Abstract
Although it is well established that the direct action of cocaine on centrally-located neural substrates is essential in mediating its reinforcing properties, cocaine induces very rapid immediate neural effects that imply cocaine’s action on peripheral neural substrates. We employed oxygen sensors coupled with high-speed amperometery to examine the effects of standard cocaine HCl that easily enters the blood-brain barrier and its blood-brain barrier-impermeable methiodide analog on oxygen levels in the nucleus accumbens in awake, freely-moving rats. Both drugs induced strong increases in nucleus accumbens oxygen levels, which displayed similarly short, second-scale latencies and a general similarity with oxygen increases induced by an auditory stimulus. This study provides additional support for the view that the immediate neural effects of iv cocaine are triggered via its direct action on peripherally located neural substrates and fast neural transmission to the central nervous system via somatosensory pathways.
Keywords: electrochemistry, cocaine methiodide, blood-brain barrier, neural activation, cerebral vasodilation, afferents of peripheral sensory nerves
It is commonly thought that the direct action of cocaine on centrally located monoamine transporters is the most important mechanism mediating the neural effects of cocaine. This view originates from classic in vitro studies that clearly demonstrate that cocaine dose-dependently inhibits reuptake of monoamines without affecting their release.1, 2 This view was supported by numerous in vivo experiments using dopamine (DA) antagonists and lesions of DA cells or its target areas, which implied the critical role of DA in mediating the reinforcing effects of cocaine.3–5 While it is undisputable that cocaine-induced changes in DA transmission are critical for mechanisms of cocaine reinforcement and drug-taking behavior,6–8 the evidence on the role of central DA and other monoamine mechanisms in mediating the acute neural and physiological effects of this drug is much more controversial.
An acute increase in arterial blood pressure is a prominent centrally-mediated effect of cocaine.9 When administered to awake, freely-moving rats at low intravenous (iv) doses optimal for self-administration behavior, cocaine elevates blood pressure with second-scale latencies and peaks within one minute.10 Such a rapid time-course is difficult to reconcile with cocaine’s pharmacokinetics and the time necessary for the drug to reach brain blood vessels, cross the blood-brain barrier (BBB), diffuse to reach and bind to monoamine transporters, and enhance monoamine neurotransmission. Furthermore, this effect is resistant to DA receptor blockade10, 11 and is mimicked by cocaine’s analog cocaine methiodide, which does not cross the blood-brain barrier (BBB).12 Both cocaine salts injected to freely-moving rats at low, behaviorally relevant doses (0.25–1.0 mg/kg) also induce rapid and strong cortical EEG desynchronization, suggesting generalized neural activation,13 which is also resistant to full DA receptor blockade.13 Finally, a critical role of peripheral actions of cocaine in mediating its acute neural effects was demonstrated by electrochemical assessment of nucleus accumbens (NAc) glucose.14 In this study, we found that iv cocaine induces a biphasic increase in NAc glucose, with the initial prominent rise occurring with 4–6 s latency and peaking 20–25 s after the injection onset. Importantly, this initial effect of cocaine HCl was only slightly attenuated by full DA receptor blockade and was mimicked by cocaine methiodide.
The goal of this study was to provide further evidence for the role of peripheral actions of cocaine in mediating its neural effects by focusing on changes to brain oxygen levels induced by cocaine HCl and its BBB-impermeable analog in awake, freely-moving rats. Similar to our previous studies, oxygen recordings were conducted in the NAc, a deep, ventrally located structure critically involved in sensorimotor integration and drug reinforcement.15, 16 Brain oxygen is an important homeostatic parameter and its efficient inflow from arterial blood into the brain’s extracellular space is essential for maintaining neuronal activity under conditions of physiological and behavioral activation. Neurovascular coupling would account for the enhancement of intra-brain inflow of oxygen during neuronal activation by inducing local cerebral vasodilation and increasing cerebral blood flow.17–19 In previous work, we showed that NAc oxygen levels are maintained at relatively stable levels under quiet resting conditions and phasically increase by arousing stimuli due to activation of this neurovascular mechanism.20 Since iv cocaine excites accumbal neurons in awake rats,21 we hypothesized that cocaine would also increase NAc oxygen levels and this increase, at least at the earliest stage, would be mimicked by cocaine methiodide, which also excites accumbal neurons.
