Abstract
This study aimed to investigate the relationship between the dietary approaches to stop hypertension (DASH) dietary patterns and bone mineral density (BMD) in adults residing in the United States. To achieve this, data from the National Health and Nutrition Examination Survey (NHANES) database for 2011–2018 were utilized. This study utilized the NHANES database from 2011 to 2018, with a sample size of 8,486 US adults, to investigate the relationship between the DASH diet and BMD. The DASH diet was assessed based on nine target nutrients: total fat, saturated fat, protein, fiber, cholesterol, calcium, magnesium, sodium and potassium. The primary outcome measures were BMD values at the total BMD, thoracic spine, lumbar spine, and pelvis. Multivariable linear models were employed to analyze the association between the DASH diet and BMD. Interaction tests, subgroup, and sensitivity analysis were also followed. A negative correlation was observed between the DASH diet and total BMD (OR: − 0.003 [95%CI: − 0.005, − 0.001), pelvic (OR: − 0.005 [95%CI: − 0.007, − 0.002]), and thoracic BMD (OR: − 0.003 [95%CI: − 0.005, − 0.001]). However, the DASH diet does not appear to have a particular effect on lumbar spine BMD (OR: − 0.002 [95%CI: − 0.004, 0.001]). Similarly, when the DASH diet was categorized into tertiles groups, the relationship with total BMD, pelvic BMD, thoracic BMD, and lumbar spine BMD remained consistent. Furthermore, we performed a sensitivity analysis by converting BMD to Z-scores, and the results remained unchanged. Subgroup analyses and interaction tests indicated no significant dependence of BMI, gender, smoking, hypertension, and diabetes on the observed association (all p for interactions > 0.05). The DASH diet has been identified as potentially reducing total BMD, while specifically impacting thoracic and pelvic BMD. However, it appears to have no significant effect on lumbar spine BMD.
Subject terms: Diseases, Gastroenterology, Risk factors
Introduction
Bone mineral density (BMD) is a crucial determinant of bone fragility as it represents the amount of bone mineral within bone tissue1. Healthy adult BMD typically falls within the range of approximately 1.045 ± 0.135 g/cm2 for males and 0.991 ± 0.107 g/cm2 for females2. The NHANES website also provides assessed BMD status for the U.S. population, for example, with BMD quartiles ranging from 1.143–1.280 g/cm2 for 20–29 years males and 1.060–1.171 g/cm2 for females of the same age3. When BMD drops below a certain threshold, osteoporosis is triggered4. In the United States, it is estimated that nearly half of individuals aged 46 and older have low BMD, with projections indicating a rise to over 3 million fractures and an annual cost of $25.3 billion due to osteoporosis by 20255. Moreover, it is anticipated that by 2030, over 70 million Americans will be diagnosed with osteoporosis6. Given the aging global population, this condition is recognized as a significant public health concern7. Numerous studies have consistently established a strong association between dietary patterns and bone health8–10. Adopting a healthy dietary pattern has the potential to impact BMD positively. In their investigation, Hsu E. et al. explored the correlation between plant-based diets and BMD, proposing mechanisms that promote bone health11. Recent studies have also indicated that adherence to the Mediterranean diet may be a preventive measure against osteoporosis12. Furthermore, a meta-analysis has proved that incorporating soy isoflavones, enriched with omega-3 fatty acids, into dietary supplementation effectively improves women's bone health during menopause13. This intervention not only mitigates bone loss caused by menopause but also enhances bone formation while reducing bone resorption14.
The Dietary Approaches to Stop Hypertension (DASH) diet, which encompasses reduced sodium and calorie intake along with a diet abundant in fruits, vegetables, low-fat dairy products, whole grains, poultry, fish, nuts, and unsaturated vegetable oils, has received endorsement from the United States Department of Agriculture's Dietary Guidelines for Americans (2020–2025)15,16. Additionally, the DASH diet has been found to have various other applications. For instance, Zhang et al.17 discovered that the DASH diet can effectively decrease both blood pressure and the incidence of osteoarthritis. Additionally, the DASH diet has been observed to have a glycemic control effect in diabetic patients18. Furthermore, a study investigating the relationship between the DASH diet and serum uric acid levels over time revealed that adherence to the DASH diet can reduce serum uric acid levels19. Therefore, research on the uses of the DASH diet should include more than just lowering high blood pressure. Considering the rising prevalence of the DASH diet in special populations everyday lives, even the smallest effects that accrue over time could have a substantial impact on our bodies. Thus, it is essential to investigate and give attention to the potential impact of this diet on BMD.
Previous research suggests that the DASH diet may decrease BMD. Recent findings indicated that moderate increases in total fat, fiber intake, and magnesium intake might improve BMD20–22. However, a study discovered that the individuals in their study who followed the DASH diet did not meet the recommended values for total fat, fiber intake, and magnesium intake23. Additionally, certain studies have shown that adhering to the DASH diet is associated with lower levels of dietary triglycerides (TG) and low-density lipoprotein cholesterol (LDL-C)24, both of which are positively associated with BMD25,26. Therefore, there may be a risk of indirectly decreasing BMD by implementing the DASH diet.
Given the extensive acceptance of this dietary pattern among the general population, it is crucial to investigate its influence on BMD. We conducted a comprehensive study using NHANES data from 2011 to 2018 to further explore the relationship between the DASH diet and BMD. Our approach involved multivariate linear regression, subgroup analysis, sensitivity analyses, and interaction tests.
Methods
Data available
The National Health and Nutrition Examination Survey (NHANES), sponsored by the National Center for Health Statistics (NCHS) within the Centers for Disease Control and Prevention (CDC), is a recurring, nationwide cross-sectional survey. It has been conducted periodically since the 1960s with the primary objective of evaluating the health and nutritional status of both children and adults in the United States. Annually, approximately 5,000 participants are recruited using a multistage stratified sampling method, ensuring a nationally representative sample from counties across the United States. Demographic information and lifestyle data including dietary habits are gathered through surveys and physical examinations. The results of this comprehensive survey are published biennially. Details about survey design and data files can be accessed publicly at https://www.cdc.gov/nchs/nhanes/. The ethics protocol has been formally approved by the Research Ethics Review Board of NCHS, and informed consent was signed by all recruited participants27. Notably, this study diligently adheres to the principles delineated by Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) regarding cross-sectional studies28.
