Abstract
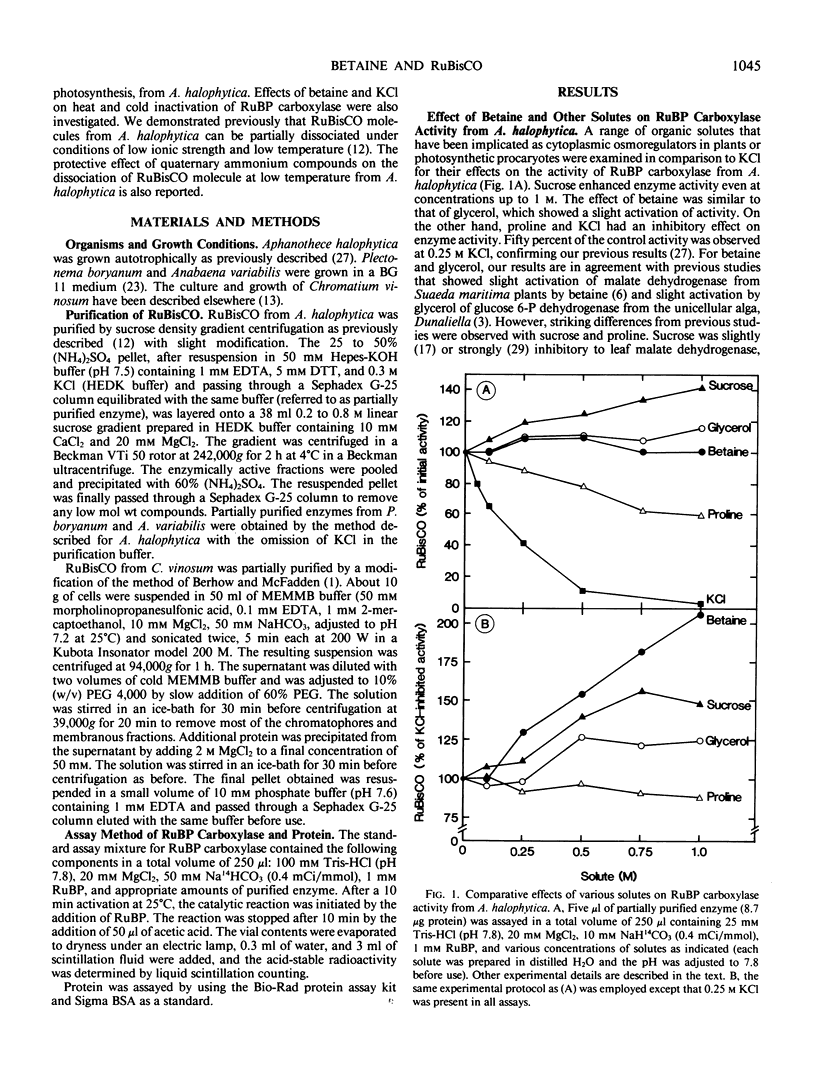
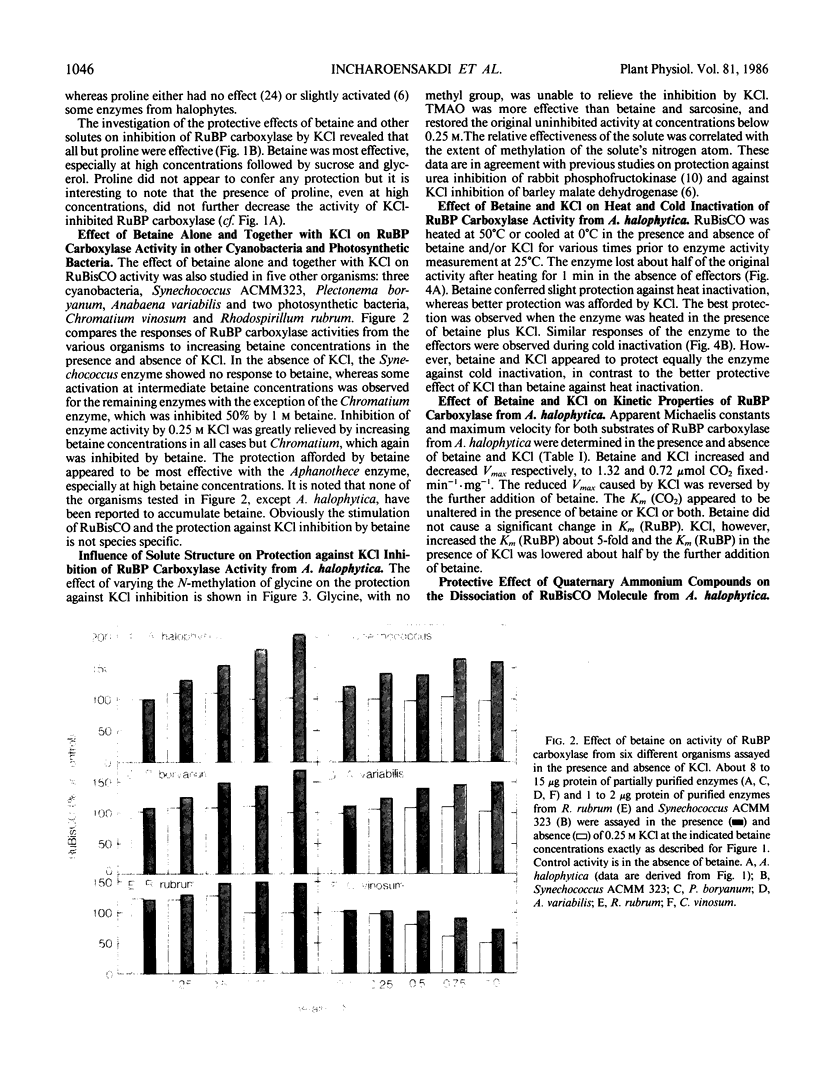
The presence of betaine, a quaternary ammonium compound, at a concentration (0.5 molar) reported to accumulate inside Aphanothece halophytica in response to increasing external salinity, slightly promoted ribulose-1,5-bisphosphate (RuBP) carboxylase activity. KCl at 0.25 molar inhibited RuBP carboxylase about 55%. Betaine relieved the inhibition by 0.25 m KCl and the original uninhibited activity was restored at 1 m betaine. Other osmoregulatory solutes such as sucrose and glycerol also reduced KCl inhibition, though to a lesser extent than betaine. Proline had no effect. The protective effect of betaine against KCl inhibition of RuBP carboxylase activity was also observed in other cyanobacteria, i.e. Synechococcus ACMM 323, Plectonema boryanum, and Anabaena variabilis, and in the photosynthetic bacterium Rhodospirillum rubrum but not in Chromatium vinosum. Apart from betaine, other quaternary ammonium compounds, i.e. sarcosine and trimethylamine-N-oxide (TMAO), but not glycine, also protected the enzyme against KCl inhibition and the effectiveness of such compounds appeared to correlate with the extent of N-methylation. Heat and cold inactivation of the enzyme could be protected by either betaine or KCl. However, best protection occurred when both betaine and KCl were present together. The Km (CO2) was not altered by either betaine or KCl, nor when they were present together. However, the Km (RuBP) was increased about 5-fold by KCl, but was unaffected by betaine. The presence of betaine together with KCl lowered the KCl-raised Km (RuBP) by about half. The extent of the dissociation of the enzyme molecule under the condition of low ionic strength was reduced by either betaine or KCl alone and more so when they were present together. Glycine, sarcosine, and TMAO were more effective than betaine or KCl in lowering the extent of the dissociation of the enzyme molecule.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumwald E., Mehlhorn R. J., Packer L. Studies of osmoregulation in salt adaptation of cyanobacteria with ESR spin-probe techniques. Proc Natl Acad Sci U S A. 1983 May;80(9):2599–2602. doi: 10.1073/pnas.80.9.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowitzka L. J., Brown A. D. The salt relations of marine and halophilic species of the unicellular green alga, Dunaliella. The role of glycerol as a compatible solute. Arch Mikrobiol. 1974 Mar 1;96(1):37–52. doi: 10.1007/BF00590161. [DOI] [PubMed] [Google Scholar]
