Abstract
Background
In traditional medicine, Xanthium strumarium is used as an anti-inflammatory and anti-arthritic plant-based medicine. Human Dental Pulp Stem Cells (hDPSCs) are an ideal in vitro model for drug and bioactive compound screening. This study assessed the potential of X. strumarium aqueous extract on hDPSCs differentiation towards the osteogenic lineage.
Materials and methods
HDPSCs were isolated and cultured by explant method and characterized by surface marker expression, Colony Forming units fibroblasts (CFU-F), Population Doubling time (PDT), and tri-lineage differentiation. X. strumarium aqueous seed extract (XSE) was prepared and its cytotoxic effect on hDPSCs was examined by MTT assay. The effect of XSE on hDPSC differentiation into osteocytes was investigated by biochemical staining and gene expression.
Results
The hDPSCs were positive for CD73, CD90, and CD105 and negative for CD34, CD45, and HLA-DR surface markers. The cells had a colony-forming ability with a PDT of 44.91 h. The hDPSCs differentiated into osteocytes, chondrocytes, and adipocytes. The XSE concentration of 15 μg/ml had a significant increase in hDPSC viability. Alizarin Red S staining revealed that XSE treatment enhanced calcium accumulation and matrix mineralization in hDPSCs. XSE treatment also increased osteonectin and IL-6 transcript expression in osteogenesis-induced hDPSCs.
Conclusion
X. strumarium aqueous extract is a suitable candidate for bone repair because it promotes osteogenic differentiation in hDPSCs. Therefore this could be explored further in the treatment of bone disorders.
Keywords: Mesenchymal stem cell, Traditional medicine, Bone regeneration, Xanthium strum
1. Introduction
The medicinal plant Xanthium strumarium (Common Cocklebur) is a member of the Asteraceae or Compositae family [1,2]. In traditional medicine of India, the entire plant is used as a diaphoretic, diuretic, and sedative as well as for the treatment of malaria fever, leukoderma, diabetes, epilepsy, and cancer [1,[3], [4], [5], [6]]. As an ethnomedicine, it is often used to manage a variety of ailments, including rhinitis, sinusitis, headaches, ulcers, hives, rheumatism, and arthritis [2]. The anti-inflammatory, anti-nociceptive, and anti-oxidative activity of extracts from X. strumarium has been exploited for controlling arthritis [[7], [8], [9]]. However, for complete tissue renewal and regeneration, it is important to study whether cellular growth and extracellular matrix (ECM) production specific to bone and cartilage are promoted or not.
Considering the affordability, availability, efficacy, non-toxicity, novelty, and safety, researchers are exploring natural herbal sources that would promote the differentiation of human Mesenchymal Stem Cells (hMSCs) into specific lineages (such as osteogenic and chondrogenic) and thus aid in tissue regeneration [10,11]. Adult stem cell proliferation and differentiation that have aided in the regeneration of disorganized and damaged tissues have been proposed to be stimulated by several plant extracts and their phytochemicals [10]. DPSCs have MSC-like properties similar to bone marrow MSCs [12]. DPSCs are easily accessible, economical sources of MSCs and are thus a prospective stem cell source for tissue regeneration and engineering [13].
The human population greatly suffers from degenerative diseases such as osteoporosis and osteoarthritis which are one of the most common and widespread diseases of the bone [11,14]. In India, factors such as the increase in life expectancy and urbanization among many others have led to an increased prevalence of osteoporosis and osteoarthritis [15,16]. Management of these skeletal diseases includes lifestyle changes such as nutrition diet, weight loss, and exercise [15]. Pharmacological remedies for osteoporosis have been hormone replacement therapy, selective estrogen receptor modulators, calcitonin, etc. whereas for osteoarthritis non-steroidal anti-inflammatory drugs, joint replacement therapy, intra-articular dosing of hyaluronic acid or glucocorticoids, etc. have been used over the years [[17], [18], [19], [20]]. However, such therapies are mostly intended to cure symptomatic issues and therefore do not address the underlying pathology and structural healing of the damaged tissue [21]. Furthermore, therapy with the foregoing methods might result in major liver damage, gastrointestinal hemorrhage, hospitalization, and mortality [22]. The aforementioned side effects and post-therapy complications are why traditional medicine resources, such as X. strumarium, are being explored for bone regeneration.
While X. strumarium is shown to have anti-inflammatory effects in treating arthritis, there have been limited studies on its effect on the regeneration of bone or cartilage tissue to treat bone disorder, with a lack of research on its proliferative and differentiation effect on stem cells towards bone cells. Additionally, X. strumarium has not been explored for the treatment of bones, the tissue closely associated with cartilage. Thus, the present study aimed to understand the effect of X. strumarium on osteogenic differentiation of hDPSCs in vitro.
