Abstract
Rice is the only crop which is well adapted to aquatic environment but, it is unable to survive if completely submerged for several weeks. Breeding rice varieties with submergence tolerance is one of the best approaches to alleviate the adverse effect of submergence which requires the introgression of Sub1 gene into elite rice varieties. Hence, the study was undertaken to introgress submergence tolerant gene into the rice variety Jaya through Marker-Assisted Backcross Breeding. Also the physiological and biochemical responses like survival percentage, underwater shoot elongation, total carbohydrate content and superoxide dismutase activity were also studied in Sub1 introgressed lines. We could develop twenty Sub1 introgressed lines with Sub1 region of 3.1–5.1mb and with 80.0- 95.3% recurrent parent genome recovery. Sub1 introgressed Jaya lines and the tolerant checks FR13A and Swarna Sub1 had lower shoot elongation under water, higher superoxide dismutase activity (about 5 times) upto 4 h after de-submergence which resulted in higher survival percentage. The reduced shoot elongation of tolerant varieties reduced the utilization of stored carbohydrate. Through our research we introgressed Sub1 gene into rice variety Jaya through Marker-Assisted Backcross Breeding and could study the physiological responses under submergence by which we confirmed the presence of Sub1 gene in these lines. These lines could be field evaluated and could be released as a new variety thus helping the farmers of flood prone areas of Kerala.
Keywords: Submergence, MABB, Shoot elongation, Survival percentage, Carbohydrate, Super oxide dismutase
Introduction
Rice (Oryza sativa L.) is the major staple food in South India and it is consumed worldwide by approximately 520.0 million tonnes in 2023/2024 which is unchanged from the 2022/2023 level (FAO 2023). Among the abiotic stresses, submergence is the third important abiotic stress affecting rice crop (Yadav et al. 2018). Submergence caused by floods due to heavy showers of south-west monsoon in coastal ecosystems of Kerala results in yield loss in rice which depends upon the plant type, crop growth stage, and the intensity and duration of submergence. When susceptible rice plants are submerged, the level of ethylene increases, plants elongate, and chlorophyll is degraded. This consumes energy and carbon and hastens plant mortality under transient submergence, particularly when the photosynthetic rate is reduced severely (Mackill et al. 2012). One way to overcome the problem of submergence is to introgress submergence tolerance genes in popular high-yielding rice varieties through Marker-Assisted Backcross Breeding. It is an easy and fast tool used for breeding that aims to introgress traits of interest into an elite variety. It has several advantages over conventional breeding like accuracy, consistency, bio-safety, speedy recovery of recurrent parent genome, and minimizes the linkage drag. Co-dominant SSR markers that are tightly linked with important traits have been identified and it is used for MABB in rice (Xu et al. 2004).
According to the duration of flooding and water depth, submergence can be classified into ‘flash flooding’ and ‘deep-water flooding’ (Bailey- Serres et al., 2010; Jackson and Ram 2003). Flash flooding lasts less than a few weeks in which the water level is shallow and is caused by continuous heavy rain. On the other hand, deep-water flooding lasts for several months, occurs during the rainy season, and the water depth reaches several meters (Hattori et al. 2011). Rice crop adapts to submergence stress by internal aeration and reduced underwater growth. For internal aeration, rice develops longitudinal aerenchyma and leaf gas films. On the other hand, some rice cultivars can survive under submergence by using special strategies of growth control: a quiescence strategy and an escape strategy. The Submergence-1A (Sub1A) gene is responsible for the quiescence strategy, which is important for survival under flash-flood conditions (Colmer and Voesenek 2009). In this case, shoot elongation is suppressed to preserve the available carbohydrates in the plant for a long period upto 10–14 days. The submergence tolerant cultivars restart their growth during de-submergence by using the preserved carbohydrates. The SNORKEL 1 (SK1) and SNORKEL 2 (SK2) genes are responsible for the escape strategy, which is important for survival under deep-water flood conditions (Bailey-Serres and Voesenek 2008; Colmer and Voesenek 2009). Fast elongation of internodes helps the leaves of the plant to rise above the water level in deep-water rice cultivars, and thereby partial photosynthesis takes place for carbohydrate synthesis (Sarkar et al. 2006).
Genome mapping of rice variety FR13A could identify the Sub1 locus on chromosome number 9 (Xu and Mackill 1996). Xu et al., (2006) found that the Sub1 locus contains Sub1A, Sub1B and Sub1C, all of which encode ethylene response factors and are upregulated under submergence. Fukao and Bailey-Serres, (2008) reported that Sub1A also enhances the expression of genes encoding Slender Rice-1 (SLR1) and SLR1 like 1 (SLRL1), which are the key repressors of gibberellin (GA) signaling in rice; it also negatively regulates the GA response to restrict shoot elongation under submergence. It was shown that Sub1A expression is also induced by drought and oxidative stress upon de-submergence (Fukao et al. 2011) and under oxidative stress, Sub1A promotes the expression of genes related to the detoxification of reactive oxygen species (ROS) and reduces the accumulation of ROS.
Flooding or submergence creates hypoxia or anoxia in the plant system due to slower diffusion of gases in water (Armstrong 1980), which produces several metabolic disturbances leading to tissue damage and gradual death of plants. ROS including superoxide, hydrogen peroxide, hydroxyl radicals and singlet oxygen are the byproducts of cell metabolism when molecular oxygen enters suddenly into the cells after exposure of plants to air during post submergence phase. The elevated levels of these free radicals of oxygen cause membrane damage through lipid peroxidation and protein denaturation if not removed by oxygen scavenging systems (Ella et al. 2003; Jackson and Ram 2003). Though oxygen scavenging systems are constitutive, it showed higher activity in response to re-exposure to oxygen after a period of anoxia induced by submergence or flooding (Srivastava et al. 2007). Twenty rice lines developed by introgressing Sub1 gene into rice variety Jaya by Marker-Assisted Backcross Breeding were used in this study. The physiological changes of these lines along with the donor parent Swarna Sub1, recurrent parent Jaya, tolerant check FR13A and the susceptible check IR64 were evaluated under submergence stress.
Materials and method
Introgression of Sub1 gene through marker-assisted backcross breeding (MABB)
Rice seeds of recurrent parent (RP) Jaya and donor parent (DP) Swarna Sub1 were raised in plastic trays and grown under natural environmental conditions in the greenhouse. Fourteen days old seedlings were transferred into well-labeled pots with four seedlings per pot. Staggered sowing of the parents was undertaken at an interval of 7 days to ensure synchronous flowering in order to produce adequate crossed seeds. Manuring, pesticide application and watering were followed as recommended in KAU, 2011. The RP Jaya was used as a female parent and crossed with DP Swarna Sub1 to raise the F1 generation by hybridization. Genotypic analysis was carried out using selected polymorphic foreground markers and the heterozygous plants in the target locus were selected for backcrossing with RP Jaya to raise BC1F1 generation to regain recurrent parent genome recovery. Genotypic analysis in each generation was carried out using selected foreground, recombinant and background polymorphic markers. The selected heterozygous BC1F1 plants were backcrossed with RP Jaya to raise BC2F1. The selected heterozygous BC2F1 progenies were backcrossed with RP Jaya and selfed to raise BC3F1 and BC2F2 generations. The selected heterozygous BC3F1 progenies were selfed to raise BC3F2 generation. Genetic analysis was done as described above and introgressed homozygous plants in the target locus with maximum background genome recovery using GGT 2.0 software (Ralph 2008) were selected for phenotypic screening and field evaluation.
In vitro imposition of submergence stress
The seeds of twenty Sub1 introgressed BC3F2 Jaya rice lines, recurrent parent Jaya and donor parent Swarna Sub1 along with tolerant and susceptible checks were surface sterilized with fungicides like Bavistin and Indofil (in the ratio of 1:1, 0.1 g/100 mL) for half an hour and seeds were washed thrice with tap water and soaked overnight. The soaked seeds were spread on moist filter paper in a petri plate and kept at room temperature in the dark for 48 h to germinate. Germinated seedlings were transferred to small pots and grown under natural environmental conditions.