Results and Discussion
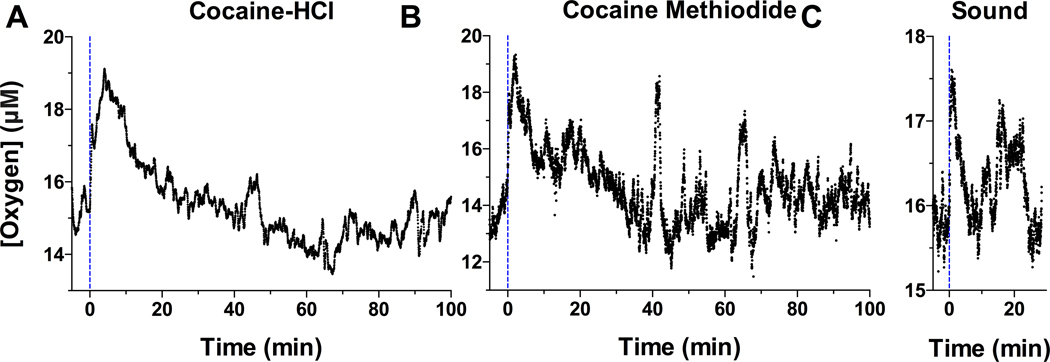
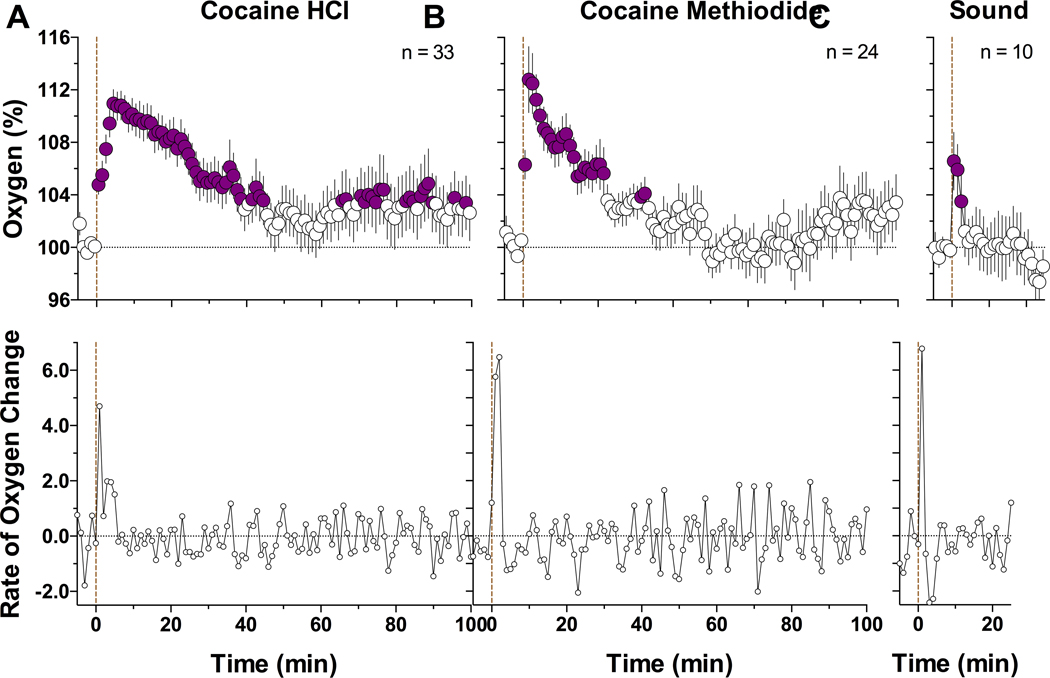
Our data were obtained in 15 rats (7 with cocaine HCl and 8 with cocaine methiodide) with histologically verified locations in the NAc shell. Consistent with our previous studies,20 NAc oxygen levels assessed in freely-moving rats under quiet resting conditions were maintained at relatively stable levels (range of basal values in different rats and sessions: 10–32 μM; mean = 17.88 ± 0.71; SD = 5.80 μM, n=15), showing transient fluctuations within 5–10% of baseline (Figure 1). When analyzed with 1-min time resolution, cocaine significantly increased NAc oxygen levels (F100, 3200 = 5.06, p<0.001; Figure 2A). The oxygen levels began to increase during the first minute from the injection onset, peaked at ~ 5 min, and slowly decreased toward the pre-injection baseline thereafter. When data were analyzed as rate of change, maximal change occurred during the first minute from injection onset. Despite the consistency of cocaine-induced oxygen increases, their magnitudes were relatively small varying within 5–15% of baseline. The oxygen response elicited by cocaine methiodide was similar (F100, 2300 = 5.12, p<0.001; Figure 2B), with a rapid rise, peaking at 2–3 min, and then slowly returning to baseline. Similarly, maximal change in concentration occurred during the first minute. While within a range of statistical variability, the oxygen response induced by cocaine methiodide was sharper and shorter in duration than that induced by cocaine HCl. Interestingly, an auditory stimulus induced an equally rapid oxygen rise (F9,81 = 5.47; Figure 2C). However, the response was much shorter and smaller than those induced by both cocaine salts. Original examples of oxygen responses induced by both cocaine analogs and an auditory signal are shown in Figure 1.
