyde MRI: magnetic resonance imaging mRS: modified Rankin Scale NIHSS: National Institutes of Health Stroke Scale RCT: randomised controlled trial SD: standard deviation SOD: serum superoxide dismutase
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Belokurov 1988 | Review only, not focused on stroke |
| Boschetty 1970 | Not a comparative study |
| Chen 2007 | Both arms received HBOT |
| Chen 2012 | Not an RCT |
| Chen 2013 | Pseudo‐randomised study |
| Dong 2005 | Not all participants had confirmed ischaemic stroke ('ischemic cerebrovascular disease') |
| Efrati 2013 | RCT with delayed treatment control arm but included participants 6 to 36 months after both ischaemic and haemorrhagic stroke |
| Efuni 1987 | Not an RCT, non‐random controls |
| Elinsky 1984 | Not an RCT, non‐random controls |
| Gusev 1990 | Not an RCT, case series only |
| Heyman 1966 | Non‐randomised and mixed aetiologies |
| Holbach 1979 | RCT, all participants received HBOT |
| Kaasik 1988 | Not an RCT |
| Kazantseva 2005 | Not an RCT |
| Kazantseva 2006 | Abstract only, not identified as an RCT |
| Lebedev 1983 | Not a randomised study |
| Oshima 1990 | Non‐random comparative study, no results given |
| Pan 1996 | Abstract only, no relevant outcomes reported, probably not RCT |
| Pravdenkova 1983 | Not a randomised study |
| Pravdenkova 1984 | Not a randomised study |
| Sarno 1972a | Not an RCT, case series only |
| Sarno 1972b | Did not study acute stroke |
| Soodan 1992 | Not an RCT |
| Vila 2005 | Not an RCT and not involving acute stroke |
| Xia 1995 | Dose of HBOT lower than inclusion criteria (1.1 ATA) |
| Xing 2007 | All groups received HBOT |
| Yang 2002 | A study about haemorrhagic stroke only |
| Zhang 2000 | Not a randomised study |
ATA: atmosphere absolute HBOT: hyperbaric oxygen therapy RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
Michalski 2008.
| Trial name or title | Combination of systemic intravenous thrombolysis and hyperbaric oxygen therapy (HBOT) in acute ischemic stroke; a randomised single‐blinded trial has started |
| Methods | Randomised single‐blinded controlled trial |
| Participants | Acute stroke |
| Interventions | HBOT and thrombolysis |
| Outcomes | Infarct size, NIHSS, Barthel Index, mRS |
| Starting date | 2008 |
| Contact information | Michalski D, Kueppers‐Tiedt L, Hobohm C, Schulz A, Wagner A, Schneider D Department of Neurology, University of Leipzig, Leipzig, Germany |
| Notes |
HBOT: hyperbaric oxygen therapy mRS: modified Rankin Scale NIHSS: National Institutes of Health Stroke Scale
Contributions of authors
MH Bennett: conception of review, background, critical appraisal, hyperbaric medicine content expert, statistical analysis, co‐author for description of studies and discussion. S Weibel: 2014 update of search, critical appraisal, clinical epidemiology expert, co‐author of 2014 update J Wasiak: search strategy and execution, critical appraisal, systematic review expert, co‐author for description of studies and discussion. C French: background to review, neurology context expert, critical appraisal. P Kranke: critical appraisal, hyperbaric content expert, text editor. A Schnabel: protocol development, critical appraisal, text editor.