Submergence screening was conducted in submergence tanks containing water of 1 m depth following standard protocols (Xu et al. 2000). The screening experiment was conducted in a Completely Randomized Design with 24 treatments (twenty introgressed lines, two tolerant check and two susceptible check varieties) of 40 plants each and three replications. Fourteen days old plants were submerged for 2 weeks and after that the plants were de-submerged and kept for recovery.
Morphological and physiological parameters after de-submergence
Survival percentage after de-submergence
The survival of the plants was scored on the 14th day after de-submergence using Standard Evaluation System (IRRI 1988) for the phenotypic analysis of survival percentage as shown in Table 1.
Table 1.
Standard Evaluation System for scoring submergence tolerance of rice
| Survival percentage | Score | Observation | Tolerance |
|---|---|---|---|
| 100% | 1 | Minor visible symptoms of injury | Highly tolerant |
| 95–99% | 3 | Some visible symptoms of injury | Tolerant |
| 75–94% | 5 | Moderate injury | Moderately tolerant |
| 50–75% | 7 | Severe injury | Susceptible |
| 0–49% | 9 | Partial to complete death | Highly susceptible |
Shoot elongation under submergence
The plant height of the rice genotypes was measured from the base of the plant to the tip of the youngest leaf. Observations were recorded before and after submergence according to the method of Das et al. 2005.
Total carbohydrate content before and after submergence
Leaf tissues were collected from the control, submerged (0th, 3rd, 7th, 10th, 14th day after submergence) and de-submerged plants (10 days after de-submergence) of Sub1 introgressed BC3F2 lines, Jaya, Jyothi, Uma, Swarna Sub1 and FR13A and were dried at 70ºC for 48 h and ground in a mortar and pestle. The total carbohydrates in leaf tissue samples were estimated as described by Fales 1951.
Superoxide dismutase assay
Activity of superoxide dismutase (SOD) was measured according to the method described by Beauchamp and Fridovich 1971. The activities of active oxygen scavenging systems like superoxide dismutase (SOD) in leaves were measured in triplicate before and after submergence. A time course measurement study was conducted after de-submerging the plants. For this, samples in triplicate were collected to measure the activity of SOD after re-exposure of the plant to normal conditions.
SOD is calculated as follows:
50% inhibition = to1 unit of enzyme. Then X% inhibition is equal to 1/50 × X = Y unit.
Y unit is from = 20 µl enzyme extract.
Total volume of enzyme extract = 5 ml = 5000 µl.
So, (Y unit/20 × 5000) = Z units of SOD.
5 ml of extract is from 0.625 g of tissue.
So, SOD units/ g give the unit value in g fresh weight.
Statistical analysis
The various parameters assessed in this study were subjected to analysis of variance using WASP 2.0 software, the first Web Based Agricultural Statistics Software Package developed by ICAR (www.icargoa.res.in).
Results and discussion
Marker-assisted backcross breeding for introgression of Sub1 gene
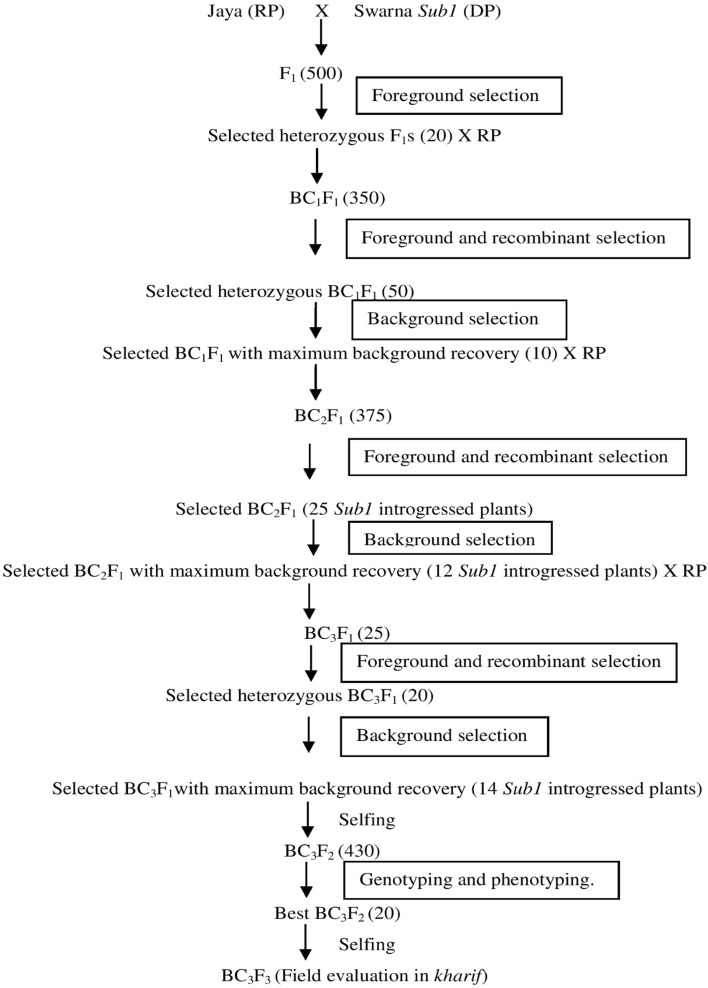
A total of 20 superior heterozygous progenies were selected from 500 progenies in F1 generation using foreground markers viz., ART5, Sub1BC2, and RM23788 (Table 2). The breeding scheme for submergence tolerance using marker-assisted backcrossing is shown in Fig. 1. In the BC1F1 generation, 155/350 plants showed clear heterozygosity using the foreground SSR markers ART5, Sub1 BC2 and RM23788 which are tightly linked to the Sub1 gene. BC1F1 plants introgressed with the Sub1 gene were screened with the above-mentioned flanking markers found on the upstream and downstream of the Sub1 locus (Table 3). Progenies with heterozygous bands expressed both the recurrent and donor-parent alleles. Fifty out of 155 plants showed clear heterozygosity using the selected polymorphic recombinant markers. Two out of 5 selected polymorphic recombinant markers viz., RM8303 (2.3 mb) and RM23770 (3.7 mb) were in the telomeric end and three markers viz, RM23917 (7.3 mb), RM23922 (7.4 mb) and RM23958 (7.9 mb) were in the centromeric end. Except for target locus, all genomic regions of the recurrent parent can be recovered in background selection using recurrent parent marker alleles (Hasan et al. 2015). In BC1F1 generation, 10 plants out of 50 plants with maximum recurrent parent recovery were selected. In this study, the percentage of recurrent parent genome recovery ranged from 39.4 to 71.3% of which BC1F1-3 had the highest recurrent parent genome recovery of 71.3% followed by BC1F1-5 (70.6%) and BC1F1-25 (70.1%) respectively. Ahmed et al. 2015 discussed that after background screening, the recurrent parent genome recovery was about 65.55–77.8% in BC1F1 generations. Similarly, Neeraja et al. 2007 reported that in the best plant no.242 recurrent parent genome recovery was 67.9%.
Table 2.