Study population
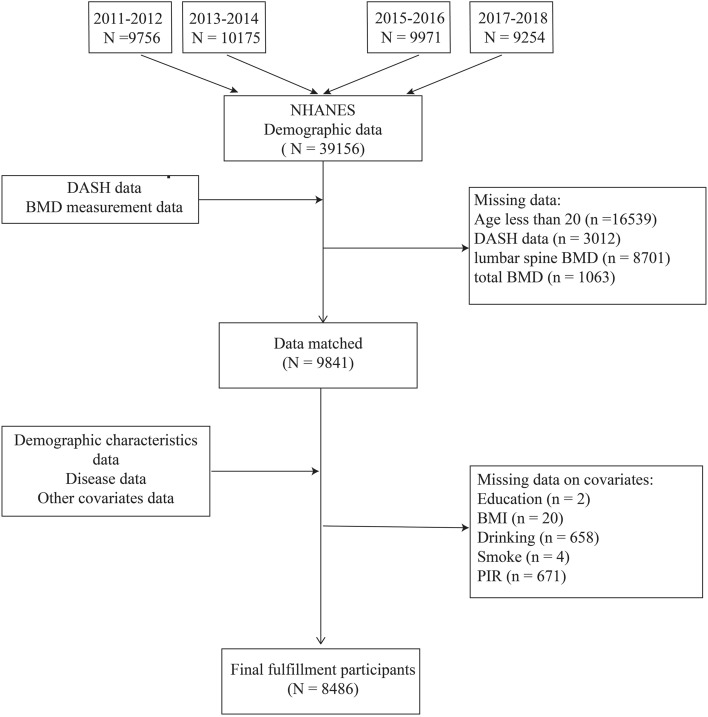
This study utilized data from four survey cycles conducted by NHANES (2011–2012, 2013–2014, 2015–2016, and 2017–2018). These specific cycles were selected due to their inclusion of data on total BMD, spanning 2011 to 2018. After a rigorous selection process, the study incorporated a total of 39,156 participants over four biennial periods. The distribution was as follows: 9756 participants from 2011–2012, 10,175 from 2013–2014, 9971 from 2015–2016, and 9254 from 2017–2018. Individuals under the age of 20 were subsequently excluded, which accounted for 16,539 of the initial cohort. Furthermore, we eliminated records that lacked DASH diet scores or BMD data, amounting to 12,776 participants. Additional data pruning included the exclusion of entries missing essential covariates such as education (n = 2), body mass index (BMI) (n = 20), smoking status (n = 4), alcohol consumption (n = 658), and poverty income ratio (PIR) (n = 671). Ultimately, this rigorous process yielded a final sample of 8,486 subjects eligible for analysis, as depicted in Fig. 1.
Figure 1.
Flowchart of the sample selection from the National Health and Nutrition Examination Survey (NHANES).
Definition of DASH
The DASH diet is a commonly followed dietary regimen incorporating a range of vital nutrients, including total fat, saturated fat, protein, fiber, cholesterol, calcium, magnesium, sodium and potassium. A detailed elucidation on the calculation of the DASH diet has been previously recorded23 and can be accessed in Supplementary Material 2 for additional information.
BMD measurement
Dual-energy X-ray absorptiometry (DXA), a widely acknowledged and extensively employed bone densitometry technique in contemporary clinical practice, offers significant merits, including expeditiousness, ease of use, and limited radiation exposure. The Hologic Discovery model A densitometer manufactured by Hologic, Inc., based in Bedford, MA, USA, was utilized for conducting the scans. The BMD measurements were performed meticulously and professionally by radiologists with training and certification. To maintain result accuracy, individuals who were pregnant, had recently used contrast media, or were overweight were excluded from the study. For more comprehensive information on the BMD measurements and the protocols employed, the NHANES website provides detailed documentation.
Covariates
We utilized multivariable adjustment models to address the possibility of confounding variables in the correlation between DASH and BMD, as previously employed in related studies29,30. The demographic variables examined in our study encompass gender (male/female), age (in years), ethnicity (Mexican American/Non-Hispanic white/Non-Hispanic black/Other races), educational level (less Than 9th grade/9-11th grade (includes 12th grade without diploma)/High School Graduate/GED or Equivalent/Some College or AA Degree/College Graduate or above), marital status (married/widowed/divorced/separated/never married/living with partner) and PIR (low-income/middle-income/high-income)31. Additionally, the study considers smoking habits (never/former/current) and patterns of alcohol consumption (never/former/heavy/mild/moderate) as outlined in an earlier report32. In addition, the research incorporates anthropometric and laboratory covariates, namely BMI (kg/m2), which is determined by dividing weight in kilograms by the square of height in meters. Health status variables encompass hypertension (Yes/No) and diabetes (Yes/No). Diabetes is defined as: (1) doctor told you have diabetes, (2) glycohemoglobin HbA1c(%) > = 6.5, (3) fasting glucose (mmol/l) > = 7.0, (4) random blood glucose (mmol/l) > = 11.1, (5) two-hour OGTT blood glucose (mmol/l) > = 11.1, or (6) use of diabetes medication or insulin33. Hypertension is defined as: taking antihypertensive medication, a doctor's diagnosis of hypertension, or having systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg on three consecutive readings34.
Statistical analysis
In our study, we adhered rigorously to the statistical analysis protocols endorsed by the CDC. Additionally, we considered the intricacies inherent in a complex multistage cluster survey design during our analytical procedures35,36. Mean values accompanied by standard errors were used to represent continuous variables, while percentages were employed for categorical variables. Subsequently, we employed a Student's t-test (for continuous variables) or a chi-square test (for categorical variables) to evaluate group disparities. Linear regression was applied to estimate the relationship between DASH diet and BMD. DASH diet served as the dependent variable and was modeled both categorically (in three categories) and continuously (scores), respectively. Meanwhile, BMD, as the independent variable, was modeled continuously, both in its raw scale and Z-score form. Estimated effects size was presented as betas (βs) along with their respective 95% confidence intervals (CIs). On one hand, our primary objective was to assess the impact of DASH dietary differences among participants on BMD. To achieve this, we categorized DASH scores into tertiles, allowing us to compare the BMD of populations in the highest tertile with those in the lowest tertile. On the other hand, we also conducted analyses using its continuous scale of the DASH scores, enabling us to explore its potential linear relationships with BMD. We accounted for potential confounding variables. Model 1 did not incorporate any additional factors. Model 2, on the other hand, included adjustments for age, sex, and race. Model 3 entailed adjustments for sex, age, race, education level, PIR, marital status, BMI, smoking status, diabetes, alcohol consumption, and hypertension. Subgroup analyses were conducted based on age, sex, BMI, smoking, hypertension, and diabetes status. Additionally, subgroup analyses as well as interaction tests were conducted to assess potential modifying effects of these variables on the relationship between DASH and BMD, and potential interactions between these variables and DASH. A significance level of less than 0.05 is commonly considered to indicate statistical significance in statistical analysis. The statistical analyses conducted in this study were executed utilizing R software (version 4.1.2; http://www.R-project.org, R Foundation for Statistical Computing, Vienna, Austria).
Ethics approval and consent to participate
The ethics review board of the National Center for Health Statistics approved all NHANES protocols.
Results
Essential characteristics of the included participants
Table 1 presents the pertinent details regarding the inclusion of 8,486 participants. The mean age of the included population was 39.07 ± 0.28 years, with males accounting for 51.82% and females accounting for 48.18%. Measurements of total BMD, thoracic spine BMD, lumbar spine BMD, and pelvic BMD yielded values of 1.11 ± 0.01 g/cm2, 0.82 ± 0.01 g/cm2, 1.04 ± 0.01 g/cm2, and 1.25 ± 0.01 g/cm2, respectively. The clinical characteristics of the participants, stratified by DASH tertiles, are presented in Table 2. This table demonstrates notable disparities in variables including age, gender, race, BMI, hypertension, education, pelvic BMD, thoracic spine BMD, and total BMD among the three DASH tertiles (all p-values < 0.05, refer to Table 2 for specific data). Our study revealed that individuals with higher DASH levels exhibited a higher likelihood of being female, older, and of Mexican American or other racial backgrounds, with these associations achieving statistical significance at p-value < 0.05 (refer to Table 2 for specific data).
Table 1.