2. Materials and methods
2.1. Sample collection
The fruits of X. strumarium were collected from the Vidarbha region near Nagpur, Maharashtra in January 2021. The fruits were transported to the Regenerative Medicine Laboratory at Dr. D. Y. Patil Dental College & Hospital, Pune, Maharashtra, and stored at room temperature until further processing.
2.2. Preparation of X. strumarium seed extraction
X. strumarium was opened and the seed was removed from the outer sheath of each fruit (Fig. 4 A and B). The seeds were then made into a coarse powder form. The extraction was carried out by using the Soxhlet apparatus. Once all the material was extracted into the solvent, the liquid was placed in a water bath and evaporated till it dried and converted into a solid form (Fig. 4 C). The Soxhlet method was carried out in a Soxhlet apparatus with 15 g for 5 h of extraction with an aqueous phase. The extracts were dried at 38 °C in a vacuum. The total extract obtained was 10–12 gm which was dissolved in sterile distilled water at the concentration of 5 mg/ml. The mixture was filter sterilized using a 0.22-micron filter and 10 ml syringe. This aqueous seed extract was stored at 4 °C for further use [23].
Fig. 4.
(A) Xanthium strumarium fruit, (B) Seeds of Xanthium strumarium fruit, (C) Extraction of Xanthium strumarium seeds, and (D) Cytotoxic assessment of XSE towards hDPSCs proliferation by MTT assay.
2.3. Isolation and culturing of human Dental Pulp Stem Cells (hDPSCs)
The present study was approved by the institutional committee for stem cell research of Dr. D. Y. Dental College and Hospital, Pune, India. The third molar was collected from adult donors (n = 10) during dental procedures as biomedical waste such as pericoronitis or orthodontic correction of teeth after obtaining informed consent. The dental pulp was extracted in a sterile manner using an air-rotor handpiece (bur chuck type) (Fig. 1 A). The opened tooth containing the healthy pulp was placed in sterile 1X PBS and further processed under aseptic conditions in a biosafety cabinet. The tissue was cut into ∼1 mm sections with the help of a sterile surgical blade (Fig. 1 B). The sections were placed in a preconditioned culture flask with FBS (Fig. 1 C). The flask was incubated in the CO2 (5%) incubator with high humidity (95%) at 37 °C for 24 h. After 24 h, 3 ml complete media (DMEM basal media, 10% FBS, 1% Antibiotic-Antimycotic cocktail) was provided, and the flask was further incubated in the same conditions. The explant culture was observed regularly, under an inverted phase-contrast microscope (Olympus CKX53) to check for the outgrowth of hDPSCs at the periphery of the explant tissue. Media was changed every 2–3 days until the outgrowth of hDPSCs reached 80–90% confluence. On reaching confluence, without disturbing the explant, the adherent hDPSCs were trypsinized (0.25% Trypsin-EDTA solution at 37 °C for 30 s) and cultured in a T-75 flask (passage 1), provided with complete media that was replaced with fresh media every 2–3 days.
Fig. 1.
Isolation and culturing of human Dental Pulp Stem Cells (hDPSCs). (A) Extraction of pulp, (B) Dental pulp cut into small sections, (D) Explant culture, scale bar = 100 μm and, (E) Outgrowth of cells from explant culture, scale bar = 100 μm.
2.4. Characterization of hDPSCs by surface marker analysis using FACS
Confluent hDPSCs at passage 5 were analyzed using Fluorescence-Activated Cell Sorting (FACS). The cells were harvested into a suspension of 1X PBS with 1% FBS. Under dark aseptic conditions, PE (phycoerythrin) conjugated anti-human monoclonal antibodies for surface markers CD73, CD90, and CD105 along with FITC (fluorescein isothiocyanate) conjugated anti-human monoclonal antibodies for surface markers CD34, CD45 and HLA-DR were added to the cell suspension and incubated for 30 min at 4 °C. Unstained cells along with corresponding isotype controls were used as negative controls. After incubation, the samples were washed with 1X PBS and run on a flow cytometer (Attune NxT, Thermo Scientific). For each sample, 10,000 events were captured.
2.5. CFU-F analysis of hDPSCs
Colony forming Units-Fibroblast (CFU-F) assay was implemented to analyze the colony-forming ability of hDPSCs. In the presence of complete media, 3.25 × 102 cells at passage 5 were seeded in each well of a sterile 6-well plate. The plate was incubated in the CO2 (5%) incubator with high humidity (95%) at 37 °C for 12 days with media change every 2–3 days. Over the incubation period, cells were regularly observed under an inverted phase-contrast microscope. After incubation, the cells were washed with 1X PBS and stained with 0.3% Crystal Violet (incubated at room temperature for 20 min). After incubation, the excess stain was removed and gently washed with sterile distilled water. The colonies were observed under the inverted phase-contrast microscope.