SES Score after 14 days of de-submergence
| Plant code./ variety | Plants before submergence (no.) | Plants after submergence (no.) | Survival percentage (%) | Submergence Score |
|---|---|---|---|---|
| BC3F2-3-2-6-2 | 40 | 38.0c | 95.2c | 3 |
| BC3F2-3-2-6-4 | 40 | 39.0a | 97.2a | 3 |
| BC3F2-3-2-6-8 | 40 | 38.6ab | 96.2b | 3 |
| BC3F2-3-2-6-10 | 40 | 38.3bc | 95.5c | 3 |
| BC3F2-3-2-11-5 | 40 | 39.0a | 97.2a | 3 |
| BC3F2-3-2-11-9 | 40 | 39.0a | 97.2a | 3 |
| BC3F2-3-2-11-10 | 40 | 38.0c | 95.2c | 3 |
| BC3F2-3-5-4-2 | 40 | 39.0a | 97.5a | 3 |
| BC3F2-3-5-4-6 | 40 | 38.0c | 95.0c | 3 |
| BC3F2-3-5-4-7 | 40 | 39.0a | 97.2a | 3 |
| BC3F2-3-5-7-2 | 40 | 38.6ab | 96.2b | 3 |
| BC3F2-3-5-7-6 | 40 | 38.6ab | 96.2b | 3 |
| BC3F2-3-5-7-7 | 40 | 38.0c | 95.2c | 3 |
| BC3F2-5-21-1-3 | 40 | 38.6ab | 96.2b | 3 |
| BC3F2-5-21-5-1 | 40 | 38.3bc | 95.5c | 3 |
| BC3F2-5-21-5-2 | 40 | 38.6ab | 96.2b | 3 |
| BC3F2-5-21-5-4 | 40 | 38.3bc | 95.5c | 3 |
| BC3F2-5-21-5-5 | 40 | 39.0a | 97.2a | 3 |
| BC3F2-5-21-5-7 | 40 | 38.6ab | 96.2b | 3 |
| BC3F2-5-21-5-9 | 40 | 38.0c | 95.2c | 3 |
| IR64 | 40 | 0.0d | 0.0d | 9 |
| Jaya | 40 | 0.0d | 0.0d | 9 |
| Swarna Sub1 | 40 | 39.0a | 97.5a | 3 |
| FR13A | 40 | 39.0a | 97.5a | 3 |
| CD (0.01)* | – | 0.862 | 0.900 | – |
| CD (0.05)* | – | 0.644 | 0.678 | – |
*CD -critical difference 0.01and 0.05. Treatments found significant at 1% and 5% level of significance
Fig. 1.
Diagrammatic representation of breeding scheme for submergence screening using marker-assisted backcross breeding
Table 3.
Plant height and elongation % of Sub1 introgressed plants before and after submergence
| Plant code/ variety | Plant height before submergence | Plant height after submergence | Elongation % |
|---|---|---|---|
| BC3F2-3-2-6-2 | 19.56hi | 21.60hi | 10.4 |
| BC3F2-3-2-6-4 | 21.26bcdef | 23.46bcdef | 10.3 |
| BC3F2-3-2-6-8 | 18.70i | 20.70i | 10.7 |
| BC3F2-3-2-6-10 | 20.26fgh | 22.43fgh | 10.7 |
| BC3F2-3-2-11-5 | 20.43fgh | 22.56fgh | 10.4 |
| BC3F2-3-2-11-9 | 19.60hi | 21.80hi | 11.2 |
| BC3F2-3-2-11-10 | 20.46fgh | 23.20fgh | 13.4 |
| BC3F2-3-5-4-2 | 20.73cdefgh | 23.26cdefgh | 12.2 |
| BC3F2-3-5-4-6 | 22.00bcd | 24.26bcd | 10.3 |
| BC3F2-3-5-4-7 | 20.66defgh | 23.36defgh | 13.1 |
| BC3F2-3-5-7-2 | 22.26b | 24.16b | 8.5 |
| BC3F2-3-5-7-6 | 20.83cdefgh | 23.43cdefgh | 12.5 |
| BC3F2-3-5-7-7 | 21.36bcdef | 23.30bcdef | 9.1 |
| BC3F2-5-21-1-3 | 19.83ghi | 22.26ghi | 12.3 |
| BC3F2-5-21-5-1 | 20.53efgh | 22.60efgh | 10.1 |
| BC3F2-5-21-5-2 | 21.36bcdef | 23.23bcdef | 8.7 |
| BC3F2-5-21-5-4 | 21.00bcdefg | 23.53bcdefg | 12.1 |
| BC3F2-5-21-5-5 | 22.033bc | 23.83bc | 8.2 |
| BC3F2-5-21-5-7 | 21.00bcdefg | 23.53bcdefg | 12.1 |
| BC3F2-5-21-5-9 | 20.26fgh | 22.63fgh | 11.7 |
| IR64 | 16.50j | 21.23j | 28.7 |
| Jaya | 21.83bcde | 26.76bcde | 22.6 |
| Swarna Sub1 | 16.86j | 18.83j | 11.7 |
| FR13A | 24.06a | 25.96a | 7.9 |
| CD (0.01)* | 1.802 | 1.837 | – |
| CD (0.05)* | 1.348 | 1.371 | – |
*CD—critical difference 0.01and 0.05. Treatments found Significant at 1% and 5% level of significance
In the BC2F1 generation, 140 out of 375 plants showed clear heterozygosity using the polymorphic foreground markers. 25 out of 140 plants showed clear heterozygosity in the target locus using the recombinant markers were selected. Progenies with heterozygous bands expressed both the recurrent and donor-parent alleles. After background selection, 12/25 plants in BC2F1 generation with maximum background recovery were selected which ranged from 64.2 to 83.2%. But, according to Ahmed et al. 2015, recurrent parent genome recovery in the BC2F1 generations was 78.79–95.5%. Also according to Neeraja et al. 2007 recurrent parent genome recovery was about 87.2–94.2% by BC2F1 generations. The plant no. BC2F1-3-2 has the highest recurrent parent genome recovery percentage of 83.2% followed by BC2F1-3-5 (80.1%) and BC2F1-5-21(77.8%) respectively. All these three plants were forwarded to BC3F1 by backcrossing with recipient parent Jaya.
In the BC3F1 generation, 25 plants were screened with polymorphic foreground markers followed by screening with polymorphic flanking markers. After foreground and recombinant screening, 20 plants showed heterozygous bands i.e., they showed both the alleles of recurrent and donor parents. The Sub1 gene introgressed plants were then screened with polymorphic background markers. After the genotypic screening, 15 plants in the BC3F1 generation with maximum background recovery were selected which ranged from 71.7 to 91.9%. According to Neeraja et al. 2007 recurrent parent genome recovery was 96.2% in BC3F1 generation. Similarly, Rahman et al. 2018 reported that genotyping of BC3F1 plants resulted in the identification of 25 positive plants having the recurrent parent genome recovery between 76.08 and 91.30%. The highest recurrent parent genome recovery percentage of 91.9% was expressed by the plant BC3F1-3-2-11 followed by BC3F1-3-5-4 (90.5%) and BC3F1-3-2-6 (89.5%) respectively.
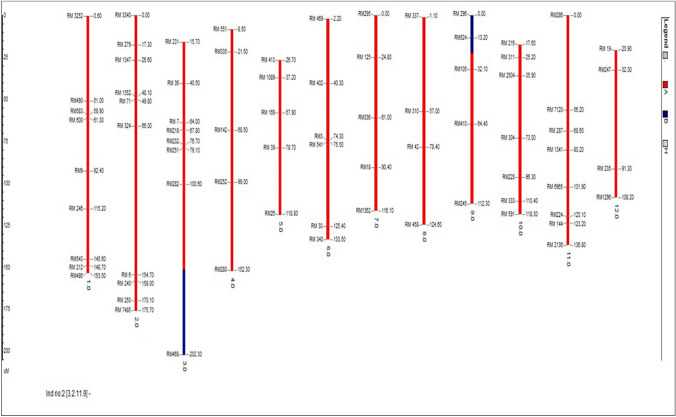
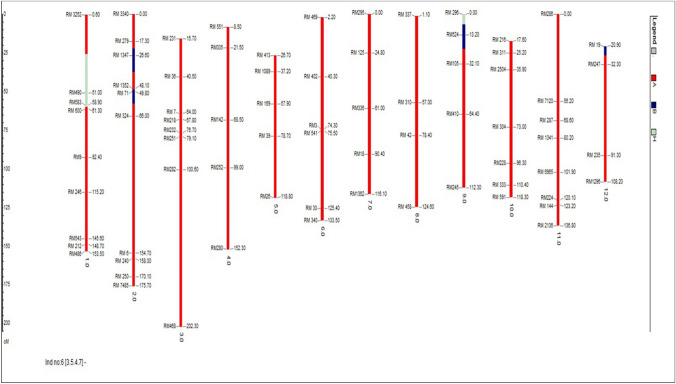
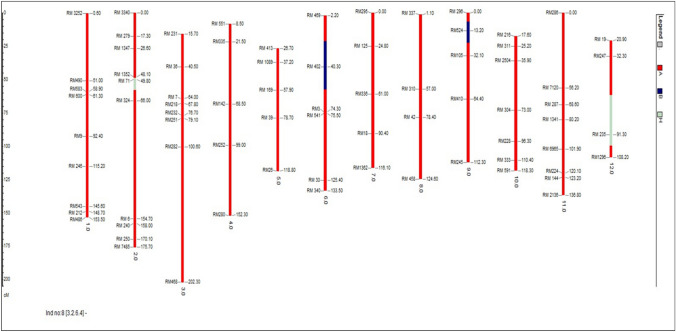
In BC3F2 generation, progenies which showed homozygous bands like that of donor parent alone were selected after the foreground screening. 340/430 plants showed clear homozygosity using the polymorphic foreground (ART5, Sub1 BC2 and RM23788) and recombinant markers (RM8303, RM23770, RM23917, RM23922 and RM23958). In selected BC3F2 plants, after screening with recombinant markers most markers showed a banding pattern like the donor parent. In BC3F2-3-2-11-9 and BC3F2-3-2-6-4, telomeric and centromeric end recombinant markers showed a homozygous alleles like that of donor parent. BC3F2-3-5-4-7, telomeric end recombinant markers showed homozygous allele like that of donor whereas centromeric end markers showed a homozygous allele like that of recipient allele with markers viz., RM23922 (7.4 mb), and RM23958 (7.9 mb) respectively. Neeraja et al. 2007 reported that the introgression size of Swarna Sub1 was 3.4 mb in BC3F2 generation using flanking markers RM316 (1.8 cM) at the proximal end and RM219 (11.7 cM) at the distal end. But in our study, the segment size of introgressed donor Sub1 region in the selected BC3F2 progenies was estimated to be 3.1–5.1 mb. The plants BC3F2-3-2-11-9 and plant BC3F2-3-2-6-4 showed 5.0 mb and 5.1 mb. While the plant BC3F2-3-5-4-7 showed a lesser donor fragment of 3.1 mb in Fig. 2.
