Abstract
The kdpFABC operon of Escherichia coli encodes the four protein subunits of the Kdp K+ transport system. Kdp is expressed when growth is limited by the availability of K+. Expression of Kdp is dependent on the products of the adjacent kdpDE operon, which encodes a pair of two-component regulators. Studies with kdp-lac fusions led to the suggestion that change in turgor pressure acts as the signal to express Kdp (L. A. Laimins, D. B. Rhoads, and W. Epstein, Proc. Natl. Acad. Sci. USA 78:464–468, 1981). More recently, effects of compatible solutes, among others, have been interpreted as inconsistent with the turgor model (H. Asha and J. Gowrishankar, J. Bacteriol. 175:4528–4537, 1993). We re-examined the effects of compatible solutes and of medium pH on expression of Kdp in studies in which growth rate was also measured. In all cases, Kdp expression correlated with the K+ concentration when growth began to slow. Making the reasonable but currently untestable assumptions that the reduction in growth rate by K+ limitation is due to a reduction in turgor and that addition of betaine does not increase turgor, we concluded that all of the data on Kdp expression are consistent with control by turgor pressure.
Many bacterial genes and some genes in eucaryotes are regulated by pairs of regulatory proteins that belong to the two-component class of signal-transducing proteins. The larger of the pair, a sensor kinase, responds to an environmental signal to control expression. In the simplest type of this system, the sensor kinase is autophosphorylated on a histidine residue, which is followed by phosphotransfer to an aspartate residue of the smaller protein, the response regulator, whose phosphorylated form stimulates transcription (for a review, see reference 14). In addition to the autokinase and phosphotransferase of the sensor kinase, two-component systems also have a phosphatase activity to dephosphorylate the response regulator and thereby terminate the response. In some cases, the phosphatase activity is mediated by the sensor kinase, while in others it is mediated by a separate protein or is an inherent property of the response regulator. Among the major questions about two-component regulators is the nature of the signal to which they respond. The signal is well established for some but is either unknown or controversial for others.
In this study, we addressed the nature of the signal that the KdpD sensor kinase and the KdpE response regulator sense. These two proteins control expression of the Kdp transport system of Escherichia coli, a P-type transport ATPase with high affinity for K+ (for a review, see reference 1). The Kdp transport system and its two regulatory proteins are found in many bacteria, both gram negative and gram positive. Kdp is one of three saturable K+ uptake systems in E. coli, the one with the highest affinity for K+ (4). Under most conditions, the other two K+ transport systems, Trk and Kup, which are expressed constitutively, satisfy the cells’ need for K+ and Kdp is not expressed. However, when the concentration of K+ ([K+]) in the medium drops sufficiently, Kdp is expressed.
Experiments performed with a transcriptional kdp::lacZ fusion led to the hypothesis that expression of Kdp occurred when turgor pressure was suboptimal (15). The fact that Kdp is expressed when the medium [K+] is low suggested that the concentration itself was sensed. However, this idea was contradicted by the finding that the [K+] below which Kdp was expressed depended on the K+ transport systems present. In a strain with both the Trk and Kup systems, expression of Kdp occurred only in medium containing less than 2 mM K+; in a mutant lacking all saturable systems, expression was seen in media containing a 50 mM or lower [K+]. Other experiments showed that the internal [K+] does not determine expression either, since when the internal [K+] was varied by changing medium osmolarity, expression could be turned on or off by varying the external [K+]. The fact that reducing the medium [K+] could turn on expression of Kdp while the specific [K+] below which expression occurred depended on the medium and the strain suggested it was not K+ per se but the cell’s need for K+ that was sensed.
The quantitative requirement for K+ is its role as a cytoplasmic osmotic solute to maintain turgor pressure. Hence, it was proposed that the need for K+ was sensed by changes in turgor pressure, with reduced turgor pressure resulting in expression of Kdp. This model was supported by showing that an osmotic upshock, which transiently reduced turgor pressure under conditions in which neither the internal nor the external [K+] was reduced, resulted in a transient burst of high-level expression of Kdp (15).
Subsequent studies have raised questions about the turgor model for control. Altered expression of Kdp was observed in medium with a constant [K+] under several conditions. When the medium pH was reduced, Kdp expression increased (2). When the osmolarity of the medium was increased by salt, Kdp expression was higher than when a similar increase in osmolarity was produced by a nonionic solute (2, 13, 22). It was also found that addition of the compatible solute glycine betaine (betaine), expected to reduce Kdp expression because it replaces part of the cell K+ pools, did not reduce expression (2).
A major difficulty in testing the turgor model is the absence of reliable methods to measure turgor pressure in E. coli and most bacteria. The only direct method, the collapse pressure of gas vesicles, is restricted to species that produce these flotation structures (24). The only firm statements about changes in turgor pressure that can be made deal with transient changes: an abrupt increase in medium osmolarity will reduce turgor, and a decrease in medium osmolarity will increase turgor. These changes last only until the cells adapt to restore turgor. During steady-state growth, no firm statements about turgor can be made. Hence, all data about Kdp expression during steady-state growth are based on indirect inferences or assumptions about effects of manipulations on turgor.
