Abstract
Background:
Hashimoto’s thyroiditis (HT) is the prevailing form of autoimmune thyroiditis and the leading cause of hypothyroidism in iodine-sufficient regions worldwide. This study aims to evaluate the efficacy of vitamin D supplementation on HT through a meta-analysis of randomized controlled trials (RCTs).
Methods:
The databases searched included PubMed, and others. We included RCTs that the treatment group received vitamin D, while the control group received either a placebo or no treatment. The studies measured the baseline and endpoint levels of 25-hydroxyvitamin D [25(OH)D], thyroid-stimulating hormone (TSH), free thyroxine (FT4), free triiodothyronine (FT3), anti-thyroid peroxidase antibody (TPO-Ab), and thyroglobulin antibody (TG-Ab). We performed a meta-analysis to calculate the standardized mean difference (SMD) and 95% confidence interval (CI).
Results:
A total of 12 studies involving 862 individuals were included. Vitamin D supplementation has a significant impact on reducing the titers of TPO-Ab (SMD = −1.084, 95% CI = −1.624 to −0.545) and TG-Ab (SMD = −0.996, 95% CI = −1.579 to −0.413) in patients with HT, and it also improves thyroid function by decreasing TSH level (SMD = −0.167, 95% CI = −0.302 to 0.031) and increasing FT3 (SMD = 0.549, 95% CI = 0.077–1.020) and FT4 (SMD = 0.734, 95% CI = 0.184–1.285) levels. Active vitamin D (calcitriol) significantly reduces the titer of TPO-Ab compared to naive forms of vitamin D (vitamin D2 or D3); treatment durations > 12 weeks result in a more effective reduction of TPO-Ab levels and a more significant increase in FT4 and FT3 levels in patients with HT (meta-regression P < .05).
Conclusion:
Vitamin D supplementation may have beneficial effects on HT patients by modulating immune responses and improving thyroid function.
Keywords: calcitriol, Hashimoto’s thyroiditis, meta-analysis, naive vitamin D, randomized controlled trial
1. Introduction
Hashimoto’s thyroiditis (HT) is the prevailing form of autoimmune thyroiditis and the leading cause of hypothyroidism in iodine-sufficient regions worldwide.[1] The etiology of HT remains uncertain but is currently attributed to genetic susceptibility and environmental factors, including excessive iodine intake, selenium deficiency, viral infections (such as hepatitis C virus), vitamin D deficiency, and others.[1–3]
The pathogenesis of HT involves a decrease in individual immune tolerance to thyroid autoantigens, which is influenced by genetic and environmental factors. Both cellular immunity and humoral immunity play a role in the development of HT. Infiltrating lymphocytes in thyroid tissue mainly consist of helper T lymphocytes (Th).[4] Th1 cells secrete interferon-γ (IFN-γ) and interleukin-2 (IL-2), which activate cytotoxic CD8 + T cells or macrophages to directly attack thyroid tissue. Th1 cells also secrete interleukin-1 (IL-1) and IFN-γ, which induce apoptosis of thyroid follicular cells.[4–7] Th2 cells, on the other hand, secrete interleukin-4 (IL-4), which inhibits the production of Th1 cells and the secretion of IFN-γ.[7] Nanba found that the balance between Th1 and Th2 cells is related to the severity of HT.[8] Studies have shown that levels of Th17 cells and their secreted cytokine interleukin-17 (IL-17) are significantly increased in HT patients. IL-17 has a negative correlation with thyroid fibrosis and can lead to hypothyroidism.[9,10] The role of regulatory T cells (Treg) is opposite to that of Th17 cells,[11,12] and the imbalance between Th17 and Treg cells is positively correlated with the severity of HT.[13] In addition to cellular immunity, humoral immunity also contributes to the development of HT. When thyroid tissue is damaged, a large number of antigens are released, leading to the production of TPO-Ab and TG-Ab. TPO-Ab can directly destroy thyroid tissue through cytotoxicity and is associated with the occurrence of hypothyroidism.[14,15] TG-Ab, on the other hand, does not directly damage thyroid cells.[15] Serum levels of TPO-Ab and TG-Ab are considered the best markers for the diagnosis of HT.
Vitamin D functions as a steroid hormone, predominantly synthesized in the skin through the activation of 7-dehydroxycholesterol upon exposure to sunlight. Dietary intake contributes minimally to circulating vitamin D levels. Currently, the assessment of vitamin D status primarily relies on measuring serum 25(OH)D, with thresholds set as follows: > 30 ng/mL for sufficiency, 20 to 30 ng/mL for insufficiency, < 20 ng/mL for deficiency, and < 10 ng/mL for severe deficiency. As the regulatory role of vitamin D in the immune system has gained recognition, researchers have investigated its relationship with HT. In HT patients, serum 25(OH)D levels were consistently lower compared to healthy individuals, with a higher prevalence of vitamin D deficiency or insufficiency.[16–18] Notably, Mazokopakis,[19] Fang[20] reported a negative correlation between serum 25(OH)D levels and serum TPO-Ab titers in HT patients with normal thyroid function. Similarly, other researchers found an inverse relationship between serum 25(OH)D and thyroid-stimulating hormone (TSH) levels in HT patients.[18,21]
Vitamin D plays a pivotal role in balancing pro-inflammatory cells such as Th1 and Th17, and anti-inflammatory cells such as Th2 and Tregs. It also exerts inhibitory effects on B cell proliferation and antigen presentation,[22–29] which potentially underlie its therapeutic potential in HT. However, the impact of vitamin D supplementation on HT remains a topic of debate. A meta-analysis of 25 observational studies conducted in 2020 suggested that the serum 25(OH)D concentration in HT patients was significantly lower than that in healthy controls, and the odds ratio of vitamin D deficiency in HT patients was 3.21.[30] Vitamin D supplementation could improve thyroid function, reduce TPO-Ab or TG-Ab titers,[19,31] reduce the Th17/Treg ratio,[32] and downregulate the production of IL-17, while promoting the synthesis of Interleukin-10 (IL-10) which inhibits Th1 production.[33–35] Conversely, some studies have reported no significant reduction in TPO-Ab and TG-Ab titers, nor improvements in thyroid function with vitamin D supplementation in HT patients.[36]
However, the current meta-analyses on vitamin D supplementation in HT have several limitations, including non-randomized controlled trials and limited literature availability.[37–41] Additionally, these meta-analyses did not consider baseline thyroid function, 25(OH)D levels, different types of vitamin D, treatment duration, or employ meta-regression analysis to explore significant factors contributing to heterogeneity. This study aims to incorporate the latest randomized controlled trials (RCTs) on vitamin D intervention in HT to address the following crucial questions: To determine whether vitamin D is beneficial to the improvement of HT. If proven effective, further clarify the appropriate type, dosage and treatment duration of vitamin D supplementation. Understanding the impact of baseline thyroid function and vitamin D levels on the ultimate outcome to determine the appropriate subjects for treatment.
2. Methods
2.1. Search trials
The study was registered into PROSPERO (CRD42023445848). A comprehensive search strategy adhering to the PICOS principle was employed to identify pertinent RCTs published from inception to August 2023. Databases such as PubMed, Embase, Cochrane Library, Web of Science, China Knowledge Network, Wanfang, VIP Database, and China Biomedical Literature Database were meticulously scoured. The search terms encompassed “Hashimoto Disease” and “Vitamin D” or “Cholecalciferol,” “Ergocalciferols,” “Calcifediol,” “Calcitriol,” or “Hydroxycholecalciferols,” combined with “Randomized controlled trial” in English or Chinese.
2.2. Inclusion and exclusion criteria
Inclusion criteria were diligently applied as follows: RCTs investigating the effects of vitamin D supplementation in patients diagnosed with HT; the treatment arm encompassing various forms of vitamin D supplementation (including vitamin D2, vitamin D3, or calcitriol), while the control group received either a placebo (e.g., paraffin oil) or no treatment, with concomitant thyroid hormone replacement therapy based on thyroid function; and thorough assessment of baseline and endpoint levels of 25(OH)D, thyroid function indicators (TSH, FT3, FT4), and autoantibodies (TPO-Ab, TG-Ab). Exclusion criteria encompassed: the inclusion of patients with autoimmune disorders other than HT; duplicate studies, reviews, conference abstracts, lectures, unpublished data, and personal communications; studies with incomplete data concerning the aforementioned outcome measures; and non-RCTs or animal studies.
2.3. Risk-of-bias assessment
Two researchers independently conducted an in-depth evaluation of the included literature’s quality employing Review Manager 5.3 software. Adhering to the Cochrane risk-of-bias tool,[42] each quality item underwent meticulous classification as low risk, high risk, or unclear risk. The 7 criteria employed to gauge bias in each trial encompassed random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and any other potential sources of bias.
2.4. Data extraction
Two researchers extracted the following key information from each study: author’s name, publication year, country of origin, sample size, pertinent demographic characteristics of the subjects, type of vitamin D administered, dosage of vitamin D, duration of vitamin D treatment, baseline and post-intervention levels of TPO-Ab, TG-Ab, TSH, FT4, FT3, and 25(OH)D, and documentation of any reported adverse drug reactions.