Declarations of interest
None known. Dr Bennett is a hyperbaric physician who does not routinely treat people with stroke.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Anderson 1991 {published data only}
- Anderson DC, Bottini AG, Jagiella WM, Westphal B, Ford S, Rockswold GL, et al. A pilot study of hyperbaric oxygen in the treatment of human stroke. Stroke 1991;22(9):1137‐42. [199100] [DOI] [PubMed] [Google Scholar]
Hong 2008 {published data only}
- Hong L, Yu S. Efficacy of hyperbaric oxygen in treatment of ischemic stroke among young patients. Journal of Rehabilitation Medicine 2008;Suppl 46:110. [Google Scholar]
Imai 2006 {published data only}
- Imai K, Mori T, Izumoto H, Takabatake N, Kunieda T, Watanabe M. Hyperbaric oxygen combined with intravenous edaravone for treatment of acute embolic stroke: a pilot clinical trial. Neurologia Medico‐Chirurgica 2006;46(8):373‐8. [DOI] [PubMed] [Google Scholar]
Li 1998 {published data only}
- Li F, Chen L, Gu D. Effect of hyperbaric oxygenation on cerebral blood flow and EEG in cerebral infarction. Chinese Journal of Physical Therapy 1998;21(2):72‐4. [Google Scholar]
Nighoghossian 1995 {published data only}
- Nighoghossian N, Trouillas M, Adeleine P, Salord F. Hyperbaric oxygen in the treatment of acute ischaemic stroke. A double‐blind pilot study. Stroke 1995;26:1369‐72. [DOI] [PubMed] [Google Scholar]
Peng 2003 {published data only}
- Peng ZR, Zhu SL, Xiao PT. Changes and efficacy evaluation of serum nitrogen monoxide and oxygen‐derived free radicals in patients with cerebral infarction before and after hyperbaric oxygen therapy. Chinese Journal of Clinical Rehabilitation 2003;7(16):2318‐9. [Google Scholar]
Rusyniak 2003 {published data only}
- Rusyniak DE, Kirk MA, May JD, Kao LW, Brizendine EJ, Welch JL, et al. Hyperbaric oxygen therapy in acute ischemic stroke: results of the Hyperbaric Oxygen in Acute Ischemic Stroke Trial Pilot Study. Stroke 2003;34(2):571‐4. [12574578] [DOI] [PubMed] [Google Scholar]
Sansone 1997 {published data only}
- Sansone A, Gulotta G, Sparacia B, Alongi A, Savola G, Sparacia GV. Effect of hyperbaric oxygen therapy on neurologic recovery after focal cerebral ischaemia. British Journal of Anaesthesia 1997;78 Suppl 1:73‐4. [Google Scholar]
Yang 2003 {published data only}
- Yang X, Li K, He T. The effect of early hyperbaric oxygenation on the ability of daily life of patients with stroke. Chinese Journal of Clinical Rehabilitation 2003;7(7):1221. [Google Scholar]
Yang 2004 {published data only}
- Yang Z, Chen H, Xian M‐J, Jiang X‐D, Yuan Li, Mai J‐H. Role of medical pressured oxygen injector in the rehabilitation of motor function and abilities of daily living at early stage of cerebral infarction. Chinese Journal of Clinical Rehabilitation 2004;8(13):2414‐5. [Google Scholar]
Zhao 2008 {published data only}
- Zhao R, Wang C, Wang Y. Changes in serum cellular adhesion molecule and matrix metalloproteinase‐9 levels in patients with cerebral infarction following hyperbaric oxygen therapy. A case and intergroup control study. Neural Regeneration Research 2008;3(11):1245‐8. [Google Scholar]
References to studies excluded from this review
Belokurov 1988 {published data only}
- Belokurov YuM, Golland AV, Kochetov KhA. Hyperbaric oxygenation in hypoxic brain injuries. Khirurgiya 1988;64(8):12‐7. [198800] [PubMed] [Google Scholar]
Boschetty 1970 {published data only}
- Boschetty V, Dostal J, Holek J. Use of hyperbaric oxygenation in acute cerebrovascular accidents. (Preliminary report). Bratislavske Lekarske Listy 1970;53(2):160‐4. [5432216] [PubMed] [Google Scholar]
Chen 2007 {published data only}
- Chen L, Li F, Gu D. Hyperbaric oxygen for cerebral blood flow and electroencephalogram in patients with acute cerebral infarction. Neural Regeneration Research 2007;2(3):171‐4. [Google Scholar]
Chen 2012 {published data only}
