Abstract
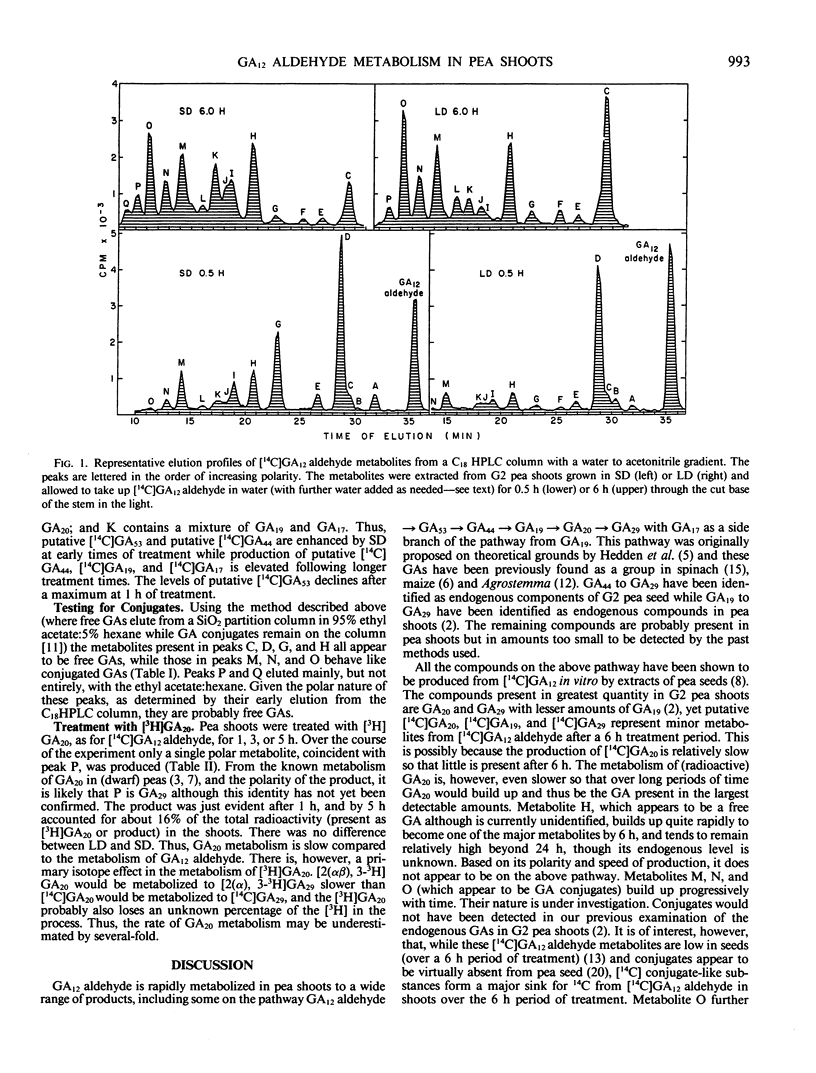
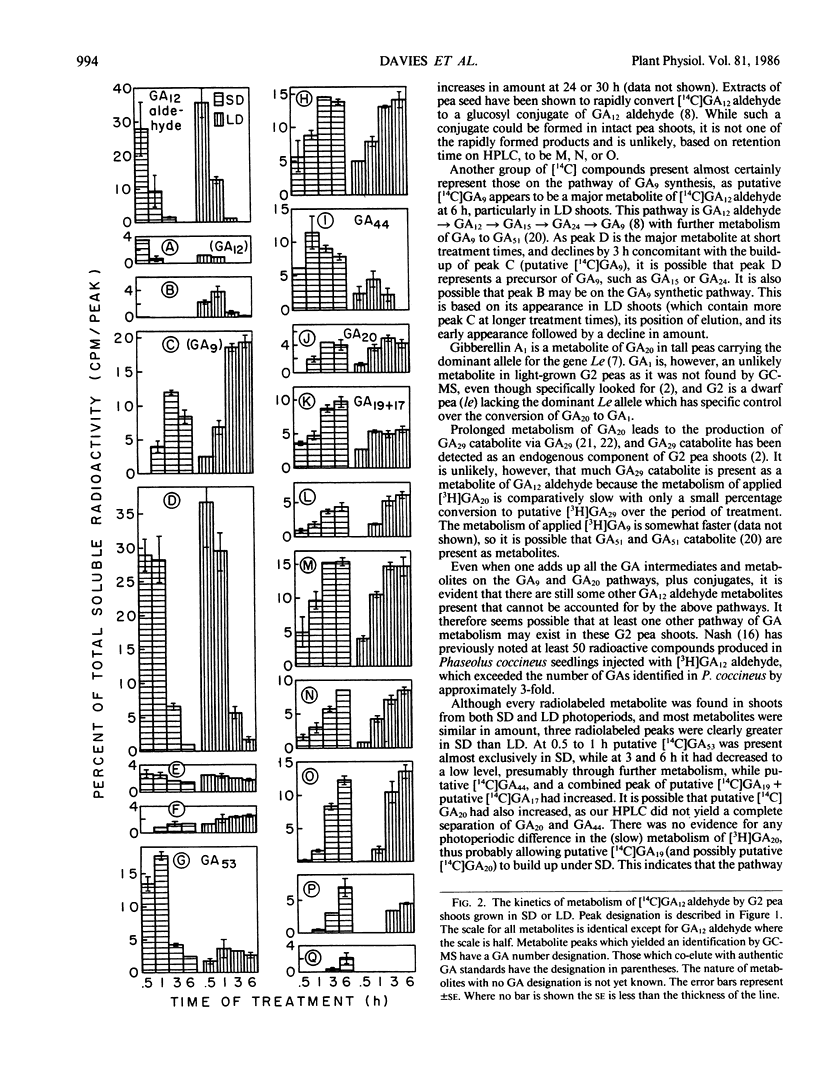
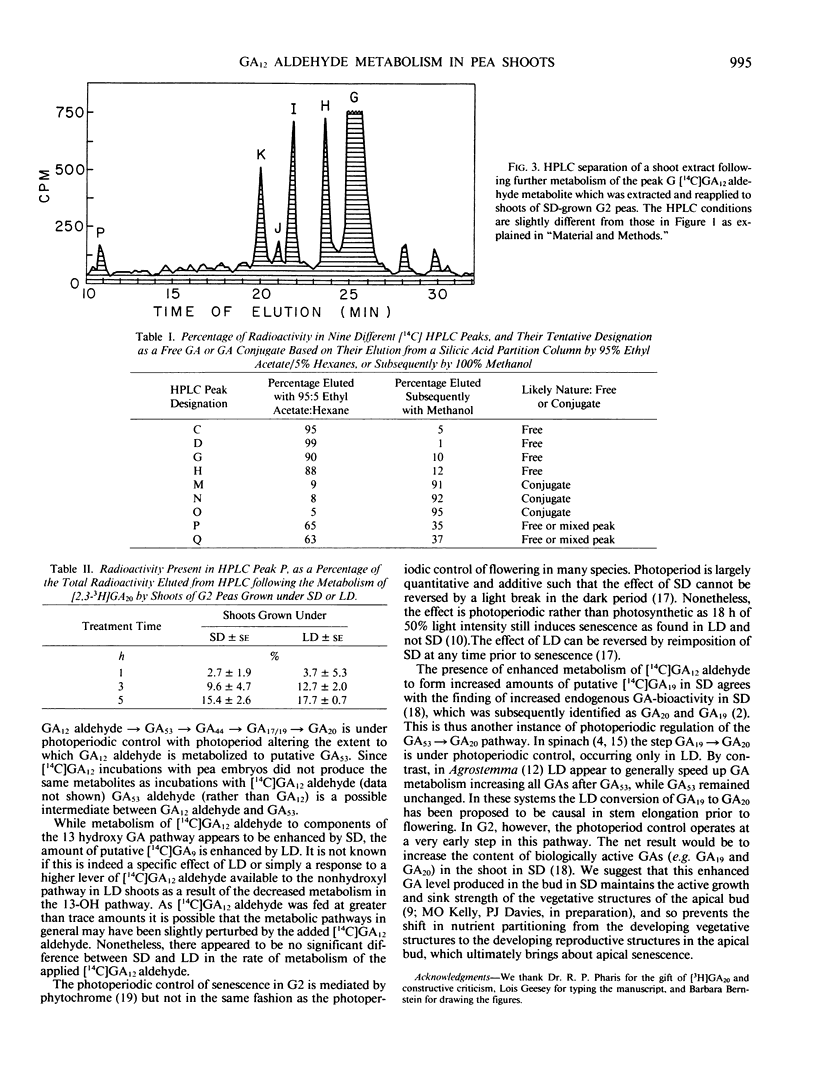
In G2 peas (Pisum sativum L.) apical senescence occurs only in long days (LD), and indeterminate growth is associated with elevated gibberellin (GA) levels in the shoot in short days (SD). Metabolism of GA12 aldehyde was investigated by feeding shoots grown in SD or LD with [14C]GA12 aldehyde through the cut end of the stem for 0.5 to 6 hours in the light and analyzing the tissue extract by high performance liquid chromatography. More radioactive products were detected than can be accounted for by the two GA metabolic pathways previously known to be present in peas. Three of the major products appear to be GA conjugates, but an additional pathway(s) of GA metabolism may be present. The levels of putative C20 GAs, [14C]GA53, [14C]GA44, [14C]GA19, and/or [14C] GA17, were all elevated in SD as compared to LD. Putative [14C]GA, was slightly higher in LD than in SD. Putative [14C]GA53 was a major metabolite after 30 minutes of treatment in SD