Abstract
Microplastic (MP) contamination is an acknowledged global problem that poses a severe risk to aquatic ecosystem biota. Nevertheless, little is known about their prevalence in animal construction. The main objective of our study was to reduce the gap information of seasonal abundance, distribution, composition, and risk assessment of MP contamination. The concentrations of MPs in sediment, Chironomus sp. larvae, and their tubes were found to be higher in site 2 (S2) than in site 1 (S1) during the four seasons of the year. However, MP concentrations ranged from 312 ± 64.7 to 470 ± 70 items/kg dry weight, 0.79 ± 0.16 to 1.1 ± 0.3 particles/individual, and 0.5 ± 0.04 to 0.9 ± 0.04 particles/tube in sediment, Chironomus, and chironomid tubes, respectively. Blue and red polyester fibers are the most dominant MPs which are distributed in sediment, Chironomus, and chironomid tubes. The length of the dominant fiber accumulates in Chironomus, and their tubes are highly varied compared to that of the substrate. Additionally, we found that the mean number of MPs/individual larvae in the fourth instar was significantly higher than that in the second instar. Risk indicators for the environment, polymer risk assessment, and pollution load were estimated, where they were higher in S2 than in S1 correlated to MPs abundance and polymer type. The seasonal fluctuation in MP concentration, characterization, and risk in the two sites could depend on the amount of sewage effluent discharged into the wastewater treatment plants (WWTPs), which was reflected by Chironomus sp. larvae. Therefore, further research should be done to adopt the applicability of Chironomus as MP bioindicators in various freshwater environments throughout the world.
Keywords: Microplastic, Wastewater, Sediment, Chironomus sp., Chironomid tube
Introduction
MP contamination has been recorded all over the world, from the poles to the equator, and from the ocean’s surface to the deepest abyss (Rochman et al. 2015; Blettler et al. 2018; Eerkes-Medrano and Thompson 2018; Peng et al. 2018a, b; Li et al. 2018; Mendoza and Balcer 2019; Tursi et al. 2022), and in the different media (Dobaradaran et al. 2018; Akhbarizadeh et al. 2020a, 2020b, 2021a, 2021b; Takdastan et al. 2021; De-la-Torre et al. 2022a, 2022b, 2023a, 2023b; Hajiouni et al. 2022; Kashfi et al. 2022, 2023; Mohammadi et al. 2022a, 2022b, 2023; Pizarro-Ortega et al. 2022; Pourfadakari et al. 2022; Cabrejos-Cardeña et al. 2023; Niari et al. 2023). MPs are particles of plastic smaller than 5 mm (Qu et al. 2023). They are transferred from terrestrial ecosystems via biotic and abiotic mechanisms, or they reach the aquatic environment directly through substances that contain MPs (Ory et al. 2017; Li et al. 2018). After that, MPs are further classified as primary MPs (plastic beads used in air blasting and cosmetic items) or secondary MPs (plastic fragments made from larger plastic particles) (Sharma et al. 2023). According to Burns et al. (2018) and Khedre et al. (2023a, b), MPs may be categorized into several form categories with names such as fragments, fibers, films, foam, and beads. The physical features of MPs, including density, shape, and size, may affect how they move across various environments and how they disperse (Hartmann et al. 2019). Stormwater runoff, industrial waste, and household rubbish are just a few of the pathways via which MPs may enter an aquatic ecosystem (Horton and Dixon 2018; Nel and Froneman 2018). Based on Li et al. (2018), the mean abundance of MPs in freshwater systems varied from almost none to several million pieces per cubic meter. Currently, there is exponential growth of MP research around the world since the first detection made around the coast of New Zealand in 1977 by Gregory (1977). However, there are very few studies on the existence of MPs in Africa.
Most of the research has classified each sampling site based on the principal human activities that qualitatively analyze the source of MPs (Lin et al. 2022). For instance, if research believes that a sample location having a high level of industrialization is typical of industrial districts, additional analysis will thus link industrial activities to the high MP content in the area surrounding the sampling site. However, this attribution is highly dubious since it ignores the temporal and hydrological aspects involved in MP transport via freshwater ecosystems (Klein et al. 2015). Residences may be found in highly industrialized areas. Furthermore, wastewater treatment plants (WWTPs) are well recognized as point sources of MPs because large volumes of MP-containing effluents are continually discharged (Grbić et al. 2020), even though the majority of the MPs are removed from the influents (Talvitie et al. 2017). Thus, WWTPs are frequently associated with significant levels of MP contamination. Recent research, for example, discovered greater MP concentrations in WWTP effluents compared to those in a reference location (Magnusson and Norén 2014).
Since MPs are readily observed in the internal tissues of animals (Prata et al. 2022), here, we use the term “internal MPs” to distinguish those MPs in either the digestive systems or internal organs of organisms from those distributed in the environment (environmental MPs). In general, there are much fewer studies on internal MPs (Lin et al. 2022) since the necessary processing steps become very complicated when comparing MPs sampled from water or sediment. However, we believe that there are both costs and benefits to investigating internal MPs.
Internal MPs also provide a longitudinal picture of the contamination of the environment with MPs. Throughout their life cycle, midge larvae are likely to accumulate MPs (Ziajahromi et al. 2018). When continuous sampling is not feasible, investigations of internal MPs offer a long-term picture of local MPs’ pollution. Because midge larvae have an intrinsic feeding strategy (i.e., they ingest MPs unintentionally with food), prior research has indicated that the quantity of MPs in sediments is related to the abundance of MPs in midge larvae (Nel et al. 2018). As an alternative, several studies have collected MPs in the environment utilizing a one-time sample technique, which may provide inaccurate information on the abundance of MPs because it only provides a snapshot of their contamination at that moment (Naji et al. 2017; Wagner and Lambert 2018). For instance, collecting MPs from the ocean’s surface at a particular moment in time was insufficient to assess the level of MP pollution since there was no MP buildup (Cheung et al. 2019). Studies on the temporal and geographical MPs distributions in the environment are accessible (Xia et al. 2021; Fan et al. 2022), but few studies specifically address MPs detected in living organisms.
In addition to ingesting, animals may integrate MPs into the structures they build. For instance, the marine polychaete Gunnarea gaimardi (de Quatrefages 1848) fixes MP particles in a biological structure by incorporating them into its habitat (Nel and Froneman 2018). Additionally, MPs may be included in the larval cases (biological structures) produced by a variety of epibenthic insects, including freshwater caddis fly (Trichoptera) species (Ehlers et al. 2019). As a result, larval cases made by aquatic insects may be used as bioindicators for MP evaluations of freshwater systems. Chironomid tubes are comparable biological formations found in watery settings. Therefore, to determine whether freshwater MPs might be absorbed into chironomid tubes, we examined whether MPs were present in those tubes and what qualities (shape, polymer type, color, and size) they possessed. So, this study provides a quantitative comparison of the spatiotemporal parameters affecting the variation in MPs dominance in WWTPs environment. Moreover, it quantifies the combined effects of seasonality and anthropogenic activities on internal MP concentrations and estimates the contributions of factors affecting internal MP pollution in two wastewater sites in Egypt, as well as studying the importance of chironomid tubes as indicators for MP freshwater pollution.
In Egypt, no data is available on the seasonal occurrence, characterization, and risk of MP pollution in sediment, water, or freshwater insects. Additionally, this highlights the use of chironomid tubes to reflect MP buildup in the aquatic environment. Two wastewater sites in the Sohag Governorate were chosen to perform this investigation. Every day, a considerable volume of wastewater is dumped there. Furthermore, wastewater sediment can be used as soil fertilizer, leading MPs to be transported to agricultural land surfaces, which is of major concern. As a result, the current study aims to (i) quantify the concentration of MPs in the current wastewater basin’s sediment, Chironomus sp. larvae, and their tubes throughout the four seasons of the year to determine possible MPs risks in the two wastewater sites; (ii) answer the following questions: (a) whether the MPs loads in Chironomus sp. larvae and their tubes differ according to the different aquatic system differences in plastic pollution level; (b) whether the MPs loads in Chironomus sp. larvae differ according to the developmental stage and size; and (c) finally, whether Chironomus sp. larvae and their tubes can be used as qualitative and quantitative bioindicators for MPs in aquatic ecosystems; (iii) to identify shapes, colors, size, and polymeric characteristics of MPs extracted from water, sediment, and aquatic insects; and (IV) to assess the potential risks of MPs through multiple indices. In addition, offering the required knowledge of MP contamination in Sohag governorate to the policymaker and stockholders will encourage them to take the necessary actions for improving plastic waste management.
Materials and methods
Study area
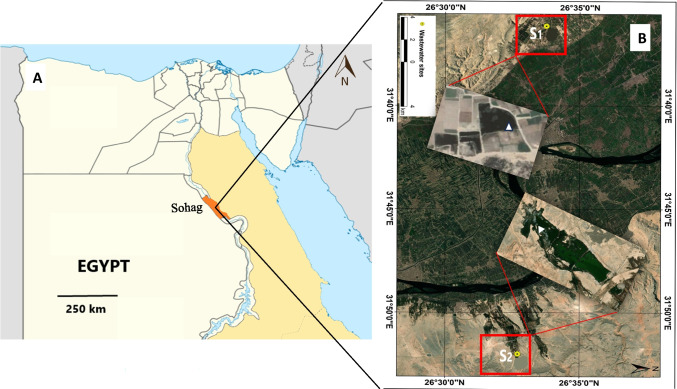
The sampling area is in Sohag City, which is in Upper Egypt, in the midst of the Nile Valley (approximately 125 km long). Sohag stretches from the southernmost point of Assiut Governorate, located at latitude 26° 57′ N, to the northernmost point of Qena Governorate, located at latitude 26° 07′ N. Between longitudes 31° 20′ and 32° 14′ E, it is enclosed (Fig. 1A). The research location is in a dry region of North Africa, which is known for its scorching summers, moderate winters, and scant rainfall. Except for the regions where there are communities, the whole valley is mostly utilized for agricultural purposes. Newly cultivated margins delineate the valley’s eastern and western flanks. Land reclamation initiatives, new urban communities, industrial zones, and wastewater disposal sites in desert zones are some of the kinds of development in the area. There are sizable plants for the textile, soft drink, and sugar sectors nearby. The industries of macaroni, sweets, onion drying, and oil dehydration are all represented by other small private firms in the study region. In Sohag Governorate, two sites have been designated for wastewater disposal. One is in the western plateau and named the West wastewater treatment plant (S1) and the other is in the east plateau named the East wastewater treatment plant (S2), which were selected for sampling (Fig. 1B) in the winter (January), summer (July), spring (April), and autumn (October) of 2022.
Fig. 1.
Egypt map showing Sohag Governorate (A). Google Earth photo showing the collecting sites (B)
Description of the collection sites
Approximately 10 km west of Sohag city, Site 1 (S1) is situated at 26° 33′ 54″ North and 31° 36′ 54″ East. It is situated in the desert region between the agricultural floodplain to the east and the Eocene limestone plateau to the west. The basin is approximately 3.786 km2 in size and has a rectangular shape. It has an average depth of 1 m and is a shallow ecology. The basin is impacted by several sources of pollution, including the ongoing discharge of wastewater from local WWTPs, human activities brought on by the expansion of adjacent agriculture, the care of animals, and sewage flow from nearby settlements. Large amounts of algae are present throughout the basin, and the water movement is so sluggish that it may be termed stagnant water. At the boundaries, various plant patches could be seen. The effluent is turbid and filthy gray in color and has a fecal or rotting stench throughout the four seasons. The gravel at the bottom of the basin is often dark in color.
Site 2 (S2) is a location in the lowland desert south of the EL cola wastewater project, which is situated at a latitude of 26° 33′ 04′′ N and longitude of 31° 50′ 55′′ E, approximately 14.6 km east of Sohag city. On a surface of approximately 4.4 km2, this location has a number of irregular basins. In the whole area, a basin of approximately 1.27 km2 was selected for sampling. Low levels of vegetation are reflected by a few isolated plant patches around the basin’s edges. The level of the water, which is approximately 1.5 m, appears to remain still. Wastewater from neighboring WWTPs has been continuously discharged into the basin. This location appears to have few human activities, most of which are brought on by the existence of a few wooden trees. Additionally, the basin has been exposed to industrial waste because an industrial district (El-Kawser) is close to the current location.
Sample collection
Sediment sampling
Through the four seasons of 2022, samples of sediment were taken from the two wastewater sampling sites (S1 and S2). Fifteen sediment samples, each weighing approximately 2 kg, were taken from the top sediment (0–5 cm depth) of each site at five randomly chosen points (three samples were taken from each point) using a stainless-steel spoon. The samples were subsequently held in glass containers in the lab at a temperature of − 4 °C to protect them from external particle contamination (sample collection was followed by a quick closure of the glass containers to prevent contamination from the air).
Chironomus sp. larvae and their tubes sampling
Chironomid larvae are the dominant genus present in the top layer of sediment in degraded habitats due to their tendency for hypoxic aquatic environments (Bere et al. 2016; Nhiwatiwa et al. 2017; Berezina et al. 2022). They deposit feeders and feed on detritus, and their associated bacteria and fungi settle in the sediment (Bertin et al. 2014). They commonly build tubes for protection (Scherer et al. 2017).
Chironomus sp. larvae were collected from the two investigated sites using a pond net (200-µm mesh size; 0.30-m aperture) at identical sediment collection points by immersing the hand net against the current while sweeping and kicking in the sediment. Chironomus sp. larvae were detected on an identification tray, and the samples were promptly preserved in 70% alcohol in 100-mL screw-capped glass vials to prevent the ejection of gut contents, which might influence the MP estimate, as described by Nel et al. (2018). The chironomid tubes were taken from sediment samples and stored in a deep freezer to extract MPs.