Figure 1.
Original records of changes in NAc oxygen levels induced by cocaine HCl (A), cocaine methiodide (B), and a short auditory stimulus (C) in awake freely-moving rats. Current values (nA) were collected with 1-s time resolution and transferred into concentration values (μM). Dotted vertical lines show the onset of iv injections and auditory stimulus presentation.
Figure 2.
Mean (±SEM) changes in NAc oxygen levels induced by cocaine HCl (A), cocaine methiodide (B), and auditory stimulus (C) in awake freely-moving rats. Data are shown with slow, 1-min time resolution as relative changes vs. the pre-stimulus baseline (=100%). Bottom graphs show rate of change. Filled symbols show values significantly different from baseline. Vertical dotted line shows the onset of injections or auditory stimulus presentation; n represents the numbers of averaged tests.
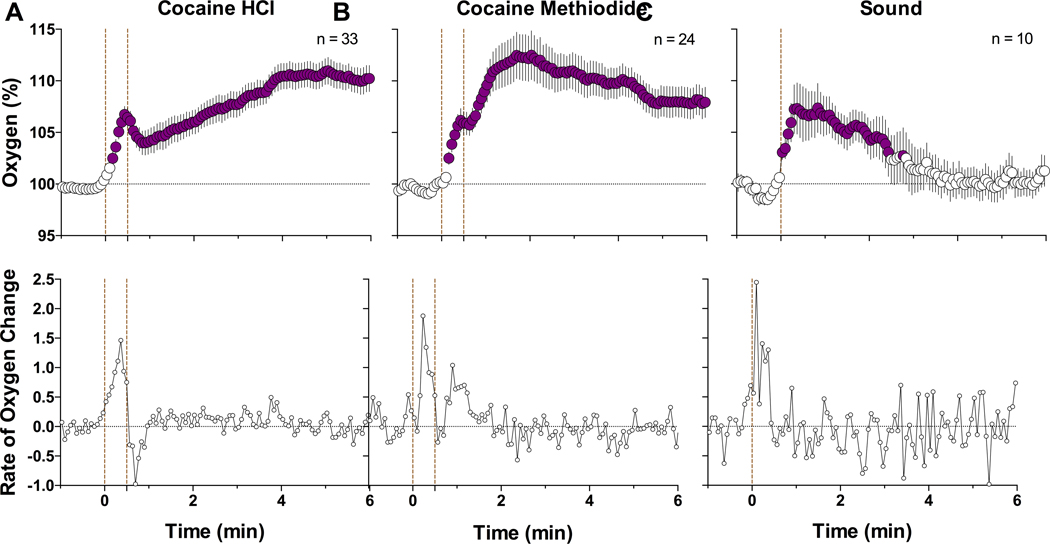
To observe more precise NAc oxygen changes elicited by cocaine and its analog, data were analyzed with high temporal resolution (Figure 3). In this case, the oxygen increase induced by cocaine HCl exhibited two clear phases: a phasic jump occurring while the injection was applied and a more prolonged and larger elevation thereafter (F32,2400 = 16.01, p<0.001). This dynamic, two-phasic response was especially evident when the data were shown as rate of change. In this case, oxygen levels increased rapidly (latency ~6–8 s) and peaked (at 25 s) during the injection duration, then decreased and slowly increased thereafter. The oxygen response elicited by cocaine methiodide was very similar, with a rapid jump during the injection, a phasic peak at its offset, and slow elevation thereafter (F23,1725 = 9.85, p<0.001). In this case, the two-phasic increase was less evident in concentration values but was more evident in rate of change. The auditory signal induced the most rapid increase in oxygen (F9,567 = 6.37, p<0.001) that peaked immediately after the stimulus onset (latency 2 s); the magnitude of this phasic increase was larger than that induced by both cocaine analogs.
