Abstract
Video Abstract
OBJECTIVES
Increased intestinal permeability seems to be a key factor in the pathogenesis of autoimmune diseases, including celiac disease (CeD). However, it is unknown whether increased permeability precedes CeD onset. This study’s objective was to determine whether intestinal permeability is altered before celiac disease autoimmunity (CDA) in at-risk children. We also examined whether environmental factors impacted zonulin, a widely used marker of gut permeability.
METHODS
We evaluated 102 children in the CDGEMM study from 2014–2022. We included 51 CDA cases and matched controls, who were enrolled for 12 months or more and consumed gluten. We measured serum zonulin from age 12 months to time of CDA onset, and the corresponding time point in controls, and examined clinical factors of interest. We ran a mixed-effects longitudinal model with dependent variable zonulin.
RESULTS
Children who developed CDA had a significant increase in zonulin in the 18.3 months (range 6–78) preceding CDA compared to those without CDA (slope differential = β = 0.1277, 95% CI: 0.001, 0.255). Among metadata considered, zonulin trajectory was only influenced by increasing number of antibiotic courses, which increased the slope of trajectory of zonulin over time in CDA subjects (P = .04).
CONCLUSIONS
Zonulin levels significantly rise in the months that precede CDA diagnosis. Exposure to a greater number of antibiotic courses was associated with an increase in zonulin levels in CDA subjects. This suggests zonulin may be used as a biomarker for preclinical CeD screening in at-risk children, and multiple antibiotic courses may increase their risk of CDA by increasing zonulin levels.
What’s Known on This Subject:
Intestinal permeability is increased in chronic inflammatory disorders including celiac disease. Increased intestinal permeability is associated with high levels of zonulin.
What This Study Adds:
Zonulin increases before celiac disease autoimmunity onset and is influenced by antibiotic exposure in at-risk children.
Intestinal permeability has been implicated in various gastrointestinal diseases, including celiac disease (CeD). CeD is a T cell-mediated autoimmune condition that occurs in individuals with a genetic predisposition and exposure to dietary gluten.1 However, most individuals with a genetic predisposition do not develop CeD; thus, the earliest steps leading to loss of tolerance to gluten are unclear.
CeD is unique because the inciting factor, gliadin, is known. Gliadin interacts with the intestinal epithelium leading to release of zonulin, an endogenous regulator of intestinal epithelial tight junctions, causing a breach in the epithelial barrier.2,3 An increase in zonulin is associated with increased intestinal permeability. Increased intestinal permeability may be a key feature of CeD pathogenesis by allowing undigested gliadin peptides to pass from the intestinal lumen into the lamina propria, reducing tolerance to gluten through immune system interactions. Although increased intestinal permeability has been associated with autoimmune diseases, including multiple sclerosis, Crohn’s disease, type I diabetes mellitus (T1D), and CeD; most studies are cross-sectional.4–10 Therefore, these studies could not distinguish whether increased permeability developed before disease or in response to the condition. One study in subjects at-risk of developing Crohn’s disease found intestinal permeability increased up to 3 years before Crohn’s disease.11 Here we aimed to determine whether an increase in intestinal permeability, as measured by serum zonulin, precedes the onset of celiac disease autoimmunity (CDA), defined as elevated celiac autoantibodies on at least 2 occurrences, in at-risk children and whether environmental factors influence zonulin. We hypothesized subjects at risk for developing CeD have increased zonulin levels before the onset of CDA, which would be mediated by infections.
Methods
To examine the earliest steps leading to loss of gluten tolerance and CDA onset, we developed a longitudinal, prospective, birth cohort study called the Celiac Disease Genomic Environmental Microbiome and Metabolomic (CD-GEMM) study.12 This study follows over 500 subjects with a first-degree relative with CeD from birth through 10 years of age and obtains blood, stool, and in-depth clinical information to monitor for CeD, taking a multiomic approach to predicting and preventing the disease.12,13 In this nested case-control study, we evaluated 102 pediatric subjects that were part of the larger CD-GEMM study and were enrolled between 2014 and 2022 in the United States and Italy.
As previously described, blood samples were drawn every 6 months for t3 years, and every 12 months thereafter, and kept frozen until analysis.12,13 During the study, parents answered monthly diaries about their child, including timing of gluten introduction and the number of servings of gluten-containing foods consumed. Every 3 months we reviewed data from parent-reported questionnaires describing respiratory and gastrointestinal viral symptoms (eg, fever, cough, rhinorrhea, vomiting, or diarrhea). Parents reported antibiotic exposure monthly for the first year after birth, then every 3 months until 18 months, and then every 6 months thereafter.