Laboratory analysis
Extraction of MPs from wastewater sediment
Bagheri et al. (2020) approach was modified somewhat to extract the MPs from sediment. The sediment samples were placed in spick-and-span glass jars, and they were then dried for 48 h in an oven set to 60 °C. In an uncontaminated 1-L beaker, 100 g of each dry sediment sample was placed. MPs were isolated from denser natural particles using a density separation approach. Before adding a freshly hypersaline solution of NaCl/NaI (0.5 g/cm3) to the sediment samples, 30 mL of 30% H2O2 was added to each beaker to assist breakdown of any organic matter that may have been present (Zhou et al. 2018) (all the materials were purchased from Sigma-Aldrich (Ontario, Canada)). Then, the beakers were shaken at 200 rpm for 2 days on an open-air, dual-action shaker table (OS-2000, JEIOTECH, Korea) to separate any MPs. The floating supernatants were moved to a second beaker and allowed to settle for 24 h. After being filtered using 0.45-µm filter paper to collect all MP particles, they were further preserved for microscopic examination. To ensure that all of the MPs had been extracted from the sediment sample, all of the aforementioned procedures were repeated several times.
Preparation of Chironomus sp. larvae and their tubes
Chironomus sp. was isolated and identified using keys by De Moor et al. (2003). Five Chironomus subsamples were obtained from each sample site to determine the presence of MPs. Each subsample consisted of ten fourth instar similar-sized Chironomus sp. individuals.
To prepare chironomid tubes for MP extraction, metal forceps were used to remove the larvae from their tubes. To proceed, the tubes were placed in glass Petri dishes and then separated into 5 samples (each sample including 10 tubes). Each sample’s wet weight was calculated. To avoid cross-contamination of the chironomid tubes with MPs, we meticulously washed our forceps between samples. Finally, we promptly covered all the Petri dishes containing chironomid tubes with aluminum foil to prevent airborne MP contamination.
MPs extraction from Chironomus sp. larvae and their tubes
Clean glass test tubes were used to hold each subsample of Chironomus sp. larvae. Each test tube contained 20 ml of H2O2 (35% V/V) and was shaken at 200 rpm for 12 h to allow for reaction (Windsor et al. 2019). The Chironomus sp. remnants were then vacuum filtered using 0.45-µm filter paper before being put in a clean petri dish for further investigation. Each filter was examined under a stereomicroscope to visually identify and count MP particles. Chironomid tubes were also processed according to the steps described above.
MPs ingestion throughout the development of Chironomus sp. larvae
To test how the developmental stage affects MP uptake, two different instars of Chironomus sp. larvae were selected (second (L2) and fourth (L4)) to analyze the correlation between body size (related to the morphological characteristics) in each instar and their mean MPs content. One hundred randomly selected larvae from each instar were obtained from the field collection in the summer season at S2. They were divided into ten replicates, each containing ten individuals. The wet weight of each instar individual was determined. Additionally, measurements of the body length, head capsule length, and width were detected. The ten replicates of each instar were analyzed as described before, and the corresponding number, size, and shape of ingested MP particles were determined.
MPs identification and characterization
Using a dissecting microscope with a digital camera (Carl Zeiss Suzhou Co.), all MPs were counted visually. MP particle shapes and colors were also identified and photographed. All MP measurements (diameter and length) were measured using the ImageJ program (version 1.53f, available at https://imagej.net/ij/). ATR-FTIR spectroscopy (Alpha Bruker Platinum, 1–211-6353) was performed on a zinc slender crystal with an incidence angle of 45 ± 15 and a scan period of 560 s (24 s) with a resolution of 4 cm−1 (range, 4000–400 cm−1) to identify the chemical composition of MPs. The experiment used MP particles of various colors and shapes. The data were modified using the OPUS program (Bruker Optics GmbH). The polymer type was established by comparing the obtained spectra to known reference spectra (Primpke et al. 2018).
Experiment quality assurance
Samples were always kept sealed in a vial or Petri dish to prevent contamination, except when suspicious plastics were selected. The experimenters used no plastic items and were outfitted in cotton lab coats and gloves. Before usage, all containers were washed with Milli-Q water. Prior to use, all solutions utilized in the study were passed through three filters. To ensure that there was no contamination from the lab environment, three procedural blanks were performed. Throughout the investigation, no MP particles were found in the blanks. Three Petri dishes were set up near the workstation for a day to collect airborne particles to calculate the amount of contamination that was airborne. The results of this investigation were not considerably impacted by procedural contamination because we only collected one cotton fiber sample.
MPs risk assessment
Pollution load index (PLI)
The following equations (Wang et al. 2020) were used to generate the pollution load index (PLI), which was used to quantify the risk of MPs contamination (Kasamesiri et al. 2023).
where is the MP concentration at sample site j and is the background MP concentration. The reference values for MPs were adopted according to worldwide records for sediments (1.79 items/kg DW) (Guo et al. 2021). was divided into four degrees of pollution by Guo et al. (2021).
Polymer risk assessment index
Li et al. (2020) calculated the polymer risk index (H) as follows:
where is the proportion of each polymer type at each sample site and is the polymer hazard score calculated by Lithner et al. (2011), with PP = 4, PES = 4, and PE = 11. Lithner et al. (2011) and Guo et al. (2021) divided H into four levels: level I, < 10; level II, 10–100; level III, 100–1000; and level IV, > 1000.
Potential ecological risk index (RI)
Has been used to assess the ecological and toxicological consequences of MPs (Peng et al. 2018a, b; Ranjani et al. 2021).
denotes the toxicity coefficient of MPs. Guo et al. (2021) identified five contamination thresholds for which are as follows: level I is less than 150, level II is 150–300, level III is 300–600, level IV is 600–1200, and level V is more than 1200.
Statistical analysis
The main characteristics of MP concentration in sediment, Chironomus sp. larvae, and their tubes collected from various seasons and sites were described using descriptive statistics (mean and standard deviation SD), which were then submitted to one-way ANOVA (analysis of variance). Differences between means were deemed significant when P < 0.05. Using a χ2 test, it was possible to compare the relative proportion of MP lengths in sediment and Chironomus sp. larvae. The connection between the independent factors and the dependent variables was examined using univariate regression and Pearson rank correlation analysis. Using IBM SPSS (ver. 22, IBM Corp., Armonk, NY, USA), data analysis was carried out.
Results
Seasonal abundance and characterization of MPs in sediment
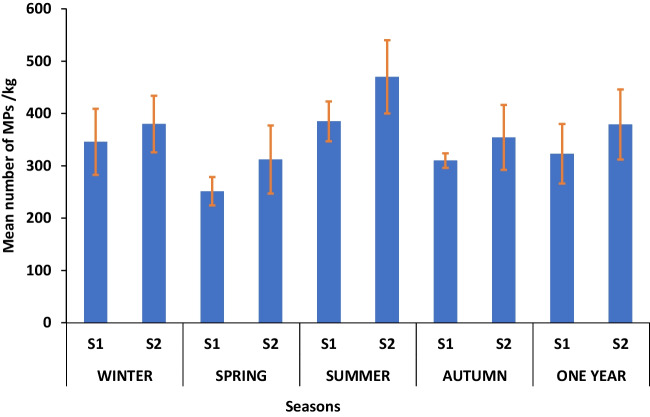
MPs were detected in all sediment samples with a 100% detection rate. Figure 2 shows the seasonal distribution of MPs in the sediment of the two wastewater sites, where the abundance of MPs in S1 and S2 during the winter season was 345.8 ± 63 and 380 ± 54 items/kg, respectively, with a mean value of 363 ± 24 items/kg. The spring season MP abundances in S1 and S2 were 251.4 ± 27.2 and 312 ± 64.7 items/kg, respectively, with a mean value of 281 ± 43 items/kg. The summer season MP abundance in S1 and S2 was 385.2 ± 38.2 and 470 ± 70 items/kg, respectively, with a mean value of 427 ± 60 items/kg. During the autumn season, the abundance of MPs in S1 and S2 was 310 ± 84 and 354.2 ± 62 items/kg, respectively, with a mean value of 332 ± 31 items/kg. Statistically, the abundance values varied significantly from season to season at the two sampling sites (P < 0.05), and the abundance of MPs was significantly higher in summer than in the other seasons (P < 0.05). Additionally, the MP abundance was significantly higher in S2 than in S1 in all seasons of the year (P < 0.05).
Fig. 2.
Mean seasonal abundance of MPs in the sediment of the two sites of wastewater
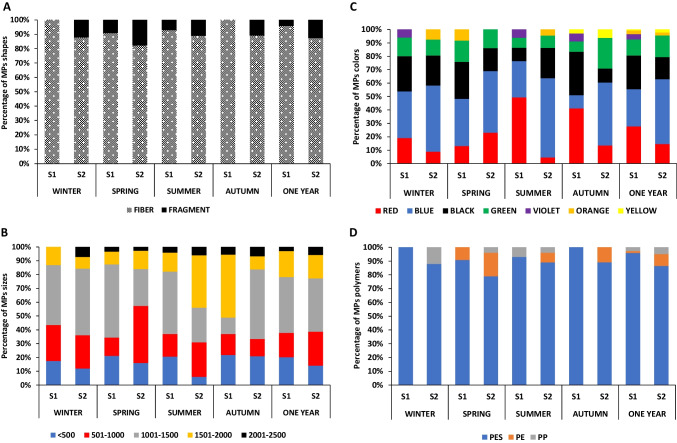
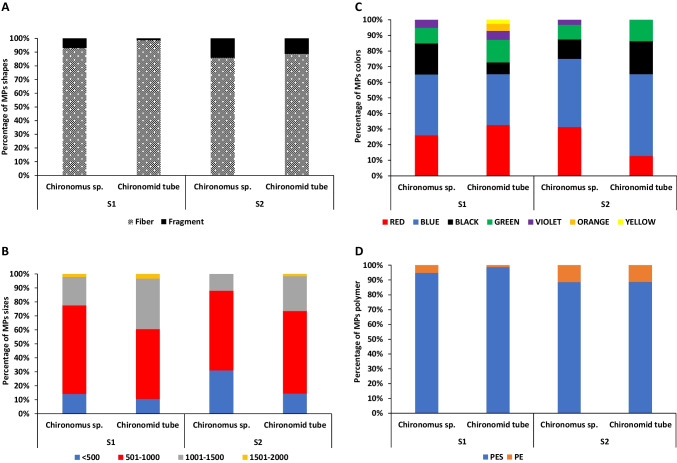
The MP shapes detected in the sediment of the two wastewater sites were fibers and fragments only (Fig. 3). Annually, fibers were the shape of the most prevalent particles observed in S1 and S2 accounting for 96% and 88%, respectively (Fig. 4A). Statistically, no significant differences in the abundance of fibers were recorded between S1 and S2 throughout the investigated year (P > 0.05). However, fragments were significantly higher in S2 than in S1 (P < 0.05).
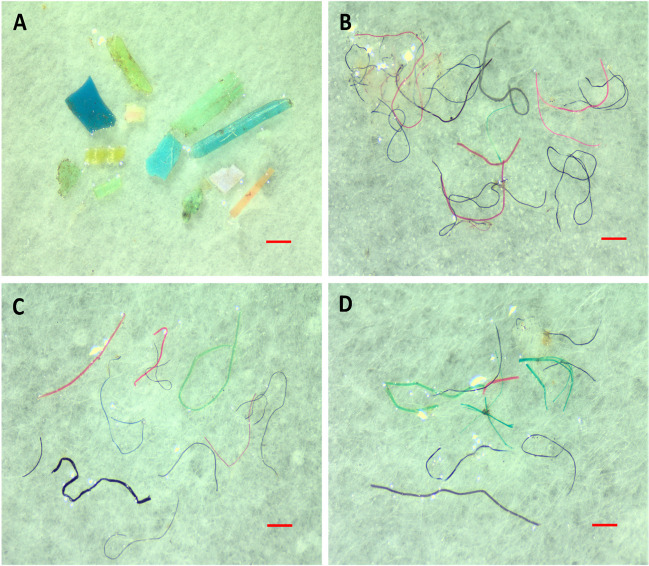
Fig. 3.
Photographs showing different shapes of microplastics obtained from sediment (A and B), Chironomus sp. (C), and chironomid tubes (D). (A) fragments and (B–D) fibers. The scale bar = 250 µm
Fig. 4.
The percentage of the different microplastic shapes (A), lengths (B) with µm, colors(C), and chemical composition (D) collected from the sediment of the two wastewater sites
Based on the length of sediment MP particles, MPs could be classified into five size classes: < 500, 501–1000, 1001–1500, 1501–2000, and 2001–2500 µm (Fig. 4B). The lengths of the fibers ranged from 684 to 2390 µm with an average of 1429 ± 495 µm and the fragments ranged from 157 to 1032 µm with an average of 554 ± 269 µm. Considering the width, the fibers ranged from 13 to 18 µm with an average of 14.6 ± 4 µm, and the fragments ranged from 64 to 632 µm with an average of 278 ± 181 µm. According to the MP size distribution during different seasons, the size in the range of 1001–1500 µm was the most dominant (39%), followed by 501–1000 µm (20%). According to the two wastewater sites, in S1, the most abundant lengths of MPs ranged from 1001–1500 µm, < 500 µm, and 1501–2000 µm (40%, 20%, and 18%, respectively); however, in S2, most MPs ranged from 1001–1500 µm, 501–1000 µm, and 1501–2000 µm (38%, 24%, and 17%, respectively) (Fig. 4B). Statistical analysis showed that the annual abundance of MP in the range of 1001–1500µm was significantly higher than that of the other MPs size classes (P < 0.05), and a significantly higher abundance of MPs size class < 500 µm was observed in S1 than in S2 (P < 0.05). However, MP size in the range of 501–1000 µm was significantly higher in S2 than in S1 (P < 0.05).
Figure 4C shows the distribution patterns of MP colors in the sediment samples. Fibers and fragments were introduced in a wide spectrum of colors, including red, blue, black, green, violet, orange, and yellow. Blue, red, black, and green colors observed in sediment samples accounted for 35%, 23%, 22%, and 13%, respectively, of the total MP particles. The distribution of MP colors displayed seasonal variation in the two sampling sites. Statistically, the red color proportion was significantly higher in S1 than in S2 (P < 0.05). However, the percentage of blue color was significantly higher in S2 than in S1 (P < 0.05).
