Abstract
Purpose of work
Enteric Adenovirus (EAdV) is recognized as one of the most commonly identified agents responsible for severe acute gastroenteritis (AGEs) in the stools of infants.
We sought to determine the rate of human adenovirus (HAdV) infections, and the genotypic characterization of circulating strains of HAdV in children under 5 years of age with AGEs in university and regional hospitals, located in the Center-East of Tunisia, from January 2014 to December 2016.
Methods
A classic PCR was performed on 582 stool samples taken within 5 days of the onset of symptoms. Chosen positive samples were sequenced, and some of the results were confirmed by the Next Generation Sequencing technique (NGS). Partial nucleotide sequences of the Hexon gene obtained in this study were compared with the NCBI GenBank database using BLAST. Multiple sequence alignment and phylogenetic analysis were conducted using MEGA6 software. The phylogenetic tree was generated using the maximum-likelihood method and bootstrap analysis was performed with 1000 replications.
Results
Out of 582 samples, 52 (8.93 %) cases were positive for HAdV, with a male predominance (57.4 %). Phylogenetic analyses showed that Tunisian HAdV strains clustered into five HAdV lineages corresponding to serotypes F41 (14/28), C2 (9/28), C5 (3/28), E4 (1/28), and A18 (1/28). HAdV was more frequent in children aged up to 12 months, as compared to the other age groups. The HAdV activity was noted in almost all the months of the year with a peak in autumn, in 2014 and 2015, and in winter in 2016.
Conclusion
This study showed that infections with HAdV species were frequent in children suffering from AGE with the predominance of HAdV F41 and C2. This result underlines the importance of regular monitoring of circulating genotypes, and it could be useful for future epidemiological research.
Keywords: Human AdV, Gastroenteritis, PCR, Sanger sequencing, NGS, Genotypes
1. Introduction
AGE is a global public health concern among children and adults. Globally, GEs cause around 1.76 million fatalities among children under the age of five [1]. AGE describes a group of basic symptoms that included varying degrees of nausea, vomiting, diarrhea, and abdominal pain. Moreover, an estimated 530,000 children under the age of five dies from diarrhea each year throughout the world [2].
Many microbial pathogens are the main causes of infectious GEs. Over 20 different types of viruses are involved, and according to the literature, the most clinically relevant ones are group A rotaviruses, caliciviruses (including Norovirus), enteric adenoviruses (EAdV), and astroviruses [3,4]. EAdV are a frequent cause of diarrhea and diarrhea-associated mortality [2] in young children, inferior only to Shigella and RV [5]. They are an important group of enteric viruses transmitted mainly by the fecal-oral route, replicate in the guts of both ill and healthy individuals, and substantial viral loads are excreted in the feces [6,7].
This pathogen is a member of the Adenoviridae family and belongs to the genus Mastadenovirus. It is a ubiquitous nonenveloped virus with a medium‐sized double-stranded DNA ranging from 34 kb to more than 37 kb, encoding around 40 genes [8]. To date, more than 100 HAdV types have been isolated, characterized, and classified into seven species (A to G) [9]. The enteric serotypes 40 and 41, which belong to the subgenus F, are the most often linked with GE caused by HAdV. More rarely, serotypes 31, 12, and 18 of subgenus A and serotypes 1, 2, 5, and 6 of subgenus C have been involved in the etiology of acute diarrhea [3]. The prevalent HAdV types circulating in a specific period vary between countries or regions and undergo changes over time. The shift from dominant viruses to new strains can occur due to the transmission of novel strains between countries.
Viral GEs occur with similar frequencies in developed and developing countries, but seasonality differs from region to region [10]. Local weather factors such as temperature, relative humidity, and precipitation have been suggested as important factors in the dissemination and seasonality of infectious AGEs [[11], [12], [13]]. Despite being present on surfaces like doorknobs, items, swimming pools, and small lakes, adenoviruses remain resilient to common disinfectants. These infections primarily affect children more than adults, although they can impact individuals of any age [14]. In most cases, these infections only lead to mild symptoms and resolve on their own within a few days. However, individuals with weakened immune systems, especially young children, face higher risks [15]. Those with compromised immune systems are particularly susceptible to severe AdV sickness. In some cases, AdV infections can go undetected in the tonsils, adenoids, and intestines of individuals, especially those with weakened immune systems [16]. These individuals can spread the virus for extended periods without exhibiting any symptoms, sometimes lasting for weeks or even months [30].
PCR-based techniques and the sequencing of the PCR products, allow the identification of HAdV species and genotypes. The occurrence of next-generation sequencing (NGS) as a research tool, to enquire into unidentified viruses, has aided in the detection of supposed novel pathogens and the documentation of the etiologic agents of several infections.
Currently in Tunisia, the knowledge of the molecular epidemiology and clinical presentation of HAdV infection is lacking. Our 3-year study provides information regarding HAdV incidence, virological and molecular epidemiological features among AGE cases collected from three university hospitals that receive patients from the center and the central coast of Tunisia.
2. Material and methods
2.1. collection of fecal samples, DNA extraction, and PCR
A total of 582 Stool samples were collected between January 2014 to December 2016 from children ≤5 years of age, who sought university hospitals of Farhat Hached-Sousse, Sahloul-Sousse, Fattouma Bourguiba-Monastir, and the regional hospital of M'saken, located in central-east Tunisia, for the treatment of AGEs during the acute phase of the disease. Two to 3 g (2–3 ml) of stool were collected in a sterile, airtight container. These samples were sent as quickly as possible to the laboratory at room temperature.
The search for HAdV was carried out the same day, using PCR technique, and if not, the sample was stored between +2 °C and +8 °C and processed within 72 h. The remainder of the sample was aliquoted into small wells and stored at −80 °C for later analysis. Various clinical and biological data were collected during the study on standardized sheets for each patient (age, sex, date of admission, the reason for hospitalization, clinical signs, duration, etc.). Ethics approval for this study was obtained from the University Hospital of Sahloul-Sousse-Tunisia.
Viral DNA was extracted from 450 μl of fecal suspension by using the phenol-chloroform method described by Shi SR in 2002 [17]. DNA was eluted in 25 μl of DNase-free water, and either immediately used or stored at −80 °C until further molecular analysis.
PCR amplification of a conserved sequence in all HAdV serotypes (Hexon gene), between base pair position 21 and position 322, was performed, using specific primers hex1deg 5′-GCC SCA RTG GKC WTA CAT GCA CAT C-3′ And hex2deg 5′-CAG CAC SCC ICG RAT GTC AAA-3′ [18].
