Abstract
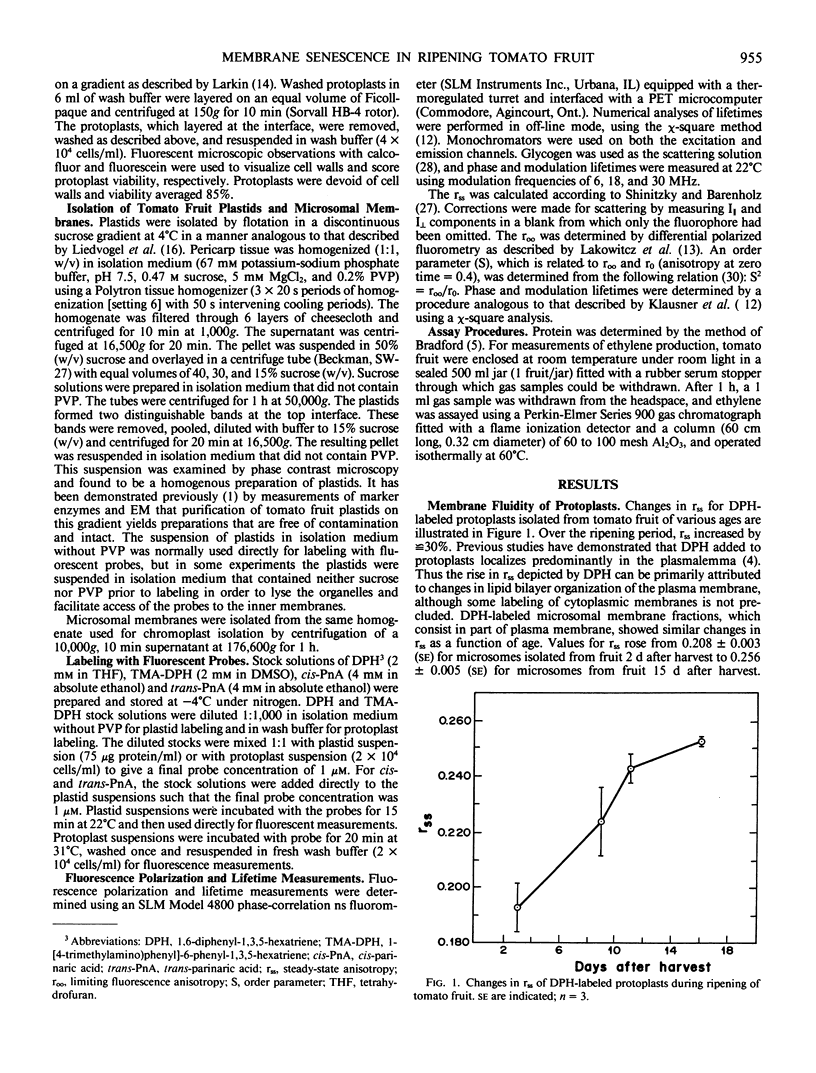
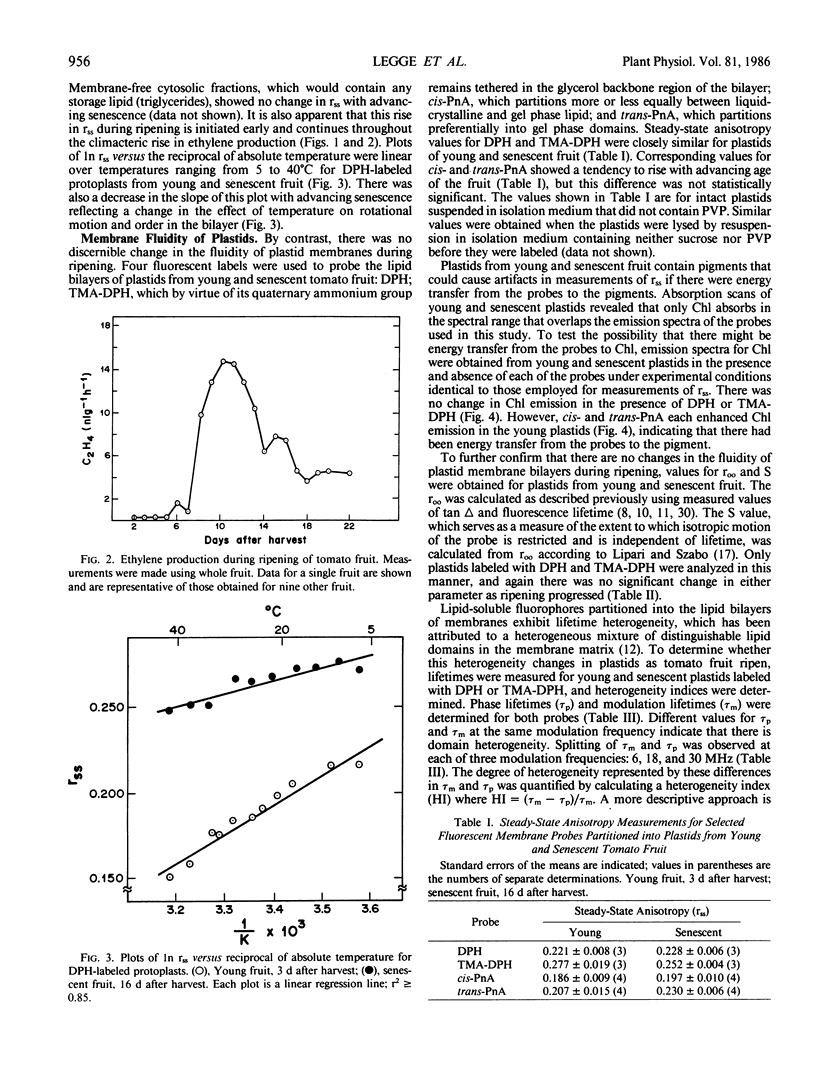
Changes in the molecular organization of membranes in pericarp cells of ripening tomato fruit were examined by fluorescence depolarization after labeling with fluorescent lipid-soluble probes. The fluorescent labels were partitioned into isolated protoplasts and purified plastids from fruit at various stages of senescence. Values for steady-state anisotropy (rss) of 1,6-diphenyl-1,3,5-hexatriene (DPH)-labeled protoplasts rose progressively during the early stages of ripening over a time frame that overlapped the climacteric rise in ethylene production. This can be interpreted as reflecting a decrease in the lipid fluidity of primarily plasma membrane. By contrast, there was no significant change during ripening in rss for plastid membranes labeled with DPH, 1-[4-trimethylamino)phenyl]-6-phenyl-1,3,5-hexatriene (TMA-DPH), and cis- or trans-parinaric acid. Nor was there any change during ripening in the limiting fluorescence anisotropy (roo) and order parameter (S) for plastids labeled with DPH or TMA-DPH, parameters that are corrected for any differences in lifetime. Some degree of lifetime heterogeneity, possibly reflecting structurally distinct domains, was discerned in both young and senescent plastids that had been labeled with DPH or TMA-DPH, but this also did not change as ripening progressed. Thus membranes of the pericarp cells sustain different fates as the tomato fruit ripens, implying that there are distinguishable mechanisms of membrane deterioration in senescing tissues.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borochov A., Halevy A. H. Microviscosity of plasmalemmas in rose petals as affected by age and environmental factors. Plant Physiol. 1978 May;61(5):812–815. doi: 10.1104/pp.61.5.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brennan T., Frenkel C. Involvement of hydrogen peroxide in the regulation of senescence in pear. Plant Physiol. 1977 Mar;59(3):411–416. doi: 10.1104/pp.59.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers D. J., Rowan K. S. The climacteric in ripening tomato fruit. Plant Physiol. 1971 Sep;48(3):235–240. doi: 10.1104/pp.48.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel L. W., Prendergast F. G. Values for and significance of order parameters and "cone angles" of fluorophore rotation in lipid bilayers. Biochemistry. 1981 Dec 22;20(26):7338–7345. doi: 10.1021/bi00529a003. [DOI] [PubMed] [Google Scholar]
- Gratton E., Jameson D. M., Hall R. D. Multifrequency phase and modulation fluorometry. Annu Rev Biophys Bioeng. 1984;13:105–124. doi: 10.1146/annurev.bb.13.060184.000541. [DOI] [PubMed] [Google Scholar]