Baseline characteristics of participants.
| Characteristics | Means (standard error) or percentage |
|---|---|
| Age (year) | 39.07 (0.28) |
| Body mass index (kg/m2) | 28.78 (0.16) |
| DASH | 2.30 (0.03) |
| LumbarSpineBMD (g/cm2) | 1.04 (0.01) |
| PelvicBMD (g/cm2) | 1.25 (0.01) |
| ThoracicSpineBMD (g/cm2) | 0.82 (0.01) |
| Total BMD (g/cm2) | 1.11 (0.01) |
| Sex (%) | |
| Male | 51.82 |
| Female | 48.18 |
| Race (%) | |
| Mexican American | 9.85 |
| Non-Hispanic White | 63.40 |
| Non-Hispanic Black | 10.79 |
| Other Race | 15.97 |
| Education level (%) | |
| Less than 9th grade | 3.16 |
| 9–11th grade (Includes 12th grade with no diploma) | 8.54 |
| High school graduate /GED or equivalent | 21.66 |
| Some college or AA degree | 33.13 |
| College graduate or above | 33.51 |
| Marital status (%) | |
| Married | 51.28 |
| Widowed | 1.12 |
| Divorced | 9.20 |
| Separated | 2.55 |
| Never married | 25.18 |
| Living with partner | 10.67 |
| Smoking status (%) | |
| Never | 60.23 |
| Former | 19.26 |
| Now | 20.50 |
| PIR (%) | |
| Low-income | 15.55 |
| Middle-income | 48.20 |
| High-income | 36.25 |
| Hypertension (%) | |
| Yes | 25.90 |
| No | 74.10 |
| Diabetes (%) | |
| Yes | 26.33 |
| No | 73.67 |
| Alcohol (%) | |
| Former | 8.11 |
| Heavy | 27.21 |
| Mild | 34.61 |
| Moderate | 20.76 |
| Never | 9.31 |
Notes: All values are presented as proportion (%), or mean(standard error).
PIR, ratio of family income to poverty; BMD, bone mineral density.
Table 2.
Baseline characteristics of participants based on DASH diet tertiles.
| Dietary approaches to stop hypertension | Tertile 1 | Tertile 2 | Tertile 3 | p for trend |
|---|---|---|---|---|
| Age (year) | 38.68 (0.30) | 39.65 (0.36) | 42.33 (0.99) | < 0.001 |
| Body mass index (kg/m2) | 29.15 (0.17) | 28.09 (0.21) | 27.70 (0.60) | < 0.001 |
| LumbarSpineBMD (g/cm2) | 1.04 (0.01) | 1.03 (0.01) | 1.03 (0.01) | 0.120 |
| PelvicBMD (g/cm2) | 1.26 (0.01) | 1.24 (0.01) | 1.20 (0.01) | < 0.001 |
| ThoracicSpineBMD (g/cm2) | 0.83 (0.01) | 0.81 (0.01) | 0.80 (0.01) | < 0.001 |
| Total BMD (g/cm2) | 1.12 (0.01) | 1.11 (0.01) | 1.10 (0.01) | < 0.001 |
| Sex (%) | < 0.001 | |||
| Male | 53.96 | 48.28 | 38.17 | |
| Female | 46.04 | 51.72 | 61.83 | |
| Race (%) | < 0.001 | |||
| Mexican American | 9.50 | 10.29 | 13.62 | |
| Non-Hispanic White | 64.04 | 62.44 | 57.98 | |
| Non-Hispanic Black | 12.22 | 7.88 | 8.46 | |
| Other Race | 14.24 | 19.39 | 19.94 | |
| Education level (%) | < 0.001 | |||
| Less than 9th grade | 2.70 | 4.02 | 4.64 | |
| 9–11th grade (Includes 12th grade with no diploma) 9.23 | 7.44 | 3.81 | ||
| High school graduate/GED or equivalent | 22.82 | 19.78 | 14.03 | |
| Some college or AA degree | 34.44 | 30.83 | 26.57 | |
| College graduate or above | 30.82 | 37.93 | 50.95 | |
| Marital status (%) | 0.250 | |||
| Married | 50.73 | 52.37 | 52.47 | |
| Widowed | 1.27 | 0.75 | 1.61 | |
| Divorced | 9.14 | 9.19 | 11.07 | |
| Separated | 2.29 | 3.24 | 1.02 | |
| Never married | 25.69 | 24.02 | 25.92 | |
| Living with partner | 10.88 | 10.44 | 7.91 | |
| Smoking status (%) | 0.110 | |||
| Never | 58.74 | 59.11 | 70.42 | |
| Former | 19.51 | 20.04 | 18.01 | |
| Now | 21.75 | 20.86 | 11.57 | |
| PIR (%) | 0.010 | |||
| Low-income | 15.83 | 15.18 | 12.47 | |
| Middle-income | 49.58 | 45.46 | 45.27 | |
| High-income | 34.59 | 39.36 | 42.26 | |
| Hypertension (%) | 0.040 | |||
| Yes | 27.28 | 24.18 | 27.82 | |
| No | 72.72 | 75.82 | 72.18 | |
| Diabetes (%) | 0.070 | |||
| Yes | 9.00 | 7.67 | 6.63 | |
| No | 91.00 | 92.33 | 93.37 | |
| Alcohol (%) | 0.01 | |||
| Former | 8.10 | 8.03 | 9.12 | |
| Heavy | 27.59 | 26.98 | 19.59 | |
| Mild | 25.28 | 33.14 | 35.02 | |
| Moderate | 20.83 | 20.72 | 19.33 | |
| Never | 8.20 | 11.13 | 16.94 | |
Significant values are in [bold].
Notes: All values are presented as proportion (%), or mean(standard error).
PIR, ratio of family income to poverty; BMD, bone mineral density.
The association between DASH and BMD
Table 3 presents the results of a linear regression analysis examining the correlation between DASH and BMD. The fully adjusted models revealed significant negative associations between DASH and total BMD (β = − 0.003, 95% CI: − 0.005, − 0.001), pelvic BMD (β = − 0.005, 95% CI: − 0.007, − 0.002), and thoracic spine BMD (β = − 0.003, 95% CI: − 0.005, − 0.001). Taking the effect size of DASH on total BMD (β = − 0.003) as an example, it suggested that for every one-point increase in DASH score, there is a corresponding decrease of 0.003 g/cm2 in total BMD. However, the relationship between the DASH diet and lumbar spine BMD did not achieve statistical significance (β = − 0.002, 95% CI: − 0.004, 0.001). We also performed a sensitivity analysis by transforming DASH from a continuous to a categorical variable (tertiles), and the outcomes remained consistent.
Table 3.