The amplification reaction begins with a denaturation step at 94 °C for 4 min followed by 32 cycles, each of which includes a denaturation step at 94 °C for 30 s, a hybridization step at 65 °C for 60 s, and an elongation step at 72 °C for 30 s. The last cycle is extended by 5 min at 72 °C.
The amplification products were revealed by electrophoretic migration on 2 % agarose gel. The size of the amplicons sought for HAdV is 301 bp.
2.2. nucleotide sequencing and phylogenetic analysis of adenovirus
Throughout the trial, twenty-eight positive samples were chosen randomly. The existence of a highly positive PCR result was the only factor used in the sample selection process (visualized by a specific band of great intensity on agarose gel).The PCR amplicons were purified with the innuPREP PCR pure kit (Analytik Jena, Jena, Germany) and sequenced using the dideoxynucleotide chain termination method with the ABI PRISM BigDye Terminator Cycle Sequencing Reaction kit (ThermoFisher Scientific, USA) on an automated sequencer ABI PRISM 3100 (ThermoFisher Scientific, USA).
Partial nucleotide sequences of the Hexon gene obtained in this study were compared with the NCBI GenBank database (http://www.ncbi.nlm.nih.gov) by using online BLAST tools to preliminarily determine the genotype. Multiple sequence alignment and phylogenetic analysis were conducted using MEGA software version 6.06. The phylogenetic tree was generated using the maximum-likelihood method and bootstrap analysis was performed with 1000 replications.
-
a.
Illumina sequencing: Random amplification, library preparation
From the 28 sequenced samples, ten were chosen to be sequenced by NGS technique.
The DNA was extracted using the QIAamp® Viral RNA Mini Kit from Qiagen according to the manufacturer's instructions.Amplification is performed for 17 cycles using a modified Whole Transcriptome Amplification (WTA2) kit procedure (Sigma–Aldrich). WTA2 products were prepared for Illumina sequencing using the Nextera XT library preparation kit (Illumina). Sequencing of the samples was performed on Illumina NextSeq 550: Up to 800 M paired-end reads for ∼80 samples.
Raw reads were filtered and trimmed for quality and adapters using Trimmomatic [19] and assembled using SPAdes assembler version 3.5.0 [20]. Scaffolds were taxonomically classified using DIAMOND (sensitive option) [21], and Amino-acid alignments of the viral sequences were performed with MUSCLE [22]. Substitution models for maximum-likelihood phylogenetic trees were calculated using MEGA6.0 [23].Phylogenetic analysis was done for the selected viruses using the protein or nucleotide sequences of suitable conserved regions and representative members of their viral family, genus, or species.
In this study, we focused on Adenoviridae [human mastadenovirus] from which near-complete genomes were obtained.
2.2.1. Nucleotide sequence Accession number
All the 28 HAdV sequences that originated from this study were submitted to GenBank.
(Accession numbers OP078713 – OP078740).
3. Results
3.1. prevalence of enteric HAdV infections and coinfections
In total, EAdV was found in fifty-two of the 582 stool samples, representing a detection rate of 8.93 % (Table 1).
Table 1.
Annual Distribution of HAdV Gastroenteritis in pediatric population on the central coast of Tunisia, between 2014 and 2016.
| 2014 | 2015 | 2016 | Total | |
|---|---|---|---|---|
| Number of samples | 55 | 132 | 395 | 582 |
| HAdV samples detected | 7 | 11 | 34 | 52 |
| % | 12,70 % | 8,33 % | 8.61 % | 8,93 % |
By using the NGS technique among 10 samples from these 52 positive samples in HADV, we found 6 samples that are coinfected with parvovirus, 2 samples with RV, 3 samples with Enterovirus, 1 with Parechovirus, 1 with Norovirus, and 1 with Sapovirus.
3.2. age and sex distribution of adenovirus infection
The age distribution among infants and children suffering from AdV infection is shown in Fig. 1. In this study, the population was divided into five age groups.
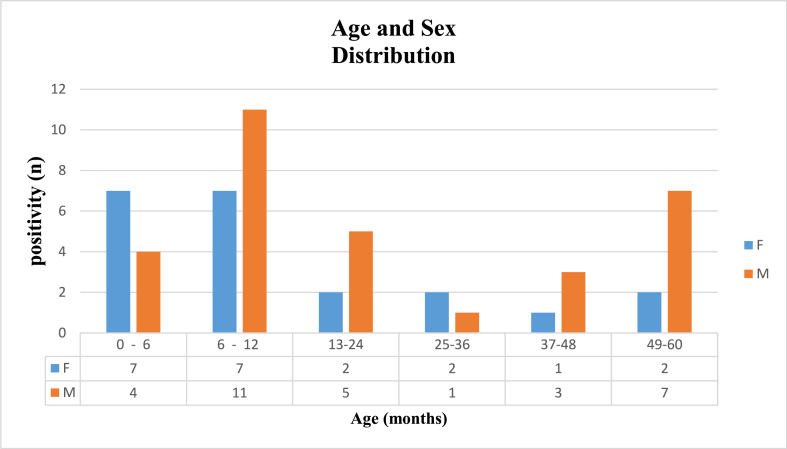
Fig. 1.
Age and Sex distribution of Enteric Adenovirus (EAdV) infections among pediatric population consulting for Acute Gastroenteritis (AGE) between 2014 and 2016 in the central coastal Tunisian hospitals. The study population is categorized into six age groups, ranging from 0 to 60 months. Each age group is represented by two parallel bars: blue bars represent female patients, while orange bars represent male patients. A data table displays the precise count of female and male patients within each age group. F: Female; M: Male. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
A predominance of AdV infection was observed up to 12 months of age (29/52), especially in the age group of 6–12 months (18/29). Also, we found an almost male predominance, from which E AdV infections affect boys (n = 30; 57.7 %) more than girls (n = 22; 42.3 %).
