Abstract
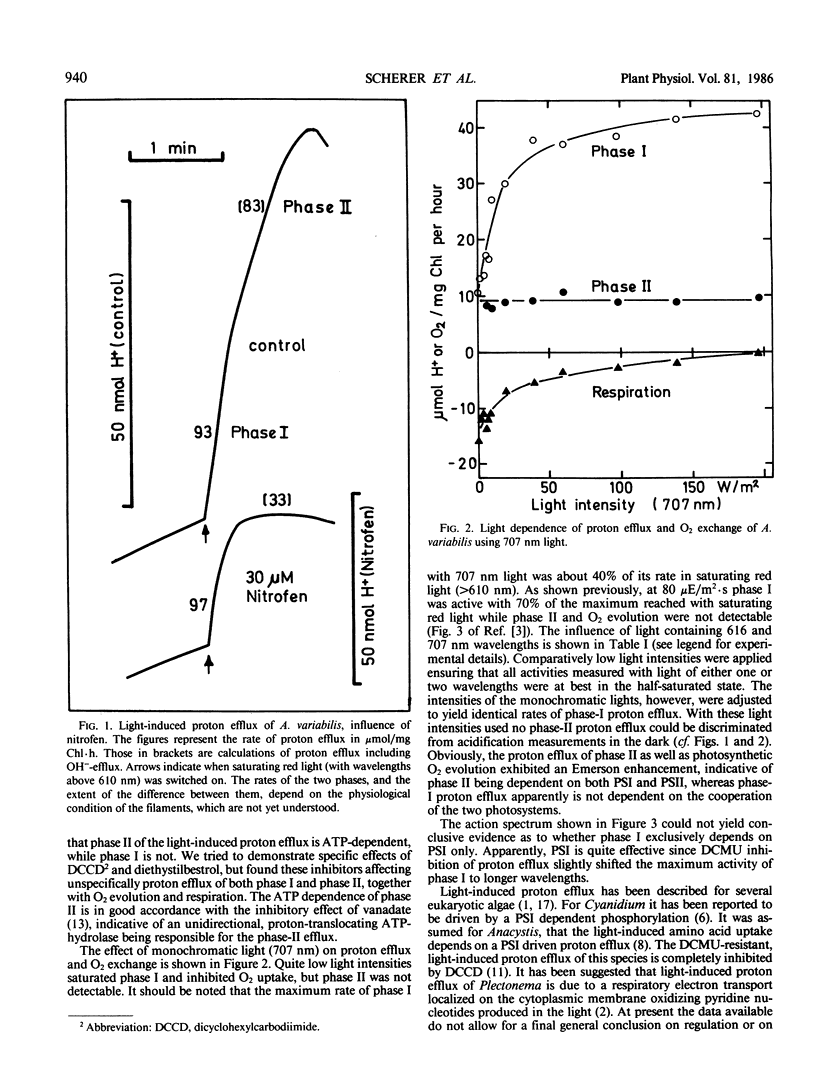
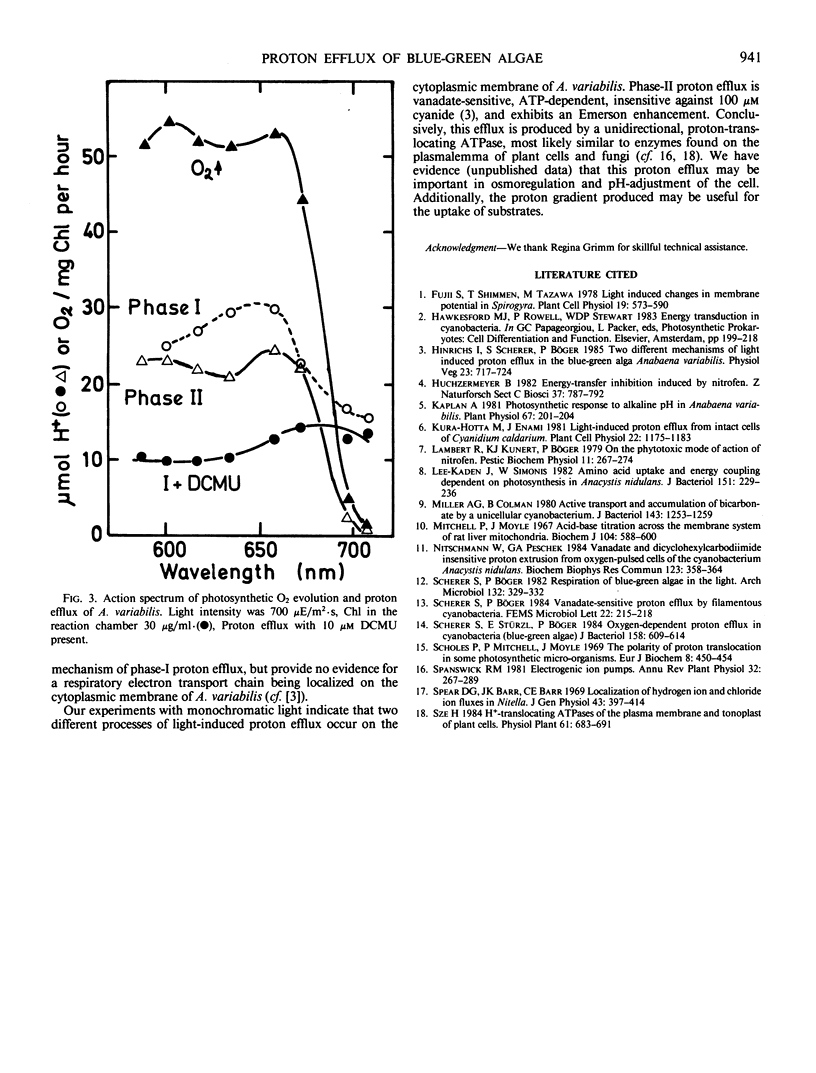
Light-induced proton efflux of Anabaena variabilis was found to be biphasic, the second phase being inhibited by the ATPase inhibitor nitrofen (2,4-dichloro-1-[4-nitrophenoxy]benzene). The first, fast phase was triggered by monochromatic light of 707 nanometers, whereas the second, slower phase was not. With 707 nanometers, light, respiratory O2 uptake was inhibited. Using light composed of two wavelengths (616 and 707 nanometers) a marked enhancement of both O2 evolution as well as the second phase of proton efflux was observed. The first phase was not enhanced. Thus, phase II is driven by both photosystems. As concluded from the action spectrum phase I is markedly determined by photosystem-I activity. Altogether the data show that two different mechanisms of light-induced proton efflux exist on the cytoplasmic membrane of Anabaena, the slower one being dependent on ATP and linear photosynthetic electron flow.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Kaplan A. Photosynthetic Response to Alkaline pH in Anabaena variabilis. Plant Physiol. 1981 Feb;67(2):201–204. doi: 10.1104/pp.67.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Kaden J., Simonis W. Amino acid uptake and energy coupling dependent on photosynthesis in Anacystis nidulans. J Bacteriol. 1982 Jul;151(1):229–236. doi: 10.1128/jb.151.1.229-236.