We re-examined Kdp regulation in the steady state under conditions that were believed to argue against control by turgor. In these studies, we also measured the growth rate, a parameter not included in some of the work that raised questions about the turgor model. We refer to the threshold [K+] as that where the growth rate starts to fall as [K+] is reduced. The results show a good correlation between the threshold [K+] and the onset of expression of Kdp. This correlation had been observed earlier in experiments in which growth rates were measured (15). A simple and parsimonious interpretation explains the drop in growth rate under these conditions as due to reduced turgor. Although other factors seem to modulate, to some extent, the quantitative level of expression, our steady-state growth data are consistent with the hypothesis that a reduction of turgor pressure is necessary and sufficient for expression of Kdp.
MATERIALS AND METHODS
Strain TK2469 (F− rha thi nagA Δ(lac)179 trkD1 Δ(trkA) Φ(kdpA24′-lacZYA) and its derivatives were used in this work. TK2469 is defective in all three saturable K+ transport systems (Kdp, Trk, and Kup) and carries a stabilized transcriptional kdp::lac fusion (18). A Kup+ (TrkD+) derivative, TK2486, was used in the experiment of Fig. 2, and a Trk+ (TrkA+) derivative, TK2470, was used for the experiments of Fig. 5. Other derivatives of TK2469 used in particular experiments are described later in the text.
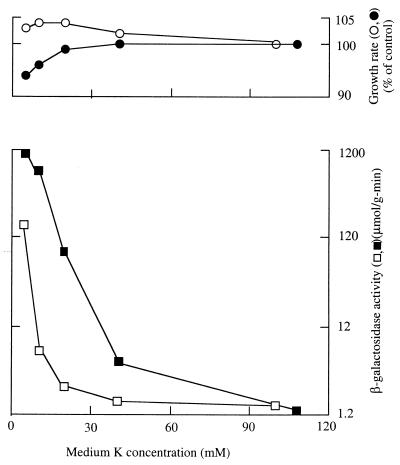
FIG. 2.
Effect of medium pH and [K+] on kdp expression in Kup+ strain TK2486. Data were obtained and plotted as described for Fig. 1; open symbols are for pH 6.3, and closed symbols are for pH 7.5. The control (100%) growth rates were 0.87 h−1 at pH 6.3 and 0.88 h−1 at pH 7.5.
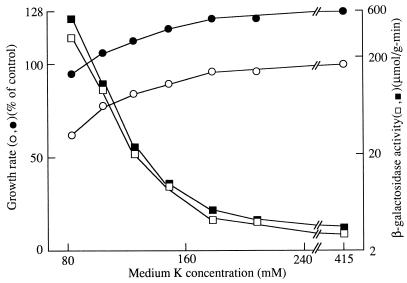
FIG. 5.
Effects of salt and sugar as osmotic solutes on the dependence of growth rate and Kdp expression on [K+] in Trk+ strain TK2470. Cells were grown in medium containing 0.3 M added salt (filled symbols) or 0.5 M added glucose (open symbols). Data were obtained as described in Materials and Methods and plotted as described for Fig. 1, except that the data for β-galactosidase are averages of four determinations and growth data are averages of two determinations. The control growth rate in the salt medium (•) was 0.70 h−1, while that in the glucose medium (○) was 0.65 h−1.
Cells were grown at 37 ± 2°C in the phosphate-buffered minimal medium described earlier (15). In all experiments, [K+] was varied without changing osmolarity by replacing K+ with equimolar concentrations of Na+ or arginine. Media of different pHs were prepared by varying the relative concentrations of mono- and dibasic phosphates while keeping osmolarity constant. The total salt concentrations (K+ plus Na+) were 119 mM in pH 7.5 medium and 105 mM in pH 6.3 medium in the experiments of Fig. 1 and 2.
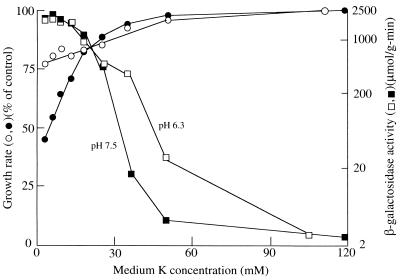
FIG. 1.
Effect of medium pH and [K+] on kdp expression in strain TK2469. Cells were grown, and average growth rates and β-galactosidase activities were measured as described in Materials and Methods. The scale of β-galactosidase activity is logarithmic to show the full range of values obtained. Open symbols are for growth at pH 6.3; closed symbols are for pH 7.5. The control (100%) growth rates were 0.65 h−1 at pH 6.3 and 0.81 h−1 at pH 7.5.