3.3. seasonal distribution of adenovirus infection
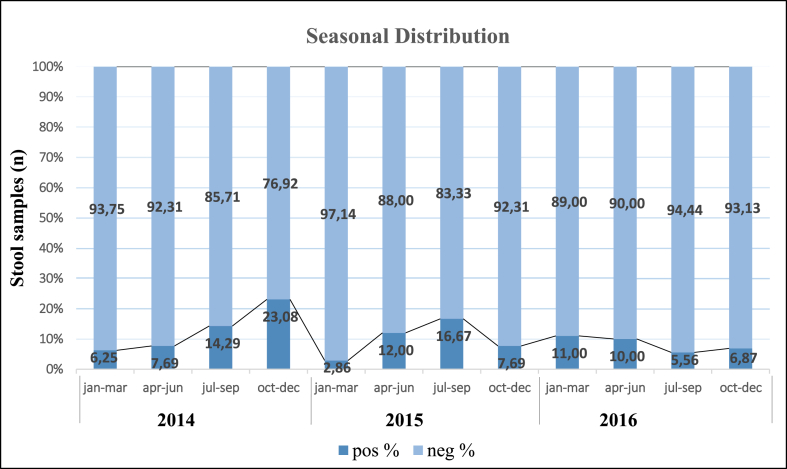
Our study detected AdV infections consistently throughout the year, as depicted in Fig. 2. However, a noteworthy seasonal pattern emerged, with a peak occurring in autumn (September and October) for the years 2014 and 2015, and in winter (January) for the year 2016. Likewise, we observed a significant monthly variation in AdV infections during these three years.
Fig. 2.
Seasonal distribution of Enteric Adenovirus (EAdV) infections during 2014, 2015, and 2016 in the pediatric population seeking medical consultation for Acute Gastroenteritis (AGE) in the central coastal Tunisian hospitals. Data are plotted as % of adeno-positive samples from the number of samples tested each month separately.
3.4. molecular characterization and phylogenetic analysis of adenovirus strains
The majority of HAdV species seem to be widespread worldwide, however, the most prevalent types vary between different countries or regions and change through time [24].
In our study, HAdV genotypes were analysed in 28 of 52 (53,84 %) positive cases, revealing that F41 (n = 14; 50 %) was the most prevalent genotype, followed by C2 (n = 9; 32.14 %), C5 (n = 3; 10.71 %), E4 (n = 1; 3.57 %), and A18 (n = 1; 3.57 %).
It was also noticed that our strains had a high identity on the nucleotide level as well as on the amino acid level with corresponding AdV reference strains previously registered in GenBank ranging from 98 % to 100 %.
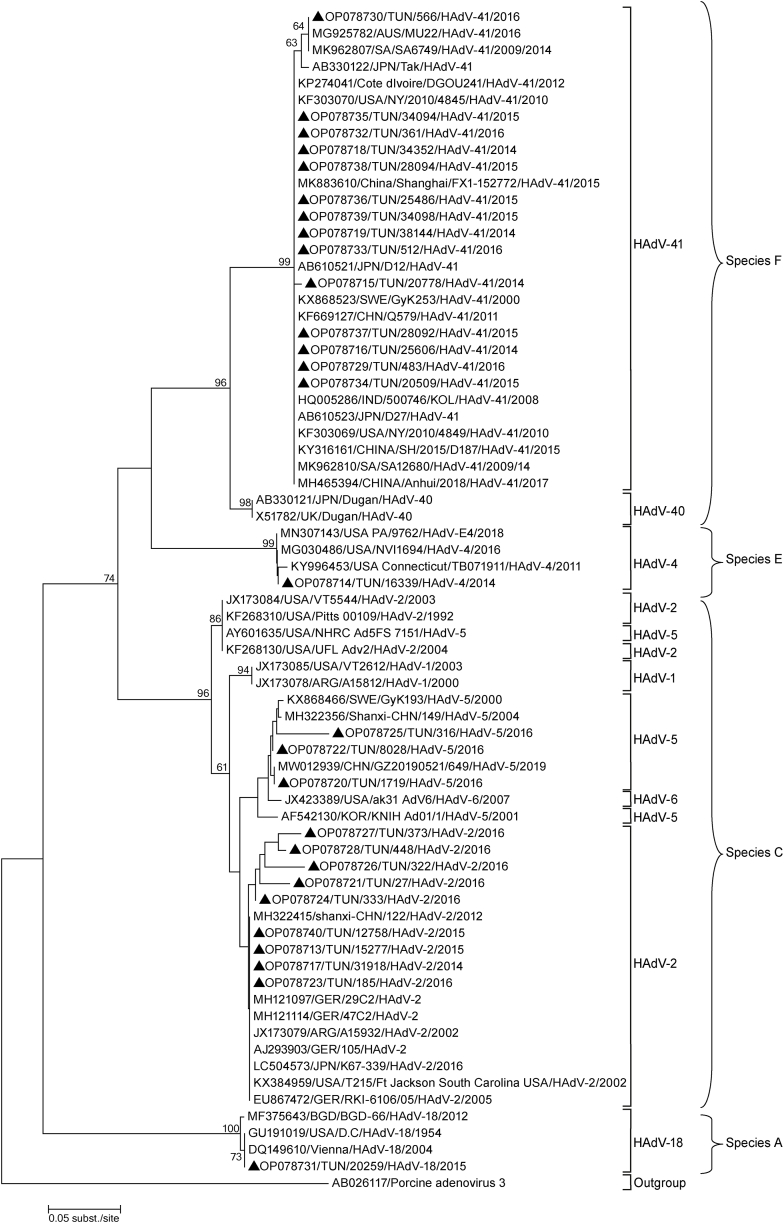
A phylogenetic tree based on the partial Hexon gene was created (Fig. 3) and revealed the presence of several HAdV strains that were more similar to those found in Europe or Asia than in Africa. The HAdV-F41 strains displayed a high degree of similarity (98–100 %) of nucleotide sequence to the HAdV-F41 strains reported from Japan, South Africa, and China.
Fig. 3.
Phylogenetic analysis of 23 partial sequences of the hexon gene (301 nucleotides) and 5 complete genomes of adenovirus type 41, 2, 5, 4, and type 18 collected in the coastal region of Tunisia from 2014 to 2016. The nucleotide sequences were aligned using Blast. The tree topology was built according to the maximum-likelihood method using the MEGA software version 6.06. The scale bars represent the frequency of nucleotide substitutions. The accuracy of the tree was assessed by 1000 bootstrap replicates. Only Bootstrap values > 50 % are presented. Tunisian sequences are indicated by a black triangle (▲).
Our HAdV A18 (OP078731) and HAdV E4 (OP078714) strains were similar (100 % nucleotide similarity) to strains from the USA.
The HAdV C2 and C5 strains in this study were closely related. These strains were compared (98 %–100 nucleotide similarity) to strains from China, the USA, and Germany.
We noticed also that other types of HAdV (C1 and C6) detected in Argentine and the USA, were closely related to our C5 and C2 strains and located in the same cluster, suggesting possible inter-genotypic recombination among co-circulating strains in central coast Tunisia.
