Abstract
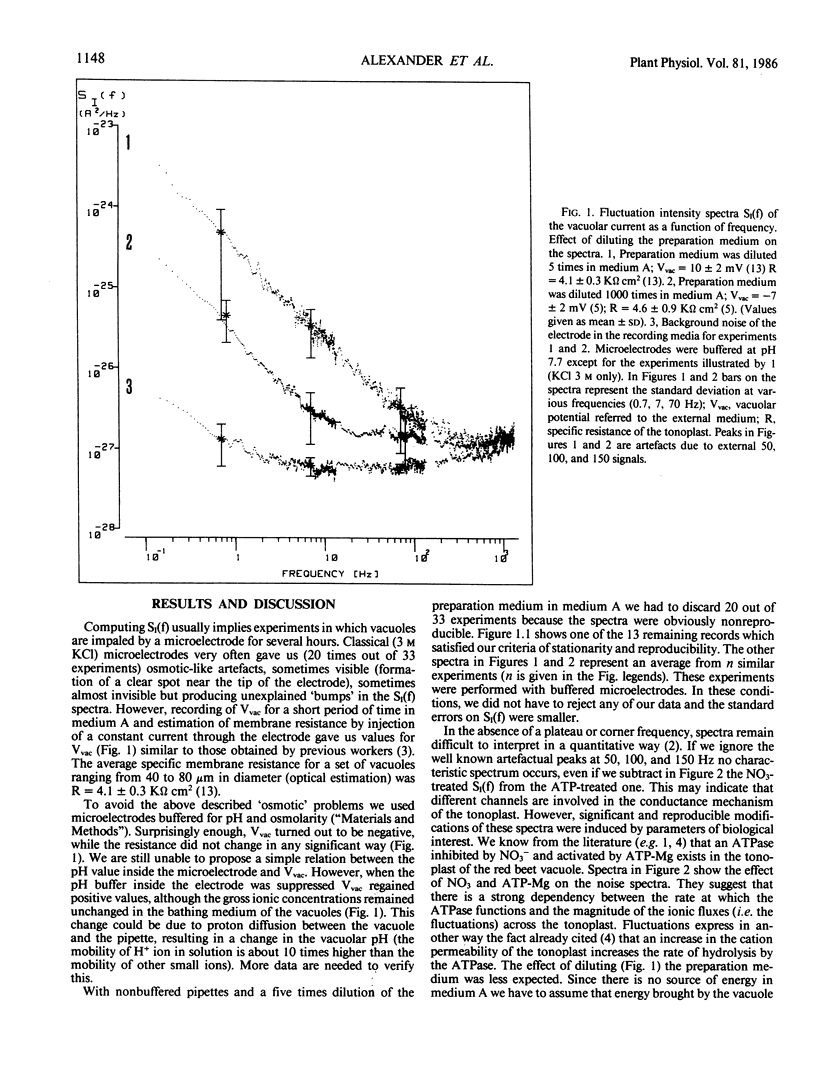
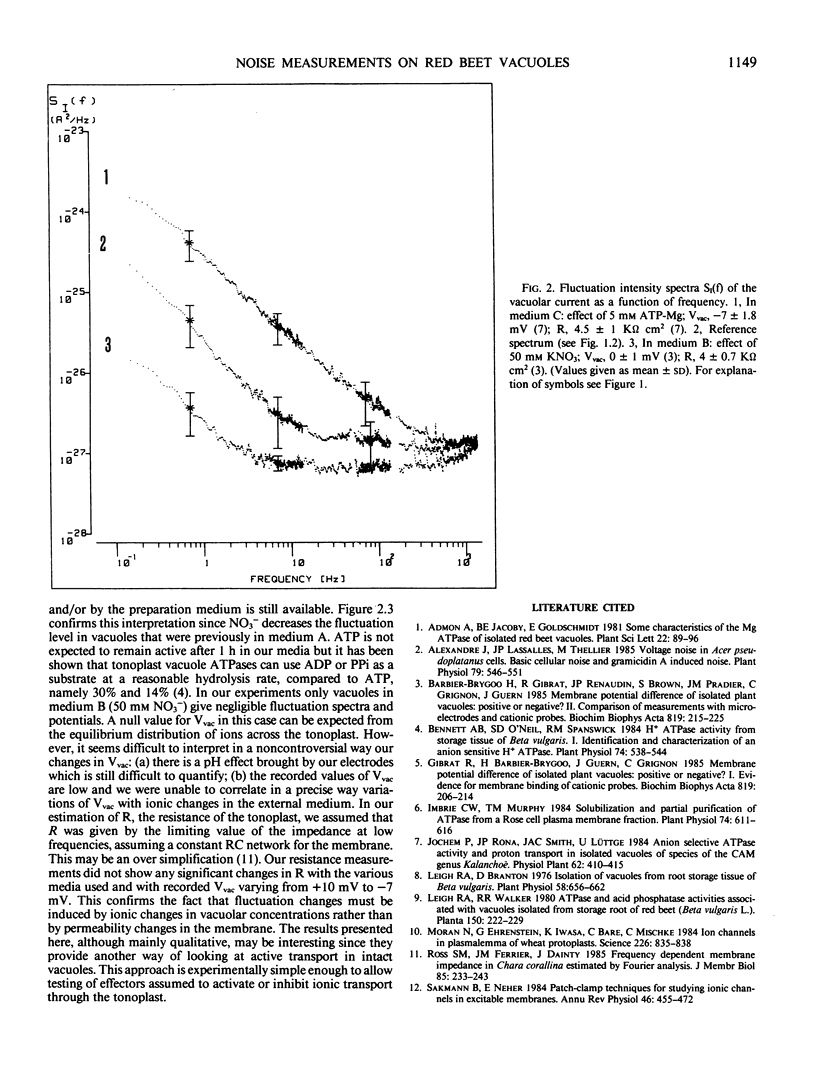
The vacuolar potential (Vvac) and its fluctuations were recorded in red beet vacuoles (Beta vulgaris L.). Measurements with vacuoles in their suspension medium gave Vvac = 10 ± 2 millivolts (referred to the external medium) when 3 molar KCl microelectrodes were used. Buffering the microelectrode filling solution at pH 7.7 reversed the sign of the potential: Vvac = −7 ± 2 millivolts. The magnitude of the potential fluctuations was lowered by dilution (5-1000 times) with the suspension medium containing components released by the cells during the mechanical preparation. Fluctuations were decreased by 50 millimolar KNO3 while they were enhanced by 5 millimolar ATP-Mg. No noticeable change in membrane resistance was detected. The presence of an ATPase bound to the tonoplast may explain the recorded noise spectra. These spectra imply a close connection between the rate of ATPase functioning and the magnitude of ionic fluxes across the tonoplast. It is suggested that noise analysis could be used to detect ATPase (or related enzyme) activity in vacuoles. Possible use of H+ diffusion through a buffered microelectrode, to modify intravacuolar pH, is also suggested.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandre J., Lassalles J. P., Thellier M. Voltage Noise in Acer pseudoplatanus Cells : Basic Cellular Noise and Gramicidin a Induced Noise. Plant Physiol. 1985 Oct;79(2):546–551. doi: 10.1104/pp.79.2.