The MP particles collected from all sediment sampling sites in this study were analyzed by FTIR spectroscopy to identify common polymers. The following polymer types were identified in the sediment: polyester (PES), polyethylene (PE), and polypropylene (PP) (Fig. 5). Polymers of MPs in the winter, spring, summer, and autumn seasons were mostly PES (93%, 86%, 91%, and 94%, respectively), followed by PP in winter (7%), PE and PP in spring (14% and 2%, respectively), PP and PE in summer (5.5% and 3.5%, respectively), and PE in autumn (6%) (Fig. 4D). Significantly, a higher abundance of PES was found in sediment when compared with other polymers (P < 0.01). PP was significantly more abundant in winter and summer (P < 0.05), and PE was more abundant in spring (P < 0.05) than in the other seasons. Considering the two sites of wastewater, the most abundant polymers of MPs were PES (96% and 87%, respectively), followed by PP and PE in S1 (3% and 1%, respectively) and PE and PP in S2 (9% and 4%, respectively) (Fig. 4D). No significant differences in the abundance of PES were found among the two sites (P > 0.05) throughout the year. However, the abundance of PE and PP was more significant in S2 than in S1 (P < 0.05).
Fig. 5.
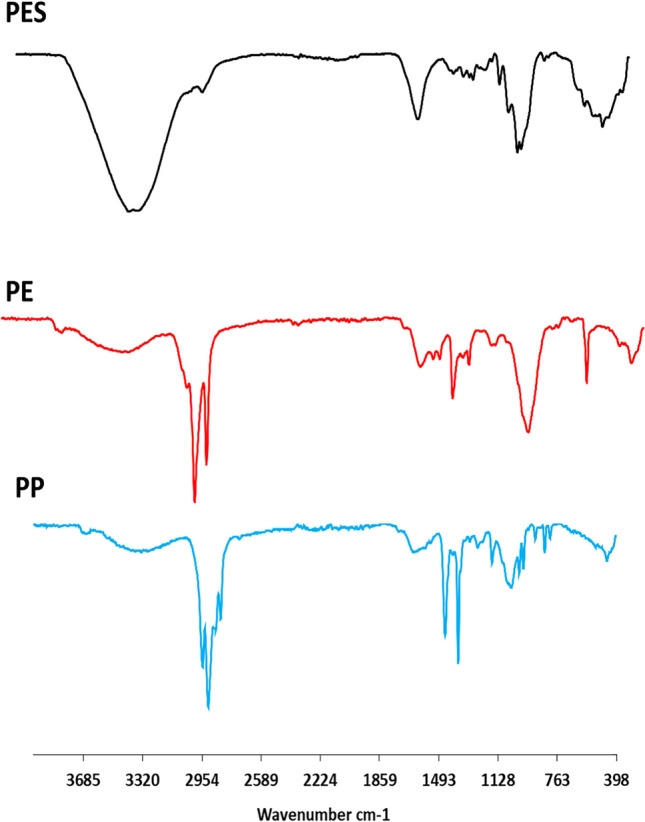
Micro-FTIR spectra of representative microplastic polymers extracted from sediment, Chironomus sp. larvae, and their tubes (PES, polyester; PE, polyethylene; and PP, polypropylene)
MPs load indices
The PLI values of MP pollution during various seasons are displayed in Table 1. As can be observed in Table 1, calculated PLI values of the two wastewater sites over various seasons were intermediate, indicating a moderate pollution discharge level (hazard category II). The greatest and lowest PLI values were also recorded in the summer and spring, respectively. Additionally, in every season, PLI values were greater in S2 than in S1. Table 1 lists the H values for MP contamination at the two wastewater sites over various seasons. In Fig. 4D, the percentages of identified polymers used to calculate H are shown. Approximately equal percentages of each polymer were found in each of the four seasons. As a result, the obtained H values were nearly comparable between seasons. A medium danger to the environment is indicated by the H values of the polymers in various seasons, which fall under category III. This is due to the toxic properties of the polymers. In the two wastewater sites, the highest H of MPs was identified during the spring. Additionally, the MP RI index of sediment from the two locations indicated a modest degree of danger (degree II).
Table 1.
Seasonal variations of microplastics impact indices in the sediments of the two wastewater sites
| Seasons | Site 1 (S1) | Site 2 (S2) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Winter | 193 | 14 | 403 | 1.16 | 223 | 212 | 15 | 400 | 1.05 | 223 |
| Spring | 141 | 12 | 463 | 1.8 | 259 | 174 | 13 | 519 | 1.7 | 290 |
| Summer | 215 | 15 | 420 | 1.04 | 223 | 263 | 16.5 | 449 | 0.96 | 251 |
| Autumn | 173 | 13 | 410 | 1.29 | 223 | 198 | 14 | 470.7 | 1.321 | 263 |
| 1 year | 181 | 13.5 (medium) | 416 (level III) | 1.28 | 232 (level II) | 212 | 15 (medium) | 460 (level III) | 1.21 | 257 (level II) |
CFj contamination factor, PLIj pollution load index, H polymer risk assessment index, Tj toxicity coefficient of MPs, and RI potential ecological risk index
Seasonal abundance and characterization of MPs in Chironomus sp. larvae and their tubes
Chironomus sp. larvae
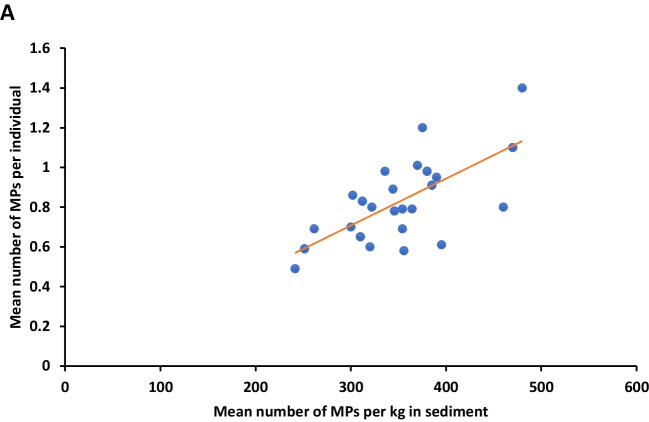
A total of 331 MP particles were extracted from 400 larvae across the two sites. The number of MP particles in S1 and S2 was 146 and 185, respectively. The maximum values of MPs per individual in S1 and S2 were observed in summer (0.91 ± 0.3 and 1.1 ± 0.3 particles/ind, respectively), followed by winter (0.78 ± 0.2 and 0.98 ± 0.028 particles/ind, respectively), but the minimum value of MPs in S1 was observed in spring (0.59 ± 0.1 particles/ind) and in autumn at S2 (0.79 ± 0.16 particles/ind) (Fig. 6A). Statistical analysis showed significant seasonal differences in MPs load per individual at both sites (P < 0.05). Moreover, the MP load per individual was significantly higher in S2 than in S1 in winter, spring, and autumn (P < 0.05). No significant difference was observed between the two sites in summer (P > 0.05). Regression analysis revealed a strong correlation between the number of MPs in the sediment and the number of MPs per individual larva in different seasons at both sites (r = 0.96, P < 0.05), as shown in Fig. 7. Higher significant seasonal differences in the size of Chironomus sp. larvae were found in S1 than in S2 (P < 0.05).
Fig. 6.
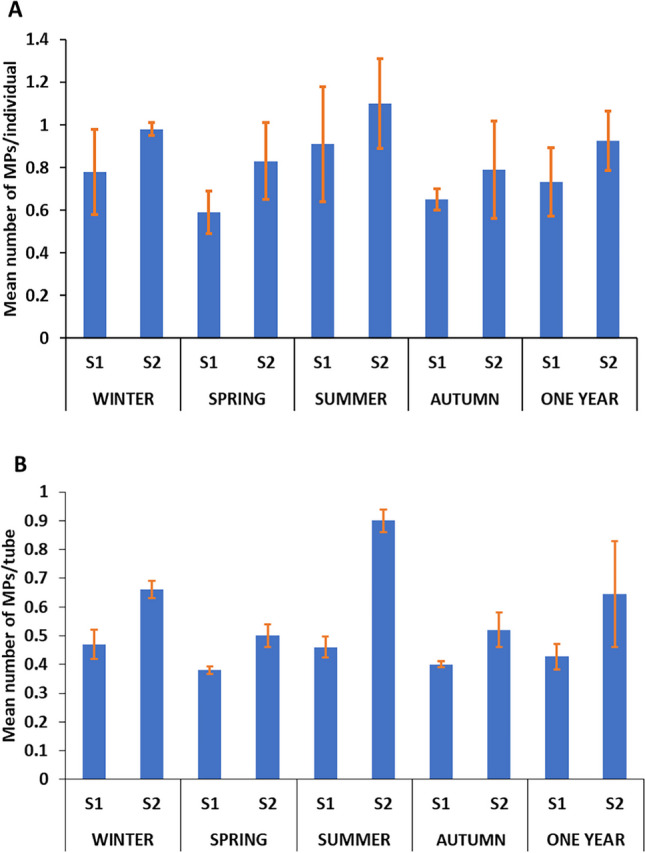
Mean seasonal abundance of MPs per individual of Chironomus sp. (A) and per chironomid tube (B) collected from the two sites of wastewater
Fig. 7.
Relationship between the mean number of MPs per kg in sediment and per individual of Chironomus sp. larvae of the two wastewater sites
Most of the particles of MPs ingested by chironomid larvae were fibers and ranged from 86 to 93% of the total ingested MP particles in the two wastewater sites. The remaining proportions were fragments (Fig. 8A). The lengths of these fibers ranged from 522 to 1400 µm with an average of 840 ± 285 µm, while the fragments were between 86 and 94 µm with an average value of 90 ± 13 µm. Additionally, the MPs in the length range of 501–1000 µm accounted for the highest percentage (61%) (Fig. 8B). According to the MPs colors, blue was the most observed color (40.5%), followed by red (28%) and black (16%) (Fig. 8C). The dominant polymer in the larvae was polyester (89.5%) followed by polyethylene (10.5%) (Fig. 8D). The percentage of chironomid larvae contaminated with MPs was 95% and 100% in S1 and S2 of all samples collected during the study period, respectively.
Fig. 8.
The percentage of the different microplastic shapes (A), lengths (B) with µm, colors(C), and chemical composition (D) collected from Chironomus sp. larvae and their tubes (chironomid tube) of the two wastewater sites
The relationship between MP size distribution across sediment and Chironomus sp. larvae
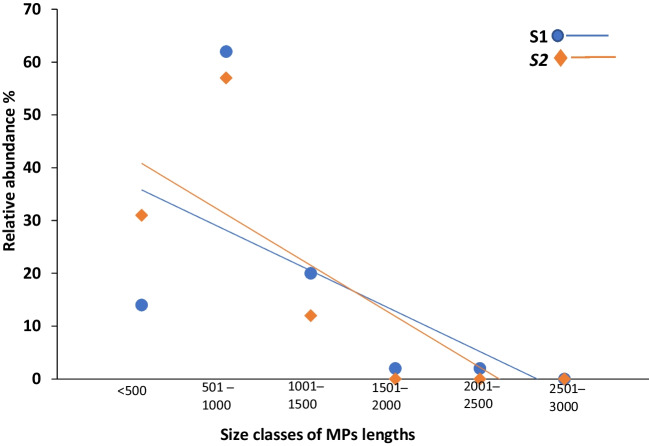
We performed a linear model analysis to examine the relationship between MP length distribution in sediment and that in chironomid larvae samples. Linear model analysis showed a linear relationship between MPs’ relative abundance and MP length class (Fig. 9). The length distribution of MPs detected in chironomid larvae demonstrated that MP concentrations decreased with increasing MP length. The percentage of MP < 1000 µm was 84% and 87% in the larvae obtained from S1 and S2, respectively. In addition to the evaluation of complete size distributions, we focused on the specific size class of 501–1000 µm, which may constitute a more favorable size for ingestion. As previously illustrated (Fig. 4B and Fig. 8B), the relative abundance of MPs particles sized 501–1000 µm was significantly higher in chironomid larvae than in their corresponding host sediments “S1 (X2 = 8.5, P < 0.05)” and “S2 (X2 = 4.7, P < 0.05)”.
Fig. 9.
The relative abundance of MP size distribution across Chironomus sp. collected from the two wastewater sites
Effect of Chironomus sp. larval size on the abundance of MPs/individual
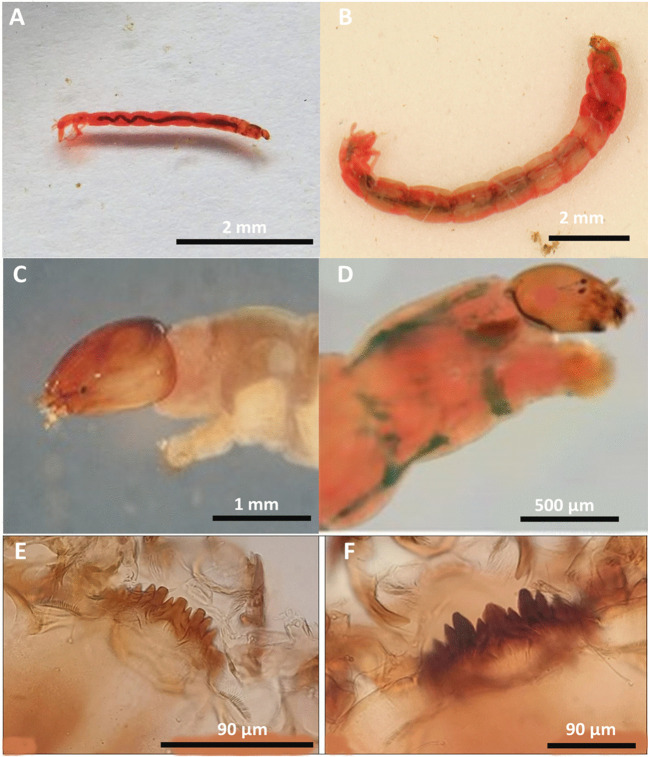
To evaluate how larval development affects MP uptake, a comparison between second (L2) and fourth (L4) instar larvae in terms of morphological characteristics was performed and is presented in Table 2. The data revealed that both body weight and length were larger in L4 than in L2 (Fig. 10). Moreover, L4 had a head capsule width of 0.55–0.74 mm with an average of 0.69 ± 0.15 mm and a corresponding mentum width of 0.158–0.176 mm with an average of 0.16 ± 0.01 mm, which was larger than those in L2. Statistically, the average body weight, body length, head capsule (width and length), and mentum width were significantly greater in L4 than in L2 (P < 0.05).