4. Discussion
4.1. detection rate of enteric AdVs in stool
EAdV continues to have a significant impact on morbidity and mortality in children under the age of five, particularly in low-income populations living in regions with insufficient or no basic infrastructure [25]. According to previous reports, EAdV has been linked to sporadic infections and outbreaks of AGEs [26,27]. In Tunisia and other North African countries, there are a limited number of studies in regard to the incidence and molecular epidemiology of HAdV in AGE cases at a national level.
In our study, EAdVs were found in 52 of the 582 stools tested (8.93 %). The detection rate of HAdV fluctuates periodically, we found that the yearly positive rate for HAdV ranged from 8.33 % to 12.70 %. A peak in the proportion of HAdV-positive cases was observed in 2014.
During this three-year surveillance, it was interesting to notice that the annual HAdV detection rate in the population aged between 0 and 60 months was significantly higher than that found in two Tunisian studies carried out between 2003 and 2007 (2.3 %) [28], and between 2002 and 2005 (6 %) [29], and in other work done in Bangladesh (2.8 %) [30] and Argentina (1.5 %) [31], as well as in recent work in Taiwan (2.5 %) [32], the Czech Republic (5 %) [33], Tanzania (3.5 %) [34], and in France (5 %) [35]. However, this rate is lower than those found in Germany (14 %) [36], the United Kingdom (15 %) [37], Australia (21 %) [38], Brazil (16 %) [39], Turkey (26.2 %) [40], and Saudi Arabia (15.5 %) [41].
Yet, our results are comparable to the other studies where HAdV rates were around 7.0 % from Aurangabad, 7.5 % from Nagpur, 9.0 % from Pune, India [42], 7.1 % from Shanghai, 8 % from Japan [27], 7.1 % from Korea [43], and 7.2 % from Thailand [44].
These data show that EAdV has an incidence that varies from one region to another, but remains among the main etiological agents in viral GE from children all over the world.
4.2. Age and Sex distribution
4.2.1. age distribution
According to the values reported by our study, a significant association between age and the occurrence of infection was observed. Infections with EAdV mainly concerned children under 12 months of age (n = 29/52; 55.7 %), with extremes ranging from 0 to 6 months (18/29).
From the literature, HAdV was detected mainly in infants aged ≤12 months old [30,42,45].
The development of a somewhat efficient immune system during childhood is most likely to be responsible for this result. Some epidemiological statistics, in previous studies, showed that the majority of primary HAdV infections occur within the first 5 years of life due to a lack of humoral immunity [24,46]. However, the multitude of types (more than 100 in humans) means that we can have GEs repeatedly.
4.2.2. sex distribution
Our results show that the proportion of boys with AGE caused by HAdV is predominant (55.76 %) comparing to the girls (44.23 %). These data are in perfect agreement with the previous studies described in Indonesia [47], Tanzania [34], Bangladesh [30], and Northern-Western Nigeria [48]. In contrast, in a study carried out in Thailand, there was an equal distribution between the two sexes [44].
According to previous reports, males frequently mount a weak immune response to viral infection when compared to females, due to physiological differences between the two sexes that may lead to weaker immune responses in males when facing viral infections, in comparison to females [49].
4.3. seasonal distribution
The incidence of HAdV infections was noted in almost every month of the year, as it is shown in Fig. 2, with a peak in autumn (2014 and 2015) and winter (2016) which corresponded to the rainy seasons in Tunisia. Longitudinal studies would be needed to determine the exact seasonality of HAdV infections.
As reported in other studies, the frequency of E AdV infection was sporadic during the winter months [45,[50], [51], [52]], and diarrheal outbreaks due to E AdV infection were reported to occur from November to January [27,53].
In a 2014 research, Lion T et al. revealed that most HAdV outbreaks were detected in immunocompetent patients throughout the winter and early spring, whereas infections in immunocompromised patients occur all year [24].
4.4. distribution of human adenovirus genotype and phylogenetic analysis
The distribution of HAdV genotypes is variable, depending on geographical, environmental, and meteorological characteristics. Certain strains may have a larger pandemic potential than others [54]. In Tunisia, there is limited information about the specific types of HAdV genotypes circulating in relation to AGE. In general, AGE is commonly linked to being infected with enteric AdV-40 and -41, along with instances of AdV-12, -18, and −31 [55], as well as AdV-52(54). Moreover, AdV-5, -31, −34, −35, and −39 have been identified as causative agents in infections among individuals with weakened immune systems, such as those who have undergone hematopoietic stem cell or solid organ transplantation [56]. Cases of hepatitis have been associated with infection by serotypes 1 to 3, 5, and 7 [57].
The characterization and comparison of HAdV circulating may provide a key to understand the spread of HAdV in the community. To identify HAdV genotypes and assess the genetic link between the 28 HAdV strains discovered in this study, and other HAdV strains previously reported worldwide, a phylogenetic tree of the partial Hexon gene [301bp] was created (Fig. 3). HAdV strains diverged into various genotypic clusters revealing the circulation of 5 genotypes of HAdV (F41, C2, C5, E4, and A18).
The overall nucleotide sequence identities of the HAdV strains obtained were extremely comparable to each other and HAdV strains previously reported from the USA, Japan, and Germany.
Of the 5 genotypes, HAdV-F41 and HAdV-C2 were the most predominant genotype, followed by HAdV-C5, HAdV-E4, and HAdV-A18. This result concord with that found in a study done in Thailand where the genotypes F41 and C2 were dominant [44].
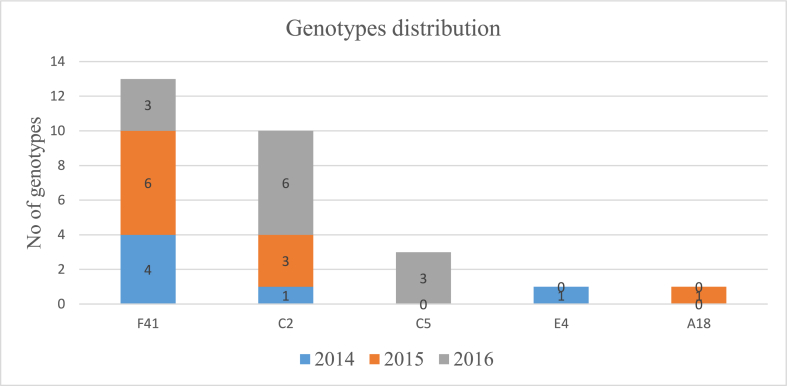
Interestingly, the HAdV-F41 and HAdV-C2 genotypes were detected in every year of our study period, with a predominance of HAdV type F41 during 2014 and 2015; and a predominance of HAdV type C2 in 2016; but HAdV-C5 and A18 were detected only in 2016 (Fig. 4).
Fig. 4.