Fig. 2.
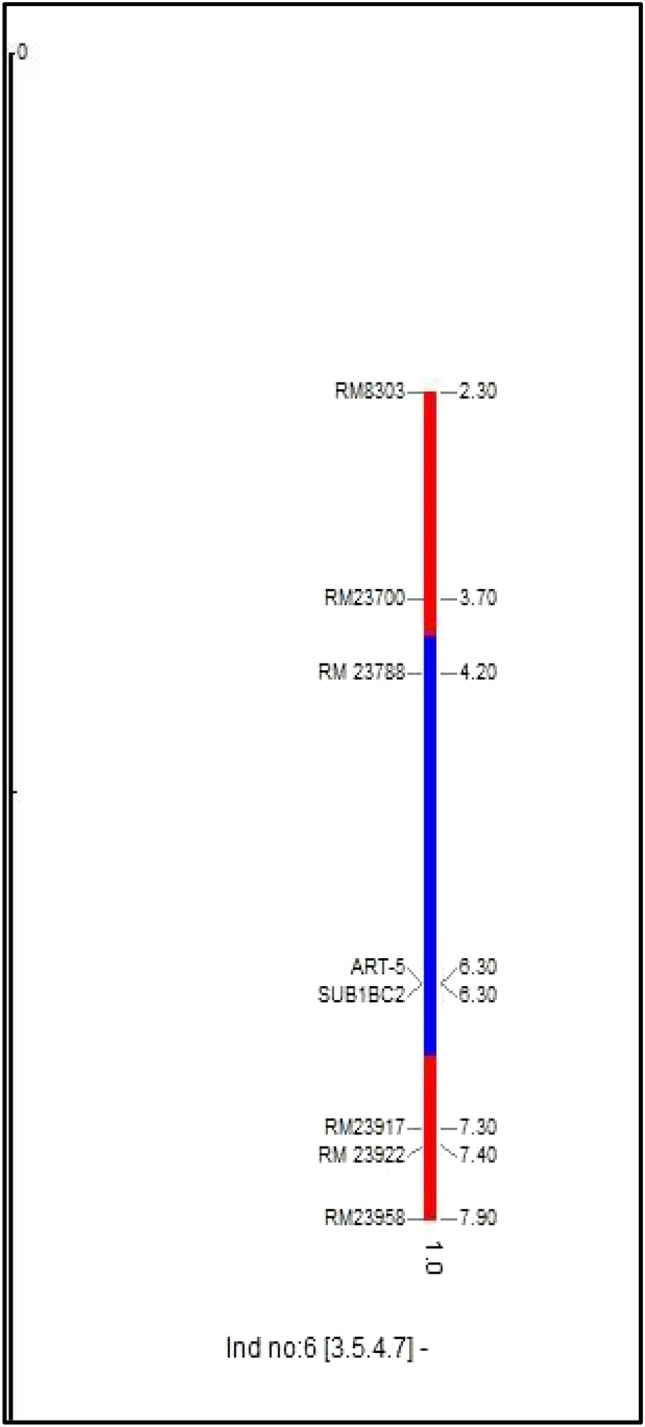
Image of donor fragment in 9th chromosome using GGT Software in plant no. BC3F2-3-5-4-7. The red-colored regions on the chromosomes indicate homozygous regions for the recipient genome while the blue-colored regions indicate homozygous regions for the donor parent
After background selection, 20 plants in BC3F2 generation were selected. Background selection of superior BC3F3 progenies using 71 SSR markers revealed the background genome recovery ranging between 94.37% and 95.78% (Rahman et al. 2018). The percentage of recurrent parent genome recovery in the BC3F2 generation ranged from 80.0 to 95.3% of which BC3F2-3-2-11-9 had the highest recurrent parent genome recovery of 95.3% followed by BC3F2-3-5-4-7 (94.4%) and BC3F2-3-2-6-4 (93.7%) respectively. In the plant BC3F2-3-2-11-9, the chromosomes no. 1, 2, 4, 5, 6, 7, 8, 10, 11, and 12 were found to be similar to the recurrent parent Jaya and the percentage of heterozygous region of the plant BC3F2-3-2-11-9 is 0. Three best plants having the highest recurrent parent genome recovery in BC3F2 -3-2-11-9, BC3F2-3-5-4-7 and BC3F2-3-2-6-4 are given in Figs. 3, 4 and 5.
Fig. 3.
Image of plant—BC3F2-3-2-11-9 (95.3%) with highest recurrent parent genome recovery using GGT software
Fig. 4.
Image of plant—BC3F2-3-5-4-7 (94.4%) with second highest recurrent parent genome recovery using GGT software
Fig. 5.
Image of plant—BC3F2-3-2-6-4 (93.7%) with third highest recurrent parent genome recovery using GGT software
Survival percentage after 14 days of submergence
The genotypically selected BC3F2 plants with Sub1 gene introgression were phenotypically screened under submergence stress for 14 days shown in Fig. 6. After de-submergence, tolerant rice plants were selected based on the visual symptoms using IRRI’s SES score for submergence tolerance screening after an establishment period of 14 days. Plants that scored ‘3’ showed a similar survival pattern similar to Swarna Sub1 were scored as tolerant thus confirming the successful introgression of Sub1 QTL (Table 4). All the Sub1 introgressed lines exhibited survival ability after 14 days of complete submergence similar to Swarna Sub1 and renewed growth and recovered after de-submergence. The recurrent parent, Jaya, and the susceptible check IR64 which lacked the Sub1 gene completely withered and died after 14 days of submergence and showed zero percentage survival. The report of Cuc et al. (2012) also supported this study in which they stated that the selected Sub1 introgressed BC2F2 and BC3F1 lines of AS996 showed submergence tolerance after submergence stress of 21 days and the tolerance score was on par with the score of donor parent IR64 Sub1.
Fig. 6.
In vitro screening for Submergence tolerance a: 14 days old plants b: de-submerged plants after 14 days of submergence. c: plants after 14 days of de-submergence. All the introgressed lines along with tolerant check varieties Swarna Sub1 and FR13A recovered from the submergence stress whereas the susceptible varieties Jaya and IR-64 could not survive after submergence
Table 4.