2.5. Statistical analysis
(1) Data Processing: Continuous variables were expressed in a standardized manner as mean ± standard deviation (SD). Whenever studies presented data in the form of standard error, conversion to SD was performed, while non-normally distributed data reported as median (P25, P75) were transformed into mean ± SD utilizing an online calculator developed by Luo et al.[43] The differences in levels and their corresponding standard deviations between baseline and endpoint measurements for TPO-Ab, TG-Ab, TSH, FT3, FT4, and 25(OH)D were calculated for each study.
(2) The comparison of continuous variables between the treatment and control groups was conducted utilizing Stata 15 software. Standardized mean difference (SMD) accompanied by their corresponding 95% CI were calculated, and the results were elegantly presented using forest plots.
(3) Publication bias was assessed via the construction of a funnel plot using Stata 15 software. Subsequent bias testing, employing Egger’s test, was conducted, and if any indication of publication bias emerged, a suitable adjustment using the trim-and-fill method was performed.
(4) Heterogeneity among the included studies was evaluated using the I2 statistic, whereby an I2 value exceeding 50% indicated substantial heterogeneity, 25% to 50% indicated moderate heterogeneity, and below 25% implied insignificant heterogeneity. A fixed-effects model was employed for I2 < 50%, whereas a random-effects model was utilized for I2 > 50%. Additionally, sensitivity analysis using Stata 15 software was conducted to assess the robustness of the pooled results. Subgroup analysis and meta-regression were carried out to identify significant factors contributing to the observed heterogeneity.
3. Results
3.1. Retrieved studies and characteristics
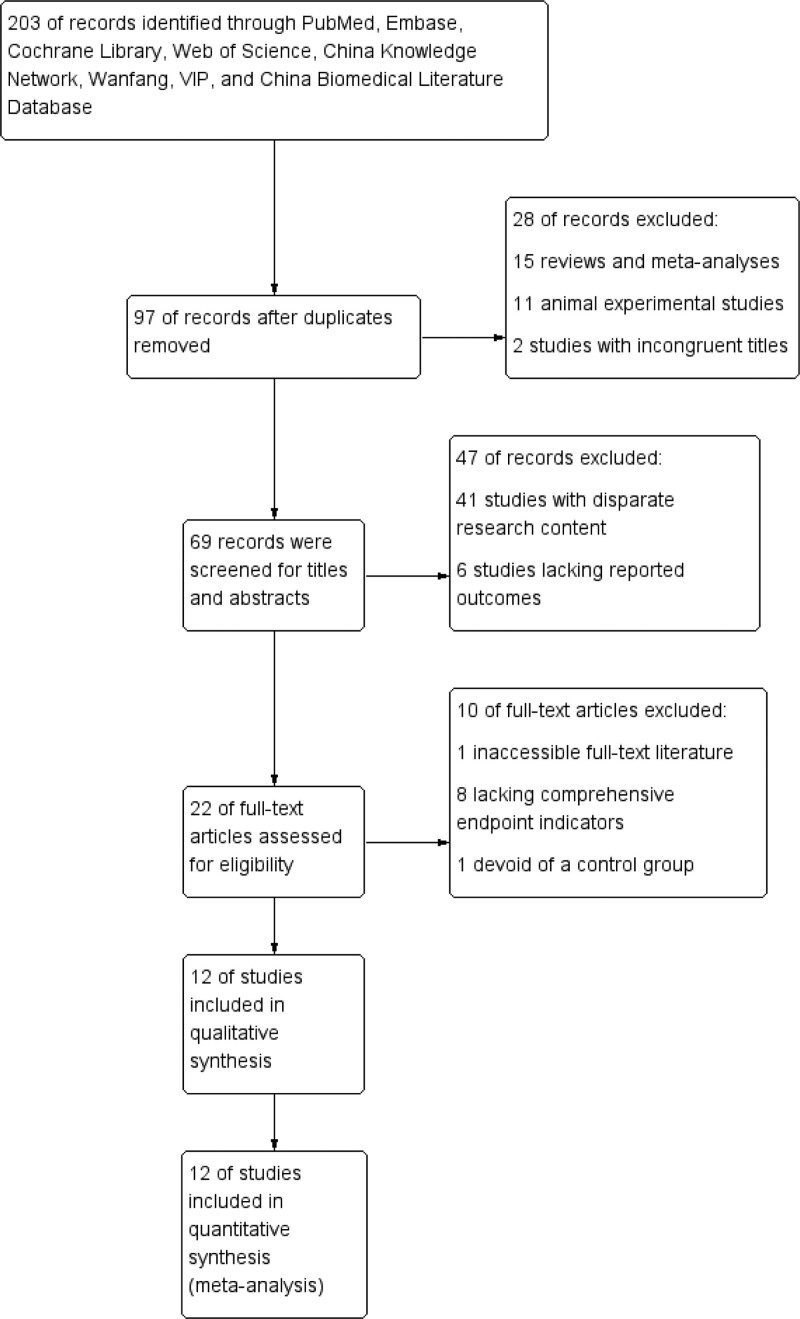
A total of 203 potentially relevant studies were retrieved through a comprehensive search. Following a rigorous screening process, 106 redundant studies, 15 reviews and meta-analyses, 11 animal experimental studies, and 2 studies with incongruent titles were systematically excluded. Subsequently, the titles and abstracts of the remaining 69 studies underwent scrutiny, resulting in the exclusion of 41 studies with disparate research content and 6 studies lacking reported outcomes. Further exclusions were made, including 1 study with inaccessible full-text literature, 8 studies lacking comprehensive endpoint indicators, and 1 study devoid of a control group. Ultimately, a refined selection of 12 RCTs[31,33,34,36,44–51] were deemed suitable for inclusion in this meta-analysis. A graphical representation of the screening process is eloquently presented in Figure 1.
Figure 1.
Literature search and screening process.
3.2. Participants
A total of 862 patients diagnosed with HT were enrolled across the 12 selected RCTs, with 429 patients in treatment group and 423 in control group. Remarkably, no statistically significant differences in baseline values of all variables were observed between the treatment and control group. Among the included studies, 7 studies explicitly recruited patients with hypothyroidism,[31,36,46–48,50,51] while the remaining studies recruited patients with euthyroidism or subclinical hypothyroidism.[33,34,44,45,49] 5 studies were designed to address vitamin D deficiency,[36,44–46,49] 4 studies targeted subjects with vitamin D insufficiency,[33,34,48,51] and the inclusion criteria of the remaining 3 studies did not explicitly delineate the vitamin D status of the enrolled patients.[31,47,50] For comprehensive elucidation, please refer to Table 1.
Table 1.
Characteristics of the included trials and participants.
| Included trials | Country | Total participants (T/C)* | Baseline thyroid function | Baseline vitamin D status† | Baseline 25(OH)D levels (T vs C) |
|---|---|---|---|---|---|
| Huang, 2013[51] | China | 32 (16/16) | Hypothyroidism | Insufficiency | 15.86 ± 3.56 vs 16.50 ± 2.81 ng/mL |
| Han et al, 2015[50] | China | 62 (32/30) | Hypothyroidism | Not restricted | 17.91 ± 6.59 vs 18.22 ± 5.19 ng/mL |
| Zhao et al, 2016[48] | China | 36 (18/18) | Hypothyroidism | Insufficiency | 16 ± 4 vs 16 ± 3 µg/L |
| Parichehr Vahabi Anaraki et al, 2017[36] | Iran | 65 (33/32) | Hypothyroidism | Deficiency | 12.76 ± 0.74 vs 13.28 ± 0.86 ng/mL |
| Zhou et al, 2018[49] | China | 122 (61/61) | Euthyroidism or Subclinical Hypothyroidism | Deficiency | 29.50 ± 19.65 vs NR‡ nmol/L |
| Zuo et al, 2018[45] | China | 70 (35/35) | Euthyroidism | Deficiency | 10.61 ± 1.30 vs 13.58 ± 1.18 nmol/L |
| Fu et al, 2019[34] | China | 70 (36/34) | Euthyroidism or Subclinical Hypothyroidism | Insufficiency | 15.08 ± 3.23 vs 13.99 ± 2.32 µg/L |
| Reza Chahardoli et al, 2019[31] | Iran | 42 (21/21) | Hypothyroidism | Not restricted | 25.38 ± 11.02 vs 19.80 ± 8.81 ng/mL |
| Jia, 2020[47] | China | 102 (51/51) | Hypothyroidism | Not restricted | 17.89 ± 6.38 vs 19.10 ± 6.51 ng/mL |
| Xiao et al, 2020[44] | China | 80 (40/40) | Euthyroidism | Deficiency | 14.60 ± 5.08 vs 15.29 ± 6.88 ng/mL |
| Zhang, 2020[46] | China | 90 (45/45) | Hypothyroidism | Deficiency | 17.16 ± 6.45 vs 18.24 ± 4.36 ng/mL |
| Zhao, 2022[33] | China | 98 (49/49) | Euthyroidism or Subclinical Hypothyroidism | Insufficiency or Deficiency | 15.64 ± 5.54 vs 16.98 ± 5.43 ng/mL |
T: Treatment Group, C: Control Group.