Table 2.
Measurement of the weight (mg), body length (mm), head capsule width (mm), head capsule length (mm), and mentum width (mm) and characterization of MPs in the second (L2) and fourth (L4) instar larvae of Chironomus sp. (mean ± SD)
| Stage | The mean weight of individual (mg) | Mean body length (mm) | Mean head capsule width (mm) | Mean head capsule length (mm) | Mean mentum width (mm) | Mean MPs particles/individual | The mean length of MPs (µm) |
|---|---|---|---|---|---|---|---|
| Second instar (L2) | 4.8 ± 1.2 | 3.2 ± 0.5 | 0.21 ± 0.0.1 | 0.213 ± 0.022 | 0.059 ± 0.004 | 0.52 ± 0.11 | Fibers 422 ± 84 |
| Fourth instar (L4) | 8.6 ± 2.4 | 14.8 ± 6.3 | 0.69 ± 0.15 | 0.37 ± 0.041 | 0.162 ± 0.013 | 0.91 ± 0.2 | Fibers 937 ± 120 |
| Fragments 73 ± 20 |
Fig. 10.
Whole body shape (A, B), head capsule (C, D), and mentum (E, F) of second (L2) and fourth (L4) instar larva of Chironomus sp., respectively
Regarding MP load/larva, the number of MP particles/L4 was significantly higher (P < 0.05) than that in L2. Regression analysis revealed a significant positive effect of head width on MP count in Chironomus sp. (r = 0.85, P < 0.05). Furthermore, fibers were the only type of MPs detected in L2 samples, ranging from 397 to 581 µm with an average length of 422 ± 84 µm. However, fibers and fragments accounted for 83% and 17%, respectively, in L4 samples. The fiber length ranged from 894 to 1132 µm with an average length of 937 ± 120 µm.
Chironomid tubes
Figure 6B shows the seasonal abundance of MPs in chironomid tubes at S1 and S2. Chironomid tubes were observed during the investigated year in both wastewater sites. A total of 215 MP particles were extracted from 400 tubes across the two sites. The number of MP particles in S1 and S2 was 130 and 85, respectively. According to the number of MPs per chironomid tube, the highest values were observed in summer in both S1 and S2 (0.47 ± 0.05 and 0.9 ± 0.04 particles/tube, respectively). However, the lowest values were recorded in spring (0.38 ± 0.01 and 0.5 ± 0.04 particles/tube, respectively). It is important to note that the mean number of MPs per gram (w.w.) of the chironomid tube (particles/g) (data from the two sites) was 6.5 ± 2.3 particles/g. Significant seasonal differences in MP load per tube were observed in both S1 and S2 (P < 0.05). Moreover, the MP’s load was significantly higher in the S2 than that in the S1 in all seasons (P < 0.05). A strong correlation was recorded between the number of MPs in sediment and the average load of MPs in chironomid tubes at both sites (r = 0.87, P < 0.05).
Based on the type of MPs, fibers were the highest proportion and ranged from 79 to 100% of the total extracted MP particles, and the remaining proportion was fragments (Fig. 8A). The length of extracted fibers ranged from 603 to 1546 µm with an average of 962 ± 287 µm, while the fragments were between 187 and 1007 µm with an average of 597 ± 386 µm. The MPs in the size range of 501–1000 µm and 1001–1500 µm accounted for the highest proportion (54% and 30.5%, respectively) (Fig. 8B). According to the MP colors, blue was the most abundant (42%), followed by red (22.5%) and black (14.5%) (Fig. 8C). Polyester was the dominant polymer in chironomid tubes (93%), followed by polyethylene (7%) (Fig. 8D).
Discussion
The main objective of this study is to highlight the seasonal abundance and characteristics variation of MPs in wastewater sediment of Sohag Governorate, Egypt that have not been reported yet. In addition, optimizing Chironomus sp. larvae and their protective structural buildings “chironomid tubes” as an effective indicator might reflect MP contamination. Considering the poorly managed WWTPs in developing countries (Mema 2010), they represent an important source of MP pollution in aquatic ecosystems (Chang 2015). WWTPs receive wastewater contaminated with MPs through domestic discharges including laundry wastewater and the uncontrolled discharge of industrial wastewater, in addition to landfill leachates (Wu et al. 2022). It is important to indicate that the study sites S1 and S2 are closely located to the main WWTPs in Sohag Governorate and continuously receive the discharged wastewater effluents. The results showed moderate seasonal abundance of MPs in both sites, while high values were recorded in summer (385.2 ± 38.2 and 470 ± 70 items/kg) in S1 and S2, respectively. Similar findings were recorded in the surface sediments of 28 stations in Sishili Bay, China (499.76 ± 370.07 items/kg d.w) (Zhang et al. 2019). However, the MP abundance was significantly higher in S2 than in S1 in all seasons of the year. This variation might be associated with the amount of sewage effluent discharged to the basins from the WWTPs. Among the dominant shapes of MPs observed in aquatic systems, fibers were the most dominant (Hajji et al. 2023). It has been reported that polyester-based cloths release over 1900 MP fibers in each single wash (Browne et al. 2011). This is consistent with our results revealing the high dominance of MP fibers in both WWTP sites accounting for 96% and 88% in S1 and S2, respectively. Considering most domestic wastewater of the majority of Sohag city citizens is discharged into WWTPs close to S1, while industrial wastewater mixed with domestic effluents is discharged into WWTPs near S2. That could explain the higher abundance of fibers in S1 than in S2. On the other hand, the strong relations between MP fragments and industrial activities (Jin et al. 2023) indicate why fragments were significantly higher in S2 than in S1. The sedimental MP fibers with lengths 1001–1500 µm are the most abundant in both sites (40 and 38%), in S1 and S2, respectively, concerning other length classes that agreed with the findings of Zhang et al. (2019). MP debris in the open environment is continuously subject to mechanical, chemical, and biological degradation (Andrady and Koongolla 2022) which could confirm the high percentage of small MP fibers detected in the current study. To obtain attractive plastic products corresponding with actual usage needs, plastic products are stained with different colors (Zhao et al. 2022). While environmental MPs appear in a wide variety of colors, blue-colored microplastics were the dominant color category (Athapaththu et al. 2020). In other findings, black, blue, and red are the most predominant colors (> 80%) (Montoto-Martinez et al. 2020). From this point, blue, red, black, and green MPs have 93% of the total MP particles collected from both sites. Notably, the consistency of MPs’ colors all over the world could reflect the popularity of plastic products and their globalization.
By FTIR, three polymers were identified: polyester (PES), polyethylene (PE), and polypropylene (PP) for the sedimental MPs. PES has a higher abundance in sediment when compared with other polymers in both WWTP sites. PES is one of the most important synthetic fibers that is widely used in a variety of other products including clothing and carpets, so it has the potential to release microplastics into the environment, especially during the manufacturing and cleaning process (Šaravanja et al. 2022). Therefore, domestic sewage derived from point sources plays an important role in MP pollution. While no significant differences in the abundance of PES in both sites, PE and PP were more significant in S2 than in S1. The wide uses of PE and PP in the industry (Li et al. 2021) confirm their higher abundance in S2. In a recent study, Ghani and coauthors found that MP fragments collected from the Red Sea belong to four plastic polymers, whereas PE and PP are the most common (Ghani et al. 2023). That could link the relative abundance of fragments with that of both PE and PP distributed in S2.
The risk assessment of MPs needs robust estimation of the characteristics, prevalence, distribution, and polymer types (Lindeque et al. 2020). Additionally, the wide variation in the abundance units of MPs led to the development of new standard parameters like pollution load index PLI, polymer risk index H, and potential ecological risk index RI. The pollution load index PLI is frequently used to explore the risk of MPs in sediment (Yin 2023). Our results revealed intermediate PLI values of the two wastewater sites over various seasons, while the greater values were recorded in the summer. Moreover, PLI values of S2 are greater than in S1 in correction with the higher abundance of MPs in this site. PLI indicated the variability of MP’s risk in different locations around the world which ranged from low (Neelavannan et al. 2022) to moderate risk (Kabir et al. 2022). In Egypt, PLI indicated moderate MPs pollution whether in the river Nile (Shabaka et al. 2022) or in the Red Sea (Ghani et al. 2023). Of note, the information submitted from abundance risk PLI is unreasonable without assessing the chemical composition of the collected MPs (Ding et al. 2022). Polymer risk assessment index H depends on the hazard score of each polymer, which greatly varies from one polymer to another (Lithner et al. 2011). Generally, the values of the H index refer to moderate risk (level III) of MPs distributed in both wastewater sites attributed to the high abundance of polyester of low hazard score (4). However, the relatively high value of the H index of S2 compared to S1 or in the spring of both sites corresponds to the increased percentage of polyethylene with 11 scores. However, the adverse effects of MPs on organisms and humans necessitate exploring their ability to internalize the organisms that inhabit such MPs-polluted environments.
Chironomids are widely distributed in wastewater sediments of Sohag Governorate (Khdre et al. 2023). Considering the high sensitivity and dominance of Chironomus larvae in polluted environments, they can be used to diagnose the ecological conditions variations in aquatic habitats. To such end, we examined the ability of Chironomus to reflect the environmental contamination with MPs at different seasons. Notably, our results showed that the mean number of MPs/individuals in chironomid larvae was significantly higher in S2 than in S1 taking a similar trend to sedimental MPs. Moreover, significant seasonal differences were observed in the MP loads within chironomid larvae at both sites. As a deposit feeder, there is a direct relationship between the accumulated MPs inside Chironomus larvae and those located in their environment (Nel et al. 2018; Khdre et al. 2023a). This relation was confirmed by regression analysis at different seasons of both sites. Since MPs accumulation has potential adverse effects on wildlife and humans (Xia et al. 2020), we observed growth inhibition of Chironomus larvae in S2 than in S1 proportionally with MPs abundance. A high abundance of MPs introduces more changes in substrate which in turn limits food uptake and therefore inhibits larval growth (Vos et al. 2002). In addition, MPs can fill the gut of Chironomus causing a negative energetic balance (Prata et al. 2023). However, malnutrition and sorbed contaminants of plastic additives are potential scenarios of impacts of MP accumulation (Narayanan 2023). As mentioned earlier for other freshwater invertebrates exposed to MPs, growth reduction was observed for Gammarus pulex (Redondo-Hasselerharm et al. 2018). To address whether MPs accumulated through development, MPs’ abundance inside two different larval instars was determined. The results revealed that the mean number of MPs/individual larvae in the fourth instar was significantly higher than that in the second instar. Also, MPs in the second instar larvae lacked fragments and had shorter fibers than those in the fourth instar larvae, which may be related to morphological restrictions in the size of the head capsule and functional mouthparts. These findings confirm the results of Redondo-Hasselerharm et al. (2018) who reported the preference in particle size and the quantity of ingested polystyrene particles changed throughout the development.
Shape, color, chemical composition, and other physical and chemical characteristics are primary factors that affect the hazards of MPs (Yin et al. 2023). Fibers were the main MPs type, accounting for 86–93% of the total MPs extracted in Chironomus sp. larvae, consistent with their higher abundance in the sediment of the two sampling sites. The regression analysis revealed that the relative abundance of MPs in Chironomus decreased as their size increased. Accordingly, the thinner width (13–18 µm) of fibers is a vital parameter stimulating their ingestion (Pirc et al. 2016). In this regard, previous studies reported the dominance of fibers in freshwater invertebrates (Naji et al. 2018; Akindele et al. 2019; Bertoli et al. 2022; Khdre et al. 2023b). On the other hand, it is necessary to pay attention to the detrimental impacts of MP colors on aquatic organisms (Chen et al. 2020). Considering the nonselective deposit-feeding of Chironomus, its larvae highly accumulated, blue-colored MPs (40.5%) compared to the other colors according to its abundance in the surrounding environment. It has been reported that daphnids are probably not able to distinguish algae from colored MPs (Chen et al. 2020). Nonetheless, blue-green algae are food resources for collector-gatherer groups (Parker et al. 2022), which may support high loads of blue color within the present larvae besides the dominance of blue MPs in their habitats. The length of fibers also affects their ingestion. Chironomus prefers low-length fibers (501–1000 µm) rather than the dominance fibers (1001–1500 µm) in the sediment. The limited dimension of the mouth apparatus and the difficulty in ingesting larger particles are the main factors that underline the small-sized microplastic selectivity of Chironomus (Prata et al. 2023). In addition, the ingestion of MPs through the alimentary canal is subject to variable conditions of physical and chemical digestion that lead to MPs-erosion and fragmentation (Sanchez-Hernandez 2021). The dominant polymer types accumulated in the Chironomus sp. larvae reflected the abundance of polymer types in the sediment (Munari et al. 2017). Therefore, Chironomus larvae accumulate polyester corresponding to its abundance in the host sediment. This suggests that Chironomus could be best employed as an MP qualitative bioindicator in freshwater. Considering the vital ecological role and important position in nutrient cycling that Chironomus has, any change in its distribution or physiological homeostasis will directly affect the higher organisms of the trophic web.
Some aquatic organisms create shelters to live inside using the available material in their environment. Since MPs are distributed in the surrounding environment, they could be used as building material (Nel and Froneman 2018). Polyvinyl chloride and polyester particles were incorporated into cases of the caddisfly (Ehlers et al. 2019). The accumulation of MPs in the tubes of Chironomus was not well highlighted. Herein, the seasonal variations of MP abundance in Chironomus tubes in both WWTP sites were detected. The results showed that MP abundance in C. tubes was significantly higher in S2 than in S1 and varied with the different seasons following the abundance pattern of MPs in sediment. Blue and red polyester fibers were the most abundant shape in the collected tubes, while a few percentages of the fragments were recorded following a similar pattern of sedimental MPs. Nonetheless, MP particles (54%) had a size range of 501–1000 µm, which were significantly higher than those observed in the sediment. This indicates the size preference of Chironomus larvae for constructing their tubes. Concerning the direct negative effects of MPs on organisms, MPs may lead to a reduction in shelter stability with an increasing number of MP particles (Ribeiro-Brasil et al. 2022). Hence, the shelter is easily transported far away by the water current, where MPs cause lightness compared with sand grains (Ehlers et al. 2020). Accordingly, Chironomus’ tubes could provide a picture round seasonal changes of MPs abundance located in their substrate as well as can be employed as a bioindicator for MPs distributed in freshwater.
