Abstract
The spatial and temporal distribution of sunlight around plants is constantly changing in natural and farmland environments. Previous studies showed that the photosynthesis of crops responds significantly to heterogeneous light conditions in fields. However, the underlying mechanisms remain unclear. In the present study, soybean plants were treated by heterogeneous light after a pre-shading (SH-HL) to simulate the light condition in relay strip intercropping. Gas exchange and nitrogen (N) of leaves were measured to evaluate the photosynthetic performance, as well as photosynthetic N- and water-use efficiency (PNUE and PWUE). Chlorophylls (Chl) and Rubisco were analyzed as representative photosynthetic N components. Results suggest that SH-HL treated soybean exhibited evident photosynthetic compensation as the net photosynthetic rate (Pn) increased significantly in unshaded leaves, from which the export of photosynthates was enhanced. Under SH-HL, leaf N concentration remained relatively stable in unshaded leaves. While Chl concentration decreased but Rubisco concentration increased in unshaded leaves, indicating preferential allocation of leaf N for CO2 fixation. Results also showed that PNUE increased and PWUE decreased in unshaded leaves under SH-HL. Therefore, we suggest that within-leaf N allocation for CO2 fixation in unshaded leaves rather than within-plant N distribution to unshaded leaves drives the photosynthetic compensation under heterogeneous light after a pre-shading. However, enhanced water loss from unshaded leaves is a cost for efficient N-use under these conditions.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12298-023-01392-8.
Keywords: CO2 fixation, Heterogeneous light, Leaf nitrogen, Photosynthetic compensation, Soybean (Glycine max L. Merr.)
Introduction
As a vital environmental factor, light plays a crucial role in plant growth and development (Kami et al. 2010). In natural and farmland conditions, the spatial and temporal distribution of sunlight around plants is constantly changing (Li et al. 2015; Sugiura and Tateno 2013; Wu et al. 2022). For example, small seedlings in fields experience nearly homogeneous light at the whole-plant level. However, upper and lower canopy layers of a mature plant are exposed to very different light conditions, i.e. heterogeneous light, due to self or mutual shading.
It is widely recognized that various life processes of plants, especially photosynthesis, have significant responses to heterogeneous light (Konert et al. 2013; Matsuda and Murakami 2016). The photosynthetic performance of leaves exposed to full sunlight is often stimulated, whereas that of shaded leaves is always restricted due to limitations in light energy availability (Araya et al. 2008; Dong et al. 2015; He and Dong 2003; Huang et al. 2019; Yamazaki and Shinomiya 2013). This phenomenon is referred to as photosynthetic compensation and represents a strategy for plants to acclimate to heterogeneous light conditions by using available light efficiently (Huang et al. 2019; Sun et al. 2019).
As an important crop, soybean (Glycine max L. Merr.) is frequently intercropped with other crops to optimize land utilization in regions with limited farmland and high populations (Du et al. 2018). In southwest China, for instance, maize-soybean relay strip intercropping is one of the most popular intercropping systems, wherein maize is sown in April and harvested in August, while soybean is sown in June and harvested in October (Wu et al. 2022). In this case, soybean plants experience a distinctive light environment characterized by two consecutive periods: a co-growth period, followed by a solo-growth period. During the co-growth period, soybean seedlings suffer from shading by taller early-planted maize. Then, during the solo-growth period, the shading is removed after maize harvesting. Previous studies revealed that the yield of soybean in relay strip intercropping is largely determined by the process of ‘recovery growth’ during the solo-growth period, which has a direct correlation with the photosynthetic capacity after shading removal (Fan et al. 2018; Wu et al. 2015, 2022). The distribution of sunlight around soybean plants during the solo-growth period shows vertical heterogeneity, which means that lower leaves suffer from self or mutual shading while upper leaves do not. Therefore, it is imperative to study the photosynthetic performance of soybean under heterogeneous light after a pre-shading.