Vitamin D Status: > 30 ng/mL (75 nmol/L) for sufficiency, 20–30 ng/mL (50–75 nmol/L) for insufficiency, < 20 ng/mL (50 nmol/L) for deficiency.
Not reported.
3.3. Intervention
In the context of patients diagnosed with hypothyroidism and HT, the intervention strategy comprised the administration of vitamin D in conjunction with thyroxine tablets. Specifically, 6 studies[31,46–48,50,51] employed calcitriol in tandem with thyroxine tablets, while 1 study[36] utilized vitamin D3 alongside thyroxine tablets. Conversely, the control groups were managed with thyroxine monotherapy or thyroxine combined with a placebo. In patients exhibiting normal thyroid function or subclinical hypothyroidism, the treatment groups exclusively received vitamin D monotherapy. Notably, among these studies, 2[34,45] employed calcitriol, 2[44,49] employed vitamin D2, and 1[33] employed vitamin D3, while the control groups were administered a placebo or received no specific treatment. The follow-up duration for 6 studies[33,45–48,50] >12 weeks, while the remaining 6 studies[31,34,36,44,49,51] adhered to a follow-up duration ≤ 12 weeks. For comprehensive details, kindly refer to Table 2.
Table 2.
Characteristics of the included trials and participants.
| Included trials | Treatment group | Control group | ||||||
|---|---|---|---|---|---|---|---|---|
| Treatment durations (weeks*) | Adverse drug reactions | Age (yr) | NO. (M/F) | Intervention | Age (yr) | NO. (M/F) | Intervention | |
| Huang, 2013[51] | 12 | NO | 45.3 ± 6.8 | 16 (2/14) | Thyroxine + Calcitriol 0.25µg, qd, p.o | 43.7 ± 8.6 | 16 (3/13) | Thyroxine |
| Han et al, 2015[50] | 24 | NR† | 34.8 ± 8.2 | 32 (5/27) | Thyroxine + Calcitriol 0.25µg, qd, p.o | 35.2 ± 7.1 | 30 (4/26) | Thyroxine |
| Zhao et al, 2016[48] | 24 | NO | 42 ± 7 | 18 (5/13) | Thyroxine + Calcitriol 0.25µg, qd, p.o | 41 ± 5 | 18 (4/14) | Thyroxine |
| Parichehr Vahabi Anaraki et al, 2017[36] | 12 | NO | 43.55 ± 8.54 | 30 (9/21) | Thyroxine + Vitamin D3 50000IU, qw, p.o | 44.12 ± 8.54 | 26 (11/15) | Thyroxine + Placebo |
| Zhou et al, 2018[49] | 12 | NR | 20–70 | 61 (NR) | Vitamin D2 7.5mg, qm, i.m | 20–70 | 61 (NR) | NO |
| Zuo et al, 2018[45] | 24 | NO | 44.3 ± 2.1 | 35 (6/29) | Calcitriol 0. 25µg, qd, p.o | 42.8 ± 2.0 | 35 (5/30) | NO |
| Fu et al, 2019[34] | 12 | NO | 49.56 ± 7.88 | 36 (2/34) | Calcitriol 0. 25µg, qd, p.o | 47.11 ± 11.42 | 34 (2/32) | NO |
| Reza Chahardoli et al, 2019[31] | 12 | NO | 36.4 ± 5.2 | 19 (0/19) | Thyroxine + Calcitriol 50000IU, qw, p.o | 35.9 ± 7.8 | 21 (0/21) | Thyroxine + Placebo |
| Jia, 2020[47] | 16 | Nausea, vomiting, and rash‡ | 41.96 ± 9.28 | 51 (22/29) | Thyroxine + Calcitriol 0.25µg, qd, p.o | 42.20 ± 8.98 | 51 (20/31) | Thyroxine |
| Xiao et al, 2020[44] | 12 | NR | 45.02 ± 7.11 | 40 (13/27) | Vitamin D2 7.5mg, qm, i.m | 46.38 ± 7.69 | 40 (11/29) | NO |
| Zhang, 2020[46] | 24 | NO | 35.2 ± 7.6 | 45 (20/25) | Thyroxine + Calcitriol 0.25µg,qd, p.o | 34.4 ± 6.3 | 45 (16/29) | Thyroxine |
| Zhao, 2022[33] | 13–15 | NR | 36.55 ± 10.21 | 46 (NR) | Vitamin D3 800-2000§ IU, qd, p.o | 36.55 ± 9.72 | 46 (NR) | NO |
1 month = 4 weeks.
Not reported.
In treatment group, there were 2 cases of nausea, 2 cases of vomiting, and 1 case of rash; in the control group, there were 2 cases of nausea, 1 case of vomiting, and 0 cases of rash. However, there was no significant difference in the incidence of adverse reactions between the treatment group and the control group.
Supplement vitamin D3 at 2000 IU/day when 25(OH)D was < 20 ng/mL, and at 800–1000 IU/d when 20 ng/mL ≤ 25(OH)D < 30 ng/mL.
3.4. Adverse drug reactions
Among the 12 included studies, 4 studies[33,44,49,50] did not report any adverse reactions. Among the remaining 8 studies,[31,34,36,45–48,51] none documented the incidence of hypercalcemia, and 7 studies[31,34,36,45,46,48,51] unequivocally reported an absence of adverse events. Notably, only 1 study[47] documented adverse reactions, specifically nausea, vomiting, and rash; however, it is worth highlighting that no statistically significant difference in the incidence of adverse reactions was discerned between the treatment and control cohorts.
3.5. Literature quality evaluation
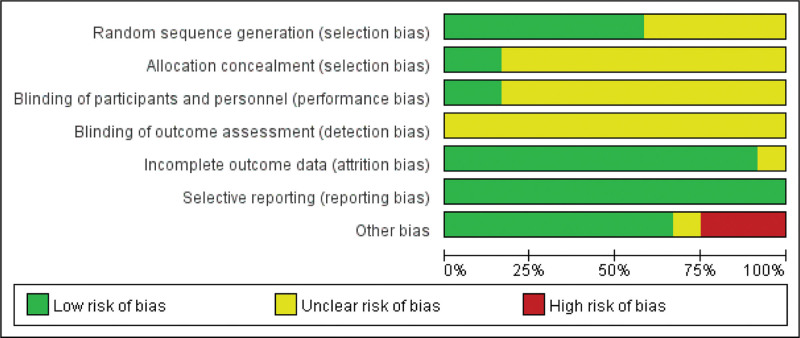
A meticulous appraisal was conducted to ascertain the quality and reliability of the included literature. The 12 included studies randomly assigned patients with HT into treatment and control groups. 7 studies[31,33,34,36,46–48] expounded upon the generation method of the random sequence, employing techniques such as the random number table method, thus exhibiting a commendable endeavor to low bias risks. Additionally, 2 studies[31,36] explicitly disclosed the concealment of the allocation method, low risk bias was considered. Furthermore, 2 studies[31,36] implemented blinding protocols for both researchers and subjects, considering low risk bias. None of the studies mentioned blinding of outcome assessors, resulting in an unclear risk of measurement bias. 1 study[36] had a relatively high rate of loss to follow-up, making it uncertain whether missing data influenced the results. In contrast, the remaining 11 studies reported minimal or no missing data, with researchers duly acknowledging and addressing this matter in their respective publications, indicating a low risk of follow-up bias. All studies reported on the predetermined variables, considering low risk reporting bias. However, it is noteworthy that 1 study[48] expressed vitamin D and autoantibody levels in integers, raising concerns about accuracy, and the other bias risks were unclear. 1 study[49] documented an identical increase in 25(OH)D level as the pretreatment 25(OH)VitD3 level, engendering doubts regarding potential recording errors, considering high other biases. The unit of TG-Ab level in 2 studies[47,50] was expressed as a percentage, casting uncertainty on the data’s precision and raising concerns of high other biases. Please refer to Figures 2 and 3 for the risk of bias assessment.
Figure 2.
Overall risk of bias assessment results for included studies.
Figure 3.
Overall risk of bias assessment results for included studies.
3.6. Results calculate
The alterations in TPO-Ab, TSH, and 25(OH)D levels among HT patients before and after vitamin D treatment were thoroughly assessed in all 12 studies. Additionally, TG-Ab levels were evaluated in 11 studies,[31,33,34,44–51] while changes in FT3 and FT4 levels following vitamin D treatment were examined in 10 studies.[31,33,34,44,46–51] Zhao[33] conducted an randomized controlled trial (RCT) investigating vitamin D intervention in HT patients afflicted with both vitamin D insufficiency and vitamin D deficiency. Due to the limited sample size of patients with vitamin D deficiency, the 2 subgroups were amalgamated into a single group to calculate. The mean ± SD between baseline and endpoint measurements of TPO-Ab, TG-Ab, TSH, FT3, and FT4 were calculated in each study. The comprehensive findings are elucidated in Table 3.
Table 3.