Total carbohydrate content (mg /100 mg of leaf) and % reduction in rice varieties during and after submergence
| Plant code/variety | Carbohydrate content (mg/100 mg leaf tissue) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before submergence | After 3 days | After 7 days | After 10 days | After 14 days | % CU | 10 days ADS | |||||||||
| NSC | S | U | NSC | S | U | NSC | S | U | NSC | S | U | ||||
| BC3F2-3-2-6-2 | 17.3 | 20.7 | 14.53 | 2.77 | 24.6 | 11.53 | 5.77 | 29.1 | 9.3 | 8.00 | 32.0 | 8.3 | 9.00 | 52.02 | 12.2 |
| BC3F2-3-2-6-4 | 16.4 | 19.6 | 13.25 | 3.15 | 23.8 | 10.5 | 5.90 | 28.3 | 8.2 | 8.20 | 31.12 | 7.2 | 9.20 | 56.09 | 11.1 |
| BC3F2-3-2-6-8 | 17.6 | 20.7 | 13.6 | 4.00 | 24.5 | 11.1 | 6.50 | 29.4 | 9.3 | 8.30 | 32.11 | 8.1 | 9.50 | 53.97 | 12.3 |
| BC3F2-3-2-6-10 | 18.3 | 21.2 | 14.5 | 3.80 | 25.3 | 12.2 | 6.10 | 31.0 | 10.3 | 8.00 | 33.1 | 9.3 | 9.00 | 49.18 | 13.4 |
| BC3F2-3-2-11-5 | 17.6 | 20.5 | 13.8 | 3.80 | 24.3 | 11.4 | 6.20 | 29.1 | 9.5 | 8.10 | 32.11 | 8.25 | 9.35 | 53.13 | 12.3 |
| BC3F2-3-2-11-9 | 16.7 | 19.6 | 13.5 | 3.20 | 23.5 | 10.23 | 6.47 | 28.4 | 8.3 | 8.40 | 31.12 | 7.3 | 9.40 | 56.28 | 11.3 |
| BC3F2-3-2-11-10 | 17.5 | 21.5 | 14.4 | 3.10 | 25.3 | 11.2 | 6.30 | 30.12 | 9.6 | 7.90 | 32.13 | 8.35 | 9.15 | 52.28 | 12.3 |
| BC3F2-3-5-4-2 | 17.4 | 21.3 | 14.3 | 3.10 | 25.4 | 11.1 | 6.00 | 30.5 | 9.6 | 7.80 | 32.14 | 8.25 | 9.15 | 52.58 | 12.5 |
| BC3F2-3-5-4-6 | 17.5 | 21.5 | 14.2 | 3.30 | 25.6 | 11.5 | 6.00 | 30.6 | 9.5 | 8.00 | 32.14 | 8.24 | 9.26 | 52.91 | 12.3 |
| BC3F2-3-5-4-7 | 17.4 | 21.5 | 14.5 | 2.90 | 25.7 | 11.6 | 5.80 | 30.14 | 9.6 | 7.80 | 32.14 | 8.26 | 9.14 | 52.52 | 12.1 |
| BC3F2-3-5-7-2 | 17.4 | 21.5 | 14.8 | 2.60 | 26.0 | 11.5 | 5.90 | 30.5 | 9.4 | 8.00 | 32.13 | 8.3 | 9.10 | 52.29 | 12.2 |
| BC3F2-3-5-7-6 | 17.3 | 21.6 | 14.53 | 2.77 | 25.8 | 11.32 | 5.98 | 30.7 | 9.12 | 8.18 | 32.11 | 8.35 | 8.95 | 51.73 | 12.0 |
| BC3F2-3-5-7-7 | 18.4 | 22.3 | 15.5 | 2.90 | 26.4 | 12.2 | 6.20 | 31.2 | 10.5 | 7.90 | 33.1 | 9.3 | 9.10 | 49.45 | 13.3 |
| BC3F2-5-21-1-3 | 16.5 | 19.3 | 13.2 | 3.30 | 24.3 | 11.3 | 5.20 | 29.1 | 9.2 | 7.20 | 31.1 | 7.4 | 9.10 | 55.15 | 11.5 |
| BC3F2-5-21-5-1 | 17.4 | 20.1 | 14.3 | 3.10 | 25.3 | 12.3 | 5.10 | 30.4 | 10.2 | 7.20 | 32.0 | 8.3 | 9.10 | 52.29 | 12.3 |
| BC3F2-5-21-5-2 | 18.6 | 21.5 | 15.6 | 3.00 | 26.8 | 13.6 | 5.00 | 31.4 | 11.5 | 7.10 | 33.12 | 9.35 | 9.25 | 49.73 | 13.3 |
| BC3F2-5-21-5-4 | 17.6 | 20.8 | 14.5 | 3.10 | 25.4 | 12.7 | 4.90 | 30.8 | 10.5 | 7.10 | 32.12 | 8.3 | 9.30 | 52.84 | 12.3 |
| BC3F2-5-21-5-5 | 16.4 | 19.4 | 13.8 | 2.60 | 24.6 | 10.5 | 3.70 | 29.4 | 8.7 | 7.7 | 31.13 | 7.29 | 9.11 | 55.54 | 11.4 |
| BC3F2-5-21-5-7 | 17.45 | 20.4 | 14.6 | 2.85 | 25.6 | 11.5 | 5.95 | 30.4 | 10.8 | 6.65 | 32.11 | 8.28 | 9.17 | 52.55 | 12.4 |
| BC3F2-5-21-5-9 | 17.6 | 20.5 | 14.7 | 2.90 | 25.7 | 11.5 | 6.10 | 30.5 | 10.8 | 6.80 | 32.13 | 8.27 | 9.33 | 53.09 | 12.3 |
| Uma | 24.65 | 33.12 | 16.05 | 8.00 | 37.2 | 9.4 | 15.25 | 39.5 | 8.8 | 15.85 | 41.09 | 6.7 | 17.95 | 72.81 | 3.25 |
| Jyothi | 22.15 | 33.75 | 15.81 | 6.34 | 36.9 | 11.8 | 10.35 | 39.78 | 8.4 | 13.75 | 40.95 | 7.4 | 14.75 | 66.59 | 4.14 |
| Jaya | 23.75 | 32.95 | 16.00 | 7.75 | 36.84 | 12.8 | 14.00 | 39.23 | 9.75 | 14.00 | 42.09 | 7.1 | 16.65 | 70.10 | 3.5 |
| Swarna Sub1 | 17.45 | 20.88 | 14.35 | 3.10 | 25.04 | 11.35 | 6.10 | 29.9 | 9.55 | 7.90 | 32.13 | 8.3 | 9.15 | 52.43 | 12.3 |
| FR13A | 20.4 | 23.83 | 19.67 | 0.73 | 28.52 | 17.06 | 3.34 | 32.5 | 12.65 | 7.75 | 36.1 | 9.29 | 11.11 | 54.46 | 13.6 |
NSC: represent without submergence; S: represent submerged treatment; U: carbohydrate content utilized under submergence. ADS: represent after de- submergence. % CU: represent percentage carbohydrate utilization after complete submergence for 14 days
The survival percentage of the tested Sub1 introgressed lines was statistically on par with the donor parent and tolerant check variety FR13A. The recurrent parent Jaya and susceptible check IR64 could not survive 14 days of complete submergence and the survival percentage was zero. The donor parent Swarna Sub1 and resistant check FR13A expressed 97.5% survival after 14 days of complete submergence whereas the survival percentage of Sub1 introgressed Jaya lines ranged from 95.0 to 97.5%. Six Sub1 introgressed lines (BC3F2-3-5-4-2, BC3F2-3-2-6-4, BC3F2-3-2-11-5, BC3F2-3-2-11-9, BC3F2-3-5-4-2, BC3F2-3-5-4-7 and BC3F2-5-21-5-5) showed the survival percentage of 97.5 similar to Swarna Sub1 and FR13A. However, all the Sub1 introgressed Jaya lines expressed ≥ 95.0% survival after 14 days of complete submergence.