Limitations
The bioaccumulation of MPs and associated contaminants (such as heavy metals and persistent organic matter) within various higher trophic levels in various aquatic systems should be carried out to comprehend the implications and risks of MPs as well as the toxicity caused by the adsorption presence of these contaminants in freshwater biota.
Conclusion
Even though MP contamination is a worldwide problem, research on the spatiotemporal distribution of freshwater MPs is still in its infancy. This is the first study to consider both human activities and seasonality in connection to internal MP contents in two wastewater sites in Egypt’s Sohag Governorate. Our findings support a prior study that revealed that Chironomus sp. might be beneficial as a freshwater MP bioindicator. Furthermore, the current study is the first to document the use of chironomid tubes as indicators for freshwater MPs. It can serve as a warning sign for MP buildup in all compartments of the aquatic environment. Furthermore, our findings were intended to provide information regarding the seasonal variation in MPs in wastewater, hence increasing the understanding of the seasonal effect on MPs pollution and risk levels in freshwater ecosystems. Consequently, our findings will help policymakers and the government, in partnership with international organizations, implement suitable management strategies to minimize the waste of plastic. Our results found that blue polyester fibers are much more prevalent than other polymers, colors, and shapes of MPs, and S2 was more highly contaminated with MPs than S1 during the four seasons of the year. Additionally, the abundance of MPs/individual was higher significantly in the fourth instar larvae (P < 0.05) than in the second instar. Further studies on the applicability of chironomid tubes as MP bioindicators in various freshwater environments throughout the world should be taken.
Author contribution
The study was organized and written by A.K. and S.R. Field sampling was carried out by A.A. and M.A. M.A. and A.A. analyzed data and contributed to the text, which was evaluated by A.K. and S.R. The published version of the work has been reviewed and approved by all authors.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The research received no external funding and depended completely upon the facilities offered by Sohag University. Article processing charge (APC) is completely covered according to Springer Nature’s fully open access agreements (Transformative Agreement between Springer Nature and Science, Technology & Innovation Funding Authority (STDF) in cooperation with Egyptian Knowledge Bank (EKB), started from 01 January 2022, and my institute (Sohag University) is one of the participating institutes.
Data availability
All data analyzed in this manuscript is included in the published article.
Declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors agree to publish their research data pertinent to the paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Akhbarizadeh R, Dobaradaran S, Nabipour I, Tajbakhsh S, Darabi AH, Spitz J. Abundance, composition, and potential intake of MPs in canned fish. Mar Pollut Bull. 2020;160:111633. doi: 10.1016/j.marpolbul.2020.111633. [DOI] [PubMed] [Google Scholar]
- Akhbarizadeh R, Dobaradaran S, Schmidt TC, Nabipour I, Spitz J. Worldwide bottled water occurrence of emerging contaminants: a review of the recent scientific literature. J Hazard Mater. 2020;392:122271. doi: 10.1016/j.jhazmat.2020.122271. [DOI] [PubMed] [Google Scholar]
- Akhbarizadeh R, Dobaradaran S, Amouei Torkmahalleh M, Saeedi R, Aibaghi R, Faraji Ghasemi F. Suspended fine particulate matter (PM2.5), MPs (MPs), and polycyclic aromatic hydrocarbons (PAHs) in air: their possible relationships and health implications. Environ Res. 2021;192:110339. doi: 10.1016/j.envres.2020.110339. [DOI] [PubMed] [Google Scholar]
- Akhbarizadeh R, Dobaradaran S, Nabipour I, Tangestani M, Abedi D, Javanfekr F, Jeddi F, Zendehboodi A. Abandoned Covid-19 personal protective equipment along the Bushehr shores, the Persian gulf: an emerging source of secondary MPs in coastlines. Mar Pollut Bull. 2021;168:112386. doi: 10.1016/j.marpolbul.2021.112386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akindele EO, Ehlers SM, Koop JH. First empirical study of freshwater MPs in West Africa using gastropods from Nigeria as bioindicators. Limnologica. 2019;78:125708. [Google Scholar]
- Akindele EO, Ehlers SM, Koop JH. Freshwater insects of different feeding guilds ingest MPs in two Gulf of Guinea tributaries in Nigeria. Environ Sci Pollut Res. 2020;27:33373–33379. doi: 10.1007/s11356-020-08763-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrady AL. MPs in the marine environment. Mar Pollut Bull. 2011;62(8):1596–1605. doi: 10.1016/j.marpolbul.2011.05.030. [DOI] [PubMed] [Google Scholar]
- Andrady AL, Koongolla B. Degradation and Fragmentation of Microplastics. Plastics and the Ocean: Origin, Characterization, Fate, and Impacts; 2022. pp. 227–268. [Google Scholar]
- Antunes J, Frias J, Sobral P. MPs on the Portuguese coast. Mar Pollut Bull. 2018;131:294–302. doi: 10.1016/j.marpolbul.2018.04.025. [DOI] [PubMed] [Google Scholar]
- Athapaththu AMAIK, Thushari GGN, Dias PCB, Abeygunawardena AP, Egodauyana KPUT, Liyanage NPP, Pitawala HMJC, Senevirathna JDM. Plastics in surface water of southern coastal belt of Sri Lanka (Northern Indian Ocean): distribution and characterization by FTIR. Mar Pollut Bull. 2020;161:111750. doi: 10.1016/j.marpolbul.2020.111750. [DOI] [PubMed] [Google Scholar]
- Bagheri T, Gholizadeh M, Abarghouei S, Zakeri M, Hedayati A, Rabaniha M, Aghaeimoghadam A, Hafezieh M. MPs distribution, abundance and composition in sediment, fishes and benthic organisms of the Gorgan Bay. Caspian Sea Chemosphere. 2020;257:127201. doi: 10.1016/j.chemosphere.2020.127201. [DOI] [PubMed] [Google Scholar]
- Bakir A, Rowland SJ, Thompson RC. Enhanced desorption of persistent organic pollutants from MPs under simulated physiological conditions. Environ Pollut. 2014;185:16–23. doi: 10.1016/j.envpol.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Ballent A, Corcoran PL, Madden O, Helm PA, Longstaffe FJ. Sources and sinks of MPs in Canadian Lake Ontario nearshore, tributary and beach sediments. Mar Pollut Bull. 2016;110(1):383–395. doi: 10.1016/j.marpolbul.2016.06.037. [DOI] [PubMed] [Google Scholar]
- Barrows APW, Cathey SE, Petersen CW. Marine environment microfiber contamination: global patterns and the diversity of microparticle origins. Environ Pollut. 2018;237:275–284. doi: 10.1016/j.envpol.2018.02.062. [DOI] [PubMed] [Google Scholar]
- Battula S, Kumar M, Panda SK, Pavan K, Rao U (2021) In-situ microplastic detection sensor based on cascaded microring resonators, OCEANS 2021: San Diego–Porto. IEEE, pp. 1–5
- Bere T, Dalu T, Mwedzi T. Detecting the impact of heavy metal contaminated sediment on benthic macroinvertebrate communities in tropical streams. Sci Total Environ. 2016;572:147–156. doi: 10.1016/j.scitotenv.2016.07.204. [DOI] [PubMed] [Google Scholar]
- Berezina NA, Tiunov AV, Petukhov VA, Gubelit YI. Benthic invertebrates abundance and trophic links in the coastal zone during Cladophora blooms. Diversity. 2022;14(12):1053. [Google Scholar]
- Bertin D, Ferrari BJ, Labadie P, Sapin A, Garric J, Budzinski H, Houde M, Babut M. Bioaccumulation of perfluoroalkyl compounds in midge (Chironomus riparius) larvae exposed to sediment. Environ Pollut. 2014;189:27–34. doi: 10.1016/j.envpol.2014.02.018. [DOI] [PubMed] [Google Scholar]
- Bertoli M, Pastorino P, Lesa D, Renzi M, Anselmi S, Prearo M, Pizzul E. MPs accumulation in functional feeding guilds and functional habit groups of freshwater macrobenthic invertebrates: novel insights in a riverine ecosystem. Sci Total Environ. 2022;804:150207. doi: 10.1016/j.scitotenv.2021.150207. [DOI] [PubMed] [Google Scholar]
- Blettler MCM, Abrial E, Khan FR, Sivri N, Espinola LA. Freshwater plastic pollution: recognizing research biases and identifying knowledge gaps. Water Res. 2018;143:416–424. doi: 10.1016/j.watres.2018.06.015. [DOI] [PubMed] [Google Scholar]
- Brahney J, Hallerud M, Heim E, Hahnenberger M, Sukumaran S. Plastic rain in protected areas of the United States. Science. 2020;368(6496):1257–1260. doi: 10.1126/science.aaz5819. [DOI] [PubMed] [Google Scholar]
- Browne MA (2015) Sources and pathways of MPs to habitats. Marine anthropogenic litter: 229–244
- Browne MA, Crump P, Niven SJ, Teuten E, Tonkin A, Galloway T, Thompson R. Accumulation of microplastic on shorelines woldwide: sources and sinks. Environ Sci Technol. 2011;45(21):9175–9179. doi: 10.1021/es201811s. [DOI] [PubMed] [Google Scholar]
- Browne MA, Niven SJ, Galloway TS, Rowland SJ, Thompson RC. Microplastic moves pollutants and additives to worms, reducing functions linked to health and biodiversity. Curr Biol. 2013;23(23):2388–2392. doi: 10.1016/j.cub.2013.10.012. [DOI] [PubMed] [Google Scholar]
- Burns EE, Boxall AB. MPs in the aquatic environment: evidence for or against adverse impacts and major knowledge gaps. Environ Toxicol Chem. 2018;37(11):2776–2796. doi: 10.1002/etc.4268. [DOI] [PubMed] [Google Scholar]
- Cabrejos-Cardeña U, De-la-Torre GE, Dobaradaran S, Rangabhashiyam S. an ecotoxicological perspective of MPs released by face masks. J Hazard Mater. 2023;443:130273. doi: 10.1016/j.jhazmat.2022.130273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesa FS, Turra A, Baruque-Ramos J. Synthetic fibers as MPs in the marine environment: a review from textile perspective with a focus on domestic washings. Sci Total Environ. 2017;598:1116–1129. doi: 10.1016/j.scitotenv.2017.04.172. [DOI] [PubMed] [Google Scholar]
- Chae D-H, Kim I-S, Kim S-K, Song YK, Shim WJ. Abundance and distribution characteristics of MPs in surface seawaters of the Incheon/Kyeonggi coastal region. Arch Environ Contam Toxicol. 2015;69:269–278. doi: 10.1007/s00244-015-0173-4. [DOI] [PubMed] [Google Scholar]
- Chang M. Reducing MPs from facial exfoliating cleansers in wastewater through treatment versus consumer product decisions. Mar Pollut Bull. 2015;101(1):330–333. doi: 10.1016/j.marpolbul.2015.10.074. [DOI] [PubMed] [Google Scholar]
- Chen Q, Li Y, Li B. Is color a matter of concern during microplastic exposure to Scenedesmus obliquus and Daphnia magna? J Hazard Mater. 2020;383:121224. doi: 10.1016/j.jhazmat.2019.121224. [DOI] [PubMed] [Google Scholar]
- Cheung PK, Hung PL, Fok L. River microplastic contamination and dynamics upon a rainfall event in Hong Kong. China Environ Process. 2019;6:253–264. [Google Scholar]
- Cole M, Lindeque P, Halsband C, Galloway TS. MPs as contaminants in the marine environment: a review. Mar Pollut Bull. 2011;62(12):2588–2597. doi: 10.1016/j.marpolbul.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Cole M, Lindeque P, Fileman E, Halsband C, Galloway TS. The impact of polystyrene MPs on feeding, function and fecundity in the marine copepod Calanus helgolandicus. Environ Sci Technol. 2015;49(2):1130–1137. doi: 10.1021/es504525u. [DOI] [PubMed] [Google Scholar]
- Conley K, Clum A, Deepe J, Lane H, Beckingham B. Wastewater treatment plants as a source of MPs to an urban estuary: removal efficiencies and loading per capita over one year. Water Res X. 2019;3:100030. doi: 10.1016/j.wroa.2019.100030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalu T, Clegg B, Marufu L, Nhiwatiwa TJP-A. The feeding habits of an introduced piscivore, Hydrocynus vittatus (Castelnau 1861) in a small tropical African reservoir. PanamJAS. 2012;7(2):85–92. [Google Scholar]
- Daniel DB, Ashraf PM, Thomas SN. Abundance, characteristics and seasonal variation of MPs in Indian white shrimps (Fenneropenaeus indicus) from coastal waters off Cochin, Kerala. India Sci Total Environ. 2020;737:139839. doi: 10.1016/j.scitotenv.2020.139839. [DOI] [PubMed] [Google Scholar]