- Chen C‐H, Chen S‐Y, Wang V, Chen C‐C, Wang K‐C, Chen C‐H, et al. Effects of repetitive hyperbaric oxygen treatment in patients with acute cerebral infarction: a pilot study. Scientific World Journal 2012;2012:Article ID 694703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2013 {published data only}
- Chen F‐P, Yu X‐X. Short‐term effect of hyperbaric oxygen combined with edaravone and ozagrel sodium in treating progressive cerebral infarction. Chinese Journal of Evidence‐Based Medicine 2013;13(4):413‐6. [Google Scholar]
Dong 2005 {published data only}
- Dong X‐L, Niu S‐Y, Xu H‐B, Han M‐Y. Effects of hyperbaric oxygenation associated with panax notoginseng saponins therapy on hemorrhology indexes in patients with ischemic cerebrovascular disease. Chinese Journal of Clinical Rehabilitation 2005;9(41):6‐7. [Google Scholar]
Efrati 2013 {published data only}
- Efrati S, Fishlev G, Bechor Y, Volkov O, Bergan J, Kliakhandler K, et al. Hyperbaric oxygen induces late neuroplasticity in post stroke patients—randomized, prospective trial. PLoS One 2013;8(1):e53716. [DOI] [PMC free article] [PubMed] [Google Scholar]
Efuni 1987 {published data only}
- Efuni SN, Lebedeva RN, Shikunova LG, Demurov EA, Mutuskina EA. Hyperbaric oxygenation in the therapy of hypoxic brain damage. Patologicheskaia Fiziologiia i Eksperimental'naia Terapiia 1987;3:24‐7. [3627829] [PubMed] [Google Scholar]
Elinsky 1984 {published data only}
- Elinsky MP, Rafikov AM, Ivanova NE, Kesaev SA. Therapeutic application of hyperbaric oxygenation in ischemic strokes. Zhurnal Nevropatologii i Psikhiatrii Imeni S.S. Korsakova 1984;84(9):1321‐5. [PubMed] [Google Scholar]
Gusev 1990 {published data only}
- Gusev EI, Kazantseva NV, Nifontova LA, Petukhov EB, Makarova LD, Zhuravlev AK, et al. On the mechanism of the therapeutic effect of hyperbaric oxygenation at minor differential pressure in patients with brain stroke. Zhurnal Nevropatologii i Psikhiatrii Imeni S.S. Korsakova 1990;90(1):34‐40. [199000] [PubMed] [Google Scholar]
Heyman 1966 {published data only}
- Heyman A, Saltzman HA, Whalen RE. The use of hyperbaric oxygenation in the treatment of cerebral ischemia and infarction. Circulation 1966;33(5 Suppl):II20‐7. [5006557] [DOI] [PubMed] [Google Scholar]
Holbach 1979 {published data only}
- Holbach KH, Wassmann H. Advantage of using hyperbaric oxygenation (HO) in combination with extra‐cranial arterial bypass (EIAB) in the treatment of completed stroke. Acta Neurochirurgica 1979;28 Suppl:309. [DOI] [PubMed] [Google Scholar]
Kaasik 1988 {published data only}
- Kaasik A‐EA, Dmitriev KK, Tomberg TA. Hyperbaric oxygenation in the treatment of patients with ischemic stroke. Zhurnal Nevropatologii i Psikhiatrii Imeni S.S. Korsakova 1988;88(9):34‐42. [198800] [PubMed] [Google Scholar]
Kazantseva 2005 {published data only}
- Kazantseva N, Volkova NA, Petukcov EB. Effect of normoxic curative compression on microcirculation in acute stroke. Journal of the Neurological Sciences 2005;238:S407. [Google Scholar]
Kazantseva 2006 {published data only}
- Kazantseva NV. Study of the effectiveness of two curative regimens of hyperbaric therapy in acute stroke. International Journal of Stroke 2006;1 Suppl 1:73. [Google Scholar]
Lebedev 1983 {published data only}
- Lebedev VV, Isakov IuV, Pravdenkova SV. Effect of hyperbaric oxygenation on the clinical course and complications of the acute period of ischemic strokes. Zhurnal Voprosy Neirokhirurgii Imeni N. N. Burdenko 1983;3:37‐42. [6613431] [PubMed] [Google Scholar]
Oshima 1990 {published data only}
- Oshima M, Sadoshima S, Kusuda K, Yao H, Yagi H, Fujishima M. Clinical application of hyperbaric oxygen therapy for acute cerebral infarction. Undersea Biomedical Research Supplement 1990;17:140. [Google Scholar]
Pan 1996 {published data only}
- Pan H. Clinical study of hyperbaric oxygen plus fonzylane on cerebral infarction. Chinese Journal of Nervous and Mental Diseases 1996;22(4):217. [Google Scholar]
