Abstract
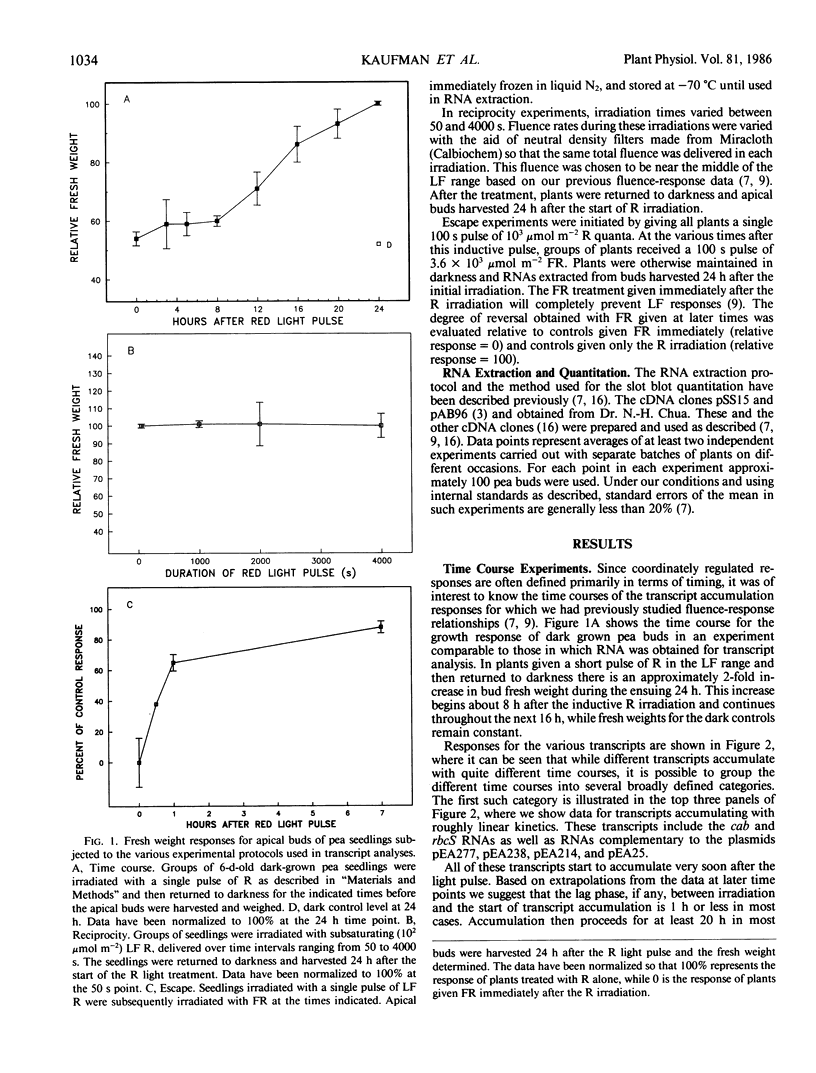
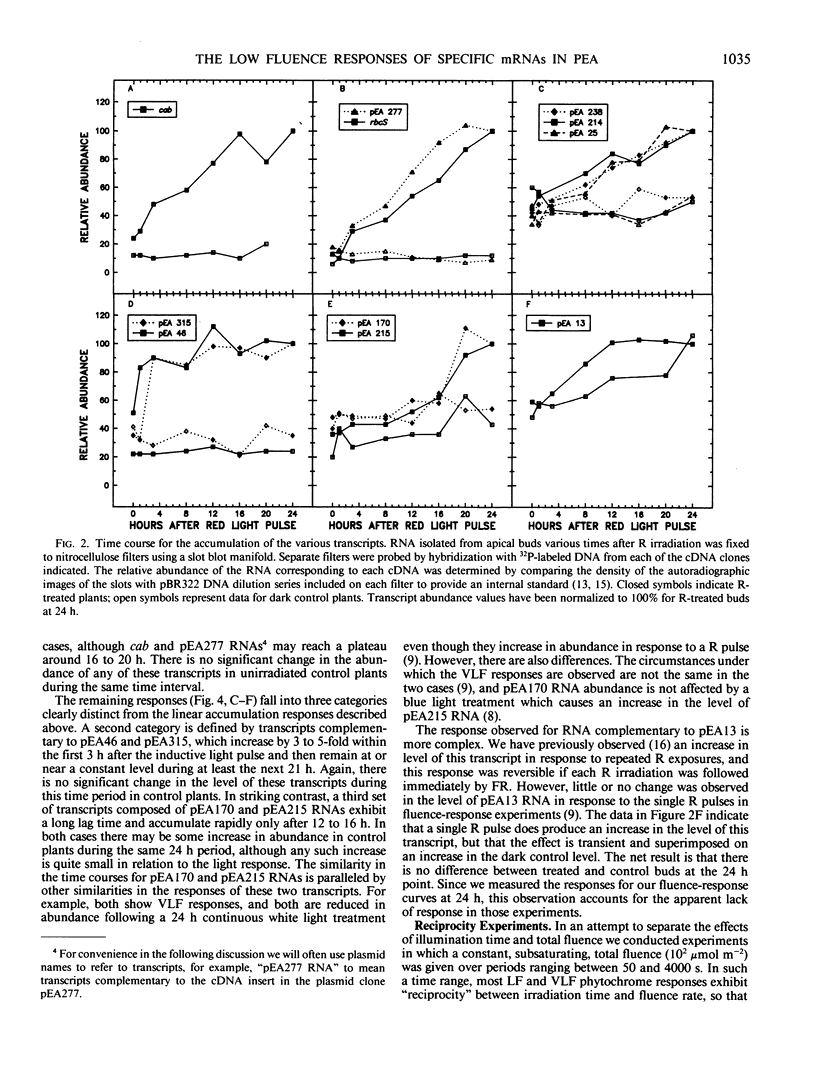
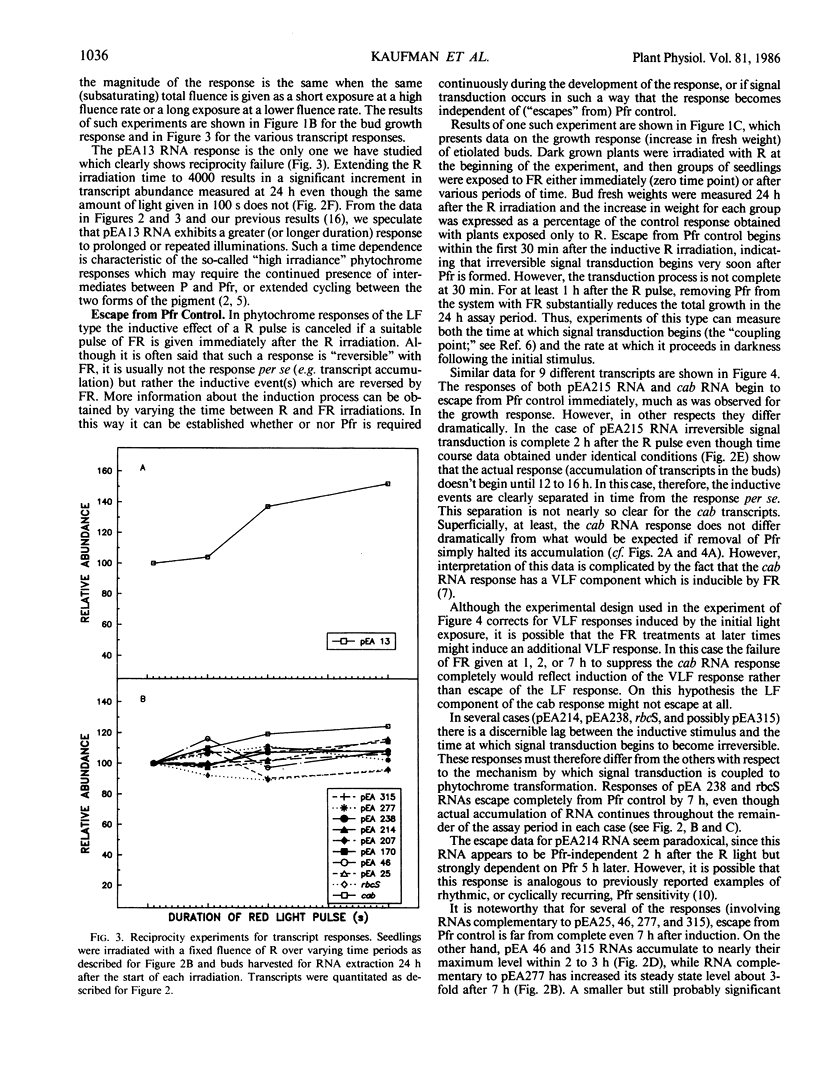
We have examined the time course for accumulation of each of 12 different nuclear gene transcripts in pea buds after irradiating dark grown seedlings with a single pulse low fluence red light (103 micromoles per square meter delivered in 100 seconds). The 12 time courses can be grouped into four general classes. Six transcripts (including RNAs coding for the chlorophyll a/b binding protein and ribulose-1,5-bisphosphate carboxylase) accumulate at a linear rate during 24 hours in darkness following the light pulse. Two transcripts increase rapidly at first but then reach a plateau after 3 hours and remain at that level for the next 21 hours. Another two transcripts exhibit a prolonged lag period before beginning to accumulate, and do not reach significant accumulation rates until 12 to 16 hours after the red light pulse. One transcript appears to undergo a transient increase in abundance in response to red light, but this is superimposed on a background of slowly increasing