2.6. Population doubling time of hDPSCs
The proliferation assay was performed by seeding 2 × 104 passage 5 cells onto each well of a sterile 24-well plate (day 0) and incubated in the CO2 (5%) incubator with high humidity (95%) at 37 °C for 9 days. Cells were harvested and cell count was taken using a hemocytometer. Without media change, the count was taken every day for 9 days. The average number of cells with the standard error of the mean was calculated for each day. A Cell Number (×104 cells/well) v/s Days graph was plotted, and the Population Doubling Time (PDT) was calculated using the exponential phase data, as per the following formula:
Where T is the total time the cells were incubated (in hours), FCC (final cell count) is the number of cells at the end of the exponential phase, and ICC (initial cell count) is the number of cells that were seeded.
2.7. Characterization of hDPSCs by tri-lineage differentiation
To assess the ability of hDPSCs towards tri-lineage differentiation such as osteogenic, chondrogenic, and adipogenic lineages, hDPSCs were induced by respective induction media. In osteogenic differentiation, hDPSCs were treated with osteogenic induction media containing DMEM with 1 μM dexamethasone, 10 mM β-glycerophosphate, and 2 mM ascorbate-2-phosphate. For chondrogenesis, the induction media contained DMEM with 1 μM dexamethasone, 2 mM ascorbate-2-phosphate, 10 μg/ml sodium pyruvate, 40 μg/ml L-proline, 1X ITS and 10 ng/ml TGF-β3. Adipogenic induction media contained DMEM with 1 μM dexamethasone, 0.5 mM 3-isobutyl-5-methylxanthine, 200 μM indomethacin, and 1.743 μM insulin. The cells were induced for 21 days with media change every 2–3 days. To confirm the differentiation of hDPSCs into osteocytes, chondrocytes, and adipocytes, the cells were stained with 0.1% Alizarin Red S, 0.1% Safranin O, and 0.3% Oil Red O respectively.
2.8. Cytotoxicity of X. strumarium seed extract on hDPSCs
MTT assay was used to check the cytotoxicity of X. strumarium seed extract on hDPSCs. hDPSCs were treated with the X. strumarium aqueous seed extract (XSE) at concentrations of 5 μg/ml, 10 μg/ml, 15 μg/ml, 20 μg/ml, 25 μg/ml, and 30 μg/ml. Treatment was given for 48 h, after which the media was replaced with 50 μl of MTT solution (5 mg/ml) (Puregene) and incubated under the same conditions for 3 h. After 3 h, the MTT solution was removed and 100 μl of Dimethyl sulfoxide was added to each well. The plate was placed in the microplate spectrophotometer (Thermo Scientific Multiskan FC) and the colorimetric absorbance was measured at 560 nm using the SkanIt Software 5.0 (Thermo Scientific).
2.8.1. Effect of X. strumarium seed extract on differentiation of hDPSCs
Osteogenic differentiation assays were performed using hDPSCs treated with XSE for 13 days and 21 days. In osteogenic differentiation, hDPSCs were treated with respective induction media containing 15 μg/ml XSE. For positive control, hDPSCs were treated with respective induction media in the absence of XSE. After 21 days of induction, osteogenesis was confirmed by staining the cells with 2% Alizarin Red S. The cells were then observed under the inverted phase-contrast microscope. Quantification was performed by colorimetric absorbance at 405 nm using the SkanIt Software 5.0 (Thermo Scientific).
2.9. Gene expression analysis
For RNA isolation, the TRIZOL method was adopted. High-capacity cDNA Reverse Transcription Kit (Applied Biosystems - Thermofisher) based first strand cDNA synthesis was performed as per the manufacturer's protocol. Real-time PCR (qPCR) was performed under the following conditions: hold stage at initial denaturation at 50 °C for 2 min, 95 °C for 10 min, 35 amplification cycles (denaturation at 95 °C for 15 s, annealing, and extension at 60 °C for 1 min) and melt curve analysis (60 °C–95 °C). Primers used were for housekeeping gene GAPDH (glyceraldehyde-3-phosphate dehydrogenase) and target genes Osteonectin and IL-6 (interleukin 6) (Table 1). Relative fold change was determined by the ΔΔCt comparative method.
Table 1.
Primers used for gene expression analysis.
| Gene | Forward primer (5′ to 3′) | Reverse primer (5′ to 3′) | Tm (°C) |
|---|---|---|---|
| GAPDH | TCCCTGAGCTGAACGGGAAG | GGAGGAGTGGGTGTCGCTGT | 60 |
| Osteonectin | GAGGAAACCGAAGAGGAGG | GGGGTGTTGTTCTCATCCAG | 59 |
| IL-6 | CCTGAGAAAGGAGACATGTAACAAGA | TGGAAGGTTCAGGTTGTTTTCTG | 60 |
| RUNX2 | TCTTCACAAATCCTCCCC | TGGATTAAAAGGACTTGG | 60 |
| ALP | AGCACTCCCACTTCATCTGGAA | GAGACCCAATAGGTAGTCCACATTG | 60 |
2.10. Statistical analysis
Statistical significance for the cytotoxicity assay was determined by one-way ANOVA and post-hoc test. For the biochemical stain quantification and gene expression analysis, an unpaired t-test was used. Data were represented as mean ± standard error of the mean.