Pravdenkova 1983 {published data only}
- Pravdenkova SV, Isakov IuV, Ioffe IuS. Therapeutic effect of hyperbaric oxygenation in the acute stage of an ischemic stroke. Anesteziologiia i Reanimatologiia 1983;6:26‐30. [6670766] [PubMed] [Google Scholar]
Pravdenkova 1984 {published data only}
- Pravdenkova SV, Romasenko MV, Shelkovskii VN. Hyperbaric oxygenation and prevention of recurrent cerebral circulatory disorders in the acute stage of a stroke. Zhurnal Nevropatologii i Psikhiatrii Imeni S.S. Korsakova 1984;84(8):1147‐51. [6495936] [PubMed] [Google Scholar]
Sarno 1972a {published data only}
- Sarno JE, Rusk HA, Diller L, Sarno MT. The effect of hyperbaric oxygen on the mental and verbal ability of stroke patients. Stroke 1972;3(1):10‐5. [5008300] [DOI] [PubMed] [Google Scholar]
Sarno 1972b {published data only}
- Sarno MT, Sarno JE, Diller L. The effect of hyperbaric oxygen on communication function in adults with aphasia secondary to stroke. Journal of Speech and Hearing Research 1972;15(1):42‐8. [5012810] [DOI] [PubMed] [Google Scholar]
Soodan 1992 {published data only}
- Soodan KS, Roy AK, Singh PK, Sing S, Hegde KP. Hyperbaric oxygen therapy in stroke cases. Indian Journal of Aerospace Medicine 1992;36(1):28‐31. [Google Scholar]
Vila 2005 {published data only}
- Vila JF, Balcarce PE, Abiusi GR, Dominguez RO, Pisarello JB. Improvement in motor and cognitive impairment after hyperbaric oxygen therapy in a selected group of patients with cerebrovascular disease: a prospective single‐blind controlled trial. Undersea and Hyperbaric Medicine 2005;32(5):341‐9. [PubMed] [Google Scholar]
Xia 1995 {published data only}
- Xia XG, Huang ZM, Zhou X. The effect of rotation magnetic field and hyperbaric oxygen on free radical metabolism in cerebral apoplexy. Chinese Journal of Physical Therapy 1995;18(3):140‐2. [Google Scholar]
Xing 2007 {published data only}
- Xing J, Wang Y, Li Y. Clinical study on acupuncture combined with hyperbaric oxygenation for improving balance function of cerebral infarction. Chinese Acupuncture and Moxibustion 2007;27(1):12‐4. [PubMed] [Google Scholar]
Yang 2002 {published data only}
- Yang S, Li B, Liu S. The therapeutic effect of the hyperbaric oxygen on diabetic cerebral haemorrhage in the aged. Guangxi Medical Journal 2002;24(3):303‐4. [Google Scholar]
Zhang 2000 {published data only}
- Zhang H, Gao P, Jia Z. The evaluation of hyperbaric oxygen therapy on neuropsychological impairments in senile cerebral infarction patients. Chinese Mental Health Journal 2000;14(3):209‐10. [Google Scholar]
References to ongoing studies
Michalski 2008 {published data only}
- Michalski D, Kueppers‐Tiedt L, Hobohm C, Schulz A, Wagner A, Schneider D. Combination of systemic intravenous thrombolysis and hyperbaric oxygen therapy (HBOT) in acute ischemic stroke, a randomized single‐blinded trial has started. International Journal of Stroke 2008;3 Suppl 1:459. [Google Scholar]
Additional references
Al‐Waili 2005
- Al‐Waili NS, Butler GJ, Beale J, Abdullah MS, Hamilton WB, Lee BY, et al. Hyperbaric oxygen in the treatment of patients with cerebral stroke, brain trauma, and neurologic disease. Adavances in Therapy 2005;22(6):659‐78. [DOI] [PubMed] [Google Scholar]
Badr 2001
- Badr AE, Yin W, Mychaskiw G, Zhang JH. Effect of hyperbaric oxygen on striatal metabolites: a microdialysis study in awake freely moving rats after MCA occlusion. Brain Research 2001;916(1‐2):85‐90. [11597594] [DOI] [PubMed] [Google Scholar]
Bamford 1991
- Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 1991;337:1521‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bath 2000
- Bath PM, Lees KR. ABC of arterial and venous disease. Acute stroke. BMJ 2000;320:920‐3. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bennett 2004
- Bennett MH, Connor D. The Database of Randomised Controlled Trials in Hyperbaric Medicine (DORCTIHM). www.hboevidence.com (accessed 14 September 2008).