Effects of vitamin D supplementation on thyroid autoantibody and thyroid function in patients with HT (T vs C).*
| Included trials | TPO-Ab (U/mL) | TG-Ab (U/mL) | TSH (mIU/L†) | FT4 | FT3 |
|---|---|---|---|---|---|
| Huang, 2013[51] | −208.81 ± 141.28 vs −84.51 ± 149.82 | −417.07 ± 452.69 vs −142.11 ± 375.4 | −87 ± 15 vs −76.4 ± 25.08 | 10.9 ± 2.72 vs 9.98 ± 2.87 pmol/L | 2.9 ± 1.04 vs 2.6 ± 1.01 pmol/L |
| Han et al, 2015[50] | −408.04 ± 61.46 vs −222.76 ± 66.39 | −55 ± 7.55 vs −31.3 ± 7.75 | −18.19 ± 7.05 vs −15.41 ± 6.48 µIU/L | 0.85 ± 0.23 vs 0.36 ± 0.28 ng/dL | 2.1 ± 0.69 vs 0.97 ± 0.79 pg/mL |
| Zhao et al, 2016[48] | −224 ± 115.05 vs −97 ± 119.53 | −270 ± 316.6 vs −178 ± 284.23 | −77.7 ± 12.91 vs −78.6 ± 11.67 | 9.8 ± 2.72 vs 9.5 ± 2.26 pmol/L | 2.2 ± 1.31 vs 1.8 ± 0.89 pmol/L |
| Parichehr Vahabi Anaraki et al, 2017[36] | −86.25 ± 553.12 vs −88.04 ± 532.1 | NR‡ | 0.58 ± 3.92 vs −0.79 ± 2.08 | NR | NR |
| Zhou et al, 2018[49] | −41.34 ± 130.09 vs 32.14 ± 103.29 | −48.87 ± 186.7 vs 35.22 ± 170.1 | −0.82 ± 2.92 vs −0.12 ± 3.41 | −0.16 ± 1.46 vs −0.02 ± 1.23 pmol/L | 0.1 ± 1.3 vs 0.25 ± 1.5 pmol/L |
| Zuo et al, 2018[45] | −10 ± 6.26 vs 2 ± 10.82 | −11 ± 15.72 vs 1 ± 8.99 | 0.19 ± 0.41 vs 0.09 ± 0.62 | NR | NR |
| Fu et al, 2019[34] | −241.13 ± 237.24 vs 27.49 ± 350.7 | −79.77 ± 86.55 vs 12.3 ± 151.12 | −0.36 ± 1.96 vs 0.42 ± 1.34 | 0.5 ± 3.01 vs 0.17 ± 1.4 pmol/L | 0.12 ± 0.55 vs 0.24 ± 0.55 pmol/L |
| Reza Chahardoli et al, 2019[31] | −13.3 ± 103.32 vs 7.5 ± 133.2 | −52.4 ± 149.95 vs −5.8 ± 160.91 | −1.17 ± 1.84 vs 0.21 ± 1.7 | −0.65 ± 1.71 vs −1.0 ± 1.8 µg/dL | 0 ± 0.35 vs −0.01 ± 0.36 ng/mL |
| Jia, 2020[47] | −358.09 ± 60.43 vs −296.97 ± 61.26 | −55.09 ± 7.36 vs −41.31 ± 7.16 | −36.39 ± 9.67 vs −36.04 ± 10.52 | 11.84 ± 1.87 vs 8.92 ± 1.68 pmol/L | 3.81 ± 0.52 vs 3.02 ± 0.57 pmol/L |
| Xiao et al, 2020[44] | −212.92 ± 2126.76 vs 247.17 ± 2309.03 | −41.73 ± 319.69 vs −36.4 ± 167.9944 | 0.05 ± 0.84 vs 0.25 ± 1.34 | 0.04 ± 0.22 vs 0.06 ± 0.19 ng/dL | −0.04 ± 0.48 vs −0.04 ± 0.39 pg/mL |
| Zhang, 2020[46] | −409.47 ± 48.72 vs −231.43 ± 39.08 | −53.48 ± 7.39 vs −32.84 ± 7.3 | −18.65 ± 6.36 vs −17.26 ± 5.35 | 0.89 ± 0.22 vs 0.37 ± 0.26 pmol/L | 2.19 ± 0.64 vs 1.03 ± 0.7 pmol/L |
| Zhao, 2022[33] | −116.61 ± 913.49 vs 120.11 ± 1179.07 | −84.15 ± 638.56 vs −164.43 ± 727.41 | 0.15 ± 2.47 vs 0.45 ± 1.89 | 1.11 ± 3.01 vs −1.72 ± 2.4 pmol/L | 0.1 ± 0.75 vs −0.17 ± 0.48 pmol/L |
T: Treatment Group, C: Control Group.
Except for Han et al in 2015,[50] TSH units were reported in mIU/L or µIU/mL. It should be noted that mIU/L is equivalent to µIU/mL.
Not reported.
3.7. Effects of vitamin D supplementation on TPO-Ab, TG-Ab, and thyroid function in patients with HT
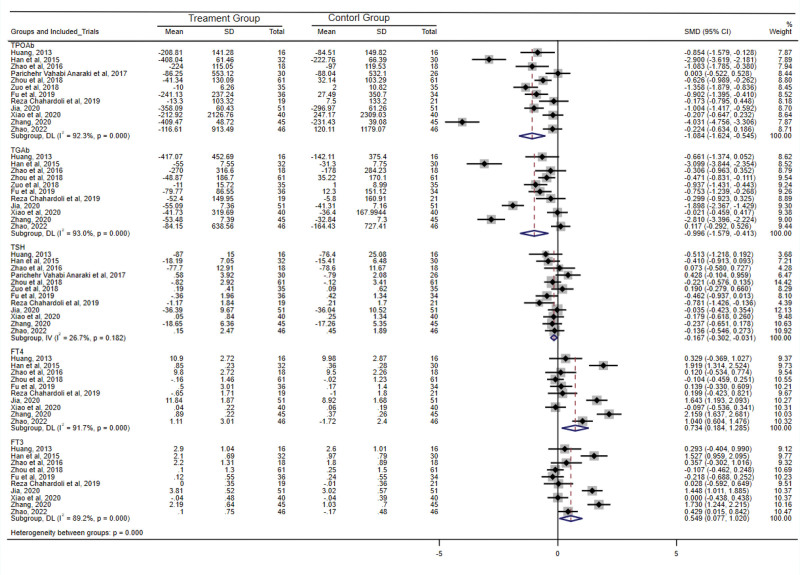
Among the 12 studies included in this analysis, 10 studies[31,33,34,45–51] consistently reported a significantly greater reduction in TPO-Ab titers within the treatment group compared to the control group. Meta-analysis of all 12 studies revealed substantial heterogeneity (I2 = 92.3%, Q test P < .001). Employing a random-effects model, the SMD and 95% CI were −1.084 (−1.624, −0.545), signifying a statistically significant difference (P < .001). These findings suggested that vitamin D supplementation exerts a considerable reduction in TPO-Ab titers among patients with HT. Sensitivity analysis further validated the consistent reduction in TPO-Ab titers following vitamin D supplementation in this patient population. Notably, both the funnel chart and Egger’s test (P = .06) revealed the absence of significant publication bias.
Within the 12 studies included, 1 study[36] did not assess TG-Ab levels, while 9 studies[31,34,45–51] consistently reported greater decrease in TG-Ab titers in the treatment group compared to the control group. Meta-analysis of the 11 studies assessing TG-Ab revealed substantial heterogeneity (I2 = 93.0%, Q test P < .001). Utilizing a random-effects model, the SMD and 95% CI were calculated as −0.996 (−1.579, −0.413), indicating a statistically significant difference (P = .001). These findings suggested that significant reduction in TG-Ab titers associated with vitamin D supplementation among patients with HT. Sensitivity analysis further supported the consistent decrease in TG-Ab titers in patients receiving vitamin D supplementation. Both the funnel chart and Egger’s test (P = .15) indicated the absence of significant publication bias.
Twelve studies analyzed, 3 studies[46,47,50] reported a noteworthy difference in the reduction of TSH levels between the treatment and control groups following intervention. The results demonstrated no substantial heterogeneity (I2 = 26.7%, Q test P = .18) between the 12 studies. Employing a fixed-effects model, the SMD and 95% CI were calculated as −0.167 (−0.302, −0.031), suggesting that vitamin D supplementation significantly decreases TSH levels in patients with HT. A sensitivity analysis was performed, and the results indicated that the combined effect size of the remaining studies after sequentially excluding each study did not reach statistical significance. This suggested that although the included studies exhibited low heterogeneity, the Meta-analysis results were unstable. Both the funnel chart and Egger’s test (P = .58) indicated the absence of significant publication bias.
Of the 12 studies, 2 studies[36,45] did not evaluate FT4 levels, while the remaining 10 studies underwent meta-analysis. The findings revealed substantial heterogeneity (I2 = 91.7%, Q test P < .001). Employing a random-effects model, the SMD and 95% CI were calculated as 0.734 (0.184, 1.285) with a statistically significant (P = .009), indicating that vitamin D supplementation significantly increases FT4 levels in patients with HT. Sensitivity analysis confirmed the stability of the result. Both the funnel chart and Egger’s test (P = .50) suggested the absence of significant publication bias.