Figure 3.
Mean (±SEM) changes in NAc oxygen levels induced by cocaine HCl (A), cocaine methiodide (B), and auditory stimulus (C) in awake freely-moving rats. Data are shown with rapid, 4-s time resolution as relative change vs. the pre-stimulus baseline (=100%). Bottom graphs show rate of change. Filled symbols show values significantly different from baseline. First vertical dotted lines show the onset of injection or auditory stimulus presentation. Second dotted line in A and B show offset of cocaine injection; n represents the numbers of averaged tests.
Our previous studies revealed that NAc oxygen levels increase following exposure to different arousing stimuli.20 While parallel excitations of ventral striatal neurons21, 22 and short-latency EEG desynchronization13 induced by these stimuli suggest neuronal activation as a cause of these increases, this mechanism has also been confirmed by the observation that NAc oxygen increases during local intra-NAc microinjections of glutamate20 known to excite accumbal neurons.23 Therefore, due to this neurovascular coupling mechanism, cerebral blood vessels become dilated and local cerebral blood flow increases, resulting in enhanced oxygen entry into brain tissue. Neurovascular coupling also appears to be responsible for rapid increases in NAc glucose elicited by arousing stimuli.24 Due to this mechanism, the inflow of oxygen and glucose into brain tissue is rapidly enhanced, thus preventing any possible metabolic deficit.
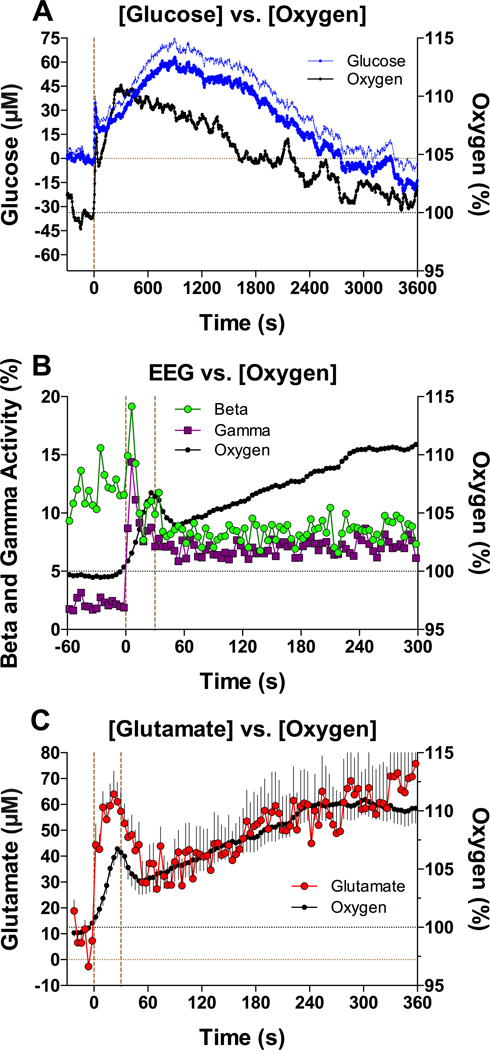
It appears that neurovascular coupling underlies the rapid cocaine-induced increases in NAc oxygen (shown in this study) and NAc glucose.14 As shown in Figure 4A, the levels of both substances increased with second-scale latencies displaying a prominent peak near the end of the injection. While the immediate changes in NAc oxygen and glucose elicited by cocaine were similar to those induced by simple sensory stimuli, pointing to the same underlying neural mechanism, cocaine also induced secondary, more powerful tonic increases. Our EEG recordings13 provided further support for this mechanism. Similar to sensory stimuli, iv cocaine induced rapid cortical EEG desynchronization as evidenced by strong decreases in EEG total power and prominent increases in beta- and gamma activities. Importantly, these indices of generalized neuronal activation showed very short latencies, with peak increases at ~6 s, clearly before the slower NAc oxygen changes (Figure 4B). Moreover, the rapid components of EEG desynchronization induced by cocaine were resistant to full DA receptor blockade but were strongly reduced during general anesthesia. Finally, iv cocaine induced significant and exceptionally rapid changes in NAc glutamate levels, with ~2–6 s latencies and peaks within the injection duration 14 (Figure 4C). Since accumbal neurons are sensitive to glutamate,23 it appears that cocaine induces rapid glutamate release onto accumbal cells leading to their excitation.
Figure 4.