Multivariate linear regression analysis of the association between the DASH diet and bone mineral density.
| Total bone mineral density (g/cm2) | Model 1a | Model 2b | Model 3c |
|---|---|---|---|
| Dietary approaches to stop hypertension | -0.006 (− 0.007, − 0.004) < 0.001 | − 0.002 (− 0.004, − 0.000) < 0.001 | − 0.003 (− 0.005, − 0.001) 0.004 |
| Q1 | Reference | Reference | Reference |
| Q2 | − 0.011 (− 0.018, − 0.005) < 0.001 | − 0.003 (− 0.009, 0.003) 0.320 | − 0.004 (− 0.011, 0.002) 0.170 |
| Q3 | − 0.020 (− 0.037, − 0.003) 0.020 | − 0.002 (− 0.020,0.015) 0.770 | − 0.009 (− 0.024, 0.007) 0.270 |
| Lumbar spine-BMD (g/cm2) | Model 1a | Model 2b | Model 3c |
|---|---|---|---|
| Dietary approaches to stop hypertension | − 0.004 (-0.006, -0.002) 0.002 | -0.002 (-0.004, 0.000) 0.100 | -0.002 (-0.004, 0.001) 0.136 |
| Q1 | Reference | Reference | Reference |
| Q2 | − 0.008 (− 0.017, 0.000) 0.046 | − 0.003 (− 0.012, 0.005) 0.424 | − 0.003 (− 0.012, 0.006) 0.471 |
| Q3 | − 0.006 (− 0.032, 0.020) 0.646 | 0.002 (− 0.023, 0.027) 0.851 | 0.003 (− 0.022, 0.027) 0.831 |
| Thoracic spine-BMD (g/cm2) | Model 1a | Model 2b | Model 3c |
|---|---|---|---|
| Dietary approaches to stop hypertension | − 0.007 (− 0.009, − 0.005) < 0.001 | − 0.005 (− 0.007, − 0.003) < 0.001 | − 0.003 (− 0.005, − 0.001) 0.003 |
| Q1 | Reference | Reference | Reference |
| Q2 | − 0.015 (− 0.021, − 0.009) < 0.001 | − 0.010 (− 0.016, − 0.004) 0.001 | − 0.008 (− 0.014, − 0.001) 0.018 |
| Q3 | − 0.022 (− 0.042, − 0.003) 0.027 | − 0.015 (− 0.034, 0.004) 0.123 | − 0.014 (− 0.031, 0.003) 0.100 |
| Pelvic-BMD (g/cm2) | Model 1a | Model 2b | Model 3c |
|---|---|---|---|
| Dietary approaches to stop hypertension | − 0.010 (− 0.013, − 0.008) < 0.001 | − 0.007 (− 0.009, − 0.004) < 0.001 | − 0.005 (− 0.007, − 0.002) < 0.001 |
| Q1 | Reference | Reference | Reference |
| Q2 | − 0.018 (− 0.028, 0.008) < 0.001 | − 0.008 (− 0.018, 0.001) 0.090 | − 0.004 (− 0.014, 0.006) 0.426 |
| Q3 | − 0.048 (− 0.041, − 0.001) 0.037 | − 0.029 (− 0.050, − 0.007) 0.011 | − 0.021 (− 0.040, − 0.001) 0.037 |
Significant values are in [bold].
Note: BMD values are presented as raw variables without any transformation.
Model 1a: no covariates were adjusted; Model 2b: adjusted for sex, age, and race; Model 3c: adjusted for age, race, sex, education, ratio of family income to poverty, marital status, body mass index, alcohol intake, smoking status, diabetes, and hypertension.
BMD, bone mineral density; 95% CI, 95% confidence interval.
Subgroup analysis
To conduct a more comprehensive investigation, we performed subgroup analyses and interaction tests to examine the potential impact of a population stratification variable on the observed relationship between DASH and BMD (as shown in Supplementary Material 3). The negative correlations between DASH and BMD remained consistent across specific subgroups. An interaction effect with age was noted in total BMD (p for interaction = 0.04), manifesting a more pronounced negative relationship between DASH and BMD in the older age groups. Among the stratification variables considered, including BMI, gender, diabetes, hypertension, and alcohol, the interaction test was not found to be significant (p for interaction > 0.05, refer to Supplementary Material 3 for specific data). This suggests that the association between DASH and BMD is not influenced by the stratification mentioned above variables.
Sensitivity analysis
The sensitivity analysis transformed BMD into a Z-score format to facilitate a more comprehensive exploration of its potential linear relationship with DASH diet (Table 4). In the full adjusted model, the β for BMD across the tertiles of DASH was were as follows: total BMD (β = − 0.026, 95% CI: − 0.044, − 0.008), pelvic BMD (β = − 0.028, 95% CI: − 0.043, − 0.013), thoracic spine BMD (β = − 0.038, 95% CI: − 0.057, − 0.020), and lumbar spine BMD (β = − 0.012, 95% CI: − 0.029, 0.004). Overall, the results demonstrated the robustness of the observed correlation between DASH and BMD.
Table 4.
DASH diet and bone mineral density correlationa Z-score linear regression analysis.
| Total bone mineral density (g/cm2) | Model 1a | Model 2b | Model 3c |
|---|---|---|---|
| Dietary approaches to stop hypertension | − 0.05 (− 0.068, − 0.035) < 0.001 | − 0.018 (− 0.033, − 0.003) 0.020 | − 0.026 (− 0.044, − 0.008) 0.005 |
| Q1 | Reference | Reference | Reference |
| Q2 | − 0.103 (− 0.162, − 0.045) < 0.001 | − 0.028 (− 0.083, 0.028) 0.320 | − 0.04 (− 0.090, 0.020) 0.100 |
| Q3 | − 0.039 (− 0.200, 0.120) 0.640 | − 0.023 (− 0.181, 0.136) 0.770 | − 0.07 (− 0.221, 0.067) 0.120 |
| Lumbar spine-BMD (g/cm2) | Model 1a | Model 2b | Model 3c |
|---|---|---|---|
| Dietary approaches to stop hypertension | − 0.025 (− 0.040, − 0.010) 0.002 | − 0.013 (− 0.029, − 0.003) 0.106 | − 0.012 (− 0.029, 0.004) 0.136 |
| Q1 | Reference | Reference | Reference |
| Q2 | − 0.050 (− 0.110, − 0.001) 0.046 | − 0.023 (− 0.079, 0.032) 0.408 | − 0.020 (− 0.078, 0.037) 0.478 |
| Q3 | − 0.039 (− 0.200, 0.120) 0.640 | 0.017 (− 0.140, 0.180) 0.838 | 0.020 (− 0.142, 0.183) 0.796 |
| Thoracic spine-BMD (g/cm2) | Model 1a | Model 2b | Model 3c |
|---|---|---|---|
| Dietary approaches to stop hypertension | − 0.060 (− 0.078, − 0.044) < 0.001 | − 0.044 (− 0.060, − 0.027) < 0.001 | − 0.038 (− 0.057, − 0.020) < 0.001 |
| Q1 | Reference | Reference | Reference |
| Q2 | − 0.130 (− 0.180, − 0.070) < 0.001 | − 0.090 (− 0.143, − 0.040) 0.001 | − 0.04 (− 0.105, 0.010) 0.110 |
| Q3 | − 0.200 (− 0.360, − 0.020) 0.027 | − 0.132 (− 0.300, 0.030) 0.124 | − 0.06 (− 0.200, 0.080) 0.407 |
| Pelvic-BMD (g/cm2) | Model 1a | Model 2b | Model 3c |
|---|---|---|---|
| Dietary approaches to stop hypertension | − 0.060 (− 0.079, − 0.048) < 0.001 | − 0.040 (− 0.055, − 0.025) < 0.001 | − 0.028 (− 0.043, − 0.013) < 0.001 |
| Q1 | Reference | Reference | Reference |
| Q2 | − 0.110 (− 0.160, 0.040) < 0.001 | − 0.050 (− 0.110, 0.010) 0.090 | − 0.024 (− 0.080, 0.030) 0.420 |
| Q3 | − 0.300 (− 0.420, − 0.160) < 0.001 | − 0.170 (− 0.310, 0.040) 0.010 | − 0.130 (− 0.290, − 0.010) 0.030 |
Significant values are in [bold].