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett A. B., O'neill S. D., Spanswick R. M. H-ATPase Activity from Storage Tissue of Beta vulgaris: I. Identification and Characterization of an Anion-Sensitive H-ATPase. Plant Physiol. 1984 Mar;74(3):538–544. doi: 10.1104/pp.74.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbrie C. W., Murphy T. M. Solubilization and partial purification of ATPase from a rose cell plasma membrane fraction. Plant Physiol. 1984 Mar;74(3):611–616. doi: 10.1104/pp.74.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh R. A., Branton D. Isolation of Vacuoles from Root Storage Tissue of Beta vulgaris L. Plant Physiol. 1976 Nov;58(5):656–662. doi: 10.1104/pp.58.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran N., Ehrenstein G., Iwasa K., Bare C., Mischke C. Ion channels in plasmalemma of wheat protoplasts. Science. 1984 Nov 16;226(4676):835–838. doi: 10.1126/science.6093255. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Neher E. Patch clamp techniques for studying ionic channels in excitable membranes. Annu Rev Physiol. 1984;46:455–472. doi: 10.1146/annurev.ph.46.030184.002323. [DOI] [PubMed] [Google Scholar]
- Smith J. A., Uribe E. G., Ball E., Heuer S., Lüttge U. Characterization of the vacuolar ATPase activity of the crassulacean-acid-metabolism plant Kalanchoë daigremontiana. Receptor modulating. Eur J Biochem. 1984 Jun 1;141(2):415–420. doi: 10.1111/j.1432-1033.1984.tb08207.x. [DOI] [PubMed] [Google Scholar]
- Stevens C. F. Principles and applications of fluctuation analysis: a nonmathematical introduction. Fed Proc. 1975 Apr;34(5):1364–1369. [PubMed] [Google Scholar]