Similarly, among the 12 studies, 2 studies[36,45] did not assess FT3 levels, while the remaining 10 studies underwent meta-analysis. The results indicated significant heterogeneity (I2 = 89.2%, Q test P < .001). Using a random-effects model, the SMD and 95% CI were calculated as 0.549 (0.077, 1.020) with a statistically significant (P = .02), demonstrating that vitamin D supplementation significantly elevates FT3 levels in patients with HT. A sensitivity analysis was conducted, and the results revealed that the combined effect size of the remaining studies, after sequentially excluding each study, did not exhibit significant statistical significance. This indicates that despite the low heterogeneity observed in the included studies, the Meta-analysis results were unstable. Both the funnel chart and Egger’s test (P = .61) indicated the absence of significant publication bias. For a comprehensive overview, please refer to Figure 4.
Figure 4.
Meta-analysis results of effects of vitamin D supplementation on anti-thyroid peroxidase antibody (TPO-Ab), thyroglobulin antibody (TG-Ab), and thyroid function in patients with Hashimoto’s thyroiditis (HT). CI = confidence interval, FT3 = free triiodothyronine, FT4 = free thyroxine, SD = standard deviation, SMD = standardized mean difference, TSH = thyroid-stimulating hormone.
3.8. Effects of treatment duration, vitamin D types, baseline thyroid function and vitamin D levels on the efficacy of vitamin D therapy in HT
Considerable heterogeneity emerged when examining the impact of the treatment group versus the control group on TPO-Ab, TG-Ab, FT4, and FT3 in patients afflicted with HT. This observed heterogeneity can be attributed to various factors, including environmental variables, racial diversity, somatotype variations, baseline vitamin D concentrations, baseline thyroid function levels, as well as the type, dosage, and duration of vitamin D supplementation.
To delve deeper into the aforementioned factors, subgroup analyses were conducted, followed by meta-regression analyses employing Stata15 software. The subgroups were delineated as follows: based on the duration of vitamin D supplementation, categorized as either ≤ 12 weeks or > 12 weeks; based on the types of vitamin D supplementation, distinguished between vitamin D2 or D3 and active calcitriol; based on baseline thyroid function, classified as hypothyroidism or normal thyroid function/subclinical hypothyroidism; and based on baseline vitamin D levels, separated into vitamin D insufficiency, vitamin D deficiency, or indeterminate. The specifics are elucidated in Table 4.
Table 4.
Effects of treatment duration, vitamin D types, baseline thyroid function, and vitamin D levels on the efficacy of vitamin D therapy in HT.
| Included trials | Weight% | SMD (95% CI) | P value | I2% | Meta-regression P value | |
|---|---|---|---|---|---|---|
| TPO-Ab | ||||||
| Duration of vitamin D supplementation | ||||||
| ≤12 wk | 6[31,34,36,44,49,51] | 50.45 | −0.454 (−0.749, −0.160) | .002 | 50.5 | .03 |
| >12 wk | 6[33,45–48,51] | 49.55 | −1.741 (−2.750, −0.732) | .001 | 95.1 | |
| Type of vitamin D | ||||||
| Naive vitamin D (vitamin D2 or D3) | 4[33,36,44,49] | 34.60 | −0.300 (−0.566, −0.034) | .027 | 35.2 | .046 |
| Calcitriol | 8[31,34,45–48,50,51] | 65.40 | −1.522 (−2.277, −0.766) | <.001 | 92.4 | |
| Baseline thyroid function | ||||||
| Euthyroidism/Subclinical hypothyroidism | 5[33,34,44,45,49] | 43.13 | −0.644 (−1.03, −0.252) | .001 | 74.8 | .27 |
| Hypothyroidism | 7[31,36,46–48,50,51] | 56.87 | −1.420 (−2.415, −0.426) | .005 | 94.7 | |
| Baseline 25(OH)D levels | ||||||
| Not restricted | 3[31,47,50] | 24.77 | −1.342 (−2.685, 0.000) | .05 | 93.9 | .97 |
| Insufficiency | 4[33,34,48,51] | 33.03 | −0.713 (−1.139, −0.287) | .001 | 56.5 | |
| Deficiency | 5[36,44–46,49] | 42.20 | −1.215 (−2.297, −0.132) | .03 | 95.9 | |
| TG-Ab | ||||||
| Duration of vitamin D supplementation | ||||||
| ≤12 wk | 5[31,34,44,49,51] | 45.70 | −0.420 (−0.683, −0.156) | .002 | 29.3 | .09 |
| >12 wk | 6[33,45–48,50] | 54.30 | −1.475 (−2.505, −0.446) | .005 | 95.5 | |
| Type of vitamin D | ||||||
| Naive vitamin D (vitamin D2 or D3) | 3[33,44,49] | 28.36 | −0.138 (−0.505, 0.229) | .46 | 60.1 | .06 |
| Calcitriol | 8[31,34,45–48,50,51] | 71.64 | −1.341 (−2.045, −0.637) | <.001 | 91.5 | |
| Baseline thyroid function | ||||||
| Euthyroidism/subclinical hypothyroidism | 5[33,34,44,45,49] | 46.86 | −0.400 (−0.783, −−0.017) | .04 | 74.4 | .06 |
| Hypothyroidism | 6[31,46–48,50,51] | 53.14 | −1.513 (−2.469, −0.557) | .002 | 93.0 | |
| Baseline 25(OH)D Levels | ||||||
| Not restricted | 3[31,47,50] | 26.72 | −1.755 (−3.184, −0.325) | .02 | 94.0 | .49 |
| Insufficiency | 4[33,34,48,51] | 36.12 | −0.373 (−0.831, 0.085) | .11 | 64.0 | |
| Deficiency | 4[44–46,49] | 37.16 | −1.043 (−2.076, −0.010) | .048 | 95.1 | |
| FT4 | ||||||
| Duration of vitamin D supplementation | ||||||
| ≤12 wk | 5[31,34,44,49,51] | 50.10 | 0.022 (−0.190, 0.234) | .84 | 0.0 | <.001 |
| >12 wk | 5[33,46–48,50] | 49.90 | 1.388 (0.756, 2.201) | <.001 | 86.4 | |
| Type of vitamin D | ||||||
| Naive vitami> D (vitamin D2 or D3) | 3[33,44,49] | 31.17 | 0.274 (−0.448, 0.997) | .46 | 89.3 | .26 |
| Calcitriol | 7[31,34,46–48,50,51] | 68.83 | 0.940 (0.241, 1.639) | .008 | 90.8 | |
| Baseline thyroid function | ||||||
| Euthyroidism/subclinical hypothyroidism | 4[33,34,44,49] | 41.38 | 0.240 (−0.289, 0.769) | .37 | 84.1 | .10 |
| Hypothyroidism | 6[31,46–48,50,51] | 58.62 | 1.080 (0.343, 1.817) | .004 | 89.7 | |
| Baseline 25(OH)D Levels | ||||||
| Not restricted | 3[31,47,50] | 29.67 | 1.264 (0.297, 2.231) | .01 | 89.0 | .39 |
| Insufficiency | 4[33,34,48,51] | 39.44 | 0.433 (−0.061, 0.927) | .09 | 69.0 | |
| Deficiency | 3[44,46,49] | 30.89 | 0.642 (−0.687, 1.971) | .34 | 96.4 | |
| FT3 | ||||||
| Duration of vitamin D supplementation | ||||||
| ≤12 wk | 5[31,34,44,49,51] | 49.92 | −0.052 (−0.264, 0.169) | .63 | 0.0 | <.001 |
| >12 wk | 5[33,46–48,50] | 50.08 | 1.107 (0.536, 1.677) | <.001 | 84.7 | |
| Type of vitamin D | ||||||
| Naive vitamin D (vitamin D2 or D3) | 3[33,44,49] | 31.53 | 0.098 (−0.226, 0.422) | .55 | 49.0 | .18 |
| Calcitriol | 7[31,34,46–48,50,51] | 68.47 | 0.750 (0.116, 1.384) | .02 | 89.4 | |
| Baseline thyroid function | ||||||
| Euthyroidism/subclinical hypothyroidism | 4[33,34,44,49] | 41.76 | 0.029 (−0.246, 0.304) | .83 | 42.7 | .02 |
| Hypothyroidism | 6[31,46–48,50,51] | 58.24 | 0.926 (0.339, 1.513) | .002 | 84.6 | |
| Baseline 25(OH)D levels | ||||||
| Not restricted | 3[31,47,50] | 29.65 | 1.018 (0.139, 1.898) | .02 | 87.5 | .44 |
| Insufficiency | 4[33,34,48,51] | 39.13 | 0.200 (−0.129, 0.529) | .23 | 33.4 | |
| Deficiency | 3[44,46,49] | 31.22 | 0.532 (−0.558, 1.622) | .34 | 95.0 | |
The administration of vitamin D for ≤ 12 weeks [SMD and 95% CI = −0.454 (−0.749, −0.160), P = .002] and > 12 weeks [SMD and 95% CI = −1.741 (−2.750, −0.732), P = .001] resulted in a substantial reduction in TPO-Ab titers among HT patients. Notably, meta-regression analysis unveiled a significant distinction between the 2 subgroups (P = .03), signifying that vitamin D supplementation > 12 weeks induced a more pronounced decline in TPO-Ab titers compared with the ≤ 12-week regimen.