Blood samples were tested for celiac autoantibodies and human leukocyte antigen (HLA) genotype, as previously described.12,13 Subjects were determined to have celiac disease autoimmunity (CDA) if they had elevated celiac autoantibodies on at least 2 occurrences.12,13 Subjects were diagnosed with CeD if they had duodenal villous atrophy on biopsy or if they met criteria for elevated celiac antibody serologies, in accordance with the North American or the revised European Society for Pediatric Gastroenterology, Hepatology, and Nutrition criteria.14,15
Since the march from genetic predisposition to CeD includes breaking tolerance to gluten marked by CDA before overt CeD, in this study, CeD and CDA were collectively referred to as CDA.16 We included all subjects with CDA and 1 matched control per case. They were matched according to mode of delivery, sex, country, and HLA genetic risk. Those negative for HLA DQ2, DQ8, or DQ7 were considered low risk, those homozygous for HLA DQ2 were considered high risk, and all others were standard risk.17,18 We included only subjects with gluten introduced during the study period, with celiac serologies beyond 12 months of age, and with HLA genetic testing. We excluded those without serum available beyond 12 months of age and those without gluten introduction. The study was approved by the MassGeneral Brigham Human Research Committee Institutional Review Board.
Zonulin Testing
Zonulin was tested on serial serum samples from 12 months of age to time of CDA diagnosis or the corresponding time point in controls (average of 4 timepoints, range 1–9). Zonulin testing was performed with the Zonulin (Serum) ELISA kit (Immundiagnostik AG), according to manufacturer instructions. Samples with zonulin levels greater than 4 standard deviations above the mean were marked discrepancy outliers and were excluded from the analysis (n = 2 samples).
Statistical Analysis
We ran a mixed effects longitudinal model with dependent variable zonulin. Subject-level (time-constant) fixed predictors were CDA diagnosis (yes, no), sex (female, male), age at CDA diagnosis or last assessment, country (United States, Italy), family member with CeD (parent, sibling, both), gene risk (low, standard, high), and gluten introduction age. Time-varying fixed predictors included the number of months before CDA diagnosis (linear, quadratic), number of antibiotic courses, total respiratory and gastrointestinal viral infections, and number of gluten-containing servings consumed per time interval. Various relevant linear and quadratic interactions were also included. The random term was subjects nested in sex, country, first-degree relative with CeD, gene risk, and CDA diagnosis group (yes, no). Higher order quadratics, interactions, and covariates were pretested and removed if they were not significant. Final model residuals from fixed effect predicted values and combined fixed and random effect predicted values were assessed for model fit and to check for reasonable conformance to model assumptions of normality. Analysis was performed using SAS software (Version 9.4; SAS Institute Inc, Cary, NC, USA).
Results
Descriptive Characteristics
We included 51 subjects in CDGEMM diagnosed with CDA, as of December 1, 2022, and 51 control subjects (63.7% female, 36.3% male, 31.4% from the United States, 68.6% from Italy) (Table 1). Within the overall CDA group, 28 had CeD and 23 had CDA. The average month of gluten introduction for controls was 7.9 months and for CDA subjects was 8.3 months. There were 26 with high-risk genetics, 73 with intermediate-risk genetics, and 3 with low-risk genetics. The average follow-up time in the study was 23 months (SD = 15.5 months).
