Abstract
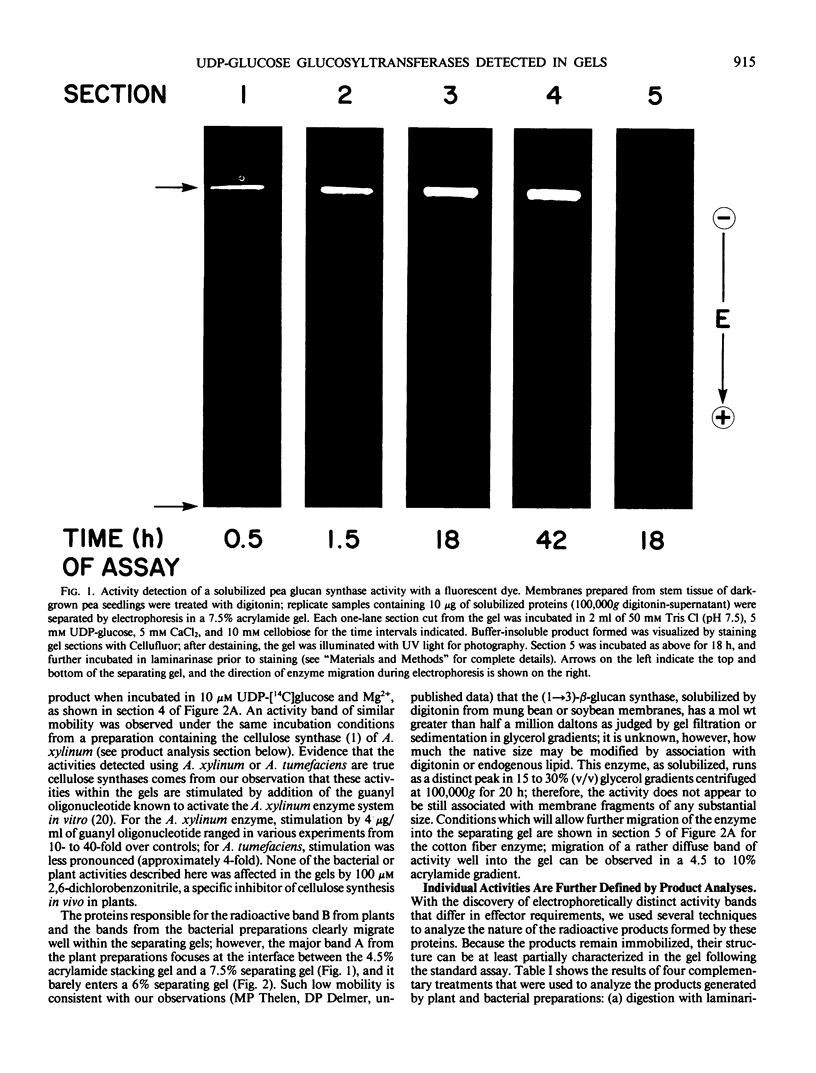
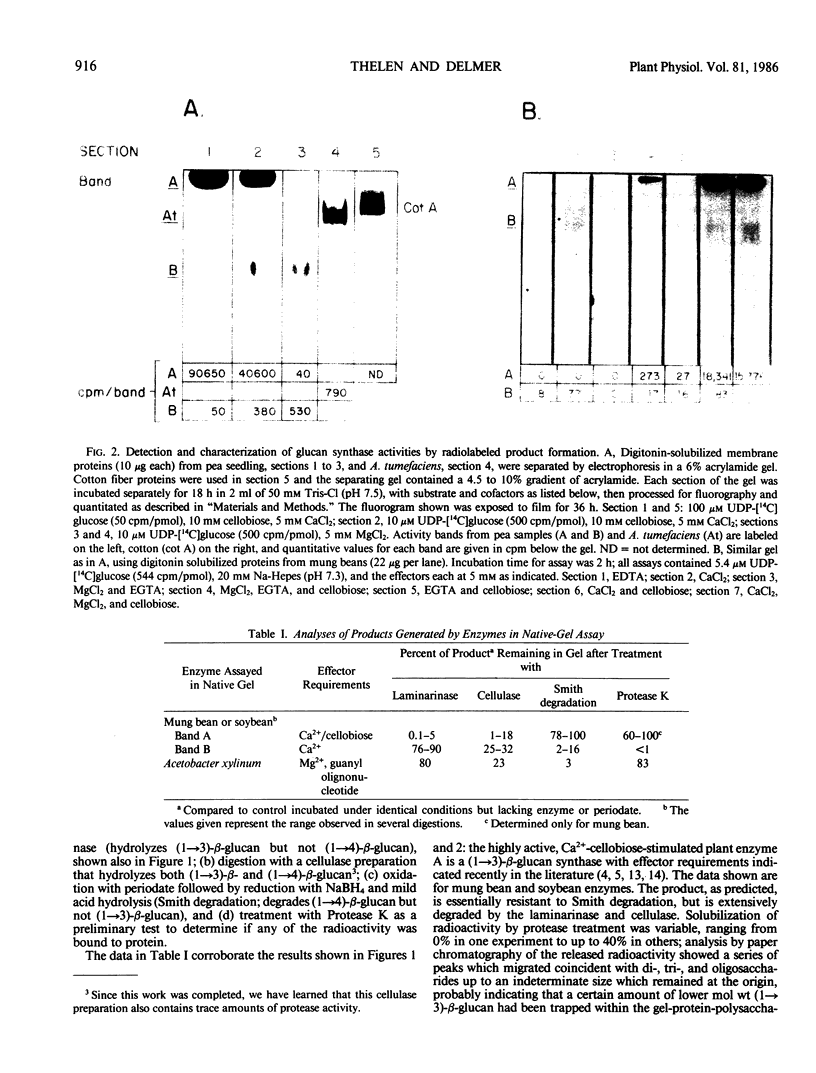
We have developed procedures for detection and characterization of UDP-glucose: glucosyltransferases following electrophoretic separation in nondenaturing polyacrylamide gels. Using digitonin-solubilized membrane protein preparations from a variety of plants and two cellulose-producing bacteria, activity can be demonstrated for several UDP-glucose:β-glucan synthases with an in situ assay following gel electrophoresis. These enzymes can be characterized within the gels with respect to effector requirements and products produced, and several advantages of this assay over solution assays are demonstrated. For example, the clear dependence of plant UDP-glucose:(1→3)-β-glucan synthase on both Ca2+ and a β-linked glucoside is shown; bacterial cellulose synthases show direct stimulation within the gel by guanyl oligonucleotide, and the Acetobacter xylinum enzyme appears more stable in the gel assay than in solution assay.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloni Y., Cohen R., Benziman M., Delmer D. Solubilization of the UDP-glucose:1,4-beta-D-glucan 4-beta-D-glucosyltransferase (cellulose synthase) from Acetobacter xylinum. A comparison of regulatory properties with those of the membrane-bound form of the enzyme. J Biol Chem. 1983 Apr 10;258(7):4419–4423. [PubMed] [Google Scholar]
- Blank A., Silber J. R., Thelen M. P., Dekker C. A. Detection of enzymatic activities in sodium dodecyl sulfate-polyacrylamide gels: DNA polymerases as model enzymes. Anal Biochem. 1983 Dec;135(2):423–430. doi: 10.1016/0003-2697(83)90705-4. [DOI] [PubMed] [Google Scholar]
- Blank A., Sugiyama R. H., Dekker C. A. Activity staining of nucleolytic enzymes after sodium dodecyl sulfate-polyacrylamide gel electrophoresis: use of aqueous isopropanol to remove detergent from gels. Anal Biochem. 1982 Mar 1;120(2):267–275. doi: 10.1016/0003-2697(82)90347-5. [DOI] [PubMed] [Google Scholar]
- Delmer D. P., Cooper G., Alexander D., Cooper J., Hayashi T., Nitsche C., Thelen M. New approaches to the study of cellulose biosynthesis. J Cell Sci Suppl. 1985;2:33–50. doi: 10.1242/jcs.1985.supplement_2.3. [DOI] [PubMed] [Google Scholar]