Osmotic solutes were added to standard medium containing 70 mM PO4. The osmotic solute used was either 0.5 M glucose, 0.3 M salt representing a mixture of NaCl and KCl, 0.3 M salt representing a mixture of arginine-HCl and KCl, or 0.2 M salt representing a mixture Na2SO4 and K2SO4. Expression of Kdp in media of high osmolarity using sulfates or arginine was measured only in the absence of betaine.
Cells were grown to mid-log phase in medium with the highest [K+] and then diluted directly (or after washing by centrifugation and suspension in medium containing no K+) to tubes of the desired [K+] at a density of 4.5 × 107 to 8 × 107/ml. Measurements of growth rate and enzyme level are averages over the subsequent 1.5 to 3 doublings, with the least growth observed in the tubes with the lowest [K+].
Growth rates were determined from periodic measurements of culture turbidity at 610 nm in a Bausch & Lomb Spectronic 20 colorimeter. Activity of β-galactosidase was assayed at 28°C as previously described after permeabilization of the cells with toluene (11). Assays were done routinely in duplicate but in triplicate for the data of Table 2 and in quadruplicate for the data of Fig. 5; average values are shown. The K+ transport measurements of Table 1 were performed at 30°C on cells grown at 30°C as described earlier, either in cells depleted of K+ by treatment with 2,4-dinitrophenol (19) or by stimulating uptake in growing cells by an osmotic upshock (20). The net uptake data of Table 2 are the rate of increase of cell K+ content during growth, the product of the average content of K+, and the growth rate constant. Duplicate measurements of K+ transport almost always agreed within 15%, so we estimate that the standard deviation of such measurements is 10% of the measured value or less. The dry weight of cells was estimated by cell turbidity and a calibration curve relating turbidity to the dry weight of cells washed twice by centrifugation with 50% ethanol and then dried to constant weight.
TABLE 2.
Relationship of growth rate and K+ flux on expression of Kdp in strain TK2469
| Carbon source and [K+] (mM) | Growth rate (h−1) | Net K+ fluxa (μmol g−1 min−1) | Kdp expressionb (μmol g−1 min−1) |
|---|---|---|---|
| Glucose | |||
| 40 | 0.67 | 4.3 | 650 |
| 44 | 0.72 | 5.0 | 310 |
| 115 | 0.83 | NDc | 2.5 |
| dl-Lactate | |||
| 70 | 0.41 | 3.7 | 1.3 |
| 115 | 0.41 | ND | 1.4 |
Rate of net increase in cell K+ content during growth, measured as described in Materials and Methods.
Values are levels of β-galactosidase from the fusion.
ND, not determined.
TABLE 1.
Effects of pH and salt on the initial rate of K+ uptake in strain TK2469
| Type of K+ uptake measurement and [K+] (mM) | Condition | K+ uptake (μmol g−1 min−1) |
|---|---|---|
| K+-depleted cellsa | ||
| 40 | pH 7.43 | 18.2 |
| 40 | pH 7.13 | 9.4 |
| 40 | pH 6.7 | 4.8 |
| Osmotic upshockb | ||
| 40 | 0.3 M NaCl added | 2.6 |
| 40 | 0.15 M NaCl + 0.25 M glucose added | 3.5 |
| 40 | 0.5 M glucose added | 6.7 |
Cells were grown in standard K115 medium, depleted of K+ by treatment with 2,4-dinitrophenol, suspended at 5 × 109/ml in 0.1 M NaCl, and added to growth medium with the indicated pH and K+ concentration.
Cells were grown in standard glucose K115 medium and diluted into medium containing the indicated additional osmotic solute(s) and a final K+ concentration of 40 mM.
RESULTS
Effects of pH on expression of Kdp.
The dependence of growth rate and Kdp expression on medium [K+] is shown in Fig. 1 for media of two different pH values: open symbols are data for pH 6.3 medium, and filled symbols are data for pH 7.5 medium. At each pH, growth was rapid and Kdp expression was at a low basal level at the highest [K+]. As the [K+] was reduced, there came a point where Kdp expression began to rise and the growth rate fell. To be able to show the wide range of Kdp expression, up to almost 1,000-fold in these experiments, the expression data are shown on a logarithmic scale. The data of Fig. 1 show that Kdp expression began at a higher medium [K+] in low-pH medium than in high-pH medium. These results are in agreement with those of Asha and Gowrishankar (2), who reported that the magnitude of Kdp expression was inversely correlated with the [K+] required for half-maximal growth. Our data extend this correlation to show that expression above the basal level begins at the threshold [K+] at which the growth rate began to decline. Hence, expression correlates with the point at which the cells’ needs for K+ are no longer fully satisfied and implies that it is the K+ need, not pH per se, that accounts for Kdp expression. A higher [K+] requirement for growth at low pH was noted for strain TK405 [pertinent genotype, Δ(kdpFAB)5 trkD1 trkA405], whose K+ transport properties are like those of strain TK2469, in an early report on K+ transport mutants (19).