TABLE 1.
Subject Characteristics
| CDA | Controls | |
|---|---|---|
| n = 51 | n = 51 | |
| Sex, n (%) | ||
| Male | 18 (17.6) | 19 (18.6) |
| Female | 33 (32.4) | 32 (31.4) |
| Country, n (%) | ||
| United States | 16 (15.7) | 16 (15.7) |
| Italy | 35 (34.2) | 35 (34.2) |
| HLA genetics, n (%) | ||
| High | 15 (14.7) | 11 (10.8) |
| Standard | 35 (34.3) | 37 (36.3) |
| Low | 0 | 3 (3) |
| Unknown | 1 (1) | 0 |
| Age at seroconversion (first positive celiac antibody) | Avg 34 mo (range 12−84 mo) | NA |
| Gluten | ||
| Age at introduction | Avg 8.34 mo (range 2−36 mo) | Avg 7.86 mo (range 5−42 mo) |
| Servings per month | Avg 36.85 (range 0−162) | Avg 37.54 (range 0−108.3) |
Longitudinal Models
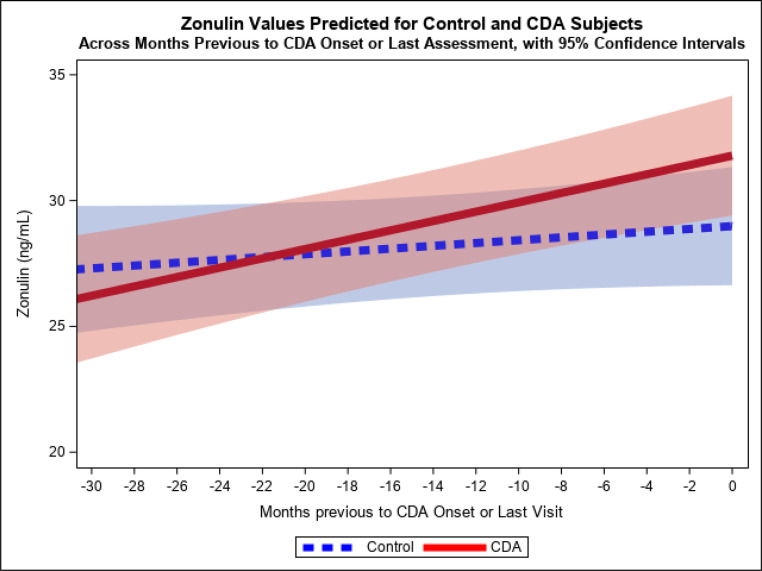
We ran a mixed effects longitudinal model with dependent variable zonulin and assessed how various factors changed zonulin over time. The final reduced model showed significant effects for months before CDA diagnosis (linear) interacting with CDA diagnosis (P = .049), such that subjects with CDA showed a significantly steeper slope of increasing zonulin in the 6 to 78 months before a CDA onset than before the last assessment in controls (slope differential = b = 0.1277, 95% confidence interval: 0.001–0.255) (Fig 1). There was also a significant main effect for country (P = .002), with subjects from Italy having a higher model-adjusted zonulin mean (30.24, SE = 0.89) than subjects from the United States (25.43, SE = 1.14), across time as a whole. We did not find a significant effect for timing of gluten introduction, nor the number of servings of gluten-containing foods consumed at each time interval evaluated. The percent variance in zonulin linearly accounted for by the fixed predictors in the model was 12.4%, whereas combined fixed and random predictors accounted for 48%. Residuals from fixed predicted values, as well as from combined fixed and random predicted values, were reasonably normal, indicating good model fit and conformance to assumptions.
FIGURE 1.
Values predicted for serum zonulin across months previous to CDA or last assessment, by the model fixed effects. (Pooled across countries. Predicted values for months less than −30 not shown because they are based on too few observations in that region - approximately 17% of total).
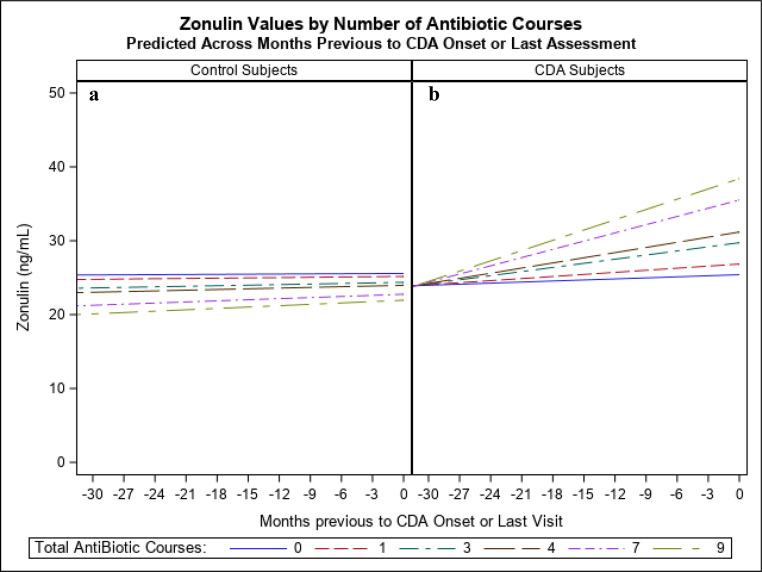
Given the interest in the relationship between infections and zonulin, as well as antibiotic courses (as a proxy for infections) and zonulin, 2 subject-level variables were created as the sum of total infections across the study per subject and the sum of antibiotic courses across the study per subject. In 2 separate analyses, these variables were entered as fixed predictors into the final model above, as well as their interactions with months before CDA diagnosis or last assessment. Effects for total viral infections were not significant. However, a significant interaction effect of antibiotic courses and months before CDA diagnosis or last assessment (P = .04) was found. Antibiotics were found to increase the slope of change across time for zonulin as the number of antibiotic courses increased (slope increase = b = 0.0243 per antibiotic course, 95% confidence interval: 0.0013–0.0473). Fixed effect predicted variance increased to 14%. Further, a 3-way interaction of CDA diagnosis, antibiotic courses, and months before CDA diagnosis or last assessment was further added and found to be marginally significant (P = .09), indicating that the association of antibiotics to increasing slope of change occurred almost exclusively for the CDA group (Fig 2). Fixed effect predicted variance further increased to 16%.
FIGURE 2.