Distribution of different genotypes (F41, C2, C5, E4, and A18) of Human Adenoviruses (HAdV), detected over three years of study (2014–2016), in children under 5 years old, consulting for acute gastroenteritis (AGE) in the central coastal Tunisian hospitals.
We noticed from Fig. 3 that the group HAdV-C shows an intermixing of HAdV-5 and HAdV-2 samples from GenBank. this issue may be partially attributed to the use of only Hexon sequences, which might not provide sufficient signal to accurately distinguish the samples. Therefore, we plan to include the study of the fiber and penton genes in our future research to enhance adenovirus detection.
The involvement of HAdV-F in the etiology of AGE has been shown worldwide, with the predominance of type 41 [[58], [59], [60], [61]]. Adenovirus type F41 is a frequent cause of severe diarrhea and diarrhea-related fatalities among young children worldwide. Gastroenteritis resulting from adenovirus F41 can be life-threatening and often leads to hospitalization. The symptoms of acute gastroenteritis caused by this specific adenovirus type are similar to those caused by other viruses. In other previous reports, a predominance of HAdV-41 over HAdV-40 has been shown [27,28,45,53,62,63], and in a recent study conducted in sub-Saharan Africa and South Asian countries to assess the impact of AGEs on children in developing countries, HAdV-F type 40 and 41 were listed among the viruses causing moderate to severe diarrhea [64]. However, in our study, we didn't detect the F40 type, which is unexpected.
Around this time, EAdV tends to have newer genotypes relative to the previous ones. Furthermore, the emergence of new genotypes is associated with the prevalence of new pathogens [44,65].
4.5. coinfections rate
Co-infections are more commonly thought of as viruses from different viral families mediating simultaneous infection, but they can also include concurrent infections by multiple viral strains or species of the same viral genus. A dual infection can be explained by the fact that, during a specific AGE episode, several viruses continue to be excreted, while another virus causes acute disease. Children with co-infection involving other pathogens are particularly at a heightened risk of experiencing severe illness [66].
By using the NGS technique on ten samples, we demonstrated that mixed infections with two or more viruses were present. Among these 10 HAdV-positive samples, 2 samples were coinfected by RV, 6 samples with Parvovirus, 3 samples with Enterovirus,1 with Norovirus, 1 with Parechovirus, and 1 with Sapovirus.
By using the PCR technique, in another study conducted in our laboratory (surveillance of RV [67]) in our 582 samples, HAdV/RV coinfection was detected in 6 samples. This type of coinfection has been described in other studies on the viral etiology of pediatric GEs [39,68,69].
5. Conclusion
The retrospective study provides baseline data about the prevalence and molecular characterization of EAdV serotypes that causes infantile AGE cases from Tunisia. To sum up, our research emphasizes that human adenovirus is prevalent within the community and serves as a significant cause of acute gastroenteritis, leading to a substantial disease burden among children under the age of five years. We revealed that non-enteric AdV (C2, C5, E4, A18) may also play an important role in gastroenteritis in this region in addition to the enteric types (F41), emphasising the importance of regular surveillance of circulating genotypes. Given the diversity of the strains identified, Genome sequencing has become necessary for very precise identification.
While this study may not fully depict the actual scenario of adenoviral gastroenteritis in Tunisia, it still manages to draw the interest of healthcare professionals. This has led to a call for organized and extensive epidemiological surveys in order to gain a thorough understanding of the true disease landscape in Tunisia. Such efforts are crucial for implementing timely interventions to address the issue effectively.
Data Availability statement
All data to support the conclusions have been either provided or are otherwise publicly available.
Funding information
This work received no specific grant from any funding agency.
Ethical approval
The study was approved by the Ethical Committee of Sahloul University Hospital.
CRediT authorship contribution statement
Asma Bouazizi: Conceptualization, Investigation, Visualization, Writing – original draft, Writing – review & editing. Mouna Ben Hadj Fredj: Software, Validation, Writing – review & editing. Haifa Bennour: Conceptualization, Resources. Amira Jerbi: Conceptualization, Resources. Ouafa kallala: Conceptualization, Resources. Imene Fodha: Resources, Writing – review & editing. Abdelhalim Trabelsi: Supervision, Validation, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We acknowledge the staff of the Microbiology Unit of Sahloul University Hospital for their technical help. We are also grateful to the nursing staff of the Paediatric Units of University Hospitals Sahloul, Farhat Hached, and Mahdia, and the regional hospital of M'saken for assisting in sample collection.
We are thankful for Pr. Jelle Matthijnssens from Laboratory of Viral Metagenomics, Department of Microbiology and Immunology, Rega Institute for Medical Research, KU Leuven – University of Leuven, B-3000 Leuven, Belgium, for giving as the opportunity to test our samples using NGS technique.