abundance of this RNA in control plants. This response, unlike all the others, exhibits reciprocity failure in experiments in which the same fluence of light is given over periods ranging between 50 and 4000 seconds.
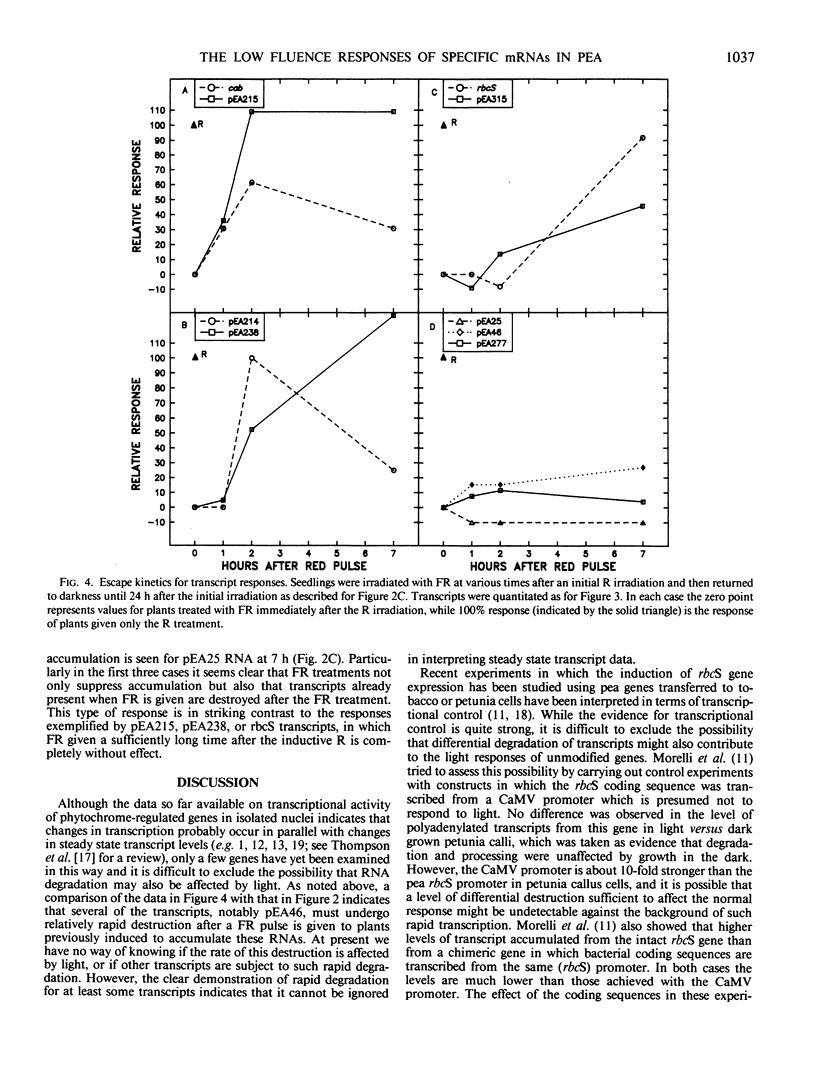
We have also examined the kinetics with which each of these 12 responses escapes from phytochrome-far-red absorbing form control by attempting to reverse the induction with far-red light given at various times after the red light pulse. Again, several different patterns are apparent for the different transcripts. The time at which far red reversibility first begins to be lost, the rate at which it is lost, and the final extent of reversibility remaining after 7 hours in the dark all differ for different transcripts. In addition, we have observed that some responses retain virtually complete photoreversibility for at least 7 hours. In some cases, a comparison of the time course and escape kinetic data indicates that relatively rapid turnover of the RNA must occur. It is not clear whether or not the rate of turnover is influenced by phytochrome.