The relationship between cocaine-induced changes in NAc oxygen and (A) NAc changes in glucose, (B) changes in beta and gamma EEG activity, and (C) changes in NAc glutamate. Small black circles in all graphs show changes in oxygen analyzed with 4-s time resolution. First dotted vertical line in all graphs shows the onset of the 30-s cocaine injection, second dotted line in B and C shows the offset of the injection. Second blue line in A shows standard errors in upright direction. See text for other explanation.
While generalized neuronal activation induced by iv cocaine appears to be the cause for rapid changes in NAc oxygen and glucose, our data with cocaine methiodide confirme the peripheral source of this activation. This cocaine analog fails to cross the BBB,12 but it induced short-latency increases in NAc oxygen that were similar to those induced by cocaine HCl. As shown previously, this BBB-impermeable cocaine analog also mimicked cocaine in its ability to induce rapid EEG desynchronization,13 to excite accumbal neurons,21 increase brain temperature,25 and induce phasic increases in NAc glutamate and glucose levels.14, 26 While the immediate effects of both cocaine analogs were surprisingly similar, there were important differences in the delayed effects of these drugs and in their behavioral outcomes. In contrast to cocaine HCl, cocaine methiodide did not induce notable locomotor activation25 and its effects on NAc glutamate were much weaker, showing within-session tolerance.14 Since cocaine methiodide cannot enter the brain, it appears that its effects are triggered from the periphery. Furthermore, the rapidity of these effects implies fast neural transmission from peripheral receptor sites to the CNS. Although cocaine HCl easily enters the CNS, the similarity of its neural effects with both its BBB-impermeable methiodide analog and sensory stimuli suggests that the rapid neural effects of iv cocaine are triggered from the periphery through fast neural transmission.
The rapid neural effects of cocaine discussed in this study are too fast to reflect direct central action. Although cocaine enters cerebral blood vessels ~10 s after the start of the iv injection, substantial time is needed to cross three cellular levels of the BBB, passively diffuse to specific neural substrates and to interact with them. The rapid changes in multiple neural parameters are also inconsistent with the slower and more prolonged increases in brain cocaine levels evaluated by PET27 and much slower and weaker changes in DA uptake induced by iv cocaine at behaviorally relevant doses (0.5–1.0 mg/kg) as assessed by fast-scan voltammetry combined with local DA microinjections;28 however, see 29.
The similarity of the rapid neural effects induced by somatosensory stimuli and both cocaine analogs suggests that they share a common underlying mechanism involving activation of peripherally located neural substrates and rapid neural transmission. However, the exact nature of these substrates and pathways transmitting this signal to the CNS remain unclear. One possibility is the interaction of cocaine with multiple ionic channels (i.e., voltage-gated Na+, K+, Ca2+, TRP channels) that are densely expressed on terminals of sensory nerves that innervate peripheral vessels.30–33 These channels are activated by multiple types of “pathological” shifts in blood chemicals and often viewed as visceral nociceptors. These substrates could be directly activated by cocaine when its levels rapidly and strongly increase after iv administration, thus generating the ascending excitatory drive to the CNS using neural pathways transmitting the effects of visceral somatosensory stimuli. While analytical research employing other techniques is necessary to determine the nature of peripheral neural substrates activated following iv cocaine administration, it is a difficult challenge to undertake because of the inability to pharmacologically block multiple neural targets and the complexity of neural pathways transmitting visceral signals to the CNS.
MATERIALS AND METHODS
Subjects
15 adult male Long-Evans rats (Charles River Laboratories) weighing 460±40 g at the time of surgery were used in this study. Rats were individually housed in a climate-controlled animal colony maintained on a 12–12 light-dark cycle (lights on at 6:00) with food and water available ad libitum. All procedures were approved by the NIDA-IRP Animal Care and Use Committee and complied with the Guide for the Care and Use of Laboratory Animals (NIH, Publication 865–23). Maximal care was taken to minimize the number of experimental animals and any possible discomfort at all stages of the study.
Overview of the study
This study describes the results of two electrochemical experiments, in which we monitored changes in NAc oxygen levels induced by iv injections of either cocaine HCl or cocaine methiodide in awake, freely-moving rats.