3. Results
3.1. Isolation and culturing of human Dental Pulp Stem Cells (hDPSCs)
Over 7 days of incubation, an outgrowth of cells was seen around the explant. The cells had spindle-shaped fibroblastic-like morphology (Fig. 1 D). On reaching confluence, hDPSCs were isolated in a new sterile T-75 flask by trypsinization. The cells were adherent and proliferated in a monolayer with its characteristic fibroblastic morphology. By the 5th to 7th day of incubation, the cells had reached confluence (Fig. 1 E) and thus were trypsinized. The cells were subcultured in T-75 flasks till passage 5, which was then further used for the current study.
3.2. Characterization of hDPSCs by surface marker analysis using FACS
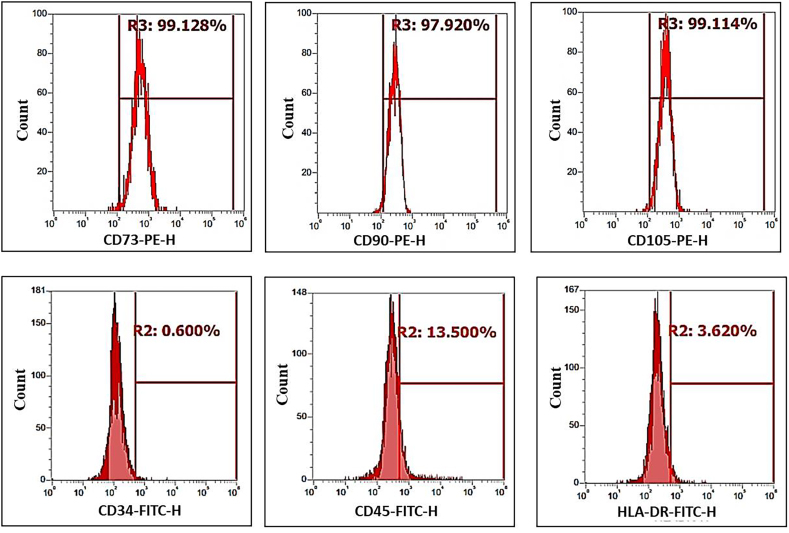
The results are depicted as the percent of cells that were stained positively in comparison to the unstained sample. The FACS histograms infer that the hDPSCs, which were isolated and cultured, expressed CD73 (99.128%), CD90 (97.920%), and CD105 (99.114%) and did not express CD34 (1.181%), CD45 (0.313%) and HLA-DR (0.531%) (Fig. 2). This indicated that the cells that were being cultured were a homogeneous population of mesenchymal-like stem cells.
Fig. 2.
FACS histograms of homogeneous population of hDPSCs positive for CD73, CD90 and CD105 surface markers and negative for CD34, CD45 and HLA-DR surface proteins.
3.3. CFU-F analysis of hDPSCs
The hDPSCs exhibited colony-forming unit-like fibroblast after 12 days of incubation. The colonies were stained using 0.3% Crystal Violet and observed under the inverted phase-contrast microscope. Colonies of hDPSCs were formed, with cells more compact and confluent towards the center and more spread out and less confluent towards the periphery since more growth space was available outwards from the colony. The staining of the cell nucleus and the cytosol highlights the multipolar spindle-shaped morphology of the cells (Fig. 3 A).
Fig. 3.
Characterization of hDPSCs by CFU-F, population doubling time and tri-lineage differentiation potential. (A) Colony forming unit like fibroblast, scale bar = 100 μm, (B) Growth curve analysis, (C) Osteogenic differentiation, scale bar = 100 μm, (D) Chondrogenic differentiation, scale bar = 100 μm and, (E) Adipogenic differentiation, scale bar = 10 μm.
3.4. Population doubling time of hDPSCs
The growth curve analysis of the hDPSCs was done to measure its Population Doubling Time (PDT). The Cell Number (×104 cells) v/s Days graph indicates the different phases of a growth curve that is log phase, lag phase, and stationary phase (Fig. 3 B). Cell number represented as mean (n = 2) ± standard error of the mean. It depicts how the number of cells increased over 6 days of incubation even without any change of media which is the log phase. PDT was 44.91 h for the hDPSCs that were cultured.
3.5. Characterization of hDPSCs by tri-lineage differentiation
After 21 days of induction with osteogenic, chondrogenic, and adipogenic induction media the cells were stained with 2% Alizarin Red S, 0.1% Safranin O, and 0.3% Oil Red O respectively, to visualize the tri-lineage differentiation. When induced with osteogenic media, hDPSCs differentiated into calcium-producing osteocytes which were stained red with 2% Alizarin Red S (Fig. 3 C). Induction with chondrogenic media promoted differentiation towards the chondrocytes that produced glycosaminoglycan containing ECM which was stained with 0.1% Safranin O (Fig. 3 D). Adipogenic media induced hDPSCs to differentiate into adipocytes which was demonstrated by 0.3% Oil Red O staining of the lipid droplet that had accumulated at the cell periphery (Fig. 3 E).