but had declined after longer treatment times to be replaced by elevated levels of putative [14C] GA44 and [14C]GA19/17. Metabolism of GA20 was slow in both photoperiods. Although GA20 and GA19 are the major endogenous GAs as determined by gas chromatography-mass spectrometry, putative [14C]GA20 and [14C]GA19 were never major products of [14C]GA12 aldehyde metabolism. Thus, photoperiod acts in G2 peas to change the rate of GA53 production from GA12 aldehyde, with the levels of the subsequent GAs on the 13-OH pathway being determined by the amount of GA53 being produced.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnberg P. R., Maki S. L., Brenner M. L., Davis G. C., Carnes M. G. An improved enzymatic synthesis of labeled gibberellin A12-aldehyde and gibberellin A12. Anal Biochem. 1986 Feb 15;153(1):1–8. doi: 10.1016/0003-2697(86)90052-7. [DOI] [PubMed] [Google Scholar]
- Gianfagna T., Zeevaart J. A., Lusk W. J. Effect of photoperiod on the metabolism of deuterium-labeled gibberellin a(53) in spinach. Plant Physiol. 1983 May;72(1):86–89. doi: 10.1104/pp.72.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshioka M., Takeno K., Beall F. D., Pharis R. P. Purification and separation of plant gibberellins from their precursors and glucosyl conjugates. Plant Physiol. 1983 Oct;73(2):398–406. doi: 10.1104/pp.73.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki S. L., Brenner M. L., Birnberg P. R., Davies P. J., Krick T. P. Identification of Pea Gibberellins by Studying [C]GA(12)-Aldehyde Metabolism. Plant Physiol. 1986 Aug;81(4):984–990. doi: 10.1104/pp.81.4.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger J. D., Zeevaart J. A. Effect of Photoperiod on the Levels of Endogenous Gibberellins in Spinach as Measured by Combined Gas Chromatography-selected Ion Current Monitoring. Plant Physiol. 1980 Nov;66(5):844–846. doi: 10.1104/pp.66.5.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proebsting W. M., Davies P. J., Marx G. A. Photoperiodic control of apical senescence in a genetic line of peas. Plant Physiol. 1976 Dec;58(6):800–802. doi: 10.1104/pp.58.6.800. [DOI] [PMC free article] [PubMed] [Google Scholar]