No marked dependence of expression on medium pH is expected for strains that express the Kup (formerly TrkD) transport system, since it was known that the K+ requirements for growth of Kup+ strains are only slightly affected by the pH of the medium (19). Effects of pH in TK2486, a Kup+ derivative of TK2469, are shown in Fig. 2. In this strain, both Kdp expression and growth began to change at a somewhat higher [K+] at low pH than at high pH, but the difference is less marked than for strain TK2469 (Fig. 1). In strain TK2486, expression was higher in 35 and 50 mM K+ medium at low pH than at high pH. However, growth was also reduced somewhat more at these [K+]s at low than at high pH, again suggesting that a reduction in growth rate is key to significant expression of Kdp. As the [K+] was further reduced, expression rose to comparable levels at the two pHs. The more modest effect of pH in Fig. 2 than in Fig. 1 is consistent with the suggestion that the effect of pH on Kdp expression is indirect, mediated by the pH dependence of the K+ requirement for growth.
Roles of compatible solutes in expression of Kdp.
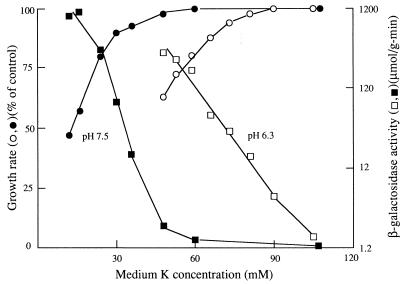
The effects of the compatible solute betaine on Kdp expression and growth are shown in Fig. 3 and 4 for strain TK2469, which requires a high [K+] for growth in all media. Since betaine is accumulated to high levels only in high-osmolarity medium, these experiments were done with medium containing either 0.3 M salt or 0.5 M sugar, creating virtually equivalent medium osmolarity. In the cultures without betaine (open symbols), Kdp expression began to rise above the basal level while the growth rate remained unchanged but did not rise above twice the basal level until the growth rate began to fall. In the presence of betaine, the results were very similar, except that the growth rate under all conditions was higher. The increase in growth rates at the highest [K+] were 28% in medium with salt and 50% in medium with sugar. The relative growth rate increase produced by betaine was larger as the growth rate became limited by a lower [K+]. Expression of Kdp was somewhat higher in the presence of betaine, but the increase was less than twofold in medium with salt and less than threefold in medium with sugar. These results are in good agreement with those of Asha and Gowrishankar (2), who noted a modest increase of expression in the presence of betaine.
FIG. 3.
Effect of betaine and [K+] on kdp expression and growth rate in strain TK2469 grown in medium containing 0.3 M added salt. Data were obtained as described in Materials and Methods and plotted as described for Fig. 1. Open symbols are for the absence of betaine, and filled symbols are for the presence of 2.5 mM betaine. The control (100%) growth rate was 0.57 h−1.
FIG. 4.
Effect of betaine and [K+] on the kdp expression and growth rate of strain TK2469 grown in medium containing 0.5 M added glucose. Data were obtained as described in Materials and Methods and plotted as described for Fig. 1. Open symbols are for the absence of betaine, and filled symbols are for the presence of 2.5 mM betaine. The control (100%) growth rate was 0.45 h−1.
We performed similar experiments and obtained very similar results with strain TK2470, which is wild type for the Trk system and hence has a much lower K+ requirement for growth (data not shown). In this strain, betaine stimulated growth by 26% in NaCl medium and 28% in glucose medium at the highest [K+], with larger stimulations as growth slowed due to reduced medium [K+]. And, as in strain TK2469, betaine stimulated the expression of Kdp up to twofold in medium with a given [K+].
Greater effectiveness of salt in expression of Kdp.
Comparison of Fig. 3 and 4 shows that Kdp expression begins at a much higher [K+] in medium in which NaCl was the osmotic solute than when glucose was used. A similar difference was seen in strain TK2470, a Trk+ derivative of TK2469 which has a much lower K+ requirement for growth (Fig. 5). Data for growth with the two types of osmotic solutes are shown in the same figure to highlight the greater effectiveness of salt. These results confirm considerable data in the literature showing that a salt such as NaCl provokes greater expression of Kdp than do osmotically equivalent concentrations of a sugar such as glucose. However, the differential effect of salts is limited to intermediate [K+]s: at a sufficiently high [K+], Kdp is not expressed in either medium, while at a sufficiently low [K+], Kdp is expressed to high levels in both media. A high level of expression in the NaCl medium occurs only at a [K+] at which substantial expression occurs in the glucose medium.
The greater effectiveness of salt in Kdp expression is correlated with a difference in the K+ requirement for the growth of strain TK2469 (compare the growth data of Fig. 3 and 4). The same appears to be true for strain TK2470 (Fig. 5), although there were only small effects on growth rate over the [K+] range examined. In fact, growth rates at 40 and 100 mM K+ in glucose medium were slightly lower than those at 10 and 20 mM and appeared to fall only at 5 mm K+. In NaCl medium, the growth rate already began to fall at 20 mM K+. At [K+]s below 5 mM, growth rates in both media fell more sharply, and in each case, the reduction was greater in NaCl medium (data not shown).