3.6. Cytotoxicity of X. strumarium seed extract on hDPSCs
To check the cytotoxicity of X. strumarium towards hDPSCs MTT assay was performed. The concentrations analyzed were 5 μg/ml, 10 μg/ml, 15 μg/ml, 20 μg/ml, 25 μg/ml and 30 μg/ml with complete media as the control. Absorbance was taken at 560 nm with data shown as mean (n = 3) ± standard error of mean. Statistical significance was determined by one-way ANOVA at p ≤ 0.05 (*, p < 0.05; **, p < 0.01). The concentrations of 15 μg/ml and 10 μg/ml had statistically significant absorbance compared to the control (Fig. 4 D). As per the absorbance, all concentrations had higher cell viability than the control cells, indicating the herbal extract promoted the proliferation of hDPSCs and was not cytotoxic.
3.7. Effect of X. strumarium seed extract on differentiation of hDPSCs
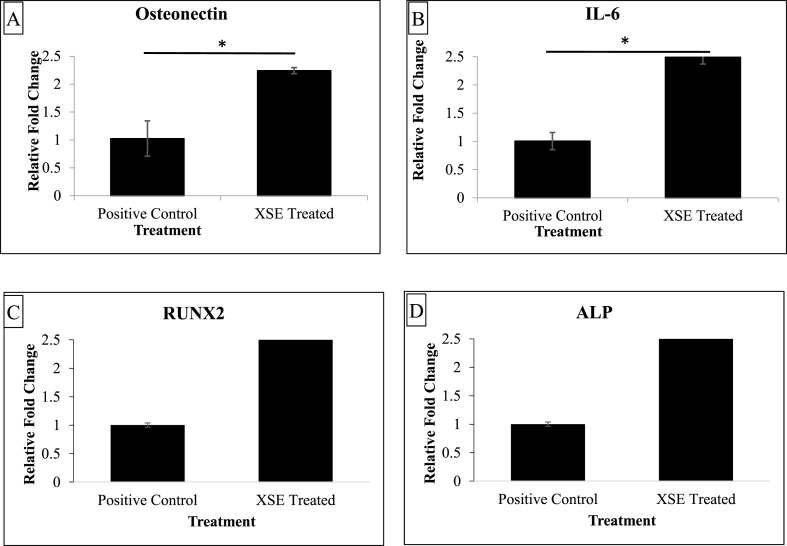
Biochemical staining after 21 days of XSE treatment showed that the induced-treated osteogenesis XSE test hDPSCs (Fig. 5 B) differentiated into the osteogenic lineage and were comparable to the respective induced-untreated positive control cells (Fig. 5 A). Thus, in the presence of induction media, the X. strumarium extract did not inhibit differentiation. Upon quantification of the stain, it was determined that inducing hDPSCs along with the treatment of XSE enhanced osteogenic matrix production as compared to the respective positive control sample (Fig. 5 C). Data are shown as mean (n = 3) ± standard error of the mean. Statistical significance was determined by unpaired t-test at p ≤ 0.05 (**, p < 0.01). This quantification corresponded with gene expression, wherein both osteonectin and IL-6 had over 2-fold upregulation in the XSE-treated differentiation of hDPSCs into osteocytes after 13 days of induction (Fig. 6). Data are shown as mean (n = 2) ± standard error of the mean. Statistical significance was determined by unpaired t-test at p ≤ 0.05 (*, p < 0.05).
Fig. 5.
Effect of Xanthium strumarium seed extract (XSE) (15 μg/ml) on osteogenic differentiation of hDPSCs. (A) Positive control for osteogenic differentiation, (B) XSE treated osteogenic differentiation and, (C) Osteogenic quantification.
Fig. 6.
Relative fold change of (A) Osteonectin, (B) IL-6, (C) RUNX2 and (D) ALP in osteogenesis induced samples, without (positive control) and with XSE treatment.
4. Discussion
Herbal medicines have been widely used to treat bone disorders for thousands of years. In recent years, herbal medicines or their formula, which are essential parts of complementary and alternative medicine for bone disorders, have been widely used for preclinical or clinical studies. The dental pulp is a suitable source of stem cells for screening and toxicological studies of drugs and bioactive phytochemical compounds. MSCs are an ideal platform for comparative research on traditional medicine and many other allopathic drugs [24,25]. hMSCs can be differentiated into the lineage of concern, and the bioactivity of traditional medicines can be investigated in this simple in vitro system [24]. X. strumarium extract is known to possess a unique phytochemical composition and anti-inflammatory potential. Phytochemicals such as Limonene, Copaene, β-Caryophyllene, Spathulenol, α-cadinol, 1,3,5-trimethyl-2[2-nitroallyl]benzene, α-Muurolene, Phytol, E,E, Z-1,3,12-Nonadecatriene-5,14-diol are known to be present in X. strumarium seed extract. β-Caryophyllene, one of the key phytochemicals is known to promote osteogenic differentiation of bone marrow cultures [26]. Due to their ease of access, lack of ethical concerns, and relative abundance, hDPSCs from tooth extracts are an alternative for mesenchymal stem cells [27].