- Heyn M. P. Determination of lipid order parameters and rotational correlation times from fluorescence depolarization experiments. FEBS Lett. 1979 Dec 15;108(2):359–364. doi: 10.1016/0014-5793(79)80564-5. [DOI] [PubMed] [Google Scholar]
- Jähnig F. Structural order of lipids and proteins in membranes: evaluation of fluorescence anisotropy data. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6361–6365. doi: 10.1073/pnas.76.12.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Kleinfeld A. M., Hoover R. L., Karnovsky M. J. Lipid domains in membranes. Evidence derived from structural perturbations induced by free fatty acids and lifetime heterogeneity analysis. J Biol Chem. 1980 Feb 25;255(4):1286–1295. [PubMed] [Google Scholar]
- Lakowicz J. R., Prendergast F. G., Hogen D. Differential polarized phase fluorometric investigations of diphenylhexatriene in lipid bilayers. Quantitation of hindered depolarizing rotations. Biochemistry. 1979 Feb 6;18(3):508–519. doi: 10.1021/bi00570a021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKersie B. D., Lepock J. R., Kruuv J., Thompson J. E. The effects of cotyledon senescence on the composition and physical properties of membrane lipid. Biochim Biophys Acta. 1978 Apr 4;508(2):197–212. doi: 10.1016/0005-2736(78)90325-5. [DOI] [PubMed] [Google Scholar]
- McKersie B. D., Thompson J. E. Phase properties of senescing plant membranes: role of the neutral lipids. Biochim Biophys Acta. 1979 Jan 5;550(1):48–58. doi: 10.1016/0005-2736(79)90114-7. [DOI] [PubMed] [Google Scholar]
- Rosso S. W. The ultrastructure of chromoplast development in red tomatoes. J Ultrastruct Res. 1968 Nov;25(3):307–322. doi: 10.1016/s0022-5320(68)80076-0. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Barenholz Y. Fluidity parameters of lipid regions determined by fluorescence polarization. Biochim Biophys Acta. 1978 Dec 15;515(4):367–394. doi: 10.1016/0304-4157(78)90010-2. [DOI] [PubMed] [Google Scholar]
- Thompson J. E., Mayak S., Shinitzky M., Halevy A. H. Acceleration of membrane senescence in cut carnation flowers by treatment with ethylene. Plant Physiol. 1982 Apr;69(4):859–863. doi: 10.1104/pp.69.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Blitterswijk W. J., Van Hoeven R. P., Van der Meer B. W. Lipid structural order parameters (reciprocal of fluidity) in biomembranes derived from steady-state fluorescence polarization measurements. Biochim Biophys Acta. 1981 Jun 22;644(2):323–332. doi: 10.1016/0005-2736(81)90390-4. [DOI] [PubMed] [Google Scholar]