In our present study, the light condition surrounding soybean plants in relay strip intercropping was simulated in a greenhouse. Leaf gas exchange suggests that soybean exhibited evident photosynthetic compensation under heterogeneous light after a pre-shading. Leaf nitrogen (N) and representative N components were measured to test that whether soybean plants can optimize the N-use efficiency during this process. The underlying mechanisms were studied by analyzing the response of both photosynthetic N- and water-use. Furthermore, the export of photosynthates was estimated for a better understanding of soybean recovery growth in relay strip intercropping.
Materials and methods
Plant material and treatments
Seeds of soybean cv. ‘Nan 032-4’ was sown in plastic pots (9 cm in diameter, 8.5 cm in height) filled with a commercial substrate (The Pindstrup Group. origin: Latvia). Each pot containing one germinated seedling was placed in a greenhouse with an irradiance of 600 µmol photons m−2 s−1 (12-h day/25 °C and 12-h night/20 °C, relative humidity 50%). Initially, all the seedlings were covered by nylon nets to an irradiance of 300 µmol photons m−2 s−1, i.e. shade (SH) treatment, until the third trifoliate leaves (TLs) emerged. Then, the second and third TLs were transferred to normal light (600 µmol photons m−2 s−1) by removing the nylon nets, while the primary leaves and first TLs remained covered by nylon nets, i.e. heterogeneous light (HL) treatment (See Fig. S1). Seedlings that were transferred to homogeneous normal light (600 µmol photons m−2 s−1) were designated as NL treatment (See Fig. S1). After ten days, the middle leaflets of the second and third TLs from both treatments were used for measurements. The seedlings were well-watered throughout the experiment.
Determination of gas exchange
Gas exchange was determined by a portable photosynthesis system (LI-6400XT, Li-COR, USA), which was equipped with red/blue LEDs (6400-02B, Li-COR, USA). Measurement was performed under 600 µmol photons m−2 s−1, 400 µmol CO2 mol−1, and 25 °C. Net photosynthetic rate (Pn), stomatal conductance (Gs), intercellular CO2 concentration (Ci), and transpiration rate (Tr) were recorded when they were steady. Photosynthetic water-use efficiency (PWUE) was calculated as the ratio of Pn to Tr.
Measurement of nitrogen (N) concentration
Leaf N was measured according to Huang et al. (2022). Leaflets were dried at 80℃ and ground. The resulting powders were digested by 98% H2SO4-30% H2O2 and then diluted with 86 mM EDTA-methyl red solution. The mixture was adjusted to pH 6. Subsequently, 106 mM phenol, 0.34 mM sodium nitroprusside, and 7.05 mM NaClO were added. Absorbance was measured at 625 nm. The concentration of N was determined from a standard curve. Photosynthetic N-use efficiency (PNUE) was calculated as the ratio of leaf mass-based Pn (Pn-LM) to N concentration.
Measurement of chlorophylls (Chl) concentration
Fresh leaflets were ground in 80% acetone. After filtration, the absorbance of the extract was read at 663 and 646 nm to determine Chl (a + b) concentration, by calculating the value of 7.15A663 + 18.71A646 (µg mL−1) according to Lichtenthaler and Buschmann (2001).
Measurement of carbohydrates concentration
Leaflets were homogenized with 80% ethanol and then centrifuged at 1000×g for 15 min. The resulting supernatant was collected for sucrose determination, while the remaining precipitate was used for starch determination. To determine sucrose, the supernatant was mixed with 2 M NaOH and incubated at 95 ℃ for 10 min. Subsequently, 0.1 M resorcinol and 10 M HCl were added. After incubation at 80 ℃ for 60 min, absorbance was measured at 500 nm. To determine starch, the precipitate was resuspended in distilled water and then mixed with 6.9 M HClO4. After centrifugation at 1000×g for 20 min, the supernatant was mixed with anthrone reagent and incubated at 95 ℃ for 15 min. Subsequently, absorbance was measured at 620 nm. The concentrations of sucrose and starch were determined from corresponding standard curves.