MSCs' ability to differentiate into osteocytes, chondrocytes, and adipocytes in specific differentiation cocktails [28]. The osteocytes mineralize the matrix and form calcium aggregates, chondrocytes are enclosed by a highly organized network of ECM that constitutes proteoglycans of high molecular mass, and adipocyte maturation results in the intracellular formation of vacuoles rich in lipids [29]. Upon 21 days of inducing hDPSCs, the cells were positively stained with respective stains thus confirming the mesenchymal-like attribute of the cultured hDPSCs.
MTT analysis for cytotoxicity gave results wherein all concentrations of X. strumarium had more viable cells than the negative control. This was statistically significant at 15 μg/ml and 10 μg/ml. Several studies on X. strumarium have highlighted that the phytochemicals present are apoptotic and anti-proliferative [2]. However, the previous studies were performed on cancer cell lines at much higher concentrations, while this is the first study to check the effect of the plant extract on hDPSCs at lower, clinically relevant concentrations.
XSE treatment, at 15 μg/ml, was given to hDPSCS induced for 21 days. A recent study analyzed the differentiation of DPSCs on days 7, 14, and 21 which showed positive results at all-time points [30]. Another study conducted the same on days 14, 17, and 21, also showed that differentiation was present on day 14 and increased till day 21 of induction [31]. Similar results are seen in the present study wherein DPSCs were induced for 20 days, and on termination with respective stain, a significant amount of differentiation was visible. The biochemical test using Alizarin Red S revealed that XSE enhanced calcium accumulation and matrix mineralization of hDPSCs differentiated into osteocytes. The activity of the seed extract led to the relative increase in gene expression of an osteogenic marker, osteonectin, and IL-6 cytokine indicating that osteogenic differentiation was enhanced upon treatment. A study by Xie et al. demonstrated that IL-6 and its membraneous receptor promote bone marrow MSCs to differentiate into osteogenic lineage in an autocrine loop via STAT3 signaling [32].
The effect of X. strumarium on bone and cartilage cells along with their respective matrix both in healthy and diseased conditions is a crucial study. In the anti-arthritic study, by Lin et al., the ethanol extract of X. strumarium significantly reduced paw swelling as well as arthritic score, increased body weight that was lost, and lowered the thymus index in the rat model [33]. Furthermore, all the treated rats' serums showed a significant reduction in TNF-alpha and IL-1beta overproduction (proinflammatory cytokines) with an upregulation of IL-10 (anti-inflammatory cytokine). Consequently, X. strumarium treatment can reverse arthritis and it could be presumed that the medicinal plant would enhance chondrogenesis. However, the augmented osteogenesis, in the current novel research, was a serendipitous finding. Therefore, it can be postulated that such upregulation could promote osteocytes for matrix production in an osteoporotic condition. In prospective studies, the effect of X. strumarium on differentiation in a diseased state will have to be studied to get a comparative analysis with the current study wherein X. strumarium was investigated in a normal, healthy in vitro condition. Understanding whether this medicinal plant promotes bone and cartilage formation will make it a candidate for osteoporosis and osteoarthritis specifically.
A study on the anti-inflammatory activity of X. strumarium has demonstrated that it downregulates IL-6 cytokine when cells are induced for pro-inflammatory cytokine expression [34]. Correlating such an outcome with the present study implies that X. strumarium discriminates between a healthy, non-inflamed environment and diseased, inflamed conditions. An unaddressed area of study is whether X. strumarium could aid in healing the bone that is associated with the cartilage. Despite all the advantages, the in vitro models have certain limitations such as finite life and senescence which make animal studies mandatory before jumping to conclusions. In vitro, cell culture may fail to account for interactions between numerous physical operations and cellular biology. Moreover, the Xanthium is not free from toxicity at higher concentrations.
5. Conclusion
We found that X srumarium is helpful in improving osteogenesis based on the aforementioned description. It can improve bone regeneration, which is important for the improvement of bone density in osteoporosis.
Sources of funding
This work is financially supported by the Dr. D. Y. Patil Vidyapeeth, Pimpri Pune, India by providing seed money.
Declaration of generative AI in scientific writing
Authors declare that AI has not been used in writing the current manuscript.