References
- 1.Parashar U.D., Hummelman E.G., Bresee J.S., Miller M.A., Glass R.I. Global illness and deaths caused by rotavirus disease in children. Emerg. Infect. Dis. 2003;9(5):565–572. doi: 10.3201/eid0905.020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Troeger C., Blacker B.F., Khalil I.A., Rao P.C., Cao S., Zimsen S.R., et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018;18(11):1211–1228. doi: 10.1016/S1473-3099(18)30362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilhelmi I., Roman E., Saânchez-Fauquier A. Viruses causing gastroenteritis. Clin. Microbiol. Infect. 2003;9:247–262. doi: 10.1046/j.1469-0691.2003.00560.x. 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jakab F., Péterfai J., Meleg E., Bányai K., Mitchell D.K., Szűcs G. Comparison of clinical characteristics between astrovirus and rotavirus infections diagnosed in 1997 to 2002 in Hungary. Acta Pædiatrica. 2005 Jun 1 doi: 10.1111/j.1651-2227.2005.tb01962.x. https://onlinelibrary.wiley.com/doi/full/10.1111/j.1651-2227.2005.tb01962.x ;94(6):667–71. Available from: [DOI] [PubMed] [Google Scholar]
- 5.Liu J., Platts-Mills J.A., Juma J., Kabir F., Nkeze J., Okoi C., et al. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet. 2016;388(10051):1291–1301. doi: 10.1016/S0140-6736(16)31529-X. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albinana-Gimenez N., Miagostovich M.P., Calgua B., Huguet J.M., Matia L., Girones R. Analysis of adenoviruses and polyomaviruses quantified by qPCR as indicators of water quality in source and drinking-water treatment plants. Water Res. 2009;43(7) doi: 10.1016/j.watres.2009.01.025. [Internet] 2011–9. [DOI] [PubMed] [Google Scholar]
- 7.Haramoto E., Katayama H., Oguma K., Ohgaki S. Quantitative analysis of human enteric adenoviruses in aquatic environments. J. Appl. Microbiol. 2007;103(6):2153–2159. doi: 10.1111/j.1365-2672.2007.03453.x. [DOI] [PubMed] [Google Scholar]
- 8.Lu X., Erdman D.D. Molecular typing of human adenoviruses by PCR and sequencing of a partial region of the hexon gene. Arch. Virol. 2006;151(8):1587–1602. doi: 10.1007/s00705-005-0722-7. [DOI] [PubMed] [Google Scholar]
- 9.Group Ha Working. HAdV Working Group. HAdV working group; 2019. http://hadvwg.gmu.edu/ [Internet] Available from: [Google Scholar]
- 10.Sumi A., Rajendran K., Ramamurthy T., Krishnan T., Nair G.B., Harigane K., et al. Effect of temperature, relative humidity and rainfall on rotavirus infections in Kolkata, India. Epidemiol. Infect. 2013;141(8):1652–1661. doi: 10.1017/S0950268812002208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Onozuka D. Effect of non-stationary climate on infectious gastroenteritis transmission in Japan. Sci. Rep. 2014;4:1–6. doi: 10.1038/srep05157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel M.M., Pitzer V., Alonso W.J., Vera D., Lopman B., Tate J., et al. Global seasonality of rotavirus disease. Pediatr. Infect. Dis. J. 2014;32(4):134–147. doi: 10.1097/INF.0b013e31827d3b68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thongprachum A., Chan-it W., Khamrin P., Saparpakorn P., Okitsu S., Takanashi S., et al. Molecular epidemiology of norovirus associated with gastroenteritis and emergence of norovirus GII.4 variant 2012 in Japanese pediatric patients. Infect. Genet. Evol. 2014 Apr 1;23:65–73. doi: 10.1016/j.meegid.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 14.Zhou X., Song X., Wan L. Case Rep Oncol; 2022 May 2. Epstein-Barr Virus Encephalitis and Disseminated Adenovirus Infection after Haploidentical Allogeneic Hematopoietic Stem Cell Transplantation for a Patient with Ph-like Acute Lymphoblastic Leukemia. [Internet] [cited 2023 Aug 4];15(1):245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kujawski S.A., Lu X., Schneider E., Blythe D., Boktor S., Farrehi J., et al. Outbreaks of adenovirus-associated respiratory illness on 5 college campuses in the United States, 2018-2019. Clin. Infect. Dis. 2021;72(11):1992–1999. doi: 10.1093/cid/ciaa465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahase E. Hepatitis in children: what's behind the outbreaks? BMJ. 2022;377(April):o1067. doi: 10.1136/bmj.o1067. [DOI] [PubMed] [Google Scholar]
- 17.Shi S.R., Cote R.J., Wu L., Liu C., Datar R., Shi Y., et al. DNA extraction from archival formalin-fixed, paraffin-embedded tissue sections based on the antigen retrieval principle: heating under the influence of pH. J. Histochem. Cytochem. 2002;50(8):1005–1011. doi: 10.1177/002215540205000802. [DOI] [PubMed] [Google Scholar]
- 18.Allard A., Albinsson B., Wadell G. Rapid typing of human adenoviruses by a general PCR combined with restriction endonuclease analysis. J. Clin. Microbiol. 2001;39(2):498–505. doi: 10.1128/JCM.39.2.498-505.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolger A.M., Lohse M., Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bankevich A., Nurk S., Antipov D., Gurevich A.A., Dvorkin M., Kulikov A.S., et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012;19(5):455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buchfink B., Xie C., Huson D.H. Fast and sensitive protein alignment using DIAMOND. Nat Methods [Internet] 2014;12(1):59–60. doi: 10.1038/nmeth.3176. [DOI] [PubMed] [Google Scholar]
- 22.Edgar R.C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30(12):2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lion T. Adenovirus infections in immunocompetent and immunocompromised patients. Clin. Microbiol. Rev. 2014 Jul;27(3):441–462. doi: 10.1128/CMR.00116-13. https://journals.asm.org/doi/10.1128/CMR.00116-13 [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farthing M., Salam M.A., Lindberg G., Dite P., Khalif I., Salazar-Lindo E., et al. Acute diarrhea in adults and children. J. Clin. Gastroenterol. 2013;47(1):12–20. doi: 10.1097/MCG.0b013e31826df662. [DOI] [PubMed] [Google Scholar]
- 26.Akihara S., Phan T.G., Nguyen T.A., Hansman G., Okitsu S., Ushijima H. Existence of multiple outbreaks of viral gastroenteritis among infants in a day care center in Japan. Arch. Virol. 2005;150(10):2061–2075. doi: 10.1007/s00705-005-0540-y. [DOI] [PubMed] [Google Scholar]
