Abstract
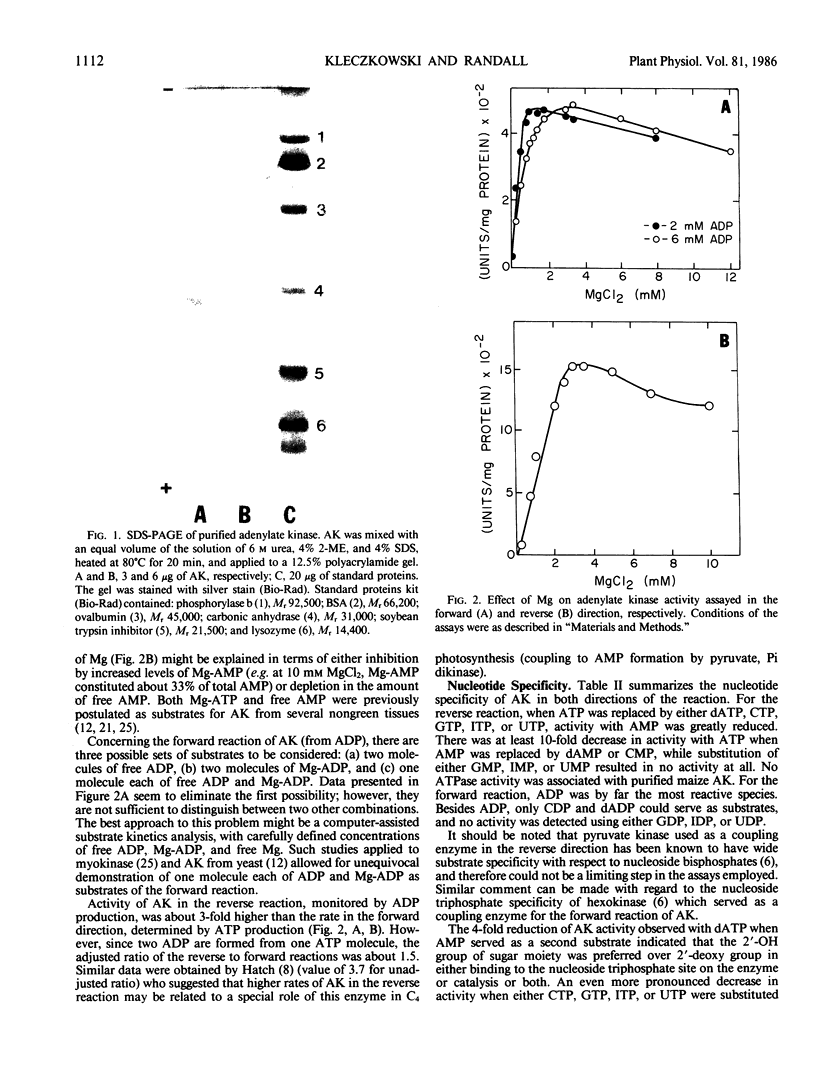
Adenylate kinase (EC 2.7.4.3) from leaves of maize (Zea mays) was purified to homogeneity using (NH4)2SO4 fractionation, followed by chromatography on DEAE-cellulose, hydroxyapatite, Sephadex G-75SF, and Green A dye-ligand columns. The purified enzyme had specific activity of about 1,550 micromoles ADP produced per minute per milligram protein, and the ratio of velocities of the reverse (utilization of ATP) to forward (formation of ATP) reaction was about 1.5. The Mr value of adenylate kinase, determined by electrophoresis in dissociating conditions and by gel filtration, was 29,000 and 31,000 respectively, suggesting monomeric nature of the enzyme. Purified preparations were stable for at least 1 month at 0 to 4°C. Magnesium ions were essential for activity of adenylate kinase in both directions of the reaction. Optimal rates in the forward direction were observed at the magnesium to ADP ratio of about 0.6 to 0.8. For the reverse reaction, ATP served as a substrate only when complexed with magnesium, while AMP reacted as a free species. The enzyme preferentially utilized adenine ribonucleotides in both directions of the reaction. The nucleoside triphosphate-binding site of adenylate kinase was fairly nonspecific with regard to nucleotide species. On the other hand, the primary amino group of either adenine and cytosine moieties was essential for effective binding to the nucleoside monophosphate site of the enzyme.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson D. E. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry. 1968 Nov;7(11):4030–4034. doi: 10.1021/bi00851a033. [DOI] [PubMed] [Google Scholar]
- Bomsel J. L., Pradet A. Study of adenosine 5'-mono-,di- and triphosphates in plant tissues. IV. Regulation of the level of nucleotides, in vivo, by adenylate kinase: theoretical and experimental study. Biochim Biophys Acta. 1968 Aug 20;162(2):230–242. doi: 10.1016/0005-2728(68)90105-9. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Hampp R., Goller M., Ziegler H. Adenylate Levels, Energy Charge, and Phosphorylation Potential during Dark-Light and Light-Dark Transition in Chloroplasts, Mitochondria, and Cytosol of Mesophyll Protoplasts from Avena sativa L. Plant Physiol. 1982 Feb;69(2):448–455. doi: 10.1104/pp.69.2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Tomasselli A. G., Noda L. H. ATP:AMP phosphotransferase from baker's yeast. Purification and properties. Eur J Biochem. 1980 Mar;105(1):85–92. doi: 10.1111/j.1432-1033.1980.tb04477.x. [DOI] [PubMed] [Google Scholar]