Author contributions
Avinash Kharat & Akshita Nagar: Experimental work, Plant extraction method and experiment designed and manuscript writing. Swapanli Sakhare: Collected the data. Avinash Sanap: manuscript writing. Supriya Kheur: Manuscript writing. Ramesh Bhonde: Data analysis and interpretation of data for the article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors wish to thank Dr. D. Y. Patil Vidyapeeth for the lab infrastructure to carry out our research.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Sahoo R.K., Tamuli K.J., Lhouvum N., Dutta D., Bordoloi M., Sharma H.K., et al. Phytochemical constituents from Xanthium strumarium L. and evaluation of their in vitro antimalarial activities. South Afr J Bot. 2020;135:35–40. doi: 10.1016/j.sajb.2020.08.006. [DOI] [Google Scholar]
- 2.Fan W., Fan L., Peng C., Zhang Q., Wang L., Li L., et al. Traditional uses, botany, phytochemistry, pharmacology, pharmacokinetics and toxicology of Xanthium strumarium L.: a review. Molecules. 2019;24:359–399. doi: 10.3390/molecules24020359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allami R.H., Qader O., Al-saadi S.A.A.M., Faraj I.M. Evaluate the influence of Xanthium strumarium L . Extract on blood sugar levels in healthy and diabetic mice. J Clin Case Rep. 2022;2022:72–81. doi: 10.46619/joccr.2022.5-2.1109. [DOI] [Google Scholar]
- 4.Khan Y., Shah S., Ullah S. Ethnomedicinal, pharmacological and phytochemical evaluation of Xanthium strumarium L. Int J Sci Eng Res. 2020;11:587–595. [Google Scholar]
- 5.Shankar R., Deb S., Sharma B. Antimalarial plants of northeast India: an overview. J Ayurveda Integr Med. 2012;3:10–16. doi: 10.4103/0975-9476.93940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh A.K., Raghubanshi A.S., Singh J.S. Medical ethnobotany of the tribals of Sonaghati of Sonbhadra district, Uttar Pradesh, India. J Ethnopharmacol. 2002;81:31–41. doi: 10.1016/S0378-8741(02)00028-4. [DOI] [PubMed] [Google Scholar]
- 7.Kim I.T., Park Y.M., Won J.H., Jung H.J., Park H.J., Choi J.W., et al. Methanol extract of Xanthium strumarium L. Possesses anti-inflammatory and anti-nociceptive activities. Biol Pharm Bull. 2005;28:94–100. doi: 10.1248/BPB.28.94. [DOI] [PubMed] [Google Scholar]
- 8.Hossen M.J., Kim M.Y., Cho J.Y. MAPK/AP-1-Targeted anti-inflammatory activities of Xanthium strumarium. Am J Chin Med. 2016;44:1111–1125. doi: 10.1142/s0192415x16500622. [DOI] [PubMed] [Google Scholar]
- 9.Scherer R., Godoy H.T. Effects of extraction methods of phenolic compounds from Xanthium strumarium L. and their antioxidant activity. Rev Bras Plantas Med. 2014;16:41–46. doi: 10.1590/S1516-05722014000100006. [DOI] [Google Scholar]
- 10.Alaribe F.N., Razwinani M., Maepa M., Shirley K., Motaung C. The potential effect of medicinal plants for cartilage regeneration. Cartil. Tissue Eng. Regen. Tech. 2019:1–11. [Google Scholar]
- 11.Buhrmann C., Honarvar A., Setayeshmehr M., Karbasi S., Shakibaei M., Valiani A. Herbal remedies as potential in cartilage tissue engineering: an overview of new therapeutic approaches and strategies. Molecules. 2020;25:3075. doi: 10.3390/MOLECULES25133075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anitua E., Troya M., Zalduendo M. Progress in the use of dental pulp stem cells in regenerative medicine. Cytotherapy. 2018;20:479–498. doi: 10.1016/J.JCYT.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Ledesma-Martínez E., Mendoza-Núñez V.M., Santiago-Osorio E. Mesenchymal stem cells derived from dental pulp: a review. Stem Cell Int. 2016;2016:1–12. doi: 10.1155/2016/4709572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salari N., Ghasemi H., Mohammadi L., hasan Behzadi M., Rabieenia E., Shohaimi S., et al. The global prevalence of osteoporosis in the world: a comprehensive systematic review and meta-analysis. J Orthop Surg Res. 2021;16:1–20. doi: 10.1186/S13018-021-02772-0/FIGURES/8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swärdh E., Jethliya G., Khatri S., Kindblom K., Opava C.H. Approaches to osteoarthritis - a qualitative study among patients in a rural setting in Central Western India. Physiother Theory Pract. 2021;2021:1–11. doi: 10.1080/09593985.2021.1872126. [DOI] [PubMed] [Google Scholar]
- 16.Bhadada S.K., Chadha M., Sriram U., Pal R., Paul T.V., Khadgawat R., et al. The Indian Society for Bone and Mineral Research (ISBMR) position statement for the diagnosis and treatment of osteoporosis in adults. Arch Osteoporosis. 2021:1–13. doi: 10.1007/S11657-021-00954-1. 161 2021;16. [DOI] [PubMed] [Google Scholar]