Model 1b: no covariates were adjusted; Model 2c: adjusted for sex, age, and race; Model 3d: adjusted for age, race, sex, education, ratio of family income to poverty, marital status, body mass index, alcohol intake, smoking status, diabetes, and hypertension.
BMD, bone mineral density; 95% CI, 95% confidence interval.
Discussion
This cross-sectional study encompassed a sample size of 8,486 individuals and aimed to investigate the correlation between the DASH diet and BMD in adults in the United States. The primary objective of this research was to ascertain whether the DASH diet was linked to BMD levels. The findings of this study suggest a negative association between the DASH diet and various aspects of BMD, including total BMD, thoracic BMD, and pelvic BMD. Sensitivity analyses were performed to confirm the findings' robustness. Furthermore, subgroup analyses and interaction tests were conducted, demonstrating that the observed correlation remained unaffected. A previous study reported that changes of about 0.050 g/cm2 (equivalent to 4–7% change, depending on the baseline BMD value) are likely to be associated with clinically significant BMD changes37. In the context of our findings, the impact of the DASH diet may not be substantial for individuals with healthy BMD. However, for those with borderline BMD, a significant adherence to the DASH diet could potentially push them into the low BMD category.
Previous studies have also suggested a possible negative correlation between the DASH diet and BMD38. First, the DASH diet is calcium-rich, but consuming too much calcium can also lead to soft tissue calcification and loss of bone mineral39. An experimental study by Doyle and Cashman found that continually feeding a DASH-type diet to rats inhibited bone formation and bone resorption, decreasing BMD40. Secondly, a reduced intake of lipids is one of the characteristics of the DASH diet, which leads to lower levels of total cholesterol (TC) and TG in the body41. Several studies have shown a positive correlation between TC and TG levels and BMD42–45. A cross-sectional study in China showed a positive association between LDL-C and BMD in women46. Also, a study from Spain found that BMD was positively associated with total cholesterol and LDL-C47. Insights from Hassoon's trial shed light on the connection between DASH, blood osteotriol concentrations, and BMD. Their findings suggest that the impact of the DASH diet on blood osteotriol concentrations could be attributed to the lower fat content, particularly saturated fat48. This insight underscores the importance of prudently managing saturated fat intake, particularly for individuals with very high DASH scores. Notably, in the DASH diet, it has been suggested that inorganic nitrate-rich foods can generate nitric oxide (NO) through a non-enzymatic process49. The effects of NO on osteoblast (OB) and osteoclast (OC) activity in vivo may vary. Specifically, the induction of pro-inflammatory cytokines, such as tumor necrosis factor-α, interleukin-1β, and interferon-gamma, can promote bone resorption by activating NOS, decreasing BMD50.
The precise mechanism underlying the association between the DASH diet and BMD remains uncertain; however, specific evidence suggests a potential negative correlation between the two. The DASH diet has been shown to have a fat-reducing effect51. Numerous studies have confirmed the impact of fat on BMD. Specifically, obesity, often accompanied by elevated fat levels, exerts a weight-bearing effect on the skeleton, potentially leading to increased BMD due to this mechanical stimulus52. Additionally, it is essential to note that fat is the primary source of aromatase, an enzyme responsible for the synthesis of estrogen53. Numerous studies have consistently demonstrated a positive association between estrogen and BMD54, indicating that a decrease in fat content will likely result in a decline in BMD.
Consequently, this comprehensive analysis supports the conclusion that implementing the DASH diet is also likely to reduce BMD through its impact on fat reduction. Previous research has indicated that the DASH diet is associated with insufficient fiber consumption23. Adequate fiber intake is crucial for maintaining BMD55. Investigative studies have demonstrated that fiber intake is protective in preserving BMD21 and mitigating bone loss, among other beneficial effects56. This phenomenon may be attributed to the alteration of gastrointestinal microorganisms by fiber intake57, which in turn influences BMD through the production of short-chain fatty acids (SCFA)58. Increased fiber consumption has been found to elevate SCFA levels, thereby promoting calcium absorption59. Insufficient dietary fiber consumption resulting from adherence to the DASH diet may contribute to a reduction in BMD via alterations in gastrointestinal microbiota. The DASH diet has resulted in insufficient magnesium intake23. Likewise, magnesium intake plays a significant role in maintaining BMD60. Inadequate magnesium intake has been linked to a decline in systemic BMD61. Animal experiments have demonstrated that animals with magnesium deficiency exhibit delayed development and reduced bone mineral content62,63. In both animals and humans, magnesium deficiency leads to reduced secretion of parathyroid hormone64 and decreased levels of serum 1,25(OH)2-vitamin D65. Insufficiency of these two hormones contributes to impaired bone formation66.
Our study's subgroup analysis and interaction test revealed a significant interaction between total BMD in relation to age. Specifically, individuals aged 50 and above exhibited a heightened vulnerability to decreased BMD when adhering to the same DASH diet. One potential factor that may impact BMD is the alteration of nutritional requirements as individuals age. This can be attributed to several reasons. Firstly, individuals over 50 tend to experience a diminished capacity to absorb calcium and vitamin D compared to their younger counterparts67. It is worth noting that both calcium and vitamin D have been found to influence BMD positively68.
Furthermore, empirical research has demonstrated that supplementing calcium and vitamin D among older adults fails to alter the prevailing pattern of age-related decline in BMD69. Consequently, there appears to be a discernible decline in BMD with age among individuals adhering to the DASH diet. Notably, protein consumption is a component of the DASH diet15; however, it is observed that digestion tends to be less efficient in older individuals70, and the overall protein intake tends to decline with age. A research investigation on sarcopenia elucidated a gradual reduction in muscle mass as individuals age71, which was found to have a positive correlation with protein consumption72. Additionally, another study demonstrated that muscle mass positively influences the maintenance and enhancement of BMD73. Therefore, as the need for protein increases with age, adhering to a long-term DASH diet may contribute to muscle loss, thereby impacting BMD. Hormonal factors were identified as significant contributors in the conducted investigations, wherein it was observed that both testosterone and estrogen levels decrease as individuals age, regardless of gender74,75. The insufficiency of testosterone and estrogen is the primary catalyst for reducing BMD among men and women74,76. Notably, the DASH diet does not prioritize hormone intake, exacerbating the decline in BMD as individuals age. The DASH diet, which emphasizes the daily consumption of dairy products for their calcium and protein content, may not be suitable for older people15. With advancing age, there is a decline in lactase production in the body, resulting in diminished digestion and absorption of lactose among older adults. This leads to lactose intolerance and necessitates reducing dairy intake77.
Consequently, as individuals age, the calcium and protein derived from dairy products, essential for maintaining BMD, become less accessible within the DASH population, potentially exacerbating the loss of BMD with increasing age. The reasons mentioned above may contribute to the significant age interaction. Nonetheless, in the case of other variables, including hypertension, diabetes mellitus, BMI, smoking, and gender, the interaction does not exhibit significance. This implies that the relationship between the DASH diet and BMD remains unaffected.