Quantification of Rubisco and sucrose transporters (SUTs)
Rubisco and SUTs were quantified from water-soluble and methanol-extracted proteins, respectively, using a double antibody sandwich ELISA with a commercially available kit (FANKEL Industrial Co., Ltd., Shanghai, China). Protein extracts were first combined with monoclonal antibodies. Subsequently, horseradish peroxidase-labeled antibody was added to form antibody-antigen-antibody complexes, and tetramethyl benzidine was used for color development. Absorbance was measured at 450 nm. The concentrations of Rubisco and SUTs were determined from corresponding standard curves.
Statistical analysis
Results were reached from three to five replicates, each with at least three different plants. Data were analyzed and statistically evaluated by Student’s t-test. A difference was considered statistically significant when P < 0.05 or very significant when P < 0.01. Histograms were created by Excel 2016.
Results and discussion
Photosynthesis of unshaded leaves is stimulated due to enhanced CO2 uptake and fixation
Photosynthetic compensation is a common phenomenon observed in plants exposed to heterogeneous light (Huang et al. 2019, 2022; Sun et al. 2019, 2020). In our present study, gas exchange measurement showed that the Pn of the second and third TLs increased significantly under HL after SH (SH-HL) by 21.0% and 24.8%, respectively, as compared to NL after SH (SH-NL) (Fig. 1a). When expressed on leaf mass basis, the Pn (Pn-LM) of both TLs also increased significantly under SH-HL (see Fig. S2). As expected, the shaded first TLs performed relatively low Pn under SH-HL (data not shown) due to limited light energy availability. These results indicate an evident photosynthetic compensation under SH-HL.
Fig. 1.
Photosynthetic performance of the second and third trifoliate leaves (TLs). a Net photosynthesis rate (Pn); b stomatal conductance (Gs); c transpiration rate (Tr); d intercellular CO2 concentration (Ci). Results are shown as mean values ± standard error. Significance of difference (**, P < 0.01; *, P < 0.05; n.s., not significant) is indicated. HL: heterogeneous light; NL: homogeneous normal light; SH, shade
In addition, Gs of the second and third TLs increased significantly under SH-HL by 73.9% and 73.5%, respectively, as compared to SH-NL (Fig. 1b). Simultaneously, Tr of the second and third TLs increased significantly by 93.4% and 86.9%, respectively (Fig. 1c). These results suggest that the CO2 uptake and the water loss of both TLs were promoted under SH-HL due to stimulated stomatal opening. Moreover, there was no significant change in Ci for both TLs under SH-HL (Fig. 1d), further indicating that the increased Pn is attributed to enhanced CO2 fixation.
Within-leaf N allocation for CO2fixation in unshaded leaves rather than within-plant N distribution to unshaded leaves is promoted
N is regarded as a crucial component involved in the response of photosynthesis to heterogeneous light (Matsuda and Murakami 2016), due to its strong positive correlation with photosynthetic performance (Yamori et al. 2011). Typically, N concentration increases in unshaded leaves, leading to enhanced photosynthesis (Araya et al. 2008; Huang et al. 2022; Sugiura and Tateno 2013). In the present study, however, N concentration did not differ between SH-HL and SH-NL in either the second or third TLs (Fig. 2a).
Fig. 2.