The greater effect of salt than sugar is not due to Na+, alkali metals, or the chloride ion, since similar experiments with strain TK2469 in which sulfate replaced chloride or arginine replaced Na+ gave results very similar to those obtained with NaCl (data not shown).
Alterations in K+ transport correlate with effects on growth and Kdp expression.
The K+ requirements for growth of mutants defective in K+ transport were found to correlate with the degree to which K+ transport was impaired (19). We found the same correlation here for strain TK2469 for the effects of pH shown in Fig. 1 and the greater effectiveness of salts than sugar in Fig. 3 and 4. The greater K+ requirement of strain TK2469 at low pH is explained by the marked pH dependence of K+ uptake in this strain (Table 1). Transport at pH 6.7 was only 26% of the rate at pH 7.43. The greater inhibition of growth and expression in strain TK2469 by high concentrations of salt is reflected in a greater inhibition of K+ uptake by salt (Table 1). The rate of uptake provoked by osmotic upshock with NaCl was only 39% of that when glucose was used as the osmotic solute and rose only slightly when upshock was performed with equal mixtures of salt and sugar.
The greater relative inhibition of growth by salt than by sugar in strain TK2470 (Fig. 5) suggests that salt is more inhibitory to K uptake in this strain. Demonstrating this has been difficult. When uptake was provoked by upshock with either salt or sugar, as for strain TK2469 in Table 1, we saw no difference in either the rate of or affinity for uptake. However, we were struck by the fact that there is a reduction in the growth rate at 5 mM K+, which is well above the Km of the Trk system and a concentration at which the Vmax, as measured by upshock, is about 100 μmol g−1 min−1 (20), at least 10-fold higher than the rate needed to sustain rapid growth. The problem is that Trk is regulated by turgor; when turgor is high, the system has a low rate of transport. During growth, it has been postulated that a very slight reduction in turgor, too slight to cause a perceptible change in growth rate or in expression of Kdp, results in a slow rate of uptake just sufficient to maintain the homeostasis of K+ pools during growth (10). We attempted to approach the conditions of growth by measuring uptake after a small upshock in cells growing in 0.3 M NaCl or 0.5 M glucose medium. In two experiments, uptake was greater in the glucose medium, but the rates of uptake were low and experimental scatter was rather large. We believe that the differential effect of salt here too is mediated by effects on transport but have not been able to obtain convincing data on this point.
Roles of growth rate and net K+ uptake.
We examined the correlation of growth rate and of net K+ uptake on expression of Kdp by comparing cells growing on dl-lactate, where the growth rate is slower, with those growing on glucose. In lactate medium, the overall pattern of expression is like that in Fig. 1, with the exception that both the basal level and the highest level of expression were lower than during growth on glucose. Table 2 shows data for growth rate, Kdp expression, and net K+ uptake for a few [K+]s during growth on lactate or glucose. These measurements were made to determine if there is a direct effect of either growth rate or net K+ uptake on expression of Kdp. Cells growing on lactate in 70 mM K+ medium had a relatively low rate of growth and of net K+ uptake yet did not express Kdp above the basal level. In contrast, cells growing on glucose in medium with either 40 or 44 mM K+ had a higher rate of growth and of net K+ uptake yet expressed Kdp at 100 to 200 times the basal levels. The data show that neither the rate of growth per se nor the rate of net K+ uptake correlates with expression of Kdp; limitation of growth rate by the medium [K+] does correlate.
Inhibition of growth in Kdp+ Trk− strains.
In a strain like TK2469 but with a wild-type copy of Kdp, it was found that expression of Kdp, as monitored by the fusion, was highest at 1 mM K+ with reduction at high concentrations so that expression resembled that in TK2469, except that the expression level was much lower (15). A recent report of Kdp expression in an analogous strain of Salmonella typhimurium confirmed the expression but noted that growth of the strain was inhibited up to 30% as the K+ was increased from 1 to 25 mM (12). The reduction in growth rate was puzzling but viewed as an apparent contradiction to control by turgor. We found the same growth phenomenon in a corresponding strain of E. coli (TK2469 with F-100 carrying wild-type kdp genes), as well as in a Kdp+ transductant of TK2469 (where the fusion is replaced by wild-type kdp genes), indicating that this effect is neither unique to Salmonella nor an artifact of the kdp::lac fusions. Growth rates are high at low (1 mM) and high (100 mM) [K+]s but reduced over the range of about 5 to 50 mM with maximum reduction at 20 to 30 mM K+. However, when such strains are grown in minimal medium in which aspartate replaces ammonium as the nitrogen source, growth rates are essentially independent of medium [K+] over the range of 1 to 100 mM. These results suggest that growth inhibition is an inhibitory effect of ammonium ions, which are inhibitory only when Kdp is expressed and the medium K content is not low. Inhibition could be due to ammonium uptake by Kdp, since it has been reported that ammonium ions are substrates of the Kdp system (6).