- De-la-Torre GE, Dioses-Salinas DC, Dobaradaran S, Spitz J, Nabipour I, Keshtkar M, Akhbarizadeh R, Tangestani M, Abedi D, Javanfekr F. release of phthalate esters (PAEs) and MPs (MPs) from face masks and gloves during the COVID-19 pandemic. Environ Res. 2022;215:114337. doi: 10.1016/j.envres.2022.114337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De-la-Torre GE, Pizarro-Ortega CI, Dioses-Salinas DC, Rakib MRJ, Ramos W, Pretell V, Ribeiro VV, Castro ÍB, Dobaradaran S. First record of plastiglomerates, pyroplastics, and plasticrusts in South America. Sci Total Environ. 2022;833:155179. doi: 10.1016/j.scitotenv.2022.155179. [DOI] [PubMed] [Google Scholar]
- De-la-Torre GE, Dioses-Salinas DC, Pizarro-Ortega CI, Forero López AD, Fernández Severini MD, Rimondino GN, Malanca FE, Dobaradaran S, Aragaw TA, Mghili B, Ayala F. Plastic and paint debris in marine protected areas of Peru. Sci Total Environ. 2023;901:165788. doi: 10.1016/j.scitotenv.2023.165788. [DOI] [PubMed] [Google Scholar]
- De-la-Torre GE, Pizarro-Ortega CI, Dioses-Salinas DC, Ribeiro VV, Urizar Garfias Reyes DF, Ben-Haddad M, Rakib MRJ, Dobaradaran S. Micro- and mesoplastic pollution along the coast of Peru. Environ Sci Pollut Res. 2023;30:71396–71408. doi: 10.1007/s11356-023-27707-6. [DOI] [PubMed] [Google Scholar]
- De Moor IJ, Day JA, De Moor FC (2003) Guides to the freshwater invertebrates of Southern Africa: Volume 7 Insecta I-Ephemeroptera, Odonata and Plecoptera. Water Research Commision
- de Quatrefages MA (1848) Études embryogéniques. Ann Sci
- Ding J, Sun Y, He C, Li J, Li F. Towards risk assessments of microplastics in bivalve mollusks globally. J Mar Sci and Eng. 2022;10(2):288. [Google Scholar]
- Dobaradaran S, Schmidt TC, Nabipour I, Khajeahmadi N, Tajbakhsh S, Saeedi R, Javad Mohammadi M, Keshtkar M, Khorsand M, Faraji Ghasemi F. Characterization of plastic debris and association of metals with MPs in coastline sediment along the Persian Gulf. Waste Manage. 2018;78:649–658. doi: 10.1016/j.wasman.2018.06.037. [DOI] [PubMed] [Google Scholar]
- Driedger AG, Dürr HH, Mitchell K, Van Cappellen P. Plastic debris in the Laurentian Great Lakes: a review. J Great Lakes Res. 2015;41(1):9–19. [Google Scholar]
- Eerkes-Medrano D, Thompson R (2018): Occurrence, fate, and effect of MPs in freshwater systems, microplastic contamination in aquatic environments. Elsevier, pp. 95–132
- Eerkes-Medrano D, Thompson RC, Aldridge DC. MPs in freshwater systems: a review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res. 2015;75:63–82. doi: 10.1016/j.watres.2015.02.012. [DOI] [PubMed] [Google Scholar]
- Ehlers SM, Manz W, Koop JH. MPs of different characteristics are incorporated into the larval cases of the freshwater caddisfly Lepidostoma basale. Aquat Biol. 2019;28:67–77. [Google Scholar]
- Ehlers SM, Al Najjar T, Taupp T, Koop JH. PVC and PET MPs in caddisfly (Lepidostoma basale) cases reduce case stability. Environ Sci Pollut Res. 2020;27:22380–22389. doi: 10.1007/s11356-020-08790-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen M, Mason S, Wilson S, Box C, Zellers A, Edwards W, Farley H, Amato S. Microplastic pollution in the surface waters of the Laurentian Great Lakes. Mar Pollut Bull. 2013;77(1–2):177–182. doi: 10.1016/j.marpolbul.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Fan J, Zou L, Zhao G. Microplastic abundance, distribution, and composition in the surface water and sediments of the Yangtze River along Chongqing City. China J Soils Sediments. 2021;21:1840–1851. [Google Scholar]
- Fan Y, Zheng J, Deng L, Rao W, Zhang Q, Liu T, Qian X. Spatiotemporal dynamics of MPs in an urban river network area. Water Res. 2022;212:118116. doi: 10.1016/j.watres.2022.118116. [DOI] [PubMed] [Google Scholar]
- Fazey FM, Ryan PG. Biofouling on buoyant marine plastics: an experimental study into the effect of size on surface longevity. Environ Pollut. 2016;210:354–360. doi: 10.1016/j.envpol.2016.01.026. [DOI] [PubMed] [Google Scholar]
- Free CM, Jensen OP, Mason SA, Eriksen M, Williamson NJ, Boldgiv B. High-levels of microplastic pollution in a large, remote, mountain lake. Mar Pollut Bull. 2014;85(1):156–163. doi: 10.1016/j.marpolbul.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Frere L, Paul-Pont I, Rinnert E, Petton S, Jaffré J, Bihannic I, Soudant P, Lambert C, Huvet A. Influence of environmental and anthropogenic factors on the composition, concentration and spatial distribution of MPs: a case study of the Bay of Brest (Brittany, France) Environ Pollut. 2017;225:211–222. doi: 10.1016/j.envpol.2017.03.023. [DOI] [PubMed] [Google Scholar]
- Gallitelli L, Cera A, Cesarini G, Pietrelli L, Scalici M. Preliminary indoor evidences of microplastic effects on freshwater benthic macroinvertebrates. Sci Rep. 2021;11(1):1–11. doi: 10.1038/s41598-020-80606-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghani SAA, Shobier AH, El-Sayed AA, Shreadah MA, Shabaka S. Quantifying microplastics pollution in the Red Sea and Gulfs of Suez and Aqaba: insights from chemical analysis and pollution load assessment. Sci Total Environ. 2023;901:166031. doi: 10.1016/j.scitotenv.2023.166031. [DOI] [PubMed] [Google Scholar]
- Grbić J, Helm P, Athey S, Rochman CM. MPs entering northwestern Lake Ontario are diverse and linked to urban sources. Water Res. 2020;174:115623. doi: 10.1016/j.watres.2020.115623. [DOI] [PubMed] [Google Scholar]
- Gregory MR. Plastic pellets on New Zealand beaches. Mar Pollut Bull. 1977;8(4):82–84. [Google Scholar]
- Guo Z, Boeing WJ, Xu Y, Borgomeo E, Mason SA, Zhu Y-G. Global meta-analysis of microplastic contamination in reservoirs with a novel framework. Water Res. 2021;207:117828. doi: 10.1016/j.watres.2021.117828. [DOI] [PubMed] [Google Scholar]
- Hajiouni S, Mohammadi A, Ramavandi B, Arfaeinia H, De-la-Torre GE, Tekle-Röttering A, Dobaradaran S. Occurrence of MPs and phthalate esters in urban runoff: a focus on the Persian Gulf coastline. Sci Total Environ. 2022;806:150559. doi: 10.1016/j.scitotenv.2021.150559. [DOI] [PubMed] [Google Scholar]
- Hajji S, Ben-Haddad M, Abelouah MR, De-la-Torre GE, Alla AA. Occurrence, characteristics, and removal of microplastics in wastewater treatment plants located on the Moroccan Atlantic: the case of Agadir metropolis. Sci Total Environ. 2023;862:160815. doi: 10.1016/j.scitotenv.2022.160815. [DOI] [PubMed] [Google Scholar]
- Hakanson L. An ecological risk index for aquatic pollution control A Sedimentological Approach. Water Res. 1980;14(8):975–1001. [Google Scholar]
- Hartmann NB, Huffer T, Thompson RC, Hassellöv M, Verschoor A, Daugaard AE, Rist S, Karlsson T, Brennholt N, Cole M. Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris: ACS Publications; 2019. [DOI] [PubMed] [Google Scholar]
- Hoellein TJ, McCormick AR, Hittie J, London MG, Scott JW, Kelly JJ. Longitudinal patterns of microplastic concentration and bacterial assemblages in surface and benthic habitats of an urban river. Freshw Sci. 2017;36(3):491–507. [Google Scholar]
- Horton AA, Dixon SJ. MPs: An introduction to environmental transport processes. Wiley Interdiscip Rev Water. 2018;5(2):e1268. [Google Scholar]
- Hurley RR, Woodward JC, Rothwell JJ. Ingestion of MPs by freshwater tubifex worms. Environ Sci Technol. 2017;51(21):12844–12851. doi: 10.1021/acs.est.7b03567. [DOI] [PubMed] [Google Scholar]
- Irfan T, Khalid S, Taneez M, Hashmi MZ. Plastic driven pollution in Pakistan: the first evidence of environmental exposure to microplastic in sediments and water of Rawal Lake. Environ Sci Pollut Res. 2020;27:15083–15092. doi: 10.1007/s11356-020-07833-1. [DOI] [PubMed] [Google Scholar]
- Jin X, Fu X, Lu W, Wang H (2023) The effects of riverside cities on microplastics in river water: a case study on the Southern Jiangsu Canal China. Sci Total Environ 858:159783 [DOI] [PubMed]
- Kabir AE, Sekine M, Imai T, Yamamoto K, Kanno A, Higuchi T. Microplastics in the sediments of small-scale Japanese rivers: abundance and distribution, characterization, sources-to-sink, and ecological risks. Sci Total Environ. 2022;812:152590. doi: 10.1016/j.scitotenv.2021.152590. [DOI] [PubMed] [Google Scholar]
- Kasamesiri P, Panchan R, Thaimuangphol W. Spatial–temporal distribution and ecological risk assessment of microplastic pollution of inland fishing ground in the ubolratana reservoir. Thailand Water. 2023;15(2):330. [Google Scholar]
- Kashfi FS, Ramavandi B, Arfaeinia H, Mohammadi A, Saeedi R, De-la-Torre GE, Dobaradaran S. Occurrence and exposure assessment of MPs in indoor dusts of buildings with different applications in Bushehr and Shiraz cities Iran. Sci Total Environ. 2022;829:154651. doi: 10.1016/j.scitotenv.2022.154651. [DOI] [PubMed] [Google Scholar]
- Kashfi FS, Mohammadi A, Rostami F, Savari A, De-la-Torre GE, Spitz J, Saeedi R, Kalantarhormozi M, Farhadi A, Dobaradaran S (2023): MPs and phthalate esters release from teabags into tea drink: Occurrence, human exposure, and health risks. [DOI] [PubMed]
- Khdre AM, Ramadan SA, Ashry A, Alaraby M. Chironomus sp as a bioindicator for assessing microplastic contamination and the heavy metals associated with It in the sediment of wastewater in Sohag Governorate Egypt. Water Air Soil Pollut. 2023;234:161. doi: 10.1007/s11270-023-06179-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khedre AM, Ramadan SA, Ashry A, Alaraby M. Pollution of freshwater ecosystems by MPs: a short review on degradation, distribution, and interaction with aquatic biota. Sohag J Sci. 2023;8:289–295. [Google Scholar]
- Khedre AM, Ramadan SA, Ashry A, Alaraby M. Ingestion and egestion of microplastic by aquatic insects in Egypt wastewater. Environ Qual Manage. 2023;33(1):135–145. [Google Scholar]
- Khedre AM, Ramadan SA, Ashry A, Alaraby M. Assessment of microplastic accumulation in aquatic insects of different feeding guilds collected from wastewater in Sohag Governorate. Egypt Mar Freshw Res. 2023;74:733–745. [Google Scholar]
- Klein S, Worch E, Knepper TP. Occurrence and spatial distribution of MPs in river shore sediments of the Rhine-Main area in Germany. Environ Sci Technol. 2015;49(10):6070–6076. doi: 10.1021/acs.est.5b00492. [DOI] [PubMed] [Google Scholar]
- Kooi M, Koelmans AA. Simplifying microplastic via continuous probability distributions for size, shape, and density. Environ Sci Technol Lett. 2019;6:551–557. [Google Scholar]
- Lagauzère S, Terrail R, Bonzom J-M. Ecotoxicity of uranium to Tubifex tubifex worms (Annelida, Clitellata, Tubificidae) exposed to contaminated sediment. Ecotoxicol Environ Saf. 2009;72(2):527–537. doi: 10.1016/j.ecoenv.2008.04.021. [DOI] [PubMed] [Google Scholar]
- Lahens L, Strady E, Kieu-Le T-C, Dris R, Boukerma K, Rinnert E, Gasperi J, Tassin B. Macroplastic and microplastic contamination assessment of a tropical river (Saigon River, Vietnam) transversed by a developing megacity. Environ Pollut. 2018;236:661–671. doi: 10.1016/j.envpol.2018.02.005. [DOI] [PubMed] [Google Scholar]
- Lei L, Wu S, Lu S, Liu M, Song Y, Fu Z, Shi H, Raley-Susman KM, He D. Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci Total Environ. 2018;619:1–8. doi: 10.1016/j.scitotenv.2017.11.103. [DOI] [PubMed] [Google Scholar]
- Li J, Liu H, Chen JP. MPs in freshwater systems: A review on occurrence, environmental effects, and methods for MPs detection. Water Res. 2018;137:362–374. doi: 10.1016/j.watres.2017.12.056. [DOI] [PubMed] [Google Scholar]
- Li R, Yu L, Chai M, Wu H, Zhu X. The distribution, characteristics and ecological risks of MPs in the mangroves of Southern China. Sci Total Environ. 2020;708:135025. doi: 10.1016/j.scitotenv.2019.135025. [DOI] [PubMed] [Google Scholar]
- Li B, Song W, Cheng Y, Zhang K, Tian H, Du Z, Wang J, Wang J, Zhang W, Zhu L. Ecotoxicological effects of different size ranges of industrial-grade polyethylene and polypropylene microplastics on earthworms Eisenia fetida. Sci Total Environ. 2021;783:147007. doi: 10.1016/j.scitotenv.2021.147007. [DOI] [PubMed] [Google Scholar]
- Lin C-T, Chiu M-C, Kuo M-H. A mini-review of strategies for quantifying anthropogenic activities in microplastic studies in aquatic environments. Polymers. 2022;14(1):198. doi: 10.3390/polym14010198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeque PK, Cole M, Coppock RL, Lewis CN, Miller RZ, Watts AJ, Wilson-McNeal A, Wright SL, Galloway TS. Are we underestimating microplastic abundance in the marine environment? A comparison of microplastic capture with nets of different mesh-size. Environ Pollut. 2020;265:114721. doi: 10.1016/j.envpol.2020.114721. [DOI] [PubMed] [Google Scholar]