- 27.Shimizu H., Phan T.G., Nishimura S., Okitsu S., Maneekarn N., Ushijima H. An outbreak of adenovirus serotype 41 infection in infants and children with acute gastroenteritis in Maizuru City, Japan. Infect. Genet. Evol. 2007;7(2):279–284. doi: 10.1016/j.meegid.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 28.Sdiri-Loulizi Khira, Gharbi-Khelifi Hakima, Rougemont Alexis de, Hassine Mouna, Chouchane Slaheddine, Sakly Nabil, Pothier Pierre, Neji Guédiche Mohamed, Aouni Mahjoub, Ambert-Balay Katia. Molecular epidemiology of human astrovirus and adenovirus serotypes 40/41 strains related to acute diarrhea in Tunisian children. J. Med. Virol. 2009;81(November 2009):1895–1902. doi: 10.1002/jmv.21586. [DOI] [PubMed] [Google Scholar]
- 29.Fodha I., Chouikha A., Peenze I., De Beer M., Dewar J., Geyer A., Messaadi F., Trabelsi A., Boujaafar N., Taylor M.B., Ds Identification of viral agents causing diarrhea among children in the eastern center of Tunisia. J. Med. Virol. 2006;78:1198–1203. doi: 10.1002/jmv.20681. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jarecki-Khan K., Tzipori S.R., Unicomb L.E. Enteric adenovirus infection among infants with diarrhea in rural Bangladesh. J. Clin. Microbiol. 1993;31(3):484–489. doi: 10.1128/jcm.31.3.484-489.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giordano M.O., Ferreyra L.J., Isa M.B., Martinez L.C., Yudowsky S.I., Nates S.V. The epidemiology of acute viral gastroenteritis in hospitalized children in Cordoba City, Argentina: an insight of disease burden. Rev. Inst. Med. Trop. Sao Paulo. 2001;43(4):193–197. doi: 10.1590/s0036-46652001000400003. [DOI] [PubMed] [Google Scholar]
- 32.Ku M.S., Sheu J.N., Lin C.P., Chao Y.H., Chen S.M. Clinical characteristics and outcome in norovirus gastroenteritis. Indian J. Pediatr. 2014;81(12):1321–1326. doi: 10.1007/s12098-014-1419-2. [DOI] [PubMed] [Google Scholar]
- 33.Fajfr M., Stěpánová V., Plíšková L., Fajfrová J. Viral gastroenteritis in eastern bohemia region of the Czech republic. Epidemiol. Mikrobiol. Imunol. 2014;63(2):88–91. [PubMed] [Google Scholar]
- 34.Moyo S.J., Hanevik K., Blomberg B., Kommedal O., Nordbø S.A., Maselle S., et al. Prevalence and molecular characterisation of human adenovirus in diarrhoeic children in Tanzania; A case control study. BMC Infect. Dis. 2014;14(1):1–9. doi: 10.1186/s12879-014-0666-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borrows C.L., Turner P.C. Seasonal screening for viral gastroenteritis in young children and elderly hospitalized patients: is it worthwhile? J Hosp Infect [Internet] 2014;87(2):98–102. doi: 10.1016/j.jhin.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 36.Oh D.Y., Gaedicke G., Schreier E. Viral agents of acute gastroenteritis in German children: prevalence and molecular diversity. J. Med. Virol. 2003;71(1):82–93. doi: 10.1002/jmv.10449. [DOI] [PubMed] [Google Scholar]
- 37.Cunliffe N.A., Booth J.A., Elliot C., Lowe S.J., Sopwith W., Kitchin N., et al. Healthcare-associated viral gastroenteritis among children in a large pediatric hospital, United Kingdom. Emerg. Infect. Dis. 2010;16(1):55–62. doi: 10.3201/eid1601.090401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fletcher S., Van Hal S., Andresen D., McLaws M.L., Stark D., Harkness J., et al. Gastrointestinal pathogen distribution in symptomatic children in Sydney, Australia. J Epidemiol Glob Health. 2013;3(1):11–21. doi: 10.1016/j.jegh.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raboni S.M., Damasio G.A.C., Ferreira C.E.O., Pereira L.A., Nogueira M.B., Vidal L.R., et al. Acute gastroenteritis and enteric viruses in hospitalised children in southern Brazil: aetiology, seasonality and clinical outcomes. Mem. Inst. Oswaldo Cruz. 2014;109(4):428–435. doi: 10.1590/0074-0276140066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ozsari T., Bora G., Kaya B., Yakut K. The prevalence of rotavirus and adenovirus in the childhood gastroenteritis. Jundishapur J. Microbiol. 2016;9(6) doi: 10.5812/jjm.34867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meqdam M.M., Thwiny I.R. Prevalence of group a rotavirus, enteric adenovirus, norovirus and astrovirus infections among children with acute gastroenteritis in Al-Qassim, Saudi Arabia. Pakistan J. Med. Sci. 2007;23(4):551–555. [Google Scholar]
- 42.Verma Harsha, Shobha D., Chitambar and GV Identification and characterization of enteric adenoviruses in infants and children hospitalized for acute gastroenteritis. J. Med. Virol. 2009;81(November 2009):60–64. doi: 10.1002/jmv.21331. [DOI] [PubMed] [Google Scholar]
- 43.Koh H., Baek S.Y., Shin J Il, Chung K.S., Jee Y.M. Coinfection of viral agents in Korean children with acute watery diarrhea. J. Kor. Med. Sci. 2008;23(6):937–940. doi: 10.3346/jkms.2008.23.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumthip K., Khamrin P., Ushijima H., Maneekarn N. Enteric and non-enteric adenoviruses associated with acute gastroenteritis in pediatric patients in Thailand, 2011 to 2017. PLoS One. 2019;14(8):1–12. doi: 10.1371/journal.pone.0220263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pereira Filho E., Da Costa Faria N.R., Fialho A.M., De Assis R.S., Almeida M.M.S., Rocha M., et al. Adenoviruses associated with acute gastroenteritis in hospitalized and community children up to 5 years old in Rio de Janeiro and Salvador, Brazil. J. Med. Microbiol. 2007;56(3):313–319. doi: 10.1099/jmm.0.46685-0. [DOI] [PubMed] [Google Scholar]
- 46.Echavarría M. Adenoviruses in immunocompromised hosts. Clin. Microbiol. Rev. 2008;21(4):704–715. doi: 10.1128/CMR.00052-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Subekti D., Lesmana M., Tjaniadi P., Safari N., Frazier E., Simanjuntak C., et al. Incidence of Norwalk-like viruses, rotavirus and adenovirus infection in patients with acute gastroenteritis in Jakarta, Indonesia. FEMS Immunol. Med. Microbiol. 2002;33(1):27–33. doi: 10.1111/j.1574-695X.2002.tb00568.x. [DOI] [PubMed] [Google Scholar]
- 48.Aminu M., Ahmad A.A., Ju Umoh, de Beer M.C., Esona M.D., Steele A.D. Adenovirus infection in children with diarrhea disease in Northwestern Nigeria. Ann. Afr. Med. 2007;6(4):168–173. doi: 10.4103/1596-3519.55702. [DOI] [PubMed] [Google Scholar]
- 49.S.L K. Sex differences in prophylaxis and therapeutic treatments for viral diseases. Handb. Exp. Pharmacol. 2012;214(12):499–522. doi: 10.1007/978-3-642-30726-3_22. [DOI] [PubMed] [Google Scholar]