Shoot elongation under submergence
The least shoot elongation (Table 5) under water was expressed by FR13A (7.9%), the original Sub1 donor, whereas Swarna Sub1, the donor parent showed underwater elongation of 11.7%. The recurrent parent Jaya expressed 22.6% elongation and the susceptible check IR64 showed a maximum elongation of 28.7%. Both Jaya and IR64 could not survive after de-submergence because these plants might have utilized a major share of stored carbohydrates for shoot elongation. After de-submergence, the carbohydrate left might not be sufficient for renewal of growth and the leaves could not synthesize carbohydrate through photosynthesis as most of the leaves were decayed under water. Among the tested Sub1 introgressed lines of Jaya, the line BC3F2-5-21-5-5 showed the least elongation of 8.2% followed by BC3F2-3-5-7-2 (8.5%) and BC3F2-5-21-5-2 (8.7%) which is less than the donor parent Swarna Sub1 but comparable to the level of FR-13A. Two introgressed lines exhibited a slightly higher elongation percentage of 13.4% (BC3F2-3-2-11-10) and BC3F2-3-5-4-7 (13.1%). In general, out of the twenty Sub1 introgressed lines screened, 13 lines showed lesser or equal elongation% (8.5 to 11.7) as that of donor parent Swarna Sub1 (11.7%) whereas 7 lines expressed slightly higher elongation (12.1–13.4%) percentage than Swarna Sub1. Srivastava et al., (2007) also observed more elongation in susceptible varieties than in Sub1 gene introgressed genotypes. Submergence tolerant rice varieties had lesser elongation under water and had better survival percentage. Fukao et al. (2006) demonstrated that genotypes lacking the Sub1 gene rapidly consumed leaf starch and soluble sugars for elongation growth during submergence so as to bring the leaves above water for affecting photosynthesis, while genotypes with Sub1 gene consumed carbohydrate energy reserves slowly during submergence and maintained growth at a rate similar to plants in air which is in line with the present study.
Table 5.
Activity of superoxide dismutase enzyme in Sub1 introgressed lines before and after submergence tolerance screening
| Plant code / variety | Before submergence | De-submerged plants after 14 days of submergence | Non- submerged plants | ||||
|---|---|---|---|---|---|---|---|
| 0 h | 2 h | 4 h | 6 h | 24 h | |||
| BC3F2-3-2-6-2 | 310.0 | 592.0bc | 1248.0bcdef | 1497.7bc | 747.0c | 297.7c | 315.0 |
| BC3F2-3-2-6-4 | 313.3 | 595.3b | 1246.3fgh | 1498.7bc | 748.7ab | 298.0c | 315.7 |
| BC3F2-3-2-6-8 | 315.7 | 593.7bc | 1246.7efgh | 1497.7bc | 748.0abc | 298.3bc | 317.3 |
| BC3F2-3-2-6-10 | 311.7 | 593.0bc | 1248.7bcd | 1498.3bc | 747.0c | 297.7c | 313.0 |
| BC3F2-3-2-11-5 | 317.0 | 593.7bc | 1249.0bc | 1498.0bc | 748.7ab | 298.3bc | 318.0 |
| BC3F2-3-2-11-9 | 316.3 | 594.3b | 1247.0defgh | 1498.0bc | 748.0abc | 297.7c | 317.0 |
| BC3F2-3-2-11-10 | 316.0 | 594.0b | 1246.0gh | 1499.0b | 747.0c | 298.3bc | 317.0 |
| BC3F2-3-5-4-2 | 315.0 | 595.3b | 1248.0bcdef | 1497.0c | 748.7ab | 298.0c | 316.3 |
| BC3F2-3-5-4-6 | 316.7 | 593.0bc | 1249.0bc | 1498.7bc | 748.0abc | 297.7c | 317.7 |
| BC3F2-3-5-4-7 | 316.0 | 594.3b | 1248.0bcdef | 1498.0bc | 747.0c | 298.0c | 317.7 |
| BC3F2-3-5-7-2 | 316.0 | 594.7b | 1245.7 h | 1497.7bc | 748.7ab | 298.3bc | 316.7 |
| BC3F2-3-5-7-6 | 316.0 | 594.0b | 1247.7cdefg | 1498.7bc | 748.0abc | 297.7c | 317.7 |
| BC3F2-3-5-7-7 | 313.0 | 595.3b | 1249.0bc | 1497.7bc | 747.0c | 298.3bc | 315.0 |
| BC3F2-5-21-1-3 | 316.0 | 593.0bc | 1248.3bcde | 1498.3bc | 748.7ab | 297.7c | 317.0 |
| BC3F2-5-21-5-1 | 316.0 | 594.3b | 1248.3bcde | 1498.0bc | 748.0abc | 298.3bc | 318.0 |
| BC3F2-5-21-5-2 | 315.3 | 594.3b | 1247.7cdefg | 1498.0bc | 748.7ab | 297.7c | 318.3 |
| BC3F2-5-21-5-4 | 315.0 | 590.7bc | 1248.0bcdef | 1499.0b | 747.7bc | 298.7abc | 315.7 |
| BC3F2-5-21-5-5 | 317.3 | 593.0bc | 1245.7 h | 1497.0c | 747.0c | 297.7c | 318.0 |
| BC3F2-5-21-5-7 | 312.7 | 595.3b | 1247.7cdefg | 1498.7bc | 748.7ab | 298.3bc | 314.3 |
| BC3F2-5-21-5-9 | 317.0 | 587.3c | 1249.0bc | 1498.0bc | 748.0abc | 298.0c | 316.7 |
| IR64 | 313.7 | 396.7d | 448.7i | 499.3d | 299.7d | 249.7d | 315.7 |
| Jaya | 315.0 | 398.7d | 447.7i | 499.7d | 299.3d | 249.0d | 316.0 |
| Swarna Sub1 | 317.7 | 596.7b | 1249.7b | 1499.0b | 749.0ab | 299.3ab | 318.7 |
| FR13A | 317.3 | 745.0a | 1499.0a | 1749.0a | 749.3a | 299.7a | 319.0 |
| CD (0.01) | – | 8.674 | 2.429 | 2.526 | 1.828 | 1.693 | – |
| CD (0.05) | – | 6.500 | 1.812 | 1.899 | 1.368 | 1.269 | – |
CD—critical difference 0.01and 0.05. Treatments found Significant at 1% and 5% level of significance
Carbohydrate utilization under submergence
In the submerged plants, the total carbohydrate content was consumed rapidly for survival underwater which resulted in reduced levels of carbohydrates whereas it increased in non- submerged plants by continuous photosynthesis along with growth. The consumption of total carbohydrate content in tolerant rice varieties viz., FR13A, Swarna Sub1 and in Sub1 introgressed BC3F2 lines were found to be lower than ( 49.18% to 56.28%) the susceptible rice varieties viz., Jaya (70.10%), Jyothi (66.59%) and Uma (72.81%). After de-submergence, the tolerant rice varieties viz., FR13A and Swarna Sub1 and Sub1 introgressed BC3F2 lines were able to synthesize and accumulate carbohydrates and thus regained their growth after de-submergence whereas the susceptible rice varieties (Jaya, Jyothi and Uma) could not synthesize and accumulate carbohydrates after de-submergence and could not survive. According to Suchimita Raha (2009), high carbohydrate content after submergence is the key factor that determines the ability of rice plants to withstand submergence stress. Kawano et al. (2009) also suggested that the shoot elongation during submergence uses energy for which it consumes carbohydrates in the leaves developed before submergence because photosynthesis is limited in leaves under water. In the present study, the submergence tolerant varieties Swarna Sub1 and FR13A utilised only 52.43% and 54.46% respectively of the stored carbohydrates during the submergence period of 14 days and could preserve 47.67% and 43.64% carbohydrate respectively for recovery after de-submergence. Das et al., (2005) reported that rice cultivars that could maintain more than 6% of their initial non—structural carbohydrate at the time of re- aeration were found to be capable of developing new leaves quickly. However, in this study, it was observed that lines that could maintain more than 40% of the carbohydrate content present at the time of submergence alone could develop new leaves quickly and regain growth as shown in Table 6. After 10 days of de—submergence the submergence tolerant varieties Swarna Sub1 and FR13A could regain their growth and produce total carbohydrate content of 12.3 mg/g and 14.6 mg/g respectively. The Sub1 introgressed lines had similar carbohydrate content before and after submergence like that of the tolerant varieties. The susceptible varieties utilised more carbohydrate for survival under water: Uma (72.81%), Jyothi (66.59%) and Jaya (70.10%) and could not preserve sufficient carbohydrate for recovery after de-submergence which might be the major reason for the zero percentage survival of these varieties. Three Sub1 introgressed lines BC3F2-3-5-7-7 utilized the least carbohydrate (49.45%) under submergence followed by BC3F2-5-21-5-2 (49.73%). These two lines could synthesize carbohydrate fast (13.3 mg/g leaf tissue) ten days after de submergence similar to the level of synthesis by the resistant donor FR13A. (13.6 mg/g leaf tissue. It is clear that the susceptible varieties Uma, Jaya and Jyothi had only 25–30% carbohydrate content compared to FR13A, Swarna Sub1 and all the Sub1 introgressed lines of Jaya developed in this study which might be the reason for non-survival under submergence.