- Lithner D, Larsson Å, Dave G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci Total Environ. 2011;409(18):3309–3324. doi: 10.1016/j.scitotenv.2011.04.038. [DOI] [PubMed] [Google Scholar]
- Liu P, Zhan X, Wu X, Li J, Wang H, Gao S. Effect of weathering on environmental behavior of MPs: properties, sorption and potential risks. Chemospher. 2020;242:125193. doi: 10.1016/j.chemosphere.2019.125193. [DOI] [PubMed] [Google Scholar]
- Liu L, Xu M, Ye Y, Zhang B. On the degradation of (micro) plastics: degradation methods, influencing factors, environmental impacts. Sci Total Environ. 2022;806:151312. doi: 10.1016/j.scitotenv.2021.151312. [DOI] [PubMed] [Google Scholar]
- Long M, Moriceau B, Gallinari M, Lambert C, Huvet A, Raffray J, Soudant P. Interactions between MPs and phytoplankton aggregates: impact on their respective fates. Mar Chem. 2015;175:39–46. [Google Scholar]
- Luo H, Xiang Y, He D, Li Y, Zhao Y, Wang S, Pan X. Leaching behavior of fluorescent additives from MPs and the toxicity of leachate to Chlorella vulgaris. Sci Total Environ. 2019;678:1–9. doi: 10.1016/j.scitotenv.2019.04.401. [DOI] [PubMed] [Google Scholar]
- Lusher AL, McHugh M, Thompson RC. Occurrence of MPs in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar Pollut Bull. 2013;67(1–2):94–99. doi: 10.1016/j.marpolbul.2012.11.028. [DOI] [PubMed] [Google Scholar]
- Magnusson K, Norén F (2014): Screening of microplastic particles in and down-stream a wastewater treatment plant
- Mani T, Burkhardt-Holm P. Seasonal MPs variation in nival and pluvial stretches of the Rhine River-From the Swiss catchment towards the North Sea. Sci Total Environ. 2020;707:135579. doi: 10.1016/j.scitotenv.2019.135579. [DOI] [PubMed] [Google Scholar]
- Mani T, Primpke S, Lorenz C, Gerdts G, Burkhardt-Holm P. Microplastic pollution in benthic midstream sediments of the Rhine River. Environ Sci Technol. 2019;53(10):6053–6062. doi: 10.1021/acs.est.9b01363. [DOI] [PubMed] [Google Scholar]
- Mason SA, Garneau D, Sutton R, Chu Y, Ehmann K, Barnes J, Fink P, Papazissimos D, Rogers DL. Microplastic pollution is widely detected in US municipal wastewater treatment plant effluent. Environ Pollut. 2016;218:1045–1054. doi: 10.1016/j.envpol.2016.08.056. [DOI] [PubMed] [Google Scholar]
- McCormick A, Hoellein TJ, Mason SA, Schluep J, Kelly JJ. Microplastic is an abundant and distinct microbial habitat in an urban river. Environ Sci Technol. 2014;48(20):11863–11871. doi: 10.1021/es503610r. [DOI] [PubMed] [Google Scholar]
- Mema V. Impact of poorly maintained wastewater sewage treatment plants-lessons from South Africa: Wastewater management. Resource. 2010;12(3):60–65. [Google Scholar]
- Mendoza LMR, Balcer M. Association of hazardous compounds with MPs in freshwater ecosystems. London, UK: MPs in Water and Wastewater; IWA Publishing; 2019. pp. 15–25. [Google Scholar]
- Mintenig SM, Int-Veen I, Löder MGJ, Primpke S, Gerdts G. Identification of microplastic in effluents of waste water treatment plants using focal plane array-based micro-Fourier-transform infrared imaging. Water Res. 2017;108:365–372. doi: 10.1016/j.watres.2016.11.015. [DOI] [PubMed] [Google Scholar]
- Mohammadi A, Dobaradaran S, Schmidt TC, Malakootian M, Spitz J. Emerging contaminants migration from pipes used in drinking water distribution systems: a review of the scientific literature. Environ Sci Pollut Res. 2022;29:75134–75160. doi: 10.1007/s11356-022-23085-7. [DOI] [PubMed] [Google Scholar]
- Mohammadi A, Malakootian M, Dobaradaran S, Hashemi M, Jaafarzadeh N. Occurrence, seasonal distribution, and ecological risk assessment of MPs and phthalate esters in leachates of a landfill site located near the marine environment: Bushehr port, Iran as a case. Sci Total Environ. 2022;842:156838. doi: 10.1016/j.scitotenv.2022.156838. [DOI] [PubMed] [Google Scholar]
- Mohammadi A, Malakootian M, Dobaradaran S, Hashemi M, Jaafarzadeh N, De-la-Torre GE. Occurrence and ecological risks of MPs and phthalate esters in organic solid wastes: in a landfill located nearby the Persian Gulf. Chemosphere. 2023;332:138910. doi: 10.1016/j.chemosphere.2023.138910. [DOI] [PubMed] [Google Scholar]
- Montoto-Martinez T, Hernández-Brito JJ, Gelado-Caballero MD. Pump-underway ship intake: an unexploited opportunity for Marine Strategy Framework Directive (MSFD) microplastic monitoring needs on coastal and oceanic waters. Plos One. 2020;15(5):e0232744. doi: 10.1371/journal.pone.0232744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munari C, Infantini V, Scoponi M, Rastelli E, Corinaldesi C, Mistri M. MPs in the sediments of terra nova bay (ross sea, Antarctica) Mar Pollut Bull. 2017;122(1–2):161–165. doi: 10.1016/j.marpolbul.2017.06.039. [DOI] [PubMed] [Google Scholar]
- Murphy F, Ewins C, Carbonnier F, Quinn B. Wastewater treatment works (WwTW) as a source of MPs in the aquatic environment. Environ Sci Technol. 2016;50(11):5800–5808. doi: 10.1021/acs.est.5b05416. [DOI] [PubMed] [Google Scholar]
- Naji A, Esmaili Z, Khan FR. Plastic debris and MPs along the beaches of the Strait of Hormuz. Persian Gulf Mar Pollut Bull. 2017;114(2):1057–1062. doi: 10.1016/j.marpolbul.2016.11.032. [DOI] [PubMed] [Google Scholar]
- Naji A, Nuri M, Vethaak AD. MPs contamination in molluscs from the northern part of the Persian Gulf. Environ Pollut. 2018;235:113–120. doi: 10.1016/j.envpol.2017.12.046. [DOI] [PubMed] [Google Scholar]
- Napper IE, Thompson RC. Release of synthetic microplastic plastic fibres from domestic washing machines: effects of fabric type and washing conditions. Mar Pollut Bull. 2016;112(1–2):39–45. doi: 10.1016/j.marpolbul.2016.09.025. [DOI] [PubMed] [Google Scholar]
- Narayanan M (2023) Origination, fate, accumulation, impact, of microplastics in a marine ecosystem and bio/technological approach for remediation: a review. Process Safety and Environmental Protection.
- Neelavannan K, Sen IS, Lone AM, Gopinath K. Microplastics in the high-altitude Himalayas: assessment of microplastic contamination in freshwater lake sediments, Northwest Himalaya (India) Chemosphere. 2022;290:133354. doi: 10.1016/j.chemosphere.2021.133354. [DOI] [PubMed] [Google Scholar]
- Nel HA, Froneman PW. Presence of MPs in the tube structure of the reef-building polychaete Gunnarea gaimardi (Quatrefages 1848) Afr J Mar Sci. 2018;40(1):87–89. [Google Scholar]
- Nel HA, Dalu T, Wasserman RJ. Sinks and sources: assessing microplastic abundance in river sediment and deposit feeders in an Austral temperate urban river system. Sci Total Environ. 2018;612:950–956. doi: 10.1016/j.scitotenv.2017.08.298. [DOI] [PubMed] [Google Scholar]
- Nguyen QAT, Nguyen HNY, Strady E, Nguyen QT, Trinh-Dang M. Characteristics of MPs in shoreline sediments from a tropical and urbanized beach (Da Nang, Vietnam) Mar Pollut Bull. 2020;161:111768. doi: 10.1016/j.marpolbul.2020.111768. [DOI] [PubMed] [Google Scholar]
- Nguyen NB, Kim M-K, Le QT, Ngo DN, Zoh K-D, Joo S-W. Spectroscopic analysis of microplastic contaminants in an urban wastewater treatment plant from Seoul South Korea. Chemosphere. 2021;263:127812. doi: 10.1016/j.chemosphere.2020.127812. [DOI] [PubMed] [Google Scholar]
- Nhiwatiwa T, Dalu T, Sithole T. Assessment of river quality in a subtropical Austral river system: a combined approach using benthic diatoms and macroinvertebrates. Appl Water Sci. 2017;7:4785–4792. [Google Scholar]
- Niari MH, Jaafarzadeh N, Dobaradaran S, Niri MV, Dargahi A. Release of MPs to the environment through wastewater treatment plants: study on four types of wastewater treatment processes. Water Air Soil Pollut. 2023;234:589. [Google Scholar]
- Ory NC, Sobral P, Ferreira JL, Thiel M. Amberstripe scad Decapterus muroadsi (Carangidae) fish ingest blue MPs resembling their copepod prey along the coast of Rapa Nui (Easter Island) in the South Pacific subtropical gyre. Sci Total Environ. 2017;586:430–437. doi: 10.1016/j.scitotenv.2017.01.175. [DOI] [PubMed] [Google Scholar]
- Pan Z, Liu Q, Jiang R, Li W, Sun X, Lin H, Jiang S, Huang H. Microplastic pollution and ecological risk assessment in an estuarine environment: the Dongshan Bay of China. Chemosphere. 2021;262:127876. doi: 10.1016/j.chemosphere.2020.127876. [DOI] [PubMed] [Google Scholar]
- Parker B, Andreou D, Pabortsava K, Barrow M, Green ID, Britton JR. Microplastic loads within riverine fishes and macroinvertebrates are not predictable from ecological or morphological characteristics. Sci Total Environ. 2022;839:156321. doi: 10.1016/j.scitotenv.2022.156321. [DOI] [PubMed] [Google Scholar]
- Patterson J, Jeyasanta KI, Laju RL, Booth AM, Sathish N, Edward JKP. Microplastic in the coral reef environments of the Gulf of Mannar, India-Characteristics, distributions, sources and ecological risks. Environ Pollut. 2022;298:118848. doi: 10.1016/j.envpol.2022.118848. [DOI] [PubMed] [Google Scholar]
- Peng G, Xu P, Zhu B, Bai M, Li D. MPs in freshwater river sediments in Shanghai, China: a case study of risk assessment in mega-cities. Environ Pollut. 2018;234:448–456. doi: 10.1016/j.envpol.2017.11.034. [DOI] [PubMed] [Google Scholar]
- Peng X, Chen M, Chen S, Dasgupta S, Xu H, Ta K, Du M, Li J, Guo Z, Bai S. MPs contaminate the deepest part of the world’s ocean. Geochem Perspect Lett. 2018;9(1):1–5. [Google Scholar]
- Pirc U, Vidmar M, Mozer A, Kržan A. Emissions of microplastic fibers from microfiber fleece during domestic washing. Environ Sci Pollut Res. 2016;23:22206–22211. doi: 10.1007/s11356-016-7703-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizarro-Ortega CI, Dioses-Salinas DC, Fernández Severini MD, Forero López AD, Rimondino GN, Benson NU, Dobaradaran S, De-la-Torre GE. Degradation of plastics associated with the COVID-19 pandemic. Mar Pollut Bull. 2022;176:113474. doi: 10.1016/j.marpolbul.2022.113474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourfadakari S, Dobaradaran S, De-la-Torre GE, Mohammadi A, Saeedi R, Spitz J. Evaluation of occurrence of organic, inorganic, and microbial contaminants in bottled drinking water and comparison with international guidelines: a worldwide review. Environ Sci Pollut Res. 2022;29:55400–55414. doi: 10.1007/s11356-022-21213-x. [DOI] [PubMed] [Google Scholar]
- Prata JC, Silva ALP, da Costa JP, Dias-Pereira P, Carvalho A, Fernandes AJS, da Costa FM, Duarte AC, Rocha-Santos T. MPs in internal tissues of companion animals from urban environments. Animals. 2022;12(15):1979. doi: 10.3390/ani12151979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primpke S, Wirth M, Lorenz C, Gerdts G. Reference database design for the automated analysis of microplastic samples based on Fourier transform infrared (FTIR) spectroscopy. Anal Bioanal Chem. 2018;410:5131–5141. doi: 10.1007/s00216-018-1156-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Wang Z, Li W, Chang X, Yang J, Yang F. MPs in the sediment of lake Ulansuhai of Yellow river basin China. Water Environ Res. 2020;92(6):829–839. doi: 10.1002/wer.1275. [DOI] [PubMed] [Google Scholar]
- Qu X, Su L, Li H, Liang M, Shi H. Assessing the relationship between the abundance and properties of MPs in water and in mussels. Sci Total Environ. 2018;621:679–686. doi: 10.1016/j.scitotenv.2017.11.284. [DOI] [PubMed] [Google Scholar]
- Qu H, Diao H, Han J, Wang B, Yu G. Understanding and addressing the environmental risk of MPs. Front Environ Sci Eng. 2023;17:12. [Google Scholar]
- Ranjani M, Veerasingam S, Venkatachalapathy R, Mugilarasan M, Bagaev A, Mukhanov V, Vethamony P. Assessment of potential ecological risk of MPs in the coastal sediments of India: a meta-analysis. Mar Pollut Bull. 2021;163:111969. doi: 10.1016/j.marpolbul.2021.111969. [DOI] [PubMed] [Google Scholar]
- Rasta M, Rahimibashar MR, Ershad A, Jafroudi HT, Kouhbane ST. Characteristics and seasonal distribution of MPs in the surface waters of southwest coast of the Caspian Sea (Guilan Province, Iran) Bull Environ Contam Toxicol. 2021;107(4):671–676. doi: 10.1007/s00128-021-03268-7. [DOI] [PubMed] [Google Scholar]