Nitrogen (N) and photosynthetic N components in the second and third trifoliate leaves (TLs). a N concentration; b concentration of total chlorophylls (Chl a + b); c Rubisco concentration; d ratio of Rubisco concentration to Chl concentration (Rubisco/Chl a + b). Results are shown as mean values ± standard error. Significance of difference (**, P < 0.01; *, P < 0.05; n.s., not significant) is indicated. HL, heterogeneous light; NL, homogeneous normal light; SH, shade
On the other hand, N allocation within a leaf has been indicated to play an important role in plant acclimation to different light conditions (Hikosaka and Terashima 1995). Our present study showed that the concentration of Chl (a + b), an essential N component for light-harvesting, decreased significantly under SH-HL in the second TLs by 12.8%, as compared to SH-NL. While there was no significant change in the third TLs (Fig. 2b). Simultaneously, the ratio of Chl a to Chl b (Chl a/b) showed no significant change in both TLs under SH-HL (see Fig. S3). In contrast, the concentration of Rubisco, an essential N component for CO2 fixation, increased significantly under SH-HL in the second and third TLs by 20.9% and 14.8%, respectively, as compared to SH-NL (Fig. 2c). These results suggest that the CO2 fixation catalyzed by Rubisco is stimulated under SH-HL in both TLs. While the capacity of light-harvesting by Chl declines, especially in the second TLs. We note that the changes are potentially correlated to the effects of heterogeneous light on leaf morphology (Matsuda and Murakami 2016; Sun et al. 2020). In our present study, the Pn-LM of both TLs increased significantly under SH-HL (Fig. S2), indicating a synchronous change of photosynthetic performance and Rubisco concentration on mass basis.
Furthermore, the ratio of Rubisco concentration to Chl concentration (Rubisco/Chl a + b) increased significantly in the second and third TLs under SH-HL by 38.7% and 23.6%, respectively, as compared to SH-NL (Fig. 2d). This result indicates that more leaf N was allocated to Rubisco rather than Chl, in the case of unaltered N concentration in both TLs under SH-HL. Consequently, it is more likely that the photosynthetic compensation under heterogeneous light after a pre-shading is a result of within-leaf N allocation for CO2 fixation in unshaded leaves rather than within-plant N distribution to unshaded leaves. Since light energy availability was not limited on the unshaded TLs, the preferential allocation of leaf N for CO2 fixation but not light-harvesting is proposed for an optimization in N-use under SH-HL.
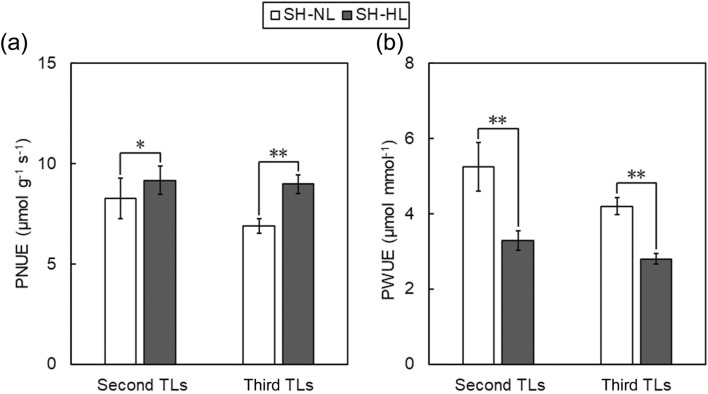
PNUE of unshaded leaves increases along with an enhanced water loss
Our present study showed that PNUE of the second and third TLs increased significantly under SH-HL by 11.0% and 30.1%, respectively, as compared to SH-NL (Fig. 3a). This is consistent with previous studies which suggested that the allocation of leaf N to photosynthetic machinery is enhanced in unshaded leaves under heterogeneous light (Dong et al. 2015; Yao et al. 2015). In contrast, PWUE of the second and third TLs decreased significantly under SH-HL by 37.4% and 33.2%, respectively, as compared to SH-NL (Fig. 3b), suggesting a trade-off between PNUE and PWUE.
Fig. 3.