Zonulin values predicted by model fixed effects, for (A) controls or (B) CDA subjects versus months previous to CDA or last assessment and number of antibiotic courses. (Illustrated for USA). (Predicted values for months less than −30 not shown because of too few observations - approximately 17% of total).
We found a diagnosis of CDA, and a greater number of antibiotic courses, had independent significant effects on increasing the slope of the trajectory of zonulin over time before CDA onset. Similar evaluations of the sum of the total number of viral infections did not have a significant effect on zonulin.
Discussion
Increased intestinal permeability is hypothesized to be a necessary feature of CeD pathophysiology, as it may be a key step in breaking mucosal tolerance.19 However, it is unknown whether increased intestinal permeability precedes onset of CeD. For the first time, we demonstrated an increased rate of rise of intestinal permeability in children that develop CDA, before disease onset, by utilizing a unique, prospective, longitudinal birth cohort of children at-risk of CeD. These changes occurred an average of 18 months before the onset of the autoimmune process.
This suggests intestinal permeability increases in the months to years before CDA. This is consistent with data from our group showing deamidated gliadin peptide immunoglobulin G rises 6 to 12 months before antitissue transglutaminase IgA seroconversion, implying intestinal permeability increases before this change in deamidated gliadin peptide immunoglobulin G as well.20 Additionally, this increase in zonulin was positively associated with a greater number of antibiotic courses in CDA subjects. We did not identify a threshold of zonulin that was significant in predicting CDA, rather we found a greater rate of rise of zonulin was predictive of CDA onset. These findings of increased intestinal permeability may result in expanded antigen trafficking of gliadin and other nonself-antigens, which could contribute to loss of tolerance to gluten in genetically susceptible individuals.21 In this study, we provide novel insights on the possible mechanisms involved in breaking mucosal tolerance before autoimmune dysfunction.
Examining the physiologic alterations in the predisease state is crucial to early intervention and disease prevention. Although studies have measured zonulin during active disease, few have explored how zonulin changes before disease onset. In addition, previous work on intestinal permeability in established diseases focuses on adults, with limited studies in pediatrics. One study found increased intestinal permeability, measured by the urinary fractional excretion of lactulose-to-mannitol ratio (LMR), is associated with later onset of Crohn’s disease in first-degree relatives of subjects with Crohn’s disease.11 Others found an elevated LMR in subjects with islet autoimmunity before T1D onset.22 Of note, many studies have used LMR as a marker of intestinal permeability; however, it is a challenging process, as subjects must drink a predetermined amount of sugar solution, collect the appropriate urine volume, and handle the urine specimen in a specific manner.23 Changes at any step may alter the test results. Here we used serum zonulin as a practical biomarker of intestinal permeability.3,24–31 Regarding the challenges in examining alterations before disease onset, there are few studies looking at predisease changes to intestinal permeability and none that we are aware of in subjects that develop CeD.11,22 Here we were able to use data from the CD-GEMM study to find that intestinal permeability increased in the months to years before CDA.
Intestinal permeability has been shown to be upregulated because of gut microbiome alterations in various disease states.27,28,31–33 Studies also show that zonulin levels are elevated in many diseases, such as CeD, T1D, Crohn’s disease, metabolic dysfunction-associated liver disease, and obesity.3,34–38 Therefore, it is not surprising that we and others have previously found gut microbiota alterations in those with CeD and other autoimmune conditions.39–43 Similarly, in T1D, 1 study found both higher intestinal permeability and imbalances in the host gut microbiome via reduced α diversity, different β diversity, and decreased abundance of antiinflammatory genus Prevotella, in children with either islet autoimmunity or T1D compared with controls.44 Previous work shows some of the strongest triggers of zonulin release are bacteria and gluten.45–47 Because of this, we adjusted for timing of gluten introduction and the number of servings of gluten-containing foods consumed per month, and we did not find a significant effect of gluten consumption on zonulin over time. Previous in vitro studies showed increased zonulin release from the small intestine in ex vivo mammalian tissue and intestinal cell monolayers mounted in Ussing chambers and exposed to enteric bacteria.46 Zonulin levels vary in the first year of life, likely because of the evolving composition of the gut microbiota.48 Zonulin pathways have been shown to be turned on by dysbiosis; therefore, antibiotics may result in dysbiosis and contribute to CDA.