Regarding thyroid function, vitamin D supplementation ≤ 12 weeks exhibited no significant increase in FT4 levels [SMD and 95% CI = 0.022 (−0.190, 0.234), P = .84] or FT3 levels [SMD and 95% CI = −0.052 (−0.264, 0.169), P = .63] among HT patients. Conversely, treatment duration > 12 weeks demonstrated a notable increase in FT4 levels [SMD and 95% CI = 1.388 (0.756, 2.201), P < .001] and FT3 levels [SMD and 95% CI = 1.107 (0.536, 1.677), P < .001] within the HT patients. Meta-regression analysis underscored a significant disparity between the 2 subgroups (P < .001), suggesting that vitamin D supplementation > 12 weeks elicited a more substantial elevation in both FT4 and FT3 levels relative to the ≤ 12-week regimen.
Regarding vitamin D types, the supplementation of vitamin D2 or D3 [SMD and 95% CI = −0.300 (−0.566, −0.034), P = .03] and calcitriol [SMD and 95% CI = −1.522 (−2.277, −0.766), P < .001] proved efficacious in significantly diminishing TPO-Ab titers among HT patients. Meta-regression analysis divulged a notable discrepancy between the 2 subgroups (P = .046), indicating that calcitriol supplementation exhibited a more pronounced reduction in TPO-Ab levels compared with naive vitamin D. For a comprehensive overview, please refer to Table 4.
4. Discussion
Numerous studies have established that patients with HT often exhibit lower levels of serum 25(OH)D or higher rates of vitamin D deficiency when compared with healthy individuals.[16–18,20] Vitamin D supplementation has shown promise in improving thyroid function, reducing levels of TPO-Ab and TG-Ab,[19,32] decreasing the ratio of Th17/Treg.[33–35] However, conflicting results have emerged, with some studies suggesting that vitamin D supplementation could not reduce TPO-Ab/TG-Ab titers, nor improve thyroid function.[36] In this study, we conducted a meta-analysis of 12 RCTs examining the effects of vitamin D intervention in HT patients. The analysis confirmed that vitamin D supplementation significantly reduced TPO-Ab and TG-Ab titers, consistent with the findings of previous meta-analyses conducted by Zhang,[37] Wang,[38] and Liu.[41] While previous only 1 meta-analyses[39] found a significant reduction in TSH levels with vitamin D supplementation, our study demonstrated that it not only lowered TSH levels but also significantly increased FT3 and FT4 levels. The discrepancy with previous meta-analyses can be attributed to the inclusion of a smaller number of studies, non-randomized controlled trials, and the inclusion of patients with other autoimmune thyroid disease. In sensitivity analysis, we observed stable results for TPO-Ab, TG-Ab, and FT4, whereas the results for TSH and FT3 were less stable. Therefore, caution should be exercised when using these indices to evaluate the effect of vitamin D in HT patients.
Our study revealed heterogeneity in the results for TPO-Ab, TG-Ab, FT3, and FT4. Subgroup analysis and meta-regression analysis were conducted to explore the factors influencing heterogeneity, including baseline thyroid function and 25(OH)D levels, type of vitamin D supplement, and treatment duration. The results indicated that baseline thyroid function status was a significant factor affecting FT3 heterogeneity, while different types of vitamin D supplement contributed to TPO-Ab heterogeneity; treatment duration emerged as a significant factor affecting TPO-Ab, FT3, and FT4 heterogeneity (meta-regression test level α = 0.05). Considering the possibility of missing important influencing factors, a study[52] suggested that the threshold for the meta-regression test level can be relaxed to 0.1. Under this standard, different types of vitamin D influenced TG-Ab heterogeneity, while baseline thyroid function status affected both TG-Ab and FT4 heterogeneity. Due to differences in vitamin D doses, frequencies, routes of administration, and lack of comparability in our study, subgroup analysis of different vitamin D supplementation doses was not performed.
Our findings demonstrated that vitamin D supplementation significantly decreased TPO-Ab and TG-Ab titers regardless of baseline thyroid function and significantly increased FT3 levels in patients with hypothyroidism. However, it should be noted that the included RCTs of hypothyroidism patients did not clearly state whether the dosage of levothyroxine (L-T4) in the treatment group was comparable to that in the control group, while L-T4 dosage was adjusted based on thyroid function before or during treatment. Moreover, calcitriol was commonly used in combination with L-T4 to treat HT in the hypothyroidism group, while a higher proportion of patients with normal or subclinical hypothyroidism received naive vitamin D. The potential impact of different vitamin D types on the study outcomes cannot be ruled out. Therefore, it has not been definitively established whether vitamin D supplementation is more effective in hypothyroid HT patients. Krysiak et al[53] found that TPO-Ab and TG-Ab titers significantly decreased after vitamin D supplementation in HT patients with subclinical hypothyroidism who were treated with L-T4 for over 6 months, but no significant changes were observed in patients with normal thyroid function. Further prospective studies are needed to ascertain whether baseline thyroid function affects the efficacy of vitamin D intervention in HT.
We did not find a significant effect of baseline 25(OH)D levels on the efficacy of vitamin D supplementation in HT patients. This finding may be attributed to the fact that patients were divided into subgroups based on their required vitamin D level status at enrollment, rather than according to actual 25(OH)D concentrations. Zhao[33] and Sahin[54] reported that TPO-Ab titers in HT patients with vitamin D deficiency (25(OH)D < 20 ng/mL) and postpartum thyroiditis were more significantly reduced following vitamin D supplementation compared to those with 25(OH)D ≥ 20 ng/mL. However, the vitamin D supplementation dose for patients with vitamin D deficiency was more than twice as high as that for patients with sufficient or normal vitamin D levels in the aforementioned studies. Another study investigating the efficacy of vitamin D supplementation in healthy adults with sufficient vitamin D levels found that lower baseline serum 25(OH)D levels correlated with greater efficacy of vitamin D supplementation,[55] but it remains unclear whether individuals with adequate vitamin D levels can benefit from supplementation.[56] In summary, additional prospective studies are required to determine whether vitamin D-deficient HT patients derive greater benefits from vitamin D supplementation.
Our study revealed that calcitriol exhibited a more pronounced effect in reducing the titers of TPO-Ab and TG-Ab, while elevating the levels of FT4 and FT3. Zhang et al[37] reached a similar conclusion, reporting a significant reduction in TPO-Ab titers with calcitriol supplementation, whereas the effect of naive vitamin D was not substantial. Mahmoudi et al[57] observed that calcitriol significantly improved insulin resistance in patients with nonalcoholic fatty liver compared to vitamin D3. Naive vitamin D is absorbed into the circulation, transported to the liver by vitamin D binding protein, and undergoes liver and kidney metabolism to produce active vitamin D, which exerts its biological effects. Calcitriol, on the other hand, binds directly to vitamin D receptors (VDRs) found in various tissues, exerting immunomodulatory effects upon binding to immune cell VDRs. Some scholars have suggested that vitamin D resistance may be associated with the development of autoimmune diseases, and the cause of vitamin D resistance may be linked to abnormal gene expression of vitamin D binding protein, cytochrome P450 enzymes, and VDRs.[58] Calcitriol may exhibit better efficacy in the presence of vitamin D resistance. Therefore, our study proposes that calcitriol is more effective in HT intervention. However, due to the increased risk of hypercalcemia compared to native vitamin D, calcitriol is not recommended for the treatment of vitamin D deficiency/insufficiency.[59,60] In summary, we contend that calcitriol is more effective in managing HT; however, the safety of calcitriol in HT requires confirmation through more clinical studies.
Regarding the duration of vitamin D supplementation, our study demonstrated that supplementation for > 12 weeks effectively reduced TPO-Ab levels and increased FT4 and FT3 levels in HT patients. Consistently, Wang,[38] Liu[41] also revealed a significant reduction in TPO-Ab levels after 6 months of vitamin D supplementation compared to the control group, while treatment durations of 1 or 3 months showed no significant effects. Chao et al[61] conducted a follow-up study on 2714 volunteers of different ages and found that supplementation for over 3 months significantly improved serum vitamin D levels compared to minimal vitamin D supplementation (1000–2000 IU, once or twice a week for 1 month). In an analysis of adverse reactions from 15 RCTs studies with long-term (≥1 year) and high-dose vitamin D supplementation, Malihi[62] found a tendency to increase hypercalcemia and hypercalciuria, but no significant increase in the risk of total adverse events or kidney stones. Thus, our study suggests that vitamin D supplementation for ≥ 3 months is more beneficial for improving the condition of HT patients.
With regard to the supplementation dosage of vitamin D, studies indicated that 25(OH)D levels should reach 20 ng/mL for calcium balance and bone health, but achieving 40 to 80 ng/mL was necessary to obtain immunomodulatory benefits.[56] Miteva et al[63] suggested supplementing vitamin D at a dose of 2000 to 4000 IU/d to achieve extra-skeletal effects, with a target serum vitamin D concentration of 30 to 45 ng/mL. Patients with hyperthyroidism or hypothyroidism should receive vitamin D supplementation of 1500 to 2000 IU/d to prevent vitamin D deficiency from exacerbating their condition. Existing evidence-based medical evidence indicates that moderate doses of vitamin D supplementation are safe,[62] but regular monitoring of blood and urine calcium is still recommended.