- Redondo-Hasselerharm PE, Falahudin D, Peeters ET, Koelmans AA. Microplastic effect thresholds for freshwater benthic macroinvertebrates. Environ Sci Technol. 2018;52(4):2278–2286. doi: 10.1021/acs.est.7b05367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezania S, Park J, Din MFM, Taib SM, Talaiekhozani A, Yadav KK, Kamyab H. MPs pollution in different aquatic environments and biota: a review of recent studies. Mar Pollut Bull. 2018;133:191–208. doi: 10.1016/j.marpolbul.2018.05.022. [DOI] [PubMed] [Google Scholar]
- Ribeiro-Brasil DRG, Brasil LS, Veloso GKO, de Matos TP, de Lima ES, Dias-Silva K. The impacts of plastics on aquatic insects. Sci Total Environ. 2022;813:152436. doi: 10.1016/j.scitotenv.2021.152436. [DOI] [PubMed] [Google Scholar]
- Rochman CM, Tahir A, Williams SL, Baxa DV, Lam R, Miller JT, Teh F-C, Werorilangi S, Teh SJ. Anthropogenic debris in seafood: plastic debris and fibers from textiles in fish and bivalves sold for human consumption. Sci Rep. 2015;5(1):1–10. doi: 10.1038/srep14340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues M, Abrantes N, Gonçalves F, Nogueira H, Marques J, Gonçalves A. Spatial and temporal distribution of MPs in water and sediments of a freshwater system (Antuã River, Portugal) Sci Total Environ. 2018;633:1549–1559. doi: 10.1016/j.scitotenv.2018.03.233. [DOI] [PubMed] [Google Scholar]
- Sanchez-Hernandez JC. A toxicological perspective of plastic biodegradation by insect larvae. Comp Biochem Physiol c: Toxicol Pharmacol. 2021;248:109117. doi: 10.1016/j.cbpc.2021.109117. [DOI] [PubMed] [Google Scholar]
- Sang W, Chen Z, Mei L, Hao S, Zhan C, bin Zhang W, Li M, Liu J. The abundance and characteristics of MPs in rainwater pipelines in Wuhan. China Sci Total Environ. 2021;755:142606. doi: 10.1016/j.scitotenv.2020.142606. [DOI] [PubMed] [Google Scholar]
- Šaravanja A, Pušić T, Dekanić T. Microplastics in wastewater by washing polyester fabrics. Materials. 2022;15(7):2683. doi: 10.3390/ma15072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer C, Brennholt N, Reifferscheid G, Wagner M. Feeding type and development drive the ingestion of MPs by freshwater invertebrates. Sci Rep. 2017;7(1):1–9. doi: 10.1038/s41598-017-17191-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt LK, Bochow M, Imhof HK, Oswald SE. Multi-temporal surveys for microplastic particles enabled by a novel and fast application of SWIR imaging spectroscopy–study of an urban watercourse traversing the city of Berlin. Germany Environ Pollut. 2018;239:579–589. doi: 10.1016/j.envpol.2018.03.097. [DOI] [PubMed] [Google Scholar]
- Shabaka S, Moawad MN, Ibrahim MI, El-Sayed AA, Ghobashy MM, Hamouda AZ, El-Alfy MA, Darwish DH, Youssef NAE. Prevalence and risk assessment of MPs in the Nile Delta estuaries:“The Plastic Nile” revisited. Sci Total Environ. 2022;852:158446. doi: 10.1016/j.scitotenv.2022.158446. [DOI] [PubMed] [Google Scholar]
- Sharma S, Bhardwaj A, Thakur M, Saini A (2023) Understanding microplastic pollution of marine ecosystem: a review. Environ Sci Pollut Res: 1–44 [DOI] [PubMed]
- Silva CJ, Silva ALP, Gravato C, Pestana JL. Ingestion of small-sized and irregularly shaped polyethylene MPs affect Chironomus riparius life-history traits. Sci Total Environ. 2019;672:862–868. doi: 10.1016/j.scitotenv.2019.04.017. [DOI] [PubMed] [Google Scholar]
- Silva-Cavalcanti JS, Silva JDB, de França EJ, de Araújo MCB, Gusmao F. MPs ingestion by a common tropical freshwater fishing resource. Environ Pollut. 2017;221:218–226. doi: 10.1016/j.envpol.2016.11.068. [DOI] [PubMed] [Google Scholar]
- Simakova A, Varenitsina A, Babkina I, Andreeva Y, Bagirov R, Yartsev V, Frank Y. Ontogenetic transfer of MPs in bloodsucking mosquitoes Aedes aegypti L.(Diptera: Culicidae) is a potential pathway for particle distribution in the environment. Water. 2022;14(12):1852. [Google Scholar]
- Singh N, Mondal A, Bagri A, Tiwari E, Khandelwal N, Monikh FA, Darbha GK. Characteristics and spatial distribution of MPs in the lower Ganga River water and sediment. Mar Pollut Bull. 2021;163:111960. doi: 10.1016/j.marpolbul.2020.111960. [DOI] [PubMed] [Google Scholar]
- Strady E, Dang TH, Dao TD, Dinh HN, Do TTD, Duong TN, Duong TT, Hoang DA, Kieu-Le TC, Le TPQ. Baseline assessment of microplastic concentrations in marine and freshwater environments of a developing Southeast Asian country. Viet Nam Mar Pollut Bull. 2021;162:111870. doi: 10.1016/j.marpolbul.2020.111870. [DOI] [PubMed] [Google Scholar]
- Su L, Xue Y, Li L, Yang D, Kolandhasamy P, Li D, Shi H. MPs in taihu lake, China. Environ Pollut. 2016;216:711–719. doi: 10.1016/j.envpol.2016.06.036. [DOI] [PubMed] [Google Scholar]
- Sun M, Zhou M, Chang Z, Li L. Polyvinyl chloride microplastics induce growth inhibition and oxidative stress in Cyprinus carpio var. larvae. Sci Total Environ. 2020;716:136479. doi: 10.1016/j.scitotenv.2019.136479. [DOI] [PubMed] [Google Scholar]
- Takdastan A, Niari MH, Babaei A, Dobaradaran S, Jorfi S, Ahmadi M. Occurrence and distribution of microplastic particles and the concentration of Di 2-ethyl hexyl phthalate (DEHP) in MPs and wastewater in the wastewater treatment plant. J Environ Manage. 2021;280:111851. doi: 10.1016/j.jenvman.2020.111851. [DOI] [PubMed] [Google Scholar]
- Talvitie J, Mikola A, Setälä O, Heinonen M, Koistinen A. How well is microlitter purified from wastewater?–A detailed study on the stepwise removal of microlitter in a tertiary level wastewater treatment plant. Water Res. 2017;109:164–172. doi: 10.1016/j.watres.2016.11.046. [DOI] [PubMed] [Google Scholar]
- Tomlinson DL, Wilson JG, Harris CR, Jeffrey DW. Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgoländer Meeresuntersuchungen. 1980;33:566–575. [Google Scholar]
- Tursi A, Baratta M, Easton T, Chatzisymeon E, Chidichimo F, De Biase M, De Filpo G. MPs in aquatic systems, a comprehensive review: origination, accumulation, impact, and removal technologies. RSC Adv. 2022;12(44):28318–28340. doi: 10.1039/d2ra04713f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Moos N, Burkhardt-Holm P, Köhler A. Uptake and effects of MPs on cells and tissue of the blue mussel Mytilus edulis L. after an experimental exposure. Environ Sci Technol. 2012;46(20):11327–11335. doi: 10.1021/es302332w. [DOI] [PubMed] [Google Scholar]
- Vos JH, Teunissen M, Postma JF, Van den Ende FP. Particle size effect on preferential settlement and growth rate of detritivorous chironomid larvae as influenced by food level. Arch Hydrobiol. 2002;154(1):103–119. [Google Scholar]
- Wagner M, Lambert S (2018): Freshwater MPs: emerging environmental contaminants? Springer Nature
- Wang S, Zhang C, Pan Z, Sun D, Zhou A, Xie S, Wang J, Zou J. MPs in wild freshwater fish of different feeding habits from Beijiang and Pearl River Delta regions, south China. Chemosphere. 2020;258:127345. doi: 10.1016/j.chemosphere.2020.127345. [DOI] [PubMed] [Google Scholar]
- Wang G, Lu J, Li W, Ning J, Zhou L, Tong Y, Liu Z, Zhou H, Xiayihazi N. Seasonal variation and risk assessment of MPs in surface water of the Manas River Basin. China Ecotoxicol Environ Saf. 2021;208:111477. doi: 10.1016/j.ecoenv.2020.111477. [DOI] [PubMed] [Google Scholar]
- Wasserman RJ. Feeding ecology of the early life-history stages of two dominant gobiid species in the headwaters of a warm-temperate estuary. Estuar Coast Shelf Sci. 2012;109:11–19. [Google Scholar]
- Watts AJR, Lewis C, Goodhead RM, Beckett SJ, Moger J, Tyler CR, Galloway TS. Uptake and retention of MPs by the shore crab Carcinus maenas. Environ Sci Technol. 2014;48(15):8823–8830. doi: 10.1021/es501090e. [DOI] [PubMed] [Google Scholar]
- Wicaksono EA, Werorilangi S, Galloway TS, Tahir A. Distribution and seasonal variation of MPs in tallo river, makassar, eastern indonesia. Toxics. 2021;9(6):129. doi: 10.3390/toxics9060129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windsor FM, Tilley RM, Tyler CR, Ormerod SJ. Microplastic ingestion by riverine macroinvertebrates. Sci Total Environ. 2019;646:68–74. doi: 10.1016/j.scitotenv.2018.07.271. [DOI] [PubMed] [Google Scholar]
- Woodall LC, Sanchez-Vidal A, Canals M, Paterson GLJ, Coppock R, Sleight V, Calafat A, Rogers AD, Narayanaswamy BE, Thompson RC. The deep sea is a major sink for microplastic debris. Royal Soc Open Sci. 2014;1(4):140317. doi: 10.1098/rsos.140317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Zhao X, Chen R, Liu P, Liang W, Wang J, Teng M, Wang X, Gao S. Wastewater treatment plants act as essential sources of microplastic formation in aquatic environments: a critical review. Water Res. 2022;221:118825. doi: 10.1016/j.watres.2022.118825. [DOI] [PubMed] [Google Scholar]
- Xia X, Sun M, Zhou M, Chang Z, Li L (2020) Polyvinyl chloride microplastics induce growth inhibition and oxidative stress in Cyprinus carpio var. larvae. Sci Total Environ 716:136479 [DOI] [PubMed]
- Xia F, Yao Q, Zhang J, Wang D. Effects of seasonal variation and resuspension on MPs in river sediments. Environ Pollut. 2021;286:117403. doi: 10.1016/j.envpol.2021.117403. [DOI] [PubMed] [Google Scholar]
- Xia X, Wu X, Zhao X, Chen R, Liu P, Liang W, Wang J, Teng M, Wang X, Gao S. Wastewater treatment plants act as essential sources of microplastic formation in aquatic environments: a critical review. Water Res. 2022;221:118825. doi: 10.1016/j.watres.2022.118825. [DOI] [PubMed] [Google Scholar]
- Xu P, Peng G, Su L, Gao Y, Gao L, Li D. Microplastic risk assessment in surface waters: a case study in the Changjiang Estuary. China Mar Pollut Bull. 2018;133:647–654. doi: 10.1016/j.marpolbul.2018.06.020. [DOI] [PubMed] [Google Scholar]
- Yin Z. The pollution of microplastics in sediments: the ecological risk assessment and pollution source analysis. Sci Total Environ. 2023;859:160323. doi: 10.1016/j.scitotenv.2022.160323. [DOI] [PubMed] [Google Scholar]
- Yin J, Long Y, Xiao W, Liu D, Tian Q, Li Y, Liu C, Chen L, Pan Y. Ecotoxicology of microplastics in Daphnia: a review focusing on microplastic properties and multiscale attributes of Daphnia. Ecotoxicol Environ Saf. 2023;249:114433. doi: 10.1016/j.ecoenv.2022.114433. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Xiang M, Liu C, Theng BK. Chronic impact of an accidental wastewater spill from a smelter, China: a study of health risk of heavy metal (loid) s via vegetable intake. Ecotoxicol Environ Saf. 2019;182:109401. doi: 10.1016/j.ecoenv.2019.109401. [DOI] [PubMed] [Google Scholar]
- Zhang K, Su J, Xiong X, Wu X, Wu C, Liu J. Microplastic pollution of lakeshore sediments from remote lakes in Tibet plateau. China Environ Pollut. 2016;219:450–455. doi: 10.1016/j.envpol.2016.05.048. [DOI] [PubMed] [Google Scholar]
- Zhang B, Wu D, Yang X, Teng J, Liu Y, Zhang C, Zhao J, Yin X, You L, Liu Y, Wang Q. Microplastic pollution in the surface sediments collected from Sishili Bay, North Yellow Sea, China. Mar Pollut Bull. 2019;141:9–15. doi: 10.1016/j.marpolbul.2019.02.021. [DOI] [PubMed] [Google Scholar]
- Zhao S, Zhu L, Li D. Microplastic in three urban estuaries. China Environ Pollut. 2015;206:597–604. doi: 10.1016/j.envpol.2015.08.027. [DOI] [PubMed] [Google Scholar]
- Zhao X, Wang J, Yee Leung KM, Wu F. Color: an important but overlooked factor for plastic photoaging and microplastic formation. Environ Sci Technol. 2022;56(13):9161–9163. doi: 10.1021/acs.est.2c02402. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Zhang H, Fu C, Zhou Y, Dai Z, Li Y, Tu C, Luo Y. The distribution and morphology of MPs in coastal soils adjacent to the Bohai Sea and the Yellow Sea. Geoderma. 2018;322:201–208. [Google Scholar]
- Ziajahromi S, Kumar A, Neale PA, Leusch FDL. Environmentally relevant concentrations of polyethylene MPs negatively impact the survival, growth and emergence of sediment-dwelling invertebrates. Environ Pollut. 2018;236:425–431. doi: 10.1016/j.envpol.2018.01.094. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data analyzed in this manuscript is included in the published article.