Carson 2005
- Carson S, McDonagh M, Russman B, Helfand M. Hyperbaric oxygen therapy for stroke: a systematic review of the evidence. Clinical Rehabilitation 2005;19:819‐833. [DOI] [PubMed] [Google Scholar]
CASTCG 1997
- Chinese Acute Stroke Trial Collaborative Group. CAST: randomised placebo‐controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. Lancet 1997;349:1641‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Clark 1982
- Clark JM. Oxygen toxicity. In: Bennett PB, Elliott DH editor(s). The Physiology and Medicine of Diving. 3rd Edition. London: Bailliere, Tindall and Cox, 1982:200‐38. [Google Scholar]
D'Olhaberriague 1996
- D'Olhaberriague L, Mitsias P. A reappraisal of reliability and validity studies in stroke. Stroke 1998;27:2331‐6. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Hart 1971
- Hart GB, Thompson RE. The treatment of cerebral ischemia with hyperbaric oxygen (OHP). Stroke 1971;2(3):247‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hills 1999
- Hills BA. A role for oxygen‐induced osmosis in hyperbaric oxygen therapy. Medical Hypotheses 1999;52(3):259‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ikeda 1990
- Ikeda Y, Long DM. The molecular basis of brain injury and brain edema: the role of oxygen free radicals. Neurosurgery 1990;27:1‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ingvar 1965
- Ingvar DH, Lassen NA. Treatment of focal cerebral ischaemia with hyperbaric oxygen. Report of 4 cases. Acta Neurologica Scandinavica 1965;41:92‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ISTCG 1997
- The International Stroke Trial Collaborative Group. The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. Lancet 1997;349:1569‐81. [MEDLINE: ] [PubMed] [Google Scholar]
Jain 1999
- Jain KK. In: Jain KK editor(s). Textbook of Hyperbaric Medicine. 3rd Edition. Seattle: Hogrefe and Huber, 1999. [MEDLINE: ] [Google Scholar]
Jain 2009
- Jain KK. Textbook of hyperbaric medicine. Textbook of Hyperbaric Medicine. 5th Edition. Seattle: Hogrefe and Huber, 2009. [ISBN: 978‐0‐88937‐361‐7] [Google Scholar]
Khan 2003
- Khan B, Evans AW, Easterbrook M. Refractive changes in patients undergoing hyperbaric oxygen therapy: a prospective study. Undersea and Hyperbaric Medicine 2003;24 Suppl:9. [Google Scholar]
Kindwall 1999
- Kindwall EP, Whelan HT. In: Kindwall EP, Whelan HT editor(s). Hyperbaric Medicine Practice. 2nd Edition. Flagstaff: Best Publishing Company, 1999. [MEDLINE: ] [Google Scholar]
Mathieu 2006
- Mathieu 2006. Handbook on Hyperbaric Medicine. 1st Edition. Dordrecht: Springer, 2006. [Google Scholar]
Mink 1995a
- Mink RB, Dutka AJ. Hyperbaric oxygen after global cerebral ischemia in rabbits does not promote brain lipid peroxidation. Critical Care Medicine 1995;23(8):1398‐404. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mink 1995b
- Mink RB, Dutka AJ. Hyperbaric oxygen after global cerebral ischemia in rabbits reduces brain vascular permeability and blood flow. Stroke 1995;26(12):2307‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Murray 1997
- Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet 1997;349:1269‐76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Neubauer 1980