5. Conclusion
Vitamin D supplementation significantly reduce TPO-Ab and TG-Ab titers among HT patients, leading to improvements in thyroid function characterized by decreased TSH levels and increased FT3 and FT4 levels.
Administration of active vitamin D exhibits superior efficacy in reducing TPO-Ab titers compared to naive vitamin D.
Prolonged duration of vitamin D supplementation (>12 weeks) results in more effective reduction of TPO-Ab levels in HT patients compared to treatment duration ≤ 12 weeks, while also leading to more significant increases in FT4 and FT3 levels.
Author contributions
Conceptualization: Fangping Li, Peng Yun.
Data curation: Jiahao Tang, Shuanghong Shan.
Formal analysis: Jiahao Tang, Shuanghong Shan.
Investigation: Jiahao Tang, Shuanghong Shan.
Methodology: Jiahao Tang, Shuanghong Shan.
Project administration: Fangping Li, Peng Yun.
Writing – original draft: Jiahao Tang, Shuanghong Shan.
Writing – review & editing: Fangping Li, Peng Yun.
Abbreviations:
- 25(OH)D
- 25-hydroxyvitamin D
- CI
- confidence interval
- FT3
- free triiodothyronine
- FT4
- free thyroxine
- HT
- Hashimoto’s thyroiditis
- IFN-γ
- interferon-γ
- IL-10
- interleukin-10
- IL-17
- interleukin-17
- IL-1
- interleukin-1
- IL-2
- interleukin-2
- IL-4
- interleukin-4
- L-T4
- levothyroxine
- RCTs
- randomized controlled trials
- SD
- standard deviation
- SMD
- standardized mean difference
- TG-Ab
- thyroglobulin antibody
- Th
- helper T lymphocytes
- TPO-Ab
- anti-thyroid peroxidase antibody
- Treg
- regulatory T cells
- TSH
- thyroid-stimulating hormone
- VDRs
- vitamin D receptors
JT and SS contributed equally to this work.
The study was funded by Sanming Project of Medicine in Shenzhen (SZSM202011007); The Hospital Research Fund of SAHSYSU (ZSQYLCKYJJ202009).
This study is a systematic review and meta-analysis, the outcomes are based on the published evidence, so examination and agreement by the ethics committee are not required in this study.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
How to cite this article: Tang J, Shan S, Li F, Yun P. Effects of vitamin D supplementation on autoantibodies and thyroid function in patients with Hashimoto’s thyroiditis: A systematic review and meta-analysis. Medicine 2023;102:52(e36759).
Contributor Information
Jiahao Tang, Email: tangjh37@mail.sysu.edu.cn.
Shuanghong Shan, Email: 963187034@qq.com.
Fangping Li, Email: 472655037@qq.com.
References
- [1].Ragusa F, Fallahi P, Elia G, et al. Hashimotos’ thyroiditis: epidemiology, pathogenesis, clinic and therapy. Best Pract Res Clin Endocrinol Metab. 2019;33:101367. [DOI] [PubMed] [Google Scholar]
- [2].McLeod DS, Cooper DS. The incidence and prevalence of thyroid autoimmunity. Endocrine. 2012;42:252–65. [DOI] [PubMed] [Google Scholar]
- [3].Ajjan RA, Weetman AP. The pathogenesis of Hashimoto’s thyroiditis: further developments in our understanding. Horm Metab Res. 2015;47:702–10. [DOI] [PubMed] [Google Scholar]
- [4].Pyzik A, Grywalska E, Matyjaszek-Matuszek B, et al. Immune disorders in Hashimoto’s thyroiditis: what do we know so far? J Immunol Res. 2015;2015:979167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Janyga S, Marek B, Kajdaniuk D, et al. CD4+ cells in autoimmune thyroid disease. Endokrynol Pol. 2021;72:572–83. [DOI] [PubMed] [Google Scholar]
- [6].Antonelli A, Ferrari SM, Giuggioli D, et al. Chemokine (C-X-C motif) ligand (CXCL)10 in autoimmune diseases. Autoimmun Rev. 2014;13:272–80. [DOI] [PubMed] [Google Scholar]
- [7].Stassi G, Zeuner A, Di Liberto D, et al. Fas-FasL in Hashimoto’s thyroiditis. J Clin Immunol. 2001;21:19–23. [DOI] [PubMed] [Google Scholar]
- [8].Nanba T, Watanabe M, Inoue N, et al. Increases of the Th1/Th2 cell ratio in severe Hashimoto’s disease and in the proportion of Th17 cells in intractable Graves’ disease. Thyroid. 2009;19:495–501. [DOI] [PubMed] [Google Scholar]
- [9].Li D, Cai W, Gu R, et al. Th17 cell plays a role in the pathogenesis of Hashimoto’s thyroiditis in patients. Clin Immunol. 2013;149:411–20. [DOI] [PubMed] [Google Scholar]
- [10].Shi Y, Wang H, Su Z, et al. Differentiation imbalance of Th1/Th17 in peripheral blood mononuclear cells might contribute to pathogenesis of Hashimoto’s thyroiditis. Scand J Immunol. 2010;72:250–5. [DOI] [PubMed] [Google Scholar]
- [11].Nakano A, Watanabe M, Iida T, et al. Apoptosis-induced decrease of intrathyroidal CD4(+)CD25(+) regulatory T cells in autoimmune thyroid diseases. Thyroid. 2007;17:25–31. [DOI] [PubMed] [Google Scholar]
- [12].Marazuela M, García-López MA, Figueroa-Vega N, et al. Regulatory T cells in human autoimmune thyroid disease. J Clin Endocrinol Metab. 2006;91:3639–46. [DOI] [PubMed] [Google Scholar]
- [13].Haibo X, Lei M, Xiuyun W, et al. The dynamic changes of Treg/Th17 cells imbalance in patients with Hashimoto thyroiditis. Chin J Clin (Electron Ed). 2012;6:2075–9. [Google Scholar]
- [14].Orgiazzi J. Thyroid autoimmunity. Presse Med. 2012;41(12 p 2):e611–25. [DOI] [PubMed] [Google Scholar]
- [15].Binjia Z, Jie Z, Dingxiang L. Role of antithyroid antibody in autoimmune thyroid disease. China Pharmacist. 2020;23:2261–5. [Google Scholar]
- [16].Tamer G, Arik S, Tamer I, et al. Relative vitamin D insufficiency in Hashimoto’s thyroiditis. Thyroid. 2011;21:891–6. [DOI] [PubMed] [Google Scholar]
- [17].Evliyaoğlu O, Acar M, Özcabi B, et al. Vitamin D deficiency and Hashimoto’s thyroiditis in children and adolescents: a critical vitamin D level for this association? J Clin Res Pediatr Endocrinol. 2015;7:128–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Metwalley KA, Farghaly HS, Sherief T, et al. Vitamin D status in children and adolescents with autoimmune thyroiditis. J Endocrinol Invest. 2016;39:793–7. [DOI] [PubMed] [Google Scholar]
- [19].Mazokopakis EE, Papadomanolaki MG, Tsekouras KC, et al. Is vitamin D related to pathogenesis and treatment of Hashimoto’s thyroiditis? Hell J Nucl Med. 2015;18:222–7. [PubMed] [Google Scholar]
- [20].Fang F, Chai Y, Wei H, et al. Vitamin D deficiency is associated with thyroid autoimmunity: results from an epidemiological survey in Tianjin, China. Endocrine. 2021;73:447–54. [DOI] [PubMed] [Google Scholar]
- [21].Chao G, Zhu Y, Fang L. Correlation between Hashimoto’s thyroiditis-related thyroid hormone levels and 25-hydroxyvitamin D. Front Endocrinol (Lausanne). 2020;11:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Baeke F, Takiishi T, Korf H, et al. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010;10:482–96. [DOI] [PubMed] [Google Scholar]
- [23].Gallo D, Mortara L, Gariboldi MB, et al. Immunomodulatory effect of vitamin D and its potential role in the prevention and treatment of thyroid autoimmunity: a narrative review. J Endocrinol Invest. 2020;43:413–29. [DOI] [PubMed] [Google Scholar]
- [24].Mattner F, Smiroldo S, Galbiati F, et al. Inhibition of Th1 development and treatment of chronic-relapsing experimental allergic encephalomyelitis by a non-hypercalcemic analogue of 1,25-dihydroxyvitamin D(3). Eur J Immunol. 2000;30:498–508. [DOI] [PubMed] [Google Scholar]
- [25].Boonstra A, Barrat FJ, Crain C, et al. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167:4974–80. [DOI] [PubMed] [Google Scholar]
- [26].Palmer MT, Lee YK, Maynard CL, et al. Lineage-specific effects of 1,25-dihydroxyvitamin D(3) on the development of effector CD4 T cells. J Biol Chem. 2011;286:997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chang SH, Chung Y, Dong C. Vitamin D suppresses Th17 cytokine production by inducing C/EBP homologous protein (CHOP) expression. J Biol Chem. 2010;285:38751–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Unger WW, Laban S, Kleijwegt FS, et al. Induction of treg by monocyte-derived DC modulated by vitamin D3 or dexamethasone: differential role for PD-L1. Eur J Immunol. 2009;39:3147–59. [DOI] [PubMed] [Google Scholar]