- 17.Jordan K.M., Arden N.K., Doherty M., Bannwarth B., Bijlsma J.W.J., Dieppe P., et al. EULAR recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a task force of the standing committee for international clinical studies including therapeutic trials (ESCISIT) Ann Rheum Dis. 2003;62:1145–1155. doi: 10.1136/ard.2003.011742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hochberg M.C., Altman R.D., April K.T., Benkhalti M., Guyatt G., McGowan J., et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2012;64:465–474. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- 19.Gambacciani M., Levancini M. Hormone replacement therapy and the prevention of postmenopausal osteoporosis. Prz Menopauzalny. 2014;13:213–220. doi: 10.5114/pm.2014.44996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kulak Júnior J., Kulak C.A.M., Taylor H.S. SERMs in the prevention and treatment of postmenopausal osteoporosis: an update. Arq Bras Endocrinol Metabol. 2010;54:200–205. doi: 10.1590/S0004-27302010000200016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rostom A., Goldkind L., Laine L. Nonsteroidal anti-inflammatory drugs and hepatic toxicity: a systematic review of randomized controlled trials in arthritis patients. Clin Gastroenterol Hepatol. 2005;3:489–498. doi: 10.1016/S1542-3565(04)00777-3. [DOI] [PubMed] [Google Scholar]
- 22.Craig E., Cappelli L.C. Gastrointestinal and hepatic disease in rheumatoid arthritis. Rheum Dis Clin N Am. 2018;44:89–111. doi: 10.1016/J.RDC.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aranjani J.M., Manuel A., Mallikarjuna Rao C., Udupa N., Rao J.V., Joy A.M., et al. Preliminary evaluation of in vitro cytotoxicity and in vivo antitumor activity of xanthium strumarium in transplantable tumors in mice. Am J Chin Med. 2013;41:145–162. doi: 10.1142/S0192415X13500110. [DOI] [PubMed] [Google Scholar]
- 24.Saud B., Malla R., Shrestha K. A Review on the effect of plant extract on mesenchymal stem cell proliferation and differentiation. Stem Cell Int. 2019;2019 doi: 10.1155/2019/7513404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhonde R., Sanap A., Joshi K. Mesenchymal stem cells as a platform for research on traditional medicine. J Ayurveda Integr Med. 2021;12:722–728. doi: 10.1016/J.JAIM.2021.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamaguchi M., Levy R.M. Β-caryophyllene promotes osteoblastic Mineralization,and suppresses osteoclastogenesis and adipogenesis in mouse bone marrow cultures in vitro. Exp Ther Med. 2016;12:3602–3606. doi: 10.3892/etm.2016.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li B., Ouchi T., Cao Y., Zhao Z., Men Y. Dental-Derived mesenchymal stem cells: state of the art. Front Cell Dev Biol. 2021;9:1310. doi: 10.3389/FCELL.2021.654559/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vater C., Kasten P., Stiehler M. Culture media for the differentiation of mesenchymal stromal cells. Acta Biomater. 2011;7:463–477. doi: 10.1016/J.ACTBIO.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 29.Chamberlain G., Fox J., Ashton B., Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cell. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 30.Labedz-Maslowska A., Bryniarska N., Kubiak A., Kaczmarzyk T., Sekula-Stryjewska M., Noga S., et al. Multilineage differentiation potential of human dental pulp stem cells—impact of 3D and hypoxic environment on osteogenesis in vitro. Int J Mol Sci. 2020;21:1–28. doi: 10.3390/IJMS21176172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eggerschwiler B., Canepa D.D., Pape H.C., Casanova E.A., Cinelli P. Automated digital image quantification of histological staining for the analysis of the trilineage differentiation potential of mesenchymal stem cells. Stem Cell Res Ther. 2019;10:1–10. doi: 10.1186/S13287-019-1170-8/FIGURES/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie Z., Tang S., Ye G., Wang P., Li J., Liu W., et al. Interleukin-6/interleukin-6 receptor complex promotes osteogenic differentiation of bone marrow-derived mesenchymal stem cells. Stem Cell Res Ther. 2018;9:13. doi: 10.1186/s13287-017-0766-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin B., Zhao Y., Han P., Yue W., Ma X.Q., Rahman K., et al. Anti-arthritic activity of Xanthium strumarium L. Extract on complete Freund's adjuvant induced arthritis in rats. J Ethnopharmacol. 2014;155:248–255. doi: 10.1016/j.jep.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y.H., Li T.H., Wu B.Q., Liu H., Shi Y.F., Feng D.Y. Protective effects of caffeoylxanthiazonoside isolated from fruits of Xanthium strumarium on sepsis mice. Pharm Biol. 2015;53:1367–1371. doi: 10.3109/13880209.2014.982300. [DOI] [PubMed] [Google Scholar]