DISCUSSION
Kdp expression is correlated with K+ limitation of growth.
We examined expression of Kdp and growth rate as a function of [K+] under conditions that were believed by some to be inconsistent with the control of Kdp expression by turgor. The marked effect of pH on the expression of Kdp (Fig. 1) is a reflection of the increased K+ requirement for growth at low pH. The fact that there is an inverse correlation between growth rate and Kdp expression was noted by Asha and Gowrishankar (2), but that statement could easily be overlooked since it was in the context of a paper that marshalled arguments against control by turgor. We confirm here the correlation noted before and, further, show that Kdp expression begins to rise near the threshold [K+] for growth. In a strain whose growth is less sensitive to pH, expression of Kdp is also less pH dependent (Fig. 2).
A major argument against turgor control was the finding that betaine did not reduce Kdp expression. Here we confirm that finding, for both strain TK2469 (Fig. 3 and 4) and its Trk+ derivative, whose behavior is qualitatively very similar. In the presence of betaine, as well as in its absence, expression of Kdp is correlated with the effects of [K+] on growth. Betaine does not reduce the K+ requirement for growth, and therefore, Kdp expression is not reduced. Our growth data for expression in the presence of betaine do not agree with those of Asha and Gowrishankar (2), who reported that during growth in the presence of betaine, an increase in expression was not correlated with a reduction in the growth rate.
The other strong argument against turgor control was the finding that salts were more effective than were equiosmolar concentrations of sugars in turning on Kdp expression. This finding is also confirmed here for strains TK2469 (Fig. 3 and 4) and TK2470 (Fig. 5). Again, by including growth rate studies, it has been possible to show that the correlation of expression with growth rate limitation by K+ applies in this case as well. Our studies reported here confirm the early inferences about Kdp expression (15) and the finding of others (2) that expression is correlated with K+ limitation of growth.
We have also shown that conditions which cause the growth rate to be limited by K+ are generally explained by effects of those conditions on the uptake of K+. There is a large effect of pH on transport in strain TK2469 (Table 1) that explains the marked effect of pH on growth, and similarly, the greater K+ requirement of strain TK2469 when growing in high-osmolarity salt medium is reflected in a greater-than-twofold reduction in the rate of K+ uptake by osmotic upshock with salt compared with upshock produced by glucose (Table 1). As noted in Results, we believe that the differential effect of salts in strain TK2470 is also mediated by effects on K+ uptake.
Effects of compatible solutes.
We have confirmed the seemingly anomalous effects of betaine on Kdp expression and shown that they fit the correlation with K+ limitation. The result is readily reconciled with what is established about the roles of compatible solutes in enteric organisms. Such solutes are synthesized or accumulated only during growth in media of elevated osmolarity and serve to replace part of the pools of K+ and glutamate that are necessary for osmotic equilibrium in the absence of compatible solutes. The increase in rate of growth produced by compatible solutes is presumably due, at least in part, to the replacement of the high concentrations of ionic solutes that are inhibitory to many enzymes with neutral molecules that inhibit enzymes little, if at all, even when present at high concentrations.
When added acutely to cells grown at high osmolarity, betaine causes an efflux of part of the cell K+ pools over a period of a few minutes (3). This efflux represents the cells’ way of reducing turgor, after the transient increase due to rapid accumulation of betaine. An increase in turgor is known to result in net K+ efflux (16). We assume that once net efflux ceases, turgor returns to the normal, control level present before the addition of betaine. The data do argue that a brief increase in turgor is produced when betaine is added to cells primed to take up this solute but provide no basis for the view that a permanent change in turgor occurs.
A somewhat different view of the effects of compatible solutes was proposed by Cayley et al. (8), who reported that compatible solutes increased the osmotically active water in cells grown at elevated osmolarity and suggested that an increase in cell water is important in determining the growth rate. Those authors also measured the major osmotic solutes in cells, as well as the osmotically active (free) cytoplasmic water. This ambitious undertaking, unfortunately, does not allow their data to be used to compare turgor, since the specific values shown required assumptions about turgor and free water that have not been tested (9). However, were those assumptions found to be correct, the data (Table 3 in reference 8) would suggest that turgor is not higher and may be lower in the presence of betaine than in its absence. There are no data that allow one to evaluate the suggestion that turgor is higher in the presence of betaine (2, 8).
In the experiments with betaine, the control cells that did not receive betaine were not devoid of compatible solutes. Growth in medium of elevated osmolarity leads to the accumulation of trehalose in E. coli by synthesis in the cell. However, virtually no trehalose is accumulated when betaine is present (10). Hence, the difference between the results for cells with and without betaine represents the difference between the use of betaine and that of trehalose as a compatible solute. The stimulation of growth by betaine reflects the greater effectiveness of betaine as a compatible solute.