Our study has several noteworthy strengths. Firstly, it uses data from the esteemed NHANES, which has been meticulously weighted to accurately depict the association between the DASH diet and BMD in US adults. Additionally, we have incorporated strategies to address confounding covariates, selecting them based on prior research to ensure the reliability of our results. However, it is important to acknowledge the inherent limitations of our study. Firstly, it is crucial to acknowledge that the study was limited to a cross-sectional design, which unfortunately hinders our ability to definitively establish causality. Secondly, this constraint also restricts our ability to evaluate participants' adherence to the DASH diet and its long-term influence on BMD. Understanding the effects of prolonged adherence to the diet could provide valuable insights into the correlation between the DASH diet and BMD. Additionally, this study found a negative correlation between the DASH diet and BMD. However, determining the specific constituents of the DASH diet that contributed to this correlation proved to be a challenging task. This complexity highlights the need for further refinement in this area. Resolving this issue and achieving improved accuracy in the field of nutrition undoubtedly require additional efforts in future research. Finally, it is of great concern that this study relied on only two 24-h dietary data to make an assessment of DASH, and that a simple average intake over one or two days may not accurately capture usual intake. Future studies, particularly longitudinal cohort or intervention trials, are urgently needed to overcome this limitation.
Conclusions
This study demonstrates a significant negative correlation between the DASH diet and BMD in various skeletal regions, encompassing total BMD, thoracic spine, and pelvic BMD within the adult population of the United States. Notably, the relationship between the DASH diet and lumbar spine BMD was not found to be significant. Further research is imperative to substantiate these findings.
Supplementary Information
Author contributions
X.-L.Z., M.-Y.T., and Q.-C.S. contributed to the study conception and design. Material preparation, data collection, and analysis were performed by XX.-L.Z., M.-Y.T., Q.-C.S., G.-P.W., and S.-X.Z. The first draft of the manuscript was written by Xiang-Long Zhai, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data availability
The survey data are publicly available on the Internet for data users and researchers throughout the world (www.cdc.gov/nchs/nhanes/).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xiang-Long Zhai and Mo-Yao Tan.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-50423-7.
References
- 1.Whiting SJ, Vatanparast H, Baxter-Jones A, Faulkner RA, Mirwald R, Bailey DA. Factors that affect bone mineral accrual in the adolescent growth spurt. J. Nutr. 2004;134(3):696S–700S. doi: 10.1093/jn/134.3.696S. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd T, Eggli DF. Measurement of bone mineral content and bone density in healthy twelve-year-old white females. J. Nucl. Med. 1992;33:1143–1145. [PubMed] [Google Scholar]
- 3.National Center for Health Statistics. NHANES 1999–2006 DXA Multiple Imputation Data Files. Centers for Disease Control and Prevention (2023, accessed Nov 2023). https://wwwn.cdc.gov/Nchs/Nhanes/Dxa/Dxa.aspx.
- 4.Kanis JA, Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Synopsis of a WHO report. Osteoporos. Int. 1994;4:368–381. doi: 10.1007/BF01622200. [DOI] [PubMed] [Google Scholar]
- 5.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J. Bone Miner. Res. 2007;22:465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 6.Clynes MA, Westbury LD, Dennison EM, Kanis JA, Javaid MK, Harvey NC, et al. Bone densitometry worldwide: A global survey by the ISCD and IOF. Osteoporos. Int. 2020;31:1779–1786. doi: 10.1007/s00198-020-05435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang L, Zhang C, Liang H, Zhou N, Huang T, Zhao Z, et al. Polyunsaturated fatty acids level and bone mineral density: A two-sample mendelian randomization study. Front. Endocrinol. 2022;13:858851. doi: 10.3389/fendo.2022.858851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neufingerl N, Eilander A. Nutrient intake and status in adults consuming plant-based diets compared to meat-eaters: A systematic review. Nutrients. 2022;14:29. doi: 10.3390/nu14010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Veronese N, Reginster JY. The effects of calorie restriction, intermittent fasting and vegetarian diets on bone health. Aging Clin. Exp. Res. 2019;31:753–758. doi: 10.1007/s40520-019-01174-x. [DOI] [PubMed] [Google Scholar]
- 10.El-Shebini SM, Ahmed NH, Rasheed EA, Kamal AN. Dietary pattern and bone health in pre and post-menopausal obese women. Pak. J. Biolog. Sci. 2020;23:602–611. doi: 10.3923/pjbs.2020.602.611. [DOI] [PubMed] [Google Scholar]
- 11.Hsu E. Plant-based diets and bone health: Sorting through the evidence. Curr. Opin. Endocrinol. Diabetes Obes. 2020;27:248–252. doi: 10.1097/MED.0000000000000552. [DOI] [PubMed] [Google Scholar]
- 12.Noori M, Jayedi A, Khan T, Moradi S, Shab-Bidar S. Mediterranean dietary pattern and bone mineral density: A systematic review and dose-response meta-analysis of observational studies. Eur. J. Clin. Nutr. 2022;76:1657–1664. doi: 10.1038/s41430-022-01093-7. [DOI] [PubMed] [Google Scholar]
- 13.Shen D, Zhang X, Li Z, Bai H, Chen L. Effects of omega-3 fatty acids on bone turnover markers in postmenopausal women: Systematic review and meta-analysis. Climacteric. 2017;20:522–527. doi: 10.1080/13697137.2017.1384952. [DOI] [PubMed] [Google Scholar]
- 14.Zheng X, Lee SK, Chun OK. Soy isoflavones and osteoporotic bone loss: A review with an emphasis on modulation of bone remodeling. J. Med. Food. 2016;19:1–14. doi: 10.1089/jmf.2015.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sacks FM, Campos H. Dietary therapy in hypertension. N. Engl. J. Med. 2010;362(22):2102–2112. doi: 10.1056/NEJMct0911013. [DOI] [PubMed] [Google Scholar]
- 16.U.S. Department of Agriculture, U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025, (9th ed) Washington, DC, (2020).
- 17.Zhang Y, Francis EC, Xia T, Kemper K, Williams J, Chen L. Adherence to DASH dietary pattern is inversely associated with osteoarthritis in Americans. Int. J. Food Sci. Nutr. 2020;71(6):750–756. doi: 10.1080/09637486.2020.1722075. [DOI] [PubMed] [Google Scholar]
- 18.Wang JS, Liu WJ, Lee CL. Associations of adherence to the DASH diet and the mediterranean diet with all-cause mortality in subjects with various glucose regulation states. Front. Nutr. 2022;9:828792. doi: 10.3389/fnut.2022.828792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang O, Miller ER, 3rd, Gelber AC, Choi HK, Appel LJ, Juraschek SP. DASH diet and change in serum uric acid over time. Clin. Rheumatol. 2017;36(6):1413–1417. doi: 10.1007/s10067-017-3613-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y. Association between obesity and bone mineral density in middle-aged adults. J. Orthop. Surg. Res. 2022;17(1):268. doi: 10.1186/s13018-022-03161-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee T, Suh HS. Associations between dietary fiber intake and bone mineral density in adult Korean population: Analysis of national health and nutrition examination survey in 2011. J. Bone Metab. 2019;26(3):151–160. doi: 10.11005/jbm.2019.26.3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carpenter TO, DeLucia MC, Zhang JH, Bejnerowicz G, Tartamella L, Dziura J, et al. A randomized controlled study of effects of dietary magnesium oxide supplementation on bone mineral content in healthy girls. J. Clin. Endocrinol. Metab. 2006;91(12):4866–4872. doi: 10.1210/jc.2006-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mellen PB, Gao SK, Vitolins MZ, Goff DC., Jr Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988–1994 and 1999–2004. Arch. Intern. Med. 2008;168(3):308–314. doi: 10.1001/archinternmed.2007.119. [DOI] [PubMed] [Google Scholar]
- 24.Akhlaghi M. Dietary Approaches to Stop Hypertension (DASH): Potential mechanisms of action against risk factors of the metabolic syndrome. Nutr. Res. Rev. 2020;33(1):1–18. doi: 10.1017/S0954422419000155. [DOI] [PubMed] [Google Scholar]
- 25.Jeong IK, Cho SW, Kim SW, Choi HJ, Park KS, Kim SY, et al. Lipid profiles and bone mineral density in pre- and postmenopausal women in Korea. Calcif. Tissue Int. 2010;87(6):507–512. doi: 10.1007/s00223-010-9427-3. [DOI] [PubMed] [Google Scholar]
- 26.Wang P, Chen C, Song C, Jia J, Wang Y, Mu W. High cholesterol and low triglycerides are associated with total lumbar bone mineral density among adults aged 50 years and over: The NHANES 2017–2020. Front. Med. (Lausanne) 2022;9:923730. doi: 10.3389/fmed.2022.923730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics. NCHS Research Ethics Review Board (ERB). Approval (2023, 7 Jun 2023). https://www.cdc.gov/nchs/nhanes/irba98.htm.