- Neubauer RA, End E. Hyperbaric oxygenation as an adjunct therapy in strokes due to thrombosis. A review of 122 patients. Stroke 1980;11(3):297‐300. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nighoghossian 1997
- Nighoghossian N, Trouillas P. Hyperbaric oxygen in the treatment of acute ischemic stroke: an unsettled issue. Journal of Neurological Sciences 1997;150(1):27‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Orgogozo 1998
- Orgogozo J‐M. Advantages and disadvantages of neurological scales. Cerebrovascular Diseases 1998;8 Suppl 2:2‐7. [DOI] [PubMed] [Google Scholar]
Oriani 1996
- Oriani G, Marroni A, Wattel F. In: Oriani G, Marroni A, Wattel F editor(s). Handbook on Hyperbaric Medicine. 1st Edition. Milan: Springer, 1996. [MEDLINE: ] [Google Scholar]
Robertson 1989
- Robertson CS, Narayan RK, Gokaslan ZL, Pahwa R, Grossman RG, Caram P Jr, et al. Cerebral arteriovenous oxygen difference as an estimate of cerebral blood flow in comatose patients. Journal of Neurosurgery 1989;70:222‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Selman 2004
- Selman WR, Lust WD, Pundik S, Zhou Y, Ratcheson RA. Compromised metabolic recovery following spontaneous spreading depression in the penumbra. Brain Research 2004;999(2):167‐74. [14759495] [DOI] [PubMed] [Google Scholar]
Siesjo 1989
- Siesjo BK, Agardh CD, Bengtsson F. Free radicals and brain damage. Cerebrovascular and Brain Metabolism Review 1989;1:165‐211. [MEDLINE: ] [PubMed] [Google Scholar]
Sudlow 1997
- Sudlow CL, Warlow CP. Comparable studies of the incidence of stroke and its pathological types: results from an international collaboration. International Stroke Incidence Collaboration. Stroke 1997;28(3):491‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sukoff 1982
- Sukoff MH, Ragatz RE. Hyperbaric oxygenation for the treatment of acute cerebral edema. Neurosurgery 1982;10(1):29‐38. [MEDLINE: ] [PubMed] [Google Scholar]
SUTC 2004
- Stroke Unit Trialists' Collaboration. Organised inpatient (stroke unit) care for stroke. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD000197.pub2] [DOI] [PubMed] [Google Scholar]
Thom 1993
- Thom SR. Functional inhibition of leukocyte B2 integrins by hyperbaric oxygen in carbon monoxide‐mediated brain injury in rats. Toxicology and Applied Pharmacology 1993;123(2):248‐56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Van Gijn 1992
- Gijn J. Measurement of outcome in stroke prevention trials. Cerebrovascular Disease 1992;2 Suppl 2:23‐34. [Google Scholar]
Wardlaw 2004
- Wardlaw JM, Keir SL, Seymour J, Lewis S, Sandercock PA, Dennis MS, et al. What is the best imaging strategy for acute stroke?. Health Technology Assessment 2004;8(1):1‐180. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yusa 1987
- Yusa T, Beckman JS, Crapo JD, Freeman BA. Hyperoxia increases H2O2 production by brain in vivo. Journal of Applied Physiology 1987;63:353‐8. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bennett 2005
- Bennett MH, Wasiak J, Schnabel A, Kranke P, French C. Hyperbaric oxygen therapy for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD004954.pub2] [DOI] [PubMed] [Google Scholar]