Photosynthetic nitrogen- and water-use efficiency of the second and third trifoliate leaves (TLs). a photosynthetic nitrogen-use efficiency (PNUE); b photosynthetic water-use efficiency (PWUE). Results are shown as mean values ± standard error. Significance of difference (**, P < 0.01; *, P < 0.05; n.s., not significant) is indicated. HL, heterogeneous light; NL, homogeneous normal light; SH, shade
Previous studies demonstrate that the xylem-carried cytokinin is an important signal in regulating photosynthetic compensation by stimulating the synthesis of photosynthetic enzymes such as Rubisco (Boonman and Pons 2007; Boonman et al. 2007), and it is plausible that cytokinin delivery to unshaded TLs would be stimulated by enhanced transpiration (Fig. 1c), resulting in improved photosynthetic performance under SH-HL. In this case, enhanced water loss could be regarded as a cost for efficient N-use in photosynthetic compensation.
Export of photosynthates from unshaded leaves is stimulated
Export of photosynthates is a crucial determinant of photosynthetic compensation under heterogeneous light (Huang et al. 2019, 2022; Sun et al. 2019, 2020). A potential correlation between the N-use and photosynthates in photosynthetic compensation was also implied (Huang et al. 2022; Sun et al. 2020). In our present study, the concentration of starch, which represents the main storage form of photosynthates, decreased significantly by 34.0% in the second TLs under SH-HL, as compared to SH-NL. While it showed no significant change in the third TLs (Fig. 4a). In contrast, the concentration of sucrose, which represents the primary transport form of photosynthates, increased significantly by 15.3% in the second TLs under SH-HL compared to SH-NL. While it showed no significant change in the third TLs (Fig. 4b). These results suggest an enhanced accumulation of photosynthates as transport form, especially in the second TLs, which may affect the photosynthetic N-use (e.g., decline in Rubisco concentration) and lead to repression of photosynthesis (Huang et al. 2022; Sun et al. 2020). However, this photosynthetic repression caused by sucrose accumulation did not happen in the unshaded TLs of present study (Fig. 1a).
Fig. 4.
Carbohydrates and sucrose transporters (SUTs) in the second and third trifoliate leaves (TLs). a Sucrose concentration; b starch concentration; c SUTs concentration; d ratio of sucrose concentration to SUTs concentration (Sucrose/SUTs). Results are shown as mean values ± standard error. Significance of difference (**, P < 0.01; *, P < 0.05; n.s., not significant) is indicated. HL, heterogeneous light; NL, homogeneous normal light; SH, shade
On the other hand, the concentration of SUTs, which are responsible for active phloem loading of sucrose (Braun 2022), increased significantly under SH-HL in the second and third TLs by 23.9% and 25.5%, respectively compared to SH-NL (Fig. 4c). This result suggests an enhanced export of photosynthates from both TLs under SH-HL. Furthermore, the ratio of sucrose concentration to SUTs concentration (Sucrose/SUTs) showed no significant change in both TLs (Fig. 4d). Therefore, the increase in SUTs concentration was in coordination with the increase in sucrose concentration, so that sucrose may not accumulated excessively in unshaded TLs to cause photosynthetic repression. In this case, it is reasonable to consider a potential effect of sucrose export and accumulation on the N-use in photosynthetic compensation.
Conclusions
Our present study indicates similar responses of the second and third TLs to SH-HL. Both unshaded TLs exhibited improved photosynthesis and enhanced export of photosynthates to perform photosynthetic compensation. Differing from the situation without pre-shading, the photosynthetic compensation under heterogeneous light after a pre-shading is proposed to be a result of within-leaf N allocation for CO2 fixation in unshaded leaves along with an increase in PNUE, rather than within-plant N distribution to unshaded leaves. In addition, a decline in PWUE of unshaded leaves should be regarded as a cost for the photosynthetic compensation under heterogeneous light after a pre-shading.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the Sichuan Science and Technology Program (Grant number 2023YFN0083) and the Sichuan Innovation Team Project of National Modern Agricultural Industry Technology System, China (Grant number SCCXTD-2023-20).