- Flowers H. M., Batra K. K., Kemp J., Hassid W. Z. Biosynthesis of Insoluble Glucans From Uridine-Diphosphate-d-Glucose With Enzyme Preparations From Phaseolus aureus and Lupinus albus. Plant Physiol. 1968 Oct;43(10):1703–1709. doi: 10.1104/pp.43.10.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry R. J., Stone B. A. Solubilization of beta-glucan synthases from the membranes of cultured ryegrass endosperm cells. Biochem J. 1982 Jun 1;203(3):629–636. doi: 10.1042/bj2030629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M. S., Elango N., Mattia E., Au-Young J., Robbins P. W., Cabib E. Isolation of chitin synthetase from Saccharomyces cerevisiae. Purification of an enzyme by entrapment in the reaction product. J Biol Chem. 1984 Dec 10;259(23):14966–14972. [PubMed] [Google Scholar]
- Köhle H., Jeblick W., Poten F., Blaschek W., Kauss H. Chitosan-elicited callose synthesis in soybean cells as a ca-dependent process. Plant Physiol. 1985 Mar;77(3):544–551. doi: 10.1104/pp.77.3.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S. A., Springhorn S. S. Renaturation of enzymes after polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate. J Biol Chem. 1980 Aug 10;255(15):7467–7473. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matthysse A. G., Holmes K. V., Gurlitz R. H. Elaboration of cellulose fibrils by Agrobacterium tumefaciens during attachment to carrot cells. J Bacteriol. 1981 Jan;145(1):583–595. doi: 10.1128/jb.145.1.583-595.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press G. A., Glazer H. S., Wasserman T. H., Aronberg D. J., Lee J. K., Sagel S. S. Thoracic wall involvement by Hodgkin disease and non-Hodgkin lymphoma: CT evaluation. Radiology. 1985 Oct;157(1):195–198. doi: 10.1148/radiology.157.1.4034966. [DOI] [PubMed] [Google Scholar]
- Skinner M. K., Griswold M. D. Fluorographic detection of radioactivity in polyacrylamide gels with 2,5-diphenyloxazole in acetic acid and its comparison with existing procedures. Biochem J. 1983 Jan 1;209(1):281–284. doi: 10.1042/bj2090281. [DOI] [PMC free article] [PubMed] [Google Scholar]