Table 6.
Polymorphic SSR markers selected for Foreground screening
| Sl. no | Primer name | Sequence | Position | Annealing temperature (Taº) | References |
|---|---|---|---|---|---|
| 1 | ART5 F | CAGGGAAAGAGATGGTGGA | 6.3 Mb | 58ºC | Septiningsih et al., (2009) |
| ART5 R | TTGGCCCTAGGTTGTTTCAG | ||||
| 2 | Sub1BC2 F | AAAACAATGGTTCCATACGAGAC | 6.3 Mb | 58ºC | Septiningsih et al., (2009) |
| Sub1BC2 R | GCCTATCAATGCGTGCTCTT | ||||
| 3 | RM23788 F | ACCTTCACATAGCAGGGTTGAATC | 4.2 Mb | 63 ºC | Neeraja et al. (2007) |
| RM23788 R | ACTCTAAGCCCCTGGATAATCTGC |
Superoxide dismutase-enzyme assay
After a period of anoxia during submergence, the rice plants were exposed to air by de-submergence and they suffered due to the production of reactive oxygen species. It was observed that fourteen days submerged rice plants appeared softened and droopy after de-submergence but the Sub1 introgressed rice lines recovered fast. The activity of SOD increased in the twenty Sub1 introgressed lines of Jaya similar to Swarna Sub1 and the tolerant check FR13A after de-submergence (Table 7). While in the non-submerged plants, there were no significant increases in the activity of SOD enzyme during the period of 14 days which indicated that the activity of SOD enzyme was increased drastically in submerged plants to nullify the toxic effect of free radicals accumulated in the plants in response to submergence stress. The increase in SOD enzyme activity was about 4–5 folds in tolerant lines but only 1–1.5 folds increase in susceptible check and in recurrent parent. After a time course measurement study, it was observed that the SOD activity increased to the maximum level after 4 h of de- submergence and gradually declined after 6 h. The maximum SOD activity was expressed by FR13A, but all the Sub1 introgressed lines showed a similar pattern of SOD activity like the donor parent Swarna Sub1 and were statistically on par. The increase in SOD activity in response to re-exposure of plants to air after submergence has been reported in rice genotypes by Srivastava et al. (2007). The time course behaviour of enzyme activity indicated that the maximum damaging effect of reactive oxygen species occurs within 24 h of re-exposure of plants to air after de- submergence. They also observed that the restoration of oxygen supply followed by a period of submergence induced hypoxia/ anoxia is potentially more damaging. Panda et al. (2012) also reported that the activity of SOD enzymes increased gradually during submergence and upon re-aeration after de-submergence Swarna Sub1 significantly increased the activity of SOD more than Swarna. Injury due to submergence in rice plants is not only during post submergence aerobic conditions but also due to anaerobiosis during submergence. Hence plants with constitutive reactive oxygen systems have the ability to synthesize them more rapidly and efficiently during post anoxia, have lesser damage and have better growth during recovery period (Jackson and Ram 2003).
Table 7.
Polymorphic SSR markers selected for Recombinant screening
| Sl. no | Primer name | Sequence | Position | Annealing temperature (Taº) | Reference |
|---|---|---|---|---|---|
| 1 | RM8303 F | AGGGGAGAGGACACACACAC | 2.3 Mb | 60 ºC | Neeraja et al. (2007) |
| RM8303 R | GGATCCTCCTGCAAAATCAA | ||||
| 2 | RM23770 F | GACCTTGTCCAGAGTGATTTTG | 3.7 Mb | 57 ºC | Neeraja et al. (2007) |
| RM23770 R | ATTTGAGAATAACTTTTCCTACTTCG | ||||
| 3 | RM23917 F | CTCAGCTGTCTGTTCAGCTCTCAC | 7.3 Mb | 63 ºC | Neeraja et al.( 2007) |
| RM23917 R | CTTTGGTGCTGAGGTAGGTATTGG | ||||
| 4 | RM23922 F | TGGAGGGAGTATCATTATTAGCCG | 7.4 Mb | 62 ºC | Neeraja et al. ( 2007) |
| RM23922 R | CTTGGATAGATTTGGTGGGATGAC | ||||
| 5 | RM23958 F | GAGACAGATGTGTACGGTTTGGTG | 7.9 Mb | 63 ºC | Neeraja et al. (2007) |
| RM23958 R | TTGACAAGGGAATTGAAGGAGAAG |
In the present study, it was observed that after de-submergence, the Sub1 introgressed Jaya lines could enhance the activity of SOD and might have acted as a scavenging system and could detoxify the ROS developed in the plant due to both submergence and oxidative stresses. In susceptible plants, the ROS might have caused membrane damage through lipid peroxidation and protein denaturation and finally resulted in death of the plants.
Conclusion
The coastal areas of Kerala are subjected to flash floods due to heavy and continuous southwest monsoons and changing climatic conditions. Because of these reasons, rice production is greatly affected in flood prone coastal areas and low lying paddy fields. In areas where typical flash flood occurs, water recedes to lower levels after complete submergence for 12–14 days. We could introgress Sub1 gene into rice variety Jaya through Marker-Assisted Backcross Breeding. The submergence tolerant plants reduce underwater shoot elongation to conserve the carbohydrate for their survival when flood water recedes. In the present study, Sub1 introgressed Jaya lines which expressed tolerance levels similar to Swarna Sub1 and FR13A were found ideal for paddy fields amenable to flash flood with stagnant water for about 1–2 weeks. These lines expressed reduced underwater shoot elongation by slowing down growth and respiration and thereby they utilized only minimum stored carbohydrate and hence could preserve more carbohydrate for survival and growth after de-submergence. In this study, it is also proved that these lines possess efficient anti-oxidative defense systems during hypoxia and after re-exposure to air due to higher activity of superoxide dismutase which can mitigate the adverse effect of reactive oxygen species developed during submergence and thereby high survival percentage when flood water recedes. Hence, a higher degree of post submergence SOD activity and low underwater shoot elongation percentage can be attributed as physiological markers of rice submergence tolerance (Tables 3 and 5).
Acknowledgements
We thank Kerala Agricultural University, Thrissur, Kerala for providing the facilities for the study.
Author’s contribution
AKV performed the experiments in her Ph.D. research work. All the experiments and data analysis were done by AKV under the supervision of SKS. SKS designed the research design. AKV wrote the manuscript and SKS rigorously edited and revised the whole manuscript. The authors do not have any conflict of interest during the entire process of conducting the experiments, data analysis, and preparation of the manuscript.
Funding
This research was funded by XIII Finance Commission, Government of Kerala and is grateful for providing the necessary financial support to carry out the study.
Data availability
File contains detailed descriptions of all supplemental files. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures and tables.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent to participate
All the authors have given their consent to participate in the submitted manuscript.