Surgical preparations
Surgical procedures for the electrochemical experiments have been described in detail elsewhere.20, 34 In brief, under general anesthesia with Equithesin (a mixture of sodium pentobarbital and chloral hydrate), the rats were unilaterally implanted in the right NAc with a BASi guide cannula (Bioanalytical Systems, West Lafayette, IN, USA) into which an oxygen sensor was later inserted. In several rats, oxygen sensors were implanted chronically. Target coordinates of the recordings in the right NAc shell were: AP +1.2 mm, ML ±0.8 mm, and DV +7.6 mm from the skull surface, according to coordinates of the rat brain atlas.35 The guide cannula or the sensor was secured with dental acrylic in a head mount anchored to the skull. During the same surgical procedure, rats were also implanted with a chronic jugular catheter, which ran subcutaneously to the head mount and was secured to the same head assembly. Rats were allowed a minimum of 5 days of post-operative recovery and at least 3 daily habituation sessions (~6 h each) to the recording environment. Jugular catheters were flushed daily with 0.2 ml heparinized saline to maintain patency.
Electrochemical detection of oxygen
We used commercially produced oxygen sensors (Model 7002–02; Pinnacle Technology, Inc., Lawrence, KS, USA) that consisted of an epoxy-sheathed disc electrode that is grounded to a fine surface using a diamond-lapping disc. These sensors are prepared from Pt-Ir wire 180 μm in diameter, with a sensing area of 0.025 mm2 at the sensor’s tip. The active electrode is incorporated with an integrated Ag/AgCl reference electrode. Dissolved oxygen is reduced on the active surface of these sensors, which is held at a stable potential of −0.6 V vs. the reference electrode, producing an amperometric current. The current from the sensor is relayed to a computer via a potentiostat (Model 3104, Pinnacle Technology) and recorded at 1-s intervals using the PAL software utility (Pinnacle Technology).
Oxygen sensors were calibrated at 37 °C by the manufacturer (Pinnacle Technology) according to a standard protocol described elsewhere.36 The sensors produced incremental current changes with increases in oxygen concentrations within the wide range of previously reported brain oxygen concentrations (0–40 μM). Substrate sensitivity of oxygen sensors varied from 0.95–1.64 nA/μM (mean = 1.34 nA/μM). Oxygen sensors were also tested by the manufacturer for their selectivity toward other electroactive substances, including dopamine (0.4 μM) and ascorbate (250 μM), none of which had significant effects on reduction currents.
Experimental procedures
At the beginning of each experimental session, rats were minimally anesthetized (<2 min) with isoflurane and the sensor (either inserted through the guide cannula or chronically implanted) was connected to the potentiostat via an electrically shielded cable and multi-channel electrical swivel. The injection port of the jugular catheter on the head mount was connected to a plastic catheter extension that allowed drug delivery from outside the cage, thus minimizing detection of iv drug injections by the rat. Testing began two hours after connecting the sensor to the potentiostat when the baseline currents stabilized.
During each session, rats received three iv injections of either cocaine HCl (1 mg/kg) or cocaine methiodide at an equimolar dose (1.33 mg/kg) and were exposed once to a brief auditory stimulus (75 dB, 0.25 s). This stimulus was presented as the first event of a session before any drug injections. Both drugs were delivered in equal 0.3–0.4 ml volumes over a 30-s injection duration. Time intervals between injections were 120 min, typically enough for the restoration of pre-stimulus current baselines. At the end of each session, rats were anesthetized with Equithesin via iv catheter and disconnected from the potentiostat. Typically, rats underwent 1 to 2 recording sessions and upon completion they were euthanized by decapitation under deep isoflurane anesthesia. In rats with chronically implanted sensors, we conducted two recording sessions with each drug. The rat brains were histologically evaluated to verify sensor location and possible brain tissue damage. In this study, we did not test the effects of saline administration. As shown previously, iv saline injections conducted from outside of the chamber via the catheter extension has negligible effects on locomotor activity, cortical EEG, brain temperatures, and NAc glutamate levels.37, 38
Data analysis.
Electrochemical data were sampled at 1 Hz and analyzed with both slow (1-min) and rapid (4-s) time resolution. First, data were analyzed as raw currents. Because each individual sensor had slightly different substrate sensitivity in vitro, currents were then transformed into concentrations and represented as relative concentration changes with the pre-stimulus baseline current set to 100%. One-way repeated measure ANOVAs (followed by Fisher LSD post-hoc tests) were used to evaluate the statistical significance of drug-induced changes in oxygen levels. Two-way ANOVA was used to compare the differences in oxygen changes induced by two cocaine analogs.