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Colman B. Active transport and accumulation of bicarbonate by a unicellular cyanobacterium. J Bacteriol. 1980 Sep;143(3):1253–1259. doi: 10.1128/jb.143.3.1253-1259.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Acid-base titration across the membrane system of rat-liver mitochondria. Catalysis by uncouplers. Biochem J. 1967 Aug;104(2):588–600. doi: 10.1042/bj1040588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschmann W. H., Peschek G. A. Vanadate and dicyclohexylcarbodiimide insensitive proton extrusion from oxygen pulsed cells of the cyanobacterium Anacystis nidulans. Biochem Biophys Res Commun. 1984 Aug 30;123(1):358–364. doi: 10.1016/0006-291x(84)90421-2. [DOI] [PubMed] [Google Scholar]
- Scherer S., Stürzl E., Böger P. Oxygen-dependent proton efflux in cyanobacteria (blue-green algae). J Bacteriol. 1984 May;158(2):609–614. doi: 10.1128/jb.158.2.609-614.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholes P., Mitchell P., Moyle J. The polarity of proton translocation in some photosynthetic microorganisms. Eur J Biochem. 1969 Apr;8(3):450–454. doi: 10.1111/j.1432-1033.1969.tb00548.x. [DOI] [PubMed] [Google Scholar]
- Spear D. G., Barr J. K., Barr C. E. Localization of hydrogen ion and chloride ion fluxes in Nitella. J Gen Physiol. 1969 Sep;54(3):397–414. doi: 10.1085/jgp.54.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]