Previous studies show that zonulin levels are increased by environmental factors, such as infections and antibiotics.49,50 This is supported by a study showing severe acute respiratory syndrome coronaviruse 2 was associated with increased serum zonulin levels in children with the autoimmune condition multisystem inflammatory syndrome in children.51 Additionally, zonulin levels were correlated with increased density of enteroviruses in small bowel biopsies of subjects with CeD.52 It is generally accepted that CeD is triggered by genetic susceptibility, loss of immune tolerance, and environmental factors. One possible environmental factor, antibiotic use, has previously been associated with increased risk of CeD.53,54 Both the Military Health System and researchers in Denmark and Norway showed that antibiotics were associated with an increased risk of CeD onset.53,54 As an objective proxy of infections, we evaluated the number of antibiotic courses each subject took during the study period. We found an association between the number of antibiotic courses and rise of serum zonulin. Subjects that go on to develop CDA who were exposed to more than 3 antibiotic courses had a greater rate of rise of zonulin before CDA onset. We did not observe the same increase in zonulin in controls, although they were taking similar numbers of antibiotic courses over time. Therefore, other unknown factors must be a part of the progression in subjects that develop CDA. Since this is an observational study and all subjects are at-risk of CeD, it is unlikely these findings are related to the study design. Based on our previously published data, we postulate microbiome alterations occur in children before CDA, particularly if exposed to multiple cycles of antibiotics, and will have microbiome-dependent epigenetic changes that upregulate zonulin-dependent intestinal permeability.39 There was no significant difference in zonulin levels when evaluating the effect of respiratory and gastrointestinal viral infections. Our finding that zonulin levels increase in subjects with CDA with increasing number of antibiotics is consistent with previous literature. Whether the number of infections or gut dysbiosis caused by the antibiotic treatment is responsible for zonulin increasing remains to be established.
Based on the literature and our findings, we hypothesize that in genetically predisposed individuals, antibiotic exposures, as a proxy of infections or as a direct effect on the gut ecosystem, lead to intestinal dysbiosis and a resulting rise in zonulin-dependent intestinal permeability. Deamidated gluten subsequently crosses into the lamina propria followed by a break in immune tolerance and subsequent onset of CDA. It is well known that antibiotics are associated with alterations in the human microbiome, and in animal studies, including decreases in beneficial commensal organisms and increases in pathogenic microorganisms.55,56 Our study raises additional concern about multiple antibiotic exposures during early childhood contributing to increased intestinal permeability with subsequent risk of onset of autoimmunity in genetically predisposed individuals. If greater antibiotic use increases the risk of intestinal permeability, and thereby risk for CeD, this serves as important guidance to families and physicians on the risk of unnecessary antibiotic use.
Our study has some limitations. Given our study population focused on subjects with a first-degree relative with CeD, we cannot necessarily extrapolate our findings to the general population. Additionally, the study was not focused on CeD alone but grouped subjects with CeD and CDA, together referred to as CDA. Serum zonulin as a marker of intestinal permeability is limited as it is a family of proteins, and commercially available assays do not currently measure all known proteins in this family.57 Another limitation is that we did not look at particular time periods of antibiotic exposure, which may be equally important. Nonetheless, all CDA cases were exposed to antibiotics in their first few years of life, the most vulnerable window when the gut microbiome programs the host immune system in determining the threshold to generate inflammation. Future studies may look at the effects of the specific timing of antibiotic use on zonulin over time.
As the rates of CeD and many other autoimmune diseases have been rising for unknown reasons, examining the predisease state may identify strategies to reverse this trend. For the first time, we assessed changes in the predisease state in children at risk for CeD and find intestinal permeability rises in the months leading to CDA onset. We also found that a greater number of antibiotic courses taken by those that develop CDA further increases the rate of rise of zonulin before disease onset. Clinically, this novel information may be used by physicians and families to help determine if a child with a strong family history of CeD or other autoimmune diseases should more carefully avoid unnecessary antibiotics or if they should consider future therapeutics that alter disease trajectory. In summary, serum zonulin may be used in the future to predict who may develop CDA among children genetically at-risk. Given our findings, there should be continued efforts to reduce unnecessary antibiotics to aid in future disease prevention.
Acknowledgments
We would like to acknowledge Dr Takumi Konno, MD, PhD, for his guidance and assistance with ELISA testing, SIGENP (Società Italiana di Gastroenterologia, Epatologia e Nutrizione Pediatrica), and the CDGEMM subjects and their families; the entire CD-GEMM Study Group for their contributions to patient enrollment and data collection; and members of the CD-GEMM Study Group: Maria Luisa Forchielli (MD), Adelaide Serretiello (MS), Corrado Vecchi (MS), Gemma Castillejo de Villsante (MD), Giorgia Venutolo (MS), Basilio Malamisura (MD), Angela Calvi (MD), Maria Elena Lionetti (MD), Chiara Maria Trovato (MD), Nicoletta Pietropaoli (MD), Michela Perrone (MD), Lidia Celeste Raguseo (MD), Carlo Catassi (MD), Fernanda Cristofori (MD), Luca Elli (PhD), Federica Malerba (MD), and Annalisa Morelli (MD).