Funding:
This research was supported by the Intramural Research Program of the NIH, NIDA (Project 1ZIADA000445-13)
REFERENCES
- [1].Heikkila RE, Orlansky H, and Cohen G. (1975) Studies on the distinction between uptake inhibition and release of (3H)dopamine in rat brain tissue slices, Biochem Pharmacol 24, 847–852. [DOI] [PubMed] [Google Scholar]
- [2].Ritz MC, Lamb RJ, Goldberg SR, and Kuhar MJ (1987) Cocaine receptors on dopamine transporters are related to self-administration of cocaine, Science 237, 1219–1223. [DOI] [PubMed] [Google Scholar]
- [3].De Wit H, and Wise RA (1977) Blockade of cocaine reinforcement in rats with the dopamine receptor blocker pimozide, but not with the noradrenergic blockers phentolamine or phenoxybenzamine, Can J Psychol 31, 195–203. [DOI] [PubMed] [Google Scholar]
- [4].Ettenberg A, Pettit HO, Bloom FE, and Koob GF (1982) Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems, Psychopharmacology (Berl) 78, 204–209. [DOI] [PubMed] [Google Scholar]
- [5].Roberts DC, and Koob GF (1982) Disruption of cocaine self-administration following 6-hydroxydopamine lesions of the ventral tegmental area in rats, Pharmacol Biochem Behav 17, 901–904. [DOI] [PubMed] [Google Scholar]
- [6].Wise RA, and Kiyatkin EA (2011) Differentiating the rapid actions of cocaine, Nat Rev Neurosci 12, 479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wise RA, and Bozarth MA (1987) A psychomotor stimulant theory of addiction, Psychol Rev 94, 469–492. [PubMed] [Google Scholar]
- [8].Wise RA, and Koob GF (2014) The development and maintenance of drug addiction, Neuropsychopharmacology 39, 254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Knuepfer MM, and Branch CA (1992) Cardiovascular responses to cocaine are initially mediated by the central nervous system in rats, J Pharmacol Exp Ther 263, 734–741. [PubMed] [Google Scholar]
- [10].Poon J, and van den Buuse M. (1998) Autonomic mechanisms in the acute cardiovascular effects of cocaine in conscious rats, Eur J Pharmacol 363, 147–152. [DOI] [PubMed] [Google Scholar]
- [11].Kiritsy-Roy JA, Halter JB, Gordon SM, Smith MJ, and Terry LC (1990) Role of the central nervous system in hemodynamic and sympathoadrenal responses to cocaine in rats, J Pharmacol Exp Ther 255, 154–160. [PubMed] [Google Scholar]
- [12].Dickerson LW, Rodak DJ, Kuhn FE, Wahlstrom SK, Tessel RE, Visner MS, Schaer GL, and Gillis RA (1999) Cocaine-induced cardiovascular effects: lack of evidence for a central nervous system site of action based on hemodynamic studies with cocaine methiodide, J Cardiovasc Pharmacol 33, 36–42. [DOI] [PubMed] [Google Scholar]
- [13].Kiyatkin EA, and Smirnov MS (2010) Rapid EEG desynchronization and EMG activation induced by intravenous cocaine in freely moving rats: a peripheral, nondopamine neural triggering, Am J Physiol Regul Integr Comp Physiol 298, R285–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wakabayashi KT, and Kiyatkin EA (2015) Central and peripheral contributions to dynamic changes in nucleus accumbens glucose induced by intravenous cocaine, Frontiers in neuroscience 9, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mogenson GJ, Jones DL, and Yim CY (1980) From motivation to action: functional interface between the limbic system and the motor system, Prog Neurobiol 14, 69–97. [DOI] [PubMed] [Google Scholar]
- [16].Wise RA (1989) The brain and reward, In The Neuropharmacological Basis of Reward (Liebman JM, and Cooper SJ, Eds.), pp 377–424, Oxford University Press, Oxford. [Google Scholar]
- [17].Fellows LK, and Boutelle MG (1993) Rapid changes in extracellular glucose levels and blood flow in the striatum of the freely moving rat, Brain Res 604, 225–231. [DOI] [PubMed] [Google Scholar]