- [29].Hewison M. Vitamin D and immune function: an overview. Proc Nutr Soc. 2012;71:50–61. [DOI] [PubMed] [Google Scholar]
- [30].Štefanić M, Tokić S. Serum 25-hydoxyvitamin D concentrations in relation to Hashimoto’s thyroiditis: a systematic review, meta-analysis and meta-regression of observational studies. Eur J Nutr. 2020;59:859–72. [DOI] [PubMed] [Google Scholar]
- [31].Chahardoli R, Saboor-Yaraghi AA, Amouzegar A, et al. Can supplementation with vitamin D modify thyroid autoantibodies (Anti-TPO Ab, Anti-Tg Ab) and Thyroid Profile (T3, T4, TSH) in Hashimoto’s thyroiditis? A double blind, randomized clinical trial. Horm Metab Res. 2019;51:296–301. [DOI] [PubMed] [Google Scholar]
- [32].Nodehi M, Ajami A, Izad M, et al. Effects of vitamin D supplements on frequency of CD4(+) T-cell subsets in women with Hashimoto’s thyroiditis: a double-blind placebo-controlled study. Eur J Clin Nutr. 2019;73:1236–43. [DOI] [PubMed] [Google Scholar]
- [33].Yaping Z. Effect of Vitamin D on Th17/Treg Transcription Factor Regulation and Thyroid Function in Patients with Hashimoto’s Thyroiditis [master thesis]. Kunming, China: Kunming Medical University; 2022. [Google Scholar]
- [34].Liping F, Jianhong J, Baofa W, et al. Impact of calcitriol on the thyroid peroxidase antibody, thyroglobulin antibody levels and Th17/Treg related cytokines in serum of patients with Hashimoto’s thyroiditis. Zhejiang Med J. 2019;41:2398–401. [Google Scholar]
- [35].Rui Z. Mechanism Analysis of Vitamin D Supplementation to Improve the Condition of Patients with Hashimoto’s Thyroiditis [master’s thesis]. Kunming, China: Kunming; Medical University; 2021. [Google Scholar]
- [36].Vahabi Anaraki P, Aminorroaya A, Amini M, et al. Effect of vitamin D deficiency treatment on thyroid function and autoimmunity markers in Hashimoto’s thyroiditis: a double-blind randomized placebo-controlled clinical trial. J Res Med Sci. 2017;22:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhang J, Chen Y, Li H, et al. Effects of vitamin D on thyroid autoimmunity markers in Hashimoto’s thyroiditis: systematic review and meta-analysis. J Int Med Res. 2021;49:3000605211060675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wang S, Wu Y, Zuo Z, et al. The effect of vitamin D supplementation on thyroid autoantibody levels in the treatment of autoimmune thyroiditis: a systematic review and a meta-analysis. Endocrine. 2018;59:499–505. [DOI] [PubMed] [Google Scholar]
- [39].Santos J, Alencar E, Martins E, et al. Vitamin D in patients with Hashimoto’s disease: a systematic review and meta-analysis. Revista de Ciências Farmacêutica Básica e Aplicadas – RCFBA. 2021;43:e758. [Google Scholar]
- [40].Jiang H, Chen X, Qian X, et al. Effects of vitamin D treatment on thyroid function and autoimmunity markers in patients with Hashimoto’s thyroiditis-a meta-analysis of randomized controlled trials. J Clin Pharm Ther. 2022;47:767–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Siqi L, Su W, Yaping W, et al. Effects of vitamin D supplementation on thyroid autoantibody levels in the treatment of autoimmune thyroiditis: a meta-analysis. J Southeast Univ (Med Ed). 2021;40:207–13. [Google Scholar]
- [42].Higgins JPT, Thomas J, Chandler J, eds, et al. Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022). Cochrane. 2022. Available at: www.training.cochrane.org/handbook [access date July 03, 2023]. [Google Scholar]
- [43].Dehui L, Xiang W, Jiming L, et al. How to estimate the sample mean and standard deviation from the sample size, median, extremes or quartiles? Chin J Evid Based Med. 2017;17:1350–6. [Google Scholar]
- [44].Yingli X, Shangnong W. Effect of vitamin D on thyroid autoantibodies in patients with Hashimoto’s thyroiditis. China Pharm. 2020;29(S01):14–5. [Google Scholar]
- [45].Zhihua Z, Danqing Z, Chong Z, et al. Effect of oral vitamin D on anti-thyroid peroxidase antibody in patients with Hashimoto’s thyroiditis. J Clin Med. 2018;35:699–701. [Google Scholar]
- [46].Hongtao Z. The effect of calctriol on FT3, FT4, TGAb and TPOAb in patients with hashimoto’s thyroiditis and hypothyroidism. Mod Med J China. 2020;22:27–30. [Google Scholar]
- [47].Ruihong J. Clinical observation of calcitriol combined with levothyroxine sodium tablets in the treatment of Hashimoto’s thyroiditis with hypothyroidism. J Med Forum. 2020;41:133–5. [Google Scholar]
- [48].Lan Z, Ling W, Bingquan F. The clinical application of calcitriol for patients of Hashimoto thyroiditis with hypothyroidism. Med Recapitulate. 2016;22:2410–2. [Google Scholar]
- [49].Jingying Z, Chongrong L, Nenghua T. Effects of vitamin D treatment on thyroid autoantibodies in patients with Hashimoto thyroiditis. J Mod Med Health. 2018;34:1656–8. [Google Scholar]
- [50].Ying H, Yujuan C, Yongmei L, et al. lmpact of calcitriol treatment on Hashimoto’s thyroiditis with hypothyroidism. Chin J Clin Res. 2015;28:17–19 + 23. [Google Scholar]
- [51].Zhengli H. The Study on Relationship Between Serum 25-hydroxyvitamin D3 Concentration and Hashimoto’s Thyroiditis [master’s thesis]. Jilin, China: Jilin University; 2013. [Google Scholar]
- [52].Xiuquan S, Zengzhen W. Application of meta-regression and subgroup analyses of heterogeneity disposal in meta-analysis. Chin J Epidemiol. 2008;29:497–501. [PubMed] [Google Scholar]
- [53].Krysiak R, Szkróbka W, Okopień B. The effect of vitamin D on thyroid autoimmunity in levothyroxine-treated women with Hashimoto’s thyroiditis and normal vitamin D status. Exp Clin Endocrinol Diabetes. 2017;125:229–33. [DOI] [PubMed] [Google Scholar]
- [54].Sahin M, Corapcioglu D. The effect of vitamin D on thyroid autoimmunity in non-lactating women with postpartum thyroiditis. Eur J Clin Nutr. 2016;70:864. [DOI] [PubMed] [Google Scholar]
- [55].Žmitek K, Hribar M, Hristov H, et al. Efficiency of vitamin D supplementation in healthy adults is associated with body mass index and baseline serum 25-hydroxyvitamin D level. Nutrients. 2020;12:1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Vanherwegen AS, Gysemans C, Mathieu C. Regulation of immune function by vitamin D and its use in diseases of immunity. Endocrinol Metab Clin North Am. 2017;46:1061–94. [DOI] [PubMed] [Google Scholar]
- [57].Mahmoudi L, Asadi S, Al-Mousavi Z, et al. A randomized controlled clinical trial comparing calcitriol versus cholecalciferol supplementation to reduce insulin resistance in patients with non-alcoholic fatty liver disease. Clin Nutr. 2021;40:2999–3005. [DOI] [PubMed] [Google Scholar]
- [58].Lemke D, Klement RJ, Schweiger F, et al. Vitamin D resistance as a possible cause of autoimmune diseases: a hypothesis confirmed by a therapeutic high-dose vitamin D protocol. Front Immunol. 2021;12:655739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Vieth R. Vitamin D supplementation: cholecalciferol, calcifediol, and calcitriol. Eur J Clin Nutr. 2020;74:1493–7. [DOI] [PubMed] [Google Scholar]
- [60].Weibo X, Zhenlin Z, Hua L. Consensus on the clinical application of vitamin D and its analoques. Chin J Endocrinol Metab. 2018;34:187–201. [Google Scholar]
- [61].Chao YS, Brunel L, Faris P, et al. The importance of dose, frequency and duration of vitamin D supplementation for plasma 25-hydroxyvitamin D. Nutrients. 2013;5:4067–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Malihi Z, Wu Z, Lawes CMM, et al. Adverse events from large dose vitamin D supplementation taken for one year or longer. J Steroid Biochem Mol Biol. 2019;188:29–37. [DOI] [PubMed] [Google Scholar]
- [63].Miteva MZ, Nonchev BI, Orbetzova MM, et al. Vitamin D and autoimmune thyroid diseases – a review. Folia Med (Plovdiv). 2020;62:223–9. [DOI] [PubMed] [Google Scholar]