Glossary
- CDA
celiac disease autoimmunity
- CD-GEMM
Celiac Disease Genomic Environmental Microbiome and Metabolomic study
- CeD
celiac disease
- DGP IgG
deamidated gliadin peptide immunoglobulin G
- HLA
human leukocyte antigen
- LMR
lactose-to-mannitol ratio
- T1D
type 1 diabetes mellitus
Footnotes
Dr DaFonte performed the experiments, conducted the initial analysis, assisted in interpreting study findings, drafted the original manuscript, and reviewed and revised the manuscript; Dr Valitutti conceptualized and designed the study and critically reviewed and revised the manuscript; Ms Kenyon contributed to the conceptualization and reviewed and revised the manuscript; Dr Locascio conducted the statistical analysis, assisted in interpreting study findings, and contributed to the draft of the original manuscript; Drs Montuori, Francavilla, Passaro, Crocco, Norsa, Piemontese, and Baldassarre conceptualized and designed the study and reviewed and revised the manuscript; Drs Leonard and Fasano conceptualized and designed the study, assisted in interpreting study findings, and critically reviewed and revised the manuscript; and all authors have approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FUNDING: Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number NIH NIDDK; R56AI169645 to Dr Fasano, DK109620 and K23DK122127 to Dr Leonard. This work was further supported by the Claflin Distinguished Scholar Award to Dr Leonard. This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR002541) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
CONFLICT OF INTEREST DISCLOSURES: Ms Kenyon serves as a consultant for Takeda Pharmaceuticals; Dr Leonard acted as a consultant for Anokion; Dr Fasano is a stockholder at Alba Therapeutics, is an advisory board member for Axial Biotherapeutics and Ubiome, and has a speaker agreement with Mead Johnson Nutrition; and the other authors have declared that no conflicts of interest exist. None of the financial disclosures above are related to this study.
References
- 1. Caio G, Volta U, Sapone A, et al. Celiac disease: a comprehensive current review. BMC Med. 2019;17(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lammers KM, Lu R, Brownley J, et al. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology. 2008;135(1):194–204.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fasano A, Not T, Wang W, et al. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet. 2000;355(9214):1518–1519 [DOI] [PubMed] [Google Scholar]
- 4. Sjöström B, Bredberg A, Mandl T, et al. Increased intestinal permeability in primary Sjögren’s syndrome and multiple sclerosis. J Transl Autoimmun. 2021;4:100082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Katz KD, Hollander D, Vadheim CM, et al. Intestinal permeability in patients with Crohn’s disease and their healthy relatives. Gastroenterology. 1989;97(4):927–931 [DOI] [PubMed] [Google Scholar]
- 6. D’Incà R, Annese V, di Leo V, et al. Increased intestinal permeability and NOD2 variants in familial and sporadic Crohn’s disease. Aliment Pharmacol Ther. 2006;23(10):1455–1461 [DOI] [PubMed] [Google Scholar]
- 7. Vaarala O, Atkinson MA, Neu J. The “perfect storm” for type 1 diabetes: the complex interplay between intestinal microbiota, gut permeability, and mucosal immunity. Diabetes. 2008;57(10):2555–2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smecuol E, Bai JC, Vazquez H, et al. Gastrointestinal permeability in celiac disease. Gastroenterology. 1997;112(4):1129–1136 [DOI] [PubMed] [Google Scholar]
- 9. Vilela EG, Torres HO, Ferrari ML, Lima AS, Cunha AS. Gut permeability to lactulose and mannitol differs in treated Crohn’s disease and celiac disease patients and healthy subjects. Braz J Med Biol Res. 2008;41(12):1105–1109 [DOI] [PubMed] [Google Scholar]
- 10. Catassi C, Fabiani E, Rätsch IM, et al. Is the sugar intestinal permeability test a reliable investigation for coeliac disease screening? Gut. 1997;40(2):215–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Turpin W, Lee SH, Raygoza Garay JA, et al. ; Crohn’s and Colitis Canada Genetic Environmental Microbial Project Research Consortium; CCC GEM Project recruitment site directors include Maria Abreu. Increased intestinal permeability is associated with later development of Crohn’s disease. Gastroenterology. 2020;159(6):2092–2100.e5 [DOI] [PubMed] [Google Scholar]
- 12. Leonard MM, Camhi S, Huedo-Medina TB, Fasano A. Celiac disease genomic, environmental, microbiome, and metabolomic (CDGEMM) study design: approach to the future of personalized prevention of celiac disease. Nutrients. 2015;7(11):9325–9336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leonard MM, Kenyon V, Valitutti F, et al. ; CDGEMM Working Group. Cohort profile: celiac disease genomic, environmental, microbiome and metabolome study; a prospective longitudinal birth cohort study of children at-risk for celiac disease. PLoS One. 2023;18(3):e0282739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hill ID, Fasano A, Guandalini S, et al. NASPGHAN clinical report on the diagnosis and treatment of gluten-related disorders. J Pediatr Gastroenterol Nutr. 2016;63(1):156–165 [DOI] [PubMed] [Google Scholar]