- [18].Fox PT, and Raichle ME (1986) Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects, Proc Natl Acad Sci U S A 83, 1140–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, and Newman EA (2010) Glial and neuronal control of brain blood flow, Nature 468, 232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Solis E Jr., Cameron-Burr KT, and Kiyatkin EA (2017) Rapid Physiological Fluctuations in Nucleus Accumbens Oxygen Levels Induced by Arousing Stimuli: Relationships with Changes in Brain Glucose and Metabolic Neural Activation, Front Integr Neurosci 11, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kiyatkin EA, and Brown PL (2007) I.v. cocaine induces rapid, transient excitation of striatal neurons via its action on peripheral neural elements: single-cell, iontophoretic study in awake and anesthetized rats, Neuroscience 148, 978–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kiyatkin EA, and Rebec GV (1996) Dopaminergic modulation of glutamate-induced excitations of neurons in the neostriatum and nucleus accumbens of awake, unrestrained rats, J Neurophysiol 75, 142–153. [DOI] [PubMed] [Google Scholar]
- [23].Kiyatkin EA, and Rebec GV (1999) Modulation of striatal neuronal activity by glutamate and GABA: iontophoresis in awake, unrestrained rats, Brain Res 822, 88–106. [DOI] [PubMed] [Google Scholar]
- [24].Kiyatkin EA, and Lenoir M. (2012) Rapid fluctuations in extracellular brain glucose levels induced by natural arousing stimuli and intravenous cocaine: fueling the brain during neural activation, J Neurophysiol 108, 1669–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Brown PL, and Kiyatkin EA (2006) The role of peripheral Na(+) channels in triggering the central excitatory effects of intravenous cocaine, Eur J Neurosci 24, 1182–1192. [DOI] [PubMed] [Google Scholar]
- [26].Wakabayashi KT, and Kiyatkin EA (2012) Rapid changes in extracellular glutamate induced by natural arousing stimuli and intravenous cocaine in the nucleus accumbens shell and core, J Neurophysiol 108, 285–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fowler JS, Volkow ND, Logan J, Gatley SJ, Pappas N, King P, Ding YS, and Wang GJ (1998) Measuring dopamine transporter occupancy by cocaine in vivo: radiotracer considerations, Synapse 28, 111–116. [DOI] [PubMed] [Google Scholar]
- [28].Kiyatkin EA, Kiyatkin DE, and Rebec GV (2000) Phasic inhibition of dopamine uptake in nucleus accumbens induced by intravenous cocaine in freely behaving rats, Neuroscience 98, 729–741. [DOI] [PubMed] [Google Scholar]
- [29].Espana RA, Roberts DC, and Jones SR (2008) Short-acting cocaine and long-acting GBR-12909 both elicit rapid dopamine uptake inhibition following intravenous delivery, Neuroscience 155, 250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lee Y, Lee CH, and Oh U. (2005) Painful channels in sensory neurons, Mol Cells 20, 315–324. [PubMed] [Google Scholar]
- [31].Premkumar LS (2005) Block of a Ca(2+)-activated potassium channel by cocaine, J Membr Biol 204, 129–136. [DOI] [PubMed] [Google Scholar]
- [32].Wu SN, Chang HD, and Sung RJ (2006) Cocaine-induced inhibition of ATP-sensitive K+ channels in rat ventricular myocytes and in heart-derived H9c2 cells, Basic Clin Pharmacol Toxicol 98, 510–517. [DOI] [PubMed] [Google Scholar]
- [33].Chen YH, Lin CH, Lin PL, and Tsai MC (2006) Cocaine elicits action potential bursts in a central snail neuron: the role of delayed rectifying K+ current, Neuroscience 138, 257–280. [DOI] [PubMed] [Google Scholar]
- [34].Solis E Jr., Cameron-Burr KT, Shaham Y, and Kiyatkin EA (2017) Intravenous heroin induces rapid brain hypoxia and hyperglycemia that precede brain metabolic response, eNeuro 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Paxinos G, and Watson C. (1998) The rat brain in stereotaxic coordinates, 4th ed., Academic Press, San Diego. [Google Scholar]
- [36].Bolger FB, McHugh SB, Bennett R, Li J, Ishiwari K, Francois J, Conway MW, Gilmour G, Bannerman DM, Fillenz M, Tricklebank M, and Lowry JP (2011) Characterisation of carbon paste electrodes for real-time amperometric monitoring of brain tissue oxygen, J Neurosci Methods 195, 135–142. [DOI] [PubMed] [Google Scholar]
- [37].Kiyatkin EA, and Lenoir M. (2011) Intravenous saline injection as an interoceptive signal in rats, Psychopharmacology (Berl) 217, 387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wakabayashi KT, and Kiyatkin EA (2014) Critical role of peripheral drug actions in experience-dependent changes in nucleus accumbens glutamate release induced by intravenous cocaine, J Neurochem 128, 672–685. [DOI] [PMC free article] [PubMed] [Google Scholar]