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry-Lowe S. L., Meagher R. B. Transcriptional regulation of a gene encoding the small subunit of ribulose-1,5-bisphosphate carboxylase in soybean tissue is linked to the phytochrome response. Mol Cell Biol. 1985 Aug;5(8):1910–1917. doi: 10.1128/mcb.5.8.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coruzzi G., Broglie R., Cashmore A., Chua N. H. Nucleotide sequences of two pea cDNA clones encoding the small subunit of ribulose 1,5-bisphosphate carboxylase and the major chlorophyll a/b-binding thylakoid polypeptide. J Biol Chem. 1983 Feb 10;258(3):1399–1402. [PubMed] [Google Scholar]
- Kaufman L. S., Briggs W. R., Thompson W. F. Phytochrome control of specific mRNA levels in developing pea buds : the presence of both very low fluence and low fluence responses. Plant Physiol. 1985 Jun;78(2):388–393. doi: 10.1104/pp.78.2.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman L. S., Thompson W. F., Briggs W. R. Different Red Light Requirements for Phytochrome-Induced Accumulation of cab RNA and rbcS RNA. Science. 1984 Dec 21;226(4681):1447–1449. doi: 10.1126/science.226.4681.1447. [DOI] [PubMed] [Google Scholar]
- Mandoli D. F., Tepperman J., Huala E., Briggs W. R. Photobiology of diagravitropic maize roots. Plant Physiol. 1984 Jun;75(2):359–363. doi: 10.1104/pp.75.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mösinger E., Batschauer A., Schäfer E., Apel K. Phytochrome control of in vitro transcription of specific genes in isolated nuclei from barley (Hordeum vulgare). Eur J Biochem. 1985 Feb 15;147(1):137–142. doi: 10.1111/j.1432-1033.1985.tb08729.x. [DOI] [PubMed] [Google Scholar]
- Silverthorne J., Tobin E. M. Demonstration of transcriptional regulation of specific genes by phytochrome action. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1112–1116. doi: 10.1073/pnas.81.4.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timko M. P., Kausch A. P., Castresana C., Fassler J., Herrera-Estrella L., Van den Broeck G., Van Montagu M., Schell J., Cashmore A. R. Light regulation of plant gene expression by an upstream enhancer-like element. Nature. 1985 Dec 12;318(6046):579–582. doi: 10.1038/318579a0. [DOI] [PubMed] [Google Scholar]