- 15. Husby S, Koletzko S, Korponay-Szabó I, et al. European Society paediatric gastroenterology, hepatology and nutrition guidelines for diagnosing coeliac disease 2020. J Pediatr Gastroenterol Nutr. 2020;70(1):141–156 [DOI] [PubMed] [Google Scholar]
- 16. Sandström O, Norström F, Carlsson A, et al. Five-year follow-up of new cases after a coeliac disease mass screening. Arch Dis Child. 2022;107(6):596–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brown NK, Guandalini S, Semrad C, Kupfer SS. A clinician’s guide to celiac disease HLA genetics. Am J Gastroenterol. 2019;114(10):1587–1592 [DOI] [PubMed] [Google Scholar]
- 18. Romanos J, Rosén A, Kumar V, et al. ; PreventCD Group. Improving coeliac disease risk prediction by testing non-HLA variants additional to HLA variants. Gut. 2014;63(3):415–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Visser J, Rozing J, Sapone A, Lammers K, Fasano A. Tight junctions, intestinal permeability, and autoimmunity: celiac disease and type 1 diabetes paradigms. Ann N Y Acad Sci. 2009;1165:195–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Valitutti F, Leonard MM, Kenyon V, et al. ; CD-GEMM Team. Early antibody dynamics in a prospective cohort of children at risk of celiac disease. Am J Gastroenterol. 2023;118(3):574–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Valitutti F, Fasano A. Breaking down barriers: how understanding celiac disease pathogenesis informed the development of novel treatments. Dig Dis Sci. 2019;64(7):1748–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bosi E, Molteni L, Radaelli MG, et al. Increased intestinal permeability precedes clinical onset of type 1 diabetes. Diabetologia. 2006;49(12):2824–2827 [DOI] [PubMed] [Google Scholar]
- 23. Musa MA, Kabir M, Hossain MI, et al. Measurement of intestinal permeability using lactulose and mannitol with conventional five hours and shortened two hours urine collection by two different methods: HPAE-PAD and LC-MSMS. PLoS One. 2019;14(8):e0220397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Drago S, El Asmar R, Di Pierro M, et al. Gliadin, zonulin and gut permeability: Effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand J Gastroenterol. 2006;41(4):408–419 [DOI] [PubMed] [Google Scholar]
- 25. Watts T, Berti I, Sapone A, et al. Role of the intestinal tight junction modulator zonulin in the pathogenesis of type I diabetes in BB diabetic-prone rats. Proc Natl Acad Sci U S A. 2005;102(8):2916–2921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tajik N, Frech M, Schulz O, et al. Targeting zonulin and intestinal epithelial barrier function to prevent onset of arthritis. Nat Commun. 2020;11(1):1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stevens BR, Goel R, Seungbum K, et al. Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut. 2018;67(8):1555–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Del Bo’ C, Bernardi S, Cherubini A, et al. A polyphenol-rich dietary pattern improves intestinal permeability, evaluated as serum zonulin levels, in older subjects: the MaPLE randomised controlled trial. Clin Nutr. 2021;40(5):3006–3018 [DOI] [PubMed] [Google Scholar]
- 29. Asbjornsdottir B, Snorradottir H, Andresdottir E, et al. Zonulin-dependent intestinal permeability in children diagnosed with mental disorders: a systematic review and meta-analysis. Nutrients. 2020;12(7):1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fasano A. All disease begins in the (leaky) gut: role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000 Res. 2020;9:F1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cayres LCF, de Salis LVV, Rodrigues GSP, et al. Detection of alterations in the gut microbiota and intestinal permeability in patients with Hashimoto thyroiditis. Front Immunol. 2021;12:579140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pellizoni FP, Leite AZ, Rodrigues NC, et al. Detection of dysbiosis and increased intestinal permeability in Brazilian patients with relapsing-remitting multiple sclerosis. Int J Environ Res Public Health. 2021;18(9):4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kinashi Y, Hase K. Partners in leaky gut syndrome: intestinal dysbiosis and autoimmunity. Front Immunol. 2021;12:673708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sapone A, de Magistris L, Pietzak M, et al. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes. 2006;55(5):1443–1449 [DOI] [PubMed] [Google Scholar]