- 28.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/s0140-6736(07)61602-x. [DOI] [PubMed] [Google Scholar]
- 29.Li Q, Zhou J. Influence of dietary patterns and physical activity on bone mineral content and density, osteoporosis among children with stimulant use. Front. Pediat. 2022;10:976258. doi: 10.3389/fped.2022.976258/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fulay AP, Rifas-Shiman SL, Oken E, Perng W. Associations of the dietary approaches to stop hypertension (DASH) diet with pregnancy complications in Project Viva. Europ. J. Clin. Nutrit. 2018;72(10):1385–1395. doi: 10.1038/s41430-017-0068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li S, et al. The role of hypertension in bone mineral density among males older than 50 years and postmenopausal females: Evidence from the US National Health and Nutrition Examination Survey, 2005–2010. Front. Public Health. 2023;11:156. doi: 10.3389/fpubh.2023.1142155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rattan P, Penrice DD, Ahn JC, et al. Inverse association of telomere length with liver disease and mortality in the US population. Hepatol. Commun. 2022;6:399–410. doi: 10.1002/hep4.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serum selenium concentrations and risk of all-cause and heart disease mortality among individuals with type 2 diabetes—PubMed (2023, accessed 25 Oct 2023). https://pubmed.ncbi.nlm.nih.gov/34664061/. [DOI] [PubMed]
- 34.Chen L, Zhang J, Zhou N, et al. Association of different obesity patterns with hypertension in US male adults: A cross-sectional study. Sci. Rep. 2023;13:10551. doi: 10.1038/s41598-023-37302-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson CL, Dohrmann SM, Burt VL, Mohadjer LK. National health and nutrition examination survey: Sample design, 2011–2014. Vital Health Stat. 2014;2(162):1–33. [PubMed] [Google Scholar]
- 36.Chen TC, Clark J, Riddles MK, Mohadjer LK, Fakhouri THI. National health and nutrition examination survey, 2015–2018: Sample design and estimation procedures. Vital Health Stat. 2020;184:1–35. [PubMed] [Google Scholar]
- 37.Phillips PJ, Phillipov G. Bone mineral density—frequently asked questions. Austr. Family Phys. 2006;35:145. [PubMed] [Google Scholar]
- 38.Monjardino T, Lucas R, Ramos E, Barros H. Associations between apriori-defined dietary patterns and longitudinal changes in bone mineral density in adolescents. Public Health Nutr. 2014;17(1):195–205. doi: 10.1017/S1368980012004879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raggi P, James G, Burke SK, Bommer J, Chasan-Taber S, Holzer H, et al. Decrease in thoracic vertebral bone attenuation with calcium-based phosphate binders in hemodialysis. J. Bone Miner. Res. 2005;20(5):764–772. doi: 10.1359/JBMR.041221. [DOI] [PubMed] [Google Scholar]
- 40.Doyle L, Cashman KD. The effect of nutrient profiles of the dietary approaches to stop hypertension (DASH) diets on blood pressure and bone metabolism and composition in normotensive and hypertensive rats. Br. J. Nutr. 2003;89:713–724. doi: 10.1359/JBMR.041221. [DOI] [PubMed] [Google Scholar]
- 41.Feng J, Huang Y, Wang H, Wang C, Xu H, Ke P, et al. Association between adherence to the Dietary Approaches to Stop Hypertension diet and serum uric acid. Sci. Rep. 2023;13:6347. doi: 10.1038/s41598-023-31762-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Makovey J, Chen JS, Hayward C, Williams FM, Sambrook PN. Association between serum cholesterol and bone mineral density. Bone. 2009;44(2):208–213. doi: 10.1016/j.bone.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 43.Kan B, Zhao Q, Wang L, Xue S, Cai H, Yang S. Association between lipid biomarkers and osteoporosis: A cross-sectional study. BMC Musculoskel. Dis. 2021;22:1–8. doi: 10.1186/s12891-021-04643-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buizert PJ, van Schoor NM, Lips P, Deeg DJ, Eekhoff EM. Lipid levels: A link between cardiovascular disease and osteoporosis? J. Bone Miner. Res. 2009;24(6):1103–1109. doi: 10.1359/jbmr.081262. [DOI] [PubMed] [Google Scholar]
- 45.Garg MK, Marwaha RK, Tandon N, et al. Relationship of lipid parameters with bone mineral density in Indian population. Indian J. Endocrinol. Metabol. 2014;18(3):325. doi: 10.4103/2230-8210.131165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Q, Zhou J, Wang Q, Lu C, Xu Y, Cao H, et al. Association between bone mineral density and lipid profile in Chinese women. Clin. Interv. Aging. 2020;15:1649–1664. doi: 10.2147/CIA.S266722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martín-González C, González-Reimers E, Quintero-Platt G. Lipid profile and bone mineral density in heavy alcoholics. Clin. Nutr. 2018;37:2137–2143. doi: 10.1016/j.clnu.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 48.Hassoon A, Michos ED, Miller ER, Crisp Z, Appel LJ. Effects of different dietary interventions on calcitriol, parathyroid hormone, calcium, and phosphorus: Results from the DASH trial. Nutrients. 2018;10:367. doi: 10.3390/nu10030367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chiavaroli L, Viguiliouk E, Nishi SK, Blanco Mejia S, Rahelić D, Kahleová H, et al. DASH dietary pattern and cardiometabolic outcomes: An umbrella review of systematic reviews and meta-analyses. Nutrients. 2019;11(2):338. doi: 10.3390/nu11020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li A, Xiao J, Xue Y. Osteoporosis and nitric oxide. Chin. J. Pathophysiol. 2001;17(2):174–179. [Google Scholar]
- 51.Chiu S, Bergeron N, Williams PT, Bray GA, Sutherland B, Krauss RM. Comparison of the DASH (Dietary Approaches to Stop Hypertension) diet and a higher-fat DASH diet on blood pressure and lipids and lipoproteins: A randomized controlled trial. Am. J. Clin. Nutr. 2016;103(2):341–347. doi: 10.3945/ajcn.115.123281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Etherington J, Harris PA, Nandra D, Hart DJ, Wolman RL, Doyle DV, et al. The effect of weight-bearing exercise on bone mineral density: A study of female ex-elite athletes and the general population. J. Bone Miner. Res. 1996;11:1333–1338. doi: 10.1002/jbmr.5650110918. [DOI] [PubMed] [Google Scholar]