- Bowlus R. D., Somero G. N. Solute compatibility with enzyme function and structure: rationales for the selection of osmotic agents and end-products of anaerobic metabolism in marine invertebrates. J Exp Zool. 1979 May;208(2):137–151. doi: 10.1002/jez.1402080202. [DOI] [PubMed] [Google Scholar]
- Brock T. D. Halophilic-blue-green algae. Arch Microbiol. 1976 Feb;107(1):109–111. doi: 10.1007/BF00427875. [DOI] [PubMed] [Google Scholar]
- Hand S. C., Somero G. N. Urea and methylamine effects on rabbit muscle phosphofructokinase. Catalytic stability and aggregation state as a function of pH and temperature. J Biol Chem. 1982 Jan 25;257(2):734–741. [PubMed] [Google Scholar]
- Incharoensakdi A., Takabe T., Akazawa T. Factors affecting the dissociation and reconstitution of ribulose-1,5-bisphosphate carboxylase/oxygenase from Aphanothece halophytica. Arch Biochem Biophys. 1985 Mar;237(2):445–453. doi: 10.1016/0003-9861(85)90298-x. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Akazawa T. Biosynthetic mechanism of ribulose-1,5-bisphosphate carboxylase in the purple photosynthetic bacterium, Chromatium vinosum. Arch Biochem Biophys. 1982 Apr 1;214(2):531–539. doi: 10.1016/0003-9861(82)90057-1. [DOI] [PubMed] [Google Scholar]
- Miller D. M., Jones J. H., Yopp J. H., Tindall D. R., Schmid W. E. Ion metabolism in a halophilic blue-green alga, Aphanothece halophytica. Arch Microbiol. 1976 Dec 1;111(1-2):145–149. doi: 10.1007/BF00446561. [DOI] [PubMed] [Google Scholar]
- Somero G. N., Neubauer M., Low P. S. Neutral salt effects on the velocity and activation volume of the lactate dehydrogenase reaction: evidence for enzyme hydration changes during catalysis. Arch Biochem Biophys. 1977 Jun;181(2):438–446. doi: 10.1016/0003-9861(77)90249-1. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Kunisawa R., Mandel M., Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev. 1971 Jun;35(2):171–205. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takabe T., Rai A. K., Akazawa T. Interaction of constituent subunits in ribulose 1,5-bisphosphate carboxylase from Aphanothece halophytica. Arch Biochem Biophys. 1984 Feb 15;229(1):202–211. doi: 10.1016/0003-9861(84)90145-0. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]