- 35. Malíčková K, Francová I, Lukáš M, et al. Fecal zonulin is elevated in Crohn’s disease and in cigarette smokers. Pract Lab Med. 2017;9:39–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hendy OM, Elsabaawy MM, Aref MM, Khalaf FM, Oda AMA, El Shazly HM. Evaluation of circulating zonulin as a potential marker in the pathogenesis of nonalcoholic fatty liver disease. APMIS. 2017;125(7):607–613 [DOI] [PubMed] [Google Scholar]
- 37. Küme T, Acar S, Tuhan H, et al. The Relationship between serum zonulin level and clinical and laboratory parameters of childhood obesity. J Clin Res Pediatr Endocrinol. 2017;9(1):31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fasano A. Zonulin, regulation of tight junctions, and autoimmune diseases. Ann N Y Acad Sci. 2012;1258(1):25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Leonard MM, Valitutti F, Karathia H, et al. ; CD-GEMM Team. Microbiome signatures of progression toward celiac disease onset in at-risk children in a longitudinal prospective cohort study. Proc Natl Acad Sci USA. 2021;118(29):e2020322118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. De Palma G, Nadal I, Medina M, et al. Intestinal dysbiosis and reduced immunoglobulin-coated bacteria associated with coeliac disease in children. BMC Microbiol. 2010;10:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ou G, Hedberg M, Hörstedt P, et al. Proximal small intestinal microbiota and identification of rod-shaped bacteria associated with childhood celiac disease. Am J Gastroenterol. 2009;104(12):3058–3067 [DOI] [PubMed] [Google Scholar]
- 42. Ochoa-Repáraz J, Mielcarz DW, Ditrio LE, et al. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J Immunol. 2009;183(10):6041–6050 [DOI] [PubMed] [Google Scholar]
- 43. Edwards CJ. Commensal gut bacteria and the etiopathogenesis of rheumatoid arthritis. J Rheumatol. 2008;35(8):1477–14797 [PubMed] [Google Scholar]
- 44. Harbison JE, Roth-Schulze AJ, Barry SC, et al. Gut microbiome dysbiosis and increased intestinal permeability in Australian children with islet autoimmunity and type 1 diabetes. Diabetes. 2018;67(Supplement_1):230-OR. [DOI] [PubMed] [Google Scholar]
- 45. Clemente MG, De Virgiliis S, Kang JS, et al. Early effects of gliadin on enterocyte intracellular signalling involved in intestinal barrier function. Gut. 2003;52(2):218–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. El Asmar R, Panigrahi P, Bamford P, et al. Host-dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology. 2002;123(5):1607–1615 [DOI] [PubMed] [Google Scholar]
- 47. Sturgeon C, Fasano A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers. 2016;4(4):e1251384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Łoniewska B, Adamek K, Węgrzyn D, et al. Analysis of faecal zonulin and calprotectin concentrations in healthy children during the first two years of life. an observational prospective cohort study. J Clin Med. 2020;9(3):777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang Y, Lee B, Thompson M, et al. Lactulose-mannitol intestinal permeability test in children with diarrhea caused by rotavirus and cryptosporidium. Diarrhea Working Group, Peru. J Pediatr Gastroenterol Nutr. 2000;31(1):16–21 [DOI] [PubMed] [Google Scholar]
- 50. Jian C, Kanerva S, Qadri S, Yki-Järvinen H, Salonen A. In vitro effects of bacterial exposure on secretion of zonulin family peptides and their detection in human tissue samples. Front Microbiol. 2022;13:848128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yonker LM, Gilboa T, Ogata AF, et al. Multisystem inflammatory syndrome in children is driven by zonulin-dependent loss of gut mucosal barrier. J Clin Invest. 2021;131(14):e149633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vorobjova T, Raikkerus H, Kadaja L, et al. Circulating zonulin correlates with density of enteroviruses and tolerogenic dendritic cells in the small bowel mucosa of celiac disease patients. Dig Dis Sci. 2017;62(2):358–371 [DOI] [PubMed] [Google Scholar]
- 53. Boechler M, Susi A, Hisle-Gorman E, Rogers PL, Nylund CM. Acid suppression and antibiotics administered during infancy are associated with celiac disease. J Pediatr. 2023;254:61–67.e1 [DOI] [PubMed] [Google Scholar]
- 54. Dydensborg Sander S, Nybo Andersen AM, Murray JA, Karlstad Ø, Husby S, Størdal K. Association between antibiotics in the first year of life and celiac disease. Gastroenterology. 2019;156(8):2217–2229 [DOI] [PubMed] [Google Scholar]
- 55. Zimmermann P, Curtis N. The effect of antibiotics on the composition of the intestinal microbiota - a systematic review. J Infect. 2019;79(6):471–489 [DOI] [PubMed] [Google Scholar]
- 56. Huang C, Feng S, Huo F, Liu H. Effects of four antibiotics on the diversity of the intestinal microbiota. Microbiol Spectr. 2022;10(2):e0190421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Konno T, Martinez EE, Ji J, Miranda-Ribera A, Fiorentino MR, Fasano A. Human coagulation factor X and CD5 antigen-like are potential new members of the zonulin family proteins. Biochem Biophys Res Commun. 2023;638:127–133 [DOI] [PMC free article] [PubMed] [Google Scholar]