- 53.Leeners B, Geary N, Tobler P, Asarian L. Ovarian hormones and obesity. Hum. Reprod. Update. 2017;23:300–321. doi: 10.1093/humupd/dmw045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Riggs BL. The mechanisms of estrogen regulation of bone resorption. J. Clin. Invest. 2000;106(10):1203–1204. doi: 10.1172/JCI11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang Y, Liu J, Zhang X, Geng B. Dietary fiber intake and femoral bone mineral density in middle-aged and older us adults: A cross-sectional study of national health and nutrition examination survey 2013–2014. Front. Nutr. 2022;9:851820. doi: 10.3389/fnut.2022.851820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dai Z, Zhang Y, Lu N, Felson DT, Kiel DP, Sahni S. Association between dietary fiber intake and bone loss in the framingham offspring study. J. Bone Miner. Res. 2018;33(2):241–249. doi: 10.1002/jbmr.3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Makki K, Deehan EC, Walter J, Bäckhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. 2018;23(6):705–715. doi: 10.1016/j.chom.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 58.So D, Whelan K, Rossi M, Morrison M, Holtmann G, Kelly JT, et al. Dietary fiber intervention on gut microbiota composition in healthy adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2018;107(6):965–983. doi: 10.1093/ajcn/nqy041. [DOI] [PubMed] [Google Scholar]
- 59.Zhou T, Sun D, Li X, Heianza Y, LeBoff MS, Bray GA, et al. Genetically determined SCFA concentration modifies the association of dietary fiber intake with changes in bone mineral density during weight loss: The Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) trial. Am. J. Clin. Nutr. 2021;114(1):42–48. doi: 10.1093/ajcn/nqab037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Farsinejad-Marj M, Saneei P, Esmaillzadeh A. Dietary magnesium intake, bone mineral density and risk of fracture: A systematic review and meta-analysis. Osteoporos. Int. 2016;27(4):1389–1399. doi: 10.1007/s00198-015-3400-y. [DOI] [PubMed] [Google Scholar]
- 61.Orchard TS, Larson JC, Alghothani N, Bout-Tabaku S, Cauley JA, Chen Z, et al. Magnesium intake, bone mineral density, and fractures: Results from the Women’s Health Initiative Observational Study. Am. J. Clin. Nutr. 2014;99(4):926–933. doi: 10.3945/ajcn.113.067488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kenney MA, McCoy H, Williams L. Effects of magnesium deficiency on strength, mass, and composition of rat femur. Calcif. Tissue Int. 1994;54(1):44–49. doi: 10.1007/BF00316289. [DOI] [PubMed] [Google Scholar]
- 63.Rude RK, Gruber HE, Norton HJ, Wei LY, Frausto A, Kilburn J. Reduction of dietary magnesium by only 50% in the rat disrupts bone and mineral metabolism. Osteoporos. Int. 2006;17(7):1022–1032. doi: 10.1007/s00198-006-0104-3. [DOI] [PubMed] [Google Scholar]
- 64.Rude RK, Oldham SB, Sharp CF, Singer FR. Parathyroid hormone secretion in magnesium deficiency. J. Clin. Endocrinol. Metab. 1978;47(4):800–806. doi: 10.1210/jcem-47-4-800. [DOI] [PubMed] [Google Scholar]
- 65.Fatemi S, Ryzen E, Flores J, Endres DB, Rude RK. Effect of experimental human magnesium depletion on parathyroid hormone secretion and 1,25- dihydroxyvitamin D metabolism. J. Clin. Endocrinol. Metab. 1991;73(5):1067–1072. doi: 10.1210/jcem-73-5-1067. [DOI] [PubMed] [Google Scholar]
- 66.Rude RK, Gruber HE, Norton HJ, Wei LY, Frausto A, Kilburn J. Dietary magnesium reduction to 25% of nutrient requirement disrupts bone and mineral metabolism in the rat. Bone. 2005;2:211–219. doi: 10.1016/j.bone.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 67.Felicetta JV. Age-related changes in calcium metabolism. Why they occur and what can be done. Postgrad. Med. 1989;85(4):85–94. doi: 10.1080/00325481.1989.11700616. [DOI] [PubMed] [Google Scholar]
- 68.Méndez-Sánchez L, Clark P, Winzenberg TM, Tugwell P, Correa-Burrows P, Costello R. Calcium and vitamin D for increasing bone mineral density in premenopausal women. Cochrane Database Syst. Rev. 2023;1(1):012664. doi: 10.1002/14651858.CD012664.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bronner F. Calcium and osteoporosis. Am. J. Clin0 Nutr. 1994;60(6):831–836. doi: 10.1093/ajcn/60.6.831. [DOI] [PubMed] [Google Scholar]
- 70.Rémond D, Shahar DR, Gille D, Pinto P, Kachal J, Peyron MA, et al. Understanding the gastrointestinal tract of the elderly to develop dietary solutions that prevent malnutrition. Oncotarget. 2015;6(16):13858–13898. doi: 10.18632/oncotarget.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Proctor DN, O’Brien PC, Atkinson EJ, Nair KS. Comparison of techniques to estimate total body skeletal muscle mass in people of different age groups. Am. J. Physiol. 1999;277(3):E489–E495. doi: 10.1152/ajpendo.1999.277.3.E489. [DOI] [PubMed] [Google Scholar]
- 72.Baumgartner RN, Koehler KM, Romero L, Garry PJ. Serum albumin is associated with skeletal muscle in elderly men and women. Am. J. Clin. Nutr. 1996;64(4):552–558. doi: 10.1093/ajcn/64.4.552. [DOI] [PubMed] [Google Scholar]
- 73.Capato LL, Cangussu-Oliveira LM, Porto JM, Jerônimo B, Ribeiro JDSG, Ferrioli E, et al. Contribution of hip abductors muscles on bone mineral density and functionality in older women. J. Clin. Densitom. 2023;26(1):97–103. doi: 10.1016/j.jocd.2022.12.007. [DOI] [PubMed] [Google Scholar]
- 74.Cauley JA. Estrogen and bone health in men and women. Steroids. 2015;99(Pt A):11–15. doi: 10.1016/j.steroids.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 75.Fink HA, Ewing SK, Ensrud KE, Barrett-Connor E, Taylor BC, Cauley JA, et al. Association of testosterone and estradiol deficiency with osteoporosis and rapid bone loss in older men. J. Clin. Endocrinol. Metab. 2006;91(10):3908–3915. doi: 10.1210/jc.2006-0173. [DOI] [PubMed] [Google Scholar]
- 76.Khosla S, Melton LJ, 3rd, Riggs BL. The unitary model for estrogen deficiency and the pathogenesis of osteoporosis: Is a revision needed? J. Bone Miner. Res. 2011;26(3):441–451. doi: 10.1002/jbmr.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wilt TJ, Shaukat A, Shamliyan T, Taylor BC, MacDonald R, Tacklind J, et al. Lactose intolerance and health. Evid. Rep. Technol. Assess. (Full Rep). 2010;192:1–410. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The survey data are publicly available on the Internet for data users and researchers throughout the world (www.cdc.gov/nchs/nhanes/).