Other factors in the expression of Kdp.
Our data suggest that factors in addition to turgor modulate the expression of Kdp. Addition of betaine increases Kdp expression nonspecifically up to threefold, both when expressed at a basal level and when induced. This stimulation indicates that the cytoplasmic environment, specifically, the solutes present, has a general influence on the relative expression of genes. There must be other genes whose expression is higher in the absence of betaine.
If the relative reduction in the growth rate reflected the relative reduction in turgor, and the magnitude of the reduction in turgor were the sole factor in expression, then the same level of expression would be expected for the same relative reduction in the growth rate. This is not the case. Expression in the experiment of Fig. 1 for a fairly large growth rate reduction is considerably less than for smaller growth rate reductions in the experiments of Fig. 2 and 5. The logarithmic scale used to plot the expression data makes these differences seem smaller than they would appear in a linear plot. Hence, it seems that a reduction in turgor is necessary and sufficient for stimulation of Kdp expression, but the exact extent of expression is affected by other factors. One of those factors could well be compatible solutes, since they increase basal and induced expression. The cytoplasmic pH may play a role, since K+ is known to have a role in pH regulation (5). Other cytoplasmic solutes, such as Na+, which rises as the medium Na+ content rises (7), may also influence expression of Kdp.
The flux model.
A number of other models for control of Kdp have been proposed, but the only one that seems consistent with the data and testable is the K+ flux model of Asha and Gowrishankar (2). Their proposal is that Kdp is expressed when K+ influx falls below some particular level. The flux model has one great advantage over the turgor model: it assumes sensing by a parameter than can be measured, which is not the case for turgor.
In all of the steady-state experiments shown here and those of others (2, 22), a reduced rate of K+ uptake produced by reducing the medium [K+] is associated with Kdp expression. Hence, the flux model appears to fit the published steady-state data about as well as the turgor model. However, the flux model is not consistent with the data of Table 1, where we find high expression at a higher rate of uptake during growth on glucose, while at a lower rate of K+ uptake during growth on lactate, there is only basal level expression.
KdpD is a plausible sensor of turgor.
The known sensor for Kdp expression, the KdpD protein, has a structure and features that make it a plausible candidate as a turgor sensor. KdpD has four membrane spans that are important in its sensing function (23, 25). A deletion variant lacking the membrane spans retains the ability to express Kdp at a low level but has lost the ability to respond to the environment (17). Mutations in and near the membrane spans result in partial constitutive expression of Kdp (21). Turgor, sensed as a stretch in the plane of the inner membrane, could alter the structure of the membrane spans, perhaps causing rotational movement that is transmitted to the kinase domain in the C terminus of KdpD.
Nothing known about KdpD makes it plausible that it senses K+ fluxes. There is no evidence that KdpD is associated with other membrane proteins, such as proteins of K+ transport systems, and no evidence that KdpD mediates K+ transport. Strains like TK2469, but in which the kdpD and kdpE genes are deleted as well, do not have lower rates of K+ transport than strains like TK2469 that express KdpD and KdpE. Overexpression of KdpD does not appear to alter K+ transport either (unpublished data).
Studies with fusions, such as those reported here and earlier, have their limitations. The measured stimulation of expression as measured here could be lower or higher than the actual value if translation efficiency or stability of the hybrid mRNA made by the fusion were different from that of the native Kdp mRNA. Hence, more direct methods, such as measurement of the level of kdp mRNA, will be useful to confirm data from fusions, as well as to extend our understanding of the regulation of Kdp.
ACKNOWLEDGMENT
R. Malli was supported in part by an Undergraduate Summer Research Award from the Howard Hughes Medical Foundation.
REFERENCES
- 1.Altendorf K, Epstein W. The Kdp-ATPase of Escherichia coli. In: Lee A G, editor. Biomembranes. Vol. 5. Greenwich, Conn: JAI Press; 1996. pp. 403–420. [Google Scholar]
- 2.Asha H, Gowrishankar J. Regulation of kdp operon expression in Escherichia coli: evidence against turgor as a signal for transcriptional control. J Bacteriol. 1993;175:4528–4537. doi: 10.1128/jb.175.14.4528-4537.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakker E P, Booth I R, Dinnbier U, Epstein W, Gajewska A. Evidence for multiple K+ export systems in Escherichia coli. J Bacteriol. 1987;169:3743–3749. doi: 10.1128/jb.169.8.3743-3749.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakker E P. Low-affinity K+ uptake systems. In: Bakker E P, editor. Alkali cation transport systems in prokaryotes. Boca Raton, Fla: CRC Press, Inc.; 1993. pp. 253–276. [Google Scholar]
- 5.Booth I R. Regulation of cytoplasmic pH in bacteria. Microbiol Rev. 1985;49:359–378. doi: 10.1128/mr.49.4.359-378.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buurman E T, Teixeira de Mattos M J, Neijssel O M. Futile cycling of ammonium ions via the high affinity potassium uptake system (Kdp) of Escherichia coli. Arch Microbiol. 1991;155:391–395. doi: 10.1007/BF00243460. [DOI] [PubMed] [Google Scholar]
- 7.Castle A M, Macnab R M, Shulman R G. Coupling between sodium and proton gradients in respiring Escherichia coli cells measured by 23Na and 31P nuclear magnetic resonance. J Biol Chem. 1986;261:7797–7806. [PubMed] [Google Scholar]
- 8.Cayley S, Lewis B A, Record M T., Jr Origins of the osmoprotective properties of betaine and proline in Escherichia coli K-12. J Bacteriol. 1992;174:1286–1295. doi: 10.1128/jb.174.5.1586-1595.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cayley, S. 1998. Personal communication.