- 50.Barnes G.L., Uren E., Stevens K.B., Bishop R.F. Etiology of acute gastroenteritis in hospitalized children in Melbourne, Australia, from April 1980 to March 1993. J. Clin. Microbiol. 1998;36(1):133–138. doi: 10.1128/jcm.36.1.133-138.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dennehy P.H., Nelson S.M., Spangenberger S., Noel J.S., Monroe S.S., Glass R.I. A prospective case-control study of the role of astrovirus in acute diarrhea among hospitalized young children. J. Infect. Dis. 2001;184(1):10–15. doi: 10.1086/321007. [DOI] [PubMed] [Google Scholar]
- 52.Soares C.C., Volota E.M., Carolina M., Albuquerque M., Fabiano M., Carvalho TRB De, et al. Prevalence of enteric adenoviruses among children.pdf. J. Clin. Virol. 2002;23:171–177. doi: 10.1016/s1386-6532(01)00220-7. [DOI] [PubMed] [Google Scholar]
- 53.Dey S.K., Shimizu H., Phan T.G., Hayakawa Y., Islam A., Salim A.F.M., et al. Molecular epidemiology of adenovirus infection among infants and children with acute gastroenteritis in Dhaka City, Bangladesh. Infect. Genet. Evol. 2009;9(4):518–522. doi: 10.1016/j.meegid.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 54.Lynch J.P., Kajon A.E. Adenovirus: epidemiology, global spread of novel serotypes, and advances in treatment and prevention. Semin. Respir. Crit. Care Med. 2016;37(4):586–602. doi: 10.1055/s-0036-1584923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim Y.J., Boeckh M., Englund J.A. Community respiratory virus infections in immunocompromised patients: hematopoietic stem cell and solid organ transplant recipients, and individuals with human immunodeficiency virus infection. Seminars in Respiratory and Critical Care Medicine. Semin Respir Crit Care Med. 2007;28 doi: 10.1055/s-2007-976494. https://pubmed.ncbi.nlm.nih.gov/17458776/ [Internet] [cited 2023 Aug 9]. pp. 222–42. Available from: [DOI] [PubMed] [Google Scholar]
- 56.Wallot M.A., Dohna-Schwake C., Auth M., Nadalin S., Fiedler M., Malagó M., et al. Pediatr Transplant; 2006 Feb. Disseminated Adenovirus Infection with Respiratory Failure in Pediatric Liver Transplant Recipients: Impact of Intravenous Cidofovir and Inhaled Nitric Oxide.https://pubmed.ncbi.nlm.nih.gov/16499602/ [Internet] [cited 2023 Aug 9];10(1):121–7. Available from: [DOI] [PubMed] [Google Scholar]
- 57.Echavarría M. Adenoviruses in immunocompromised hosts. Clin. Microbiol. Rev. 2008 Oct doi: 10.1128/CMR.00052-07. https://pubmed.ncbi.nlm.nih.gov/18854488/ ;21(4):704–15. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lennon G., Cashman O., Lane K., Cryan B., O'Shea H. Prevalence and characterization of enteric adenoviruses in the South of Ireland G. J. Med. Virol. 2007;79(November 2007):1518–1526. doi: 10.1002/jmv.20975. [DOI] [PubMed] [Google Scholar]
- 59.Dey R.S., Ghosh S., Chawla-Sarkar M., Panchalingam S., Nataro J.P., Sur D., et al. Circulation of a novel pattern of infections by enteric adenovirus serotype 41 among children below 5 years of age in Kolkata, India. J. Clin. Microbiol. 2011;49(2):500–505. doi: 10.1128/JCM.01834-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fukuda S., Kuwayama M., Takao S., Shimazu Y., Miyazaki K. Molecular epidemiology of subgenus F adenoviruses associated with pediatric gastroenteritis during eight years in Hiroshima Prefecture as a limited area. Arch. Virol. 2006;151(12):2511–2517. doi: 10.1007/s00705-006-0816-x. [DOI] [PubMed] [Google Scholar]
- 61.Bányai K., Kisfali P., Á Bogdán, Martella V., Melegh B., Erdman D., et al. Adenovirus gastroenteritis in Hungary, 2003-2006. Eur. J. Clin. Microbiol. Infect. Dis. 2009;28(8):997–999. doi: 10.1007/s10096-009-0722-8. [DOI] [PubMed] [Google Scholar]
- 62.Kamel A.H., Ali M.A., El-Nady H.G., De Rougemont A., Pothier P., Belliot G. Predominance and circulation of enteric viruses in the region of greater Cairo, Egypt. J. Clin. Microbiol. 2009;47(4):1037–1045. doi: 10.1128/JCM.01381-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mayindou Gontran, Ngokana Berge, Sidibe Anissa, Moundel Victoire, Koukouikila-Koussounda Felix, Christevy Vouvoungui Jeannhey, Kwedi Nolna Sylvie, Thirumalaisamy P., Velavan and F Molecular epidemiology and surveillance of circulating rotavirus and adenovirus in Congolese children with gastroenteritis. J. Med. Virol. 2016;88:596–605. doi: 10.1002/jmv.24382. 2016. [DOI] [PubMed] [Google Scholar]
- 64.Kotloff K.L., Nataro J.P., Blackwelder W.C., Nasrin D., Farag T.H., Panchalingam S., et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382(9888) doi: 10.1016/S0140-6736(13)60844-2. [Internet] 209–22. [DOI] [PubMed] [Google Scholar]
- 65.Oka T., Wang Q., Katayama K., Saifb L.J. Comprehensive review of human sapoviruses. Clin. Microbiol. Rev. 2015;28(1):32–53. doi: 10.1128/CMR.00011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alharbi S., Van Caeseele P., Consunji-Araneta R., Zoubeidi T., Fanella S., Souid A.K., et al. Epidemiology of severe pediatric adenovirus lower respiratory tract infections in Manitoba, Canada, 1991-2005. BMC Infect. Dis. 2012;12 doi: 10.1186/1471-2334-12-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bennour H., Bouazizi A., Fodha I., Ben Hadj Fredj M., Ben Hamida-Rebai M., Jerbi A., et al. Unexpected predominance of rotavirus G9P[8] strain in Tunisian adult diarrheal patients. J. Med. Microbiol. 2020 Feb 1 doi: 10.1099/jmm.0.001156. https://www.microbiologyresearch.org/content/journal/jmm/10.1099/jmm.0.001156 69(2):280–9. Available from: [DOI] [PubMed] [Google Scholar]
- 68.Amaral M.S.C., Estevam G.K., Penatti M., Lafontaine R., Lima I.C.G., Spada P.K.P., et al. Mem Inst Oswaldo Cruz; 2015 Apr 1. The Prevalence of Norovirus, Astrovirus and Adenovirus Infections Among Hospitalised Children with Acute Gastroenteritis in Porto Velho, State of Rondônia, Western Brazilian Amazon.https://pubmed.ncbi.nlm.nih.gov/25946245/ [Internet] [cited 2022 Sep 19];110(2):215–21. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tran A., Talmud D., Lejeune B., Jovenin N., Renois F., Payan C., et al. Prevalence of rotavirus, adenovirus, norovirus, and astrovirus infections and coinfections among hospitalized children in northern France. J. Clin. Microbiol. 2010 May doi: 10.1128/JCM.02181-09. https://pubmed.ncbi.nlm.nih.gov/20305010/ ;48(5):1943–6. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data to support the conclusions have been either provided or are otherwise publicly available.