Abbreviations
- Ci
Intercellular CO2 concentration
- Chl
Chlorophylls
- Gs
Stomatal conductance
- HL
Heterogeneous light
- N
Nitrogen
- NL
Normal light
- Pn
Net photosynthetic rate
- PNUE
Photosynthetic nitrogen-use efficiency
- PWUE
Photosynthetic water-use efficiency
- SH
Shade
- SUTs
Sucrose transporters
- TLs
Trifoliate leaves
- Tr
Transpiration rate
Author contributions
XS and JBD conceived and designed the experiments; XS, EZZ, and LY performed the experiments; XS, EZZ, and JBD analyzed the data and wrote the paper; WYY supervised the text. All authors approved the final manuscript.
Declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xin Sun, Email: sunxin529@sicau.edu.cn.
Jun-Bo Du, Email: junbodu@sicau.edu.cn.
References
- Araya T, Noguchi KO, Terashima I. Manipulation of light and CO2 environments of the primary leaves of bean (Phaseolus vulgaris L.) affects photosynthesis in both the primary and the first trifoliate leaves: involvement of systemic regulation. Plant Cell Environ. 2008;31:50–61. doi: 10.1111/j.1365-3040.2007.01736.x. [DOI] [PubMed] [Google Scholar]
- Boonman A, Pons TL. Canopy light gradient perception by cytokinin. Plant Signal Behav. 2007;2:489–491. doi: 10.4161/psb.2.6.4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonman A, Prinsen E, Gilmer F, Schurr U, Peeters AJM, et al. Cytokinin import rate as a signal for photosynthetic acclimation to canopy light gradients. Plant Physiol. 2007;143:1841–1852. doi: 10.1104/pp.106.094631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun DM. Phloem loading and unloading of sucrose: what a long, strange trip from source to sink. Annu Rev Plant Biol. 2022;73:553–584. doi: 10.1146/annurev-arplant-070721-083240. [DOI] [PubMed] [Google Scholar]
- Dong TF, Li JY, Zhang YB, Korpelainen H, Niinemets Ü, et al. Partial shading of lateral branches affects growth, and foliage nitrogen- and water-use efficiencies in the conifer Cunninghamia lanceolata growing in a warm monsoon climate. Tree Physiol. 2015;35:632–643. doi: 10.1093/treephys/tpv036. [DOI] [PubMed] [Google Scholar]
- Du JB, Han TF, Gai JY, Yong TW, Sun X, et al. Maize-soybean strip intercropping: achieved a balance between high productivity and sustainability. J Integr Agr. 2018;17:747–754. doi: 10.1016/S2095-3119(17)61789-1. [DOI] [Google Scholar]
- Fan Y, Chen J, Cheng Y, Raza MA, Wu X, et al. Effect of shading and light recovery on the growth, leaf structure, and photosynthetic performance of soybean in a maize-soybean relay-strip intercropping system. PLoS One. 2018;13:e0198159. doi: 10.1371/journal.pone.0198159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He WM, Dong M. Physiological acclimation and growth response to partial shading in Salix matsudana in the Mu Us Sandland in China. Trees. 2003;17:87–93. doi: 10.1007/s00468-002-0217-z. [DOI] [PubMed] [Google Scholar]
- Hikosaka K, Terashima I. A model of the acclimation of photosynthesis in the leaves of C3 plants to sun and shade with respect to nitrogen use. Plant Cell Environ. 1995;18:605–618. doi: 10.1111/j.1365-3040.1995.tb00562.x. [DOI] [Google Scholar]
- Huang SR, Ai Y, Du JB, Yu L, Wang XC, et al. Photosynthetic compensation of maize in heterogeneous light is impaired by restricted photosynthate export. Plant Physiol Biochem. 2022;192:50–56. doi: 10.1016/j.plaphy.2022.09.026. [DOI] [PubMed] [Google Scholar]