Consent for publication
All the authors have given their consent for publication of the submitted manuscript in physiology and molecular biology of plants.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmed F, Rafii MY, Ismail MR, Juraimi AS, Rahim HA, Tanweer FA, Latif MA. Recurrent parent genome recovery in different populations with the introgression of Sub1 gene from a cross between MR219 and Swarna Sub1. Euphytica. 2015;207:605–618. doi: 10.1007/s10681-015-1554-5). [DOI] [Google Scholar]
- Armstrong W. Aeration in higher plants. Adv Bot Res. 1980;7:225–332. doi: 10.1016/S0065-2296(08)60089-0. [DOI] [Google Scholar]
- Bailey-Serres J, Voesenek LACJ. Flooding stress: acclimations and genetic diversity. Annu Rev Plant Biol. 2008;59:313–339. doi: 10.1146/annurev.arplant.59.032607.092752. [DOI] [PubMed] [Google Scholar]
- Bailey-Serres J, Fukao T, Ronald P, Ismail A, Heuer S, Mackill D. Submergence tolerant rice: SUB1’s journey from landrace to modern cultivar. Rice. 2010;3:138–147. doi: 10.1007/s12284-010-9048-5. [DOI] [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Colmer TD, Voesenek LACJ. Flooding tolerance: suites of plant traits in variable environments. Funct Plant Biol. 2009;36:665–681. doi: 10.1071/FP09144. [DOI] [PubMed] [Google Scholar]
- Cuc Luu M, Huyen Luu TN, Hien Pham TM, Hang Vu TT, Dam Nguyen Q, Mui Pham T, Quang VuD, Ismail Abdelbagi M, Le Ham H. Application of marker assisted backcrossing to introgress the submergence tolerance QTL SUB1 into the Vietnam elite rice variety - AS996. Am J Plant Sci. 2012;3:528–536. doi: 10.4236/ajps.2012.34063. [DOI] [Google Scholar]
- Das KK, Sarkar RK, Ismail AM. Elongation ability and non-structural carbohydrate levels in relation to submergence tolerance in rice. Plant Sci. 2005;168:131–136. doi: 10.1016/j.plantsci.2004.07.023. [DOI] [Google Scholar]
- Ella ES, Kawano N, Ito O. Importance of active oxygen scavenging system on the recovery of rice seedling after submergence. Plant Sci. 2003;165:85–93. doi: 10.1016/S0168-9452(03)00146-8. [DOI] [Google Scholar]
- Fales FW. The assimilation and degradation of carbohydrate by yeast cells. The J Biol Chem. 1951;193:113–124. doi: 10.1016/S0021-9258(19)52433-4. [DOI] [PubMed] [Google Scholar]
- FAO, 2023. https://www.fao.org/worldfoodsituation/csdb/en/
- Fukao T, Bailey-Serres J. Submergence tolerance conferred by Sub1A is mediated by SLR1 and SLRL1 restriction of gibberellins responses in rice. Proc Natl Acad Sci. 2008;105:16814–16819. doi: 10.1073/pnas.0807821105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao T, Xu K, Ronald PC, Bailey-Serres J. A variable cluster of ethylene response factor-like genes regulates metabolic and developmental acclimation responses to submergence in rice. Plant Cell. 2006;18:2021–2034. doi: 10.1105/tpc.106.043000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao T, Yeung E, Bailey-Serres J. The submergence tolerance regulator SUB1A mediates crosstalk between submergence and drought tolerance in rice. Plant Cell. 2011;23:412–427. doi: 10.1105/tpc.110.080325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan MM, Rafi MY, Ismail MR, Mahmood M, Rahim HA, Alam MA, Ashkani S, Malek MA, Latif MA. Marker - assisted backcrossing: A useful method for rice improvement. Biotechnol Biotechnol Equip. 2015;29(2):237–254. doi: 10.1080/13102818.2014.995920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori Y, Nagai K, Ashikari M. Rice growth adapting to deep-water. Curr Opin Plant Biol. 2011;14:100–105. doi: 10.1016/j.pbi.2010.09.008. [DOI] [PubMed] [Google Scholar]
- IRRI (1988) Standard evaluation system for rice testing programme (IRTP), Rice Manual 3rd Ed, International Rice Research Institute, Manila (Philippines), pp19.
- Jackson MB, Ram PC. Physiological and molecular basis of susceptibility and tolerance of rice plants to complete submergence. Ann Bot. 2003;91:227–241. doi: 10.1093/aob/mcf242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano INO, Sakagami J. Morphological and physiological responses of rice seedlings to complete submergence (flash flooding) Ann Bot. 2009;103:161–169. doi: 10.1093/aob/mcn171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackill DJ, Ismail AM, Singh US, Labios RV, Paris TR. Development and rapid adoption of submergence-tolerant (Sub1) rice varieties. Adv Agron. 2012;115:299–352. doi: 10.1016/B978-0-12-394276-0.00006-8. [DOI] [Google Scholar]
- Neeraja C, Maghirang RR, Pamplona A, Heuer S, Collard BCY, Septiningsih EM, Vergara G, Sanchez D, Xu K, Ismail AM, Mackill DJ. A marker-assisted backcross approach for developing submergence-tolerant rice cultivars. Theor Appl Genet. 2007;115(6):767–776. doi: 10.1007/s00122-007-0607-0. [DOI] [PubMed] [Google Scholar]
- Panda D, Sarkar RK. Leaf photosynthetic activity and antioxidant defense associated with Sub1 QTL in rice subjected to submergence and subsequent re-aeration. Rice Sci. 2012 doi: 10.1016/s1672-6308(12)60029-8. [DOI] [Google Scholar]
- Suchimita Raha (2009) Marker assisted introgression of Sub1 locus in rice. MSc Thesis 1–82 TNAU.
- Rahman H, Dakshinamurthi V, Ramasamy S, Manickam S, Kaliyaperumal AK, Raha S, Panneerselvam N, Ramanathan V, Nallathambi J, Sabariappan R, Raveendran M. Introgression of submergence tolerance into CO 43, a popular rice variety of India, through marker-assisted backcross breeding. Czech J Genet Plant Breed. 2018;54:101–108. doi: 10.17221/149/2017-cjgpb. [DOI] [Google Scholar]
- Ralph VB. GGT 2.0: Versatile software for visualization and analysis of genetic data. J Hered. 2008;99(2):232–236. doi: 10.1093/jhered/esm109. [DOI] [PubMed] [Google Scholar]
- Sarkar RK, Reddy JN, Sharma SG, Ismail AM. Physiological basis of submergence tolerance in rice and implications for crop improvement. Curr Sci. 2006;91:899–906. [Google Scholar]
- Srivastava AK, Singh PN, Kumar S, Ram PC, Ismail A. Physiological changes associated with submergence tolerance in genetically diverse lowland rice genotypes. Trop Agric Res. 2007;19:240–253. [Google Scholar]
- Xu K, Mackill DJ. A major locus for submergence tolerance mapped on rice chromosome 9. Mol Breed. 1996;2:219–224. doi: 10.1007/bf00564199. [DOI] [Google Scholar]
- Xu K, Xu X, Ronald PC, Mackill DJ. A high-resolution linkage map in the vicinity of the rice submergence tolerance locus Sub1. Mol Genet. 2000;263:681–689. doi: 10.1007/s004380051217. [DOI] [PubMed] [Google Scholar]
- Xu K, Deb R, Mackill DJ. A microsatellite marker and a codominant PCR-based marker for marker-assisted selection of submergence tolerance in rice. Crop Sci. 2004;44:248–253. doi: 10.2135/cropsci2004.2480. [DOI] [Google Scholar]
- Xu K, Xia X, Fukao T, Canlas P, Maghirang-Rodriguez R, Heuer S, Ismail AM, Bailey-Serres J, Ronald PC, Mackill DJ. Sub1A is an ethylene response factor-like gene that confers submergence tolerance to rice. Nature. 2006;442:705–708. doi: 10.1038/nature04920. [DOI] [PubMed] [Google Scholar]
- Yadav V, Prasad S, Singh S, Verma OP. Effect of submergence stress on yield and yield components of various rice (Oryza sativa L.) genotypes with its mapping population. J Pharmacogn Phytochem. 2018;7(6):2386–2389. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
File contains detailed descriptions of all supplemental files. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures and tables.