- 10.Csonka L N, Epstein W. Osmoregulation. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C: ASM Press; 1996. pp. 1210–1223. [Google Scholar]
- 11.Epstein W. Transposition of the lac region of Escherichia coli. IV. Escape from repression in bacteriophage-carried lac genes. J Mol Biol. 1967;30:529–543. doi: 10.1016/0022-2836(67)90366-x. [DOI] [PubMed] [Google Scholar]
- 12.Frymier J S, Reed T D, Fletcher S A, Csonka L N. Characterization of transcriptional regulation of the kdp operon of Salmonella typhimurium. J Bacteriol. 1997;179:3061–3063. doi: 10.1128/jb.179.9.3061-3063.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gowrishankar J. Identification of osmoresponsible genes in Escherichia coli: evidence for participation of potassium and proline transport systems in osmoregulation. J Bacteriol. 1985;164:434–445. doi: 10.1128/jb.164.1.434-445.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C: ASM Press; 1995. [Google Scholar]
- 15.Laimins L A, Rhoads D B, Epstein W. Osmotic control of kdp operon expression in Escherichia coli. Proc Natl Acad Sci USA. 1981;78:464–468. doi: 10.1073/pnas.78.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meury J, Robin A, Monnier-Champeix P. Turgor-controlled K+ fluxes and their pathways in Escherichia coli. Eur J Biochem. 1985;151:614–619. doi: 10.1111/j.1432-1033.1985.tb09148.x. [DOI] [PubMed] [Google Scholar]
- 17.Nakashima K, Sugiura A, Kanamaru K, Mizuno T. Signal transduction between the two regulatory components involved in the regulation of the kdpABC operon of Escherichia coli: phosphorylation-dependent functioning of the positive regulator, KdpE. Mol Microbiol. 1993;7:109–116. doi: 10.1111/j.1365-2958.1993.tb01102.x. [DOI] [PubMed] [Google Scholar]
- 18.Polarek J W, Williams G, Epstein W. The products of the kdpDE operon are required for expression of the Kdp ATPase of Escherichia coli. J Bacteriol. 1992;174:2145–2151. doi: 10.1128/jb.174.7.2145-2151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhoads D B, Waters F W, Epstein W. Cation transport in Escherichia coli. VIII. Potassium transport mutants. J Gen Physiol. 1976;67:325–341. doi: 10.1085/jgp.67.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rhoads D B, Epstein W. Cation transport in Escherichia coli. IX. Regulation of K transport. J Gen Physiol. 1978;72:283–295. doi: 10.1085/jgp.72.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugiura A, Hirokawa K, Nakashima K, Mizuno T. Signal-sensing mechanisms of the putative osmosensor KdpD in Escherichia coli. Mol Microbiol. 1994;14:929–938. doi: 10.1111/j.1365-2958.1994.tb01328.x. [DOI] [PubMed] [Google Scholar]
- 22.Sutherland L, Cairney J, Elmore M J, Booth I R, Higgins C F. Osmotic regulation of transcription: induction of the proU transport gene is dependent on accumulation of intracellular potassium. J Bacteriol. 1986;168:804–814. doi: 10.1128/jb.168.2.805-814.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walderhaug M O, Polarek J W, Voelkner P, Daniel J M, Hesse J E, Altendorf K, Epstein W. KdpD and KdpE, proteins that control expression of the kdpABC operon, are members of the two-component sensor-effector class of regulators. J Bacteriol. 1992;174:2152–2159. doi: 10.1128/jb.174.7.2152-2159.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walsby A E. Gas vesicles. Microbiol Rev. 1994;58:94–144. doi: 10.1128/mr.58.1.94-144.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zimmann P, Puppe W, Altendorf K. Membrane topology analysis of the sensor kinase KdpD of Escherichia coli. J Biol Chem. 1995;270:28282–28288. doi: 10.1074/jbc.270.47.28282. [DOI] [PubMed] [Google Scholar]