- Huang SR, Du JB, Wang XC, Sun X, Yang WY. Involvement of carbohydrates in long-term light-dependent systemic regulation on photosynthesis of maize under light heterogeneity. Plant Signal Behav. 2019;14:e1629266. doi: 10.1080/15592324.2019.1629266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kami C, Lorrain S, Hornitschek P, Fankhauser C. Light-regulated plant growth and development. Curr Top Dev Biol. 2010;91:29–66. doi: 10.1016/S0070-2153(10)91002-8. [DOI] [PubMed] [Google Scholar]
- Konert G, Rahikainen M, Trotta A, Kangasjärvi S. Systemic signaling in light acclimation of leaves. In: Baluška F, editor. Long-distance systemic signaling and communication in plants. Berlin Heidelberg: Springer-Verlag; 2013. pp. 231–250. [Google Scholar]
- Li T, Liu Y, Shi L, Jiang CD. Systemic regulation of photosynthetic function in field-grown sorghum. Plant Physiol Biochem. 2015;94:86–94. doi: 10.1016/j.plaphy.2015.05.008. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK, Buschmann C. Chlorophylls and carotenoids: measurement and characterization by UV–Vis spectroscopy. In: Wrolstad RE, editor. Current protocols in food analytical chemistry. Chichester, UK: Wiley; 2001. pp. F4.3.1–F4.3.8. [Google Scholar]
- Matsuda R, Murakami K. Light- and CO2-dependent systemic regulation of photosynthesis. In: Lüttge U, Cánovas F, Matyssek R, editors. Progress in Botany 77. Cham: Springer; 2016. pp. 151–166. [Google Scholar]
- Sugiura D, Tateno M. Concentrative nitrogen allocation to sun-lit branches and the effects on whole-plant growth under heterogeneous light environments. Oecologia. 2013;172:949–960. doi: 10.1007/s00442-012-2558-7. [DOI] [PubMed] [Google Scholar]
- Sun X, Lu J, Yang MY, Huang SR, Du JB, et al. Light-induced systemic signalling down-regulates photosynthetic performance of soybean leaves with different directional effects. Plant Biol. 2019;21:891–898. doi: 10.1111/plb.12980. [DOI] [PubMed] [Google Scholar]
- Sun X, Huang SR, Ai Y, Zhang EZ, Wang XC, et al. Comparative study on the different responses of maize photosynthesis to systemic regulation under light heterogeneity. Plant Sci. 2020;301:110666. doi: 10.1016/j.plantsci.2020.110666. [DOI] [PubMed] [Google Scholar]
- Wu Y, Gong W, Yang F, Wang X, Yong T, et al. Responses to shade and subsequent recovery of soya bean in maize-soya bean relay strip intercropping. Plant Prod Sci. 2015;19:206–214. doi: 10.1080/1343943X.2015.1128095. [DOI] [Google Scholar]
- Wu Y, Gong W, Yang F, Wang X, Yong T, et al. Dynamic of recovery growth of intercropped soybean after maize harvest in maize-soybean relay strip intercropping system. Food Energy Secur. 2022;11:e350. doi: 10.1002/fes3.350. [DOI] [Google Scholar]
- Yamazaki J, Shinomiya Y. Effect of partial shading on the photosynthetic apparatus and photosystem stoichiometry in sunflower leaves. Photosynthetica. 2013;51:3–12. doi: 10.1007/s11099-012-0073-z. [DOI] [Google Scholar]
- Yamori W, Nagai T, Makino A. The rate-limiting step for CO2 assimilation at different temperatures is influenced by the leaf nitrogen content in several C3 crop species. Plant Cell Environ. 2011;34:764–777. doi: 10.1111/j.1365-3040.2011.02280.x. [DOI] [PubMed] [Google Scholar]
- Yao H, Zhang Y, Yi X, Hu Y, Luo H, et al. Plant density alters nitrogen partitioning among photosynthetic components, leaf photosynthetic capacity and photosynthetic nitrogen use efficiency in field-grown cotton. Field Crop Res. 2015;184:39–49. doi: 10.1016/j.fcr.2015.09.005. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.