- Karu A. E., Moudrianakis E. N. Fractionation and comparative studies of enzymes in aqueous extracts of spinach chloroplasts. Arch Biochem Biophys. 1969 Feb;129(2):655–671. doi: 10.1016/0003-9861(69)90226-4. [DOI] [PubMed] [Google Scholar]
- Khoo J. C., Russell P. J., Jr Adenylate kinase from bakers' yeast. IV. Substrate and inhibitor structurll requirements. J Biol Chem. 1970 Aug 25;245(16):4163–4167. [PubMed] [Google Scholar]
- Kleczkowski L. A., Randall D. D. Light and thiol activation of maize leaf glycerate kinase : the stimulating effect of reduced thioredoxins and ATP. Plant Physiol. 1985 Sep;79(1):274–277. doi: 10.1104/pp.79.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mazelis M. Particulate Adenylic Kinase in Higher Plants. Plant Physiol. 1956 Jan;31(1):37–43. doi: 10.1104/pp.31.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami S., Strotmann H. Adenylate kinase bound to the envelope membranes of spinach chloroplasts. Arch Biochem Biophys. 1978 Jan 15;185(1):30–38. doi: 10.1016/0003-9861(78)90140-6. [DOI] [PubMed] [Google Scholar]
- O'Sullivan W. J., Smithers G. W. Stability constants for biologically important metal-ligand complexes. Methods Enzymol. 1979;63:294–336. doi: 10.1016/0076-6879(79)63014-8. [DOI] [PubMed] [Google Scholar]
- Purich D. L., Fromm H. J. Studies on factors influencing enzyme responses to adenylate energy charge. J Biol Chem. 1972 Jan 10;247(1):249–255. [PubMed] [Google Scholar]
- Rhoads D. G., Lowenstein J. M. Initial velocity and equilibrium kinetics of myokinase. J Biol Chem. 1968 Jul 25;243(14):3963–3972. [PubMed] [Google Scholar]
- Stitt M., Lilley R. M., Heldt H. W. Adenine nucleotide levels in the cytosol, chloroplasts, and mitochondria of wheat leaf protoplasts. Plant Physiol. 1982 Oct;70(4):971–977. doi: 10.1104/pp.70.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terai H. Adenylate kinase from Pseudomonas denitrificans. I. Purification and antiserum inhibition. J Biochem. 1974 May;75(5):1027–1036. doi: 10.1093/oxfordjournals.jbchem.a130474. [DOI] [PubMed] [Google Scholar]
- Tsuboi K. K., Chervenka C. H. Adenylate kinase of human erythrocyte. Isolation and properties of the predominant inherited form. J Biol Chem. 1975 Jan 10;250(1):132–140. [PubMed] [Google Scholar]