Abstract
Among the leading causes of vision impairment and blindness globally, diabetic retinopathy (DR) is one of the most important causes. There is increasing evidence of DR prevalence in the prediabetic population. This systematic review presents collective data on retinopathy in the prediabetic population. This review article aimed to estimate the reported prevalence of retinopathy in prediabetes, impaired glucose tolerance test (GTT) without diabetes mellitus, and the risk factors involved and to summarize it. Literature searches were done using the Web of Science, CINAHL, Google Scholar, Cochrane, EMBASE, and PubMed databases from inception to April 2023. Our search included the words prediabetes, DR, and risk factors. All searches were looked at for methodological quality and evidence. Thirty-one studies were included after the screening. Population-based data were used in 23 studies (82.1%). The prediabetic population screened was 10,539. The prevalence of retinopathy ranged between 0.3% and 20.9%, showing a median of 8.1% with an interquartile range (IQR) of 4.2-11%, showing great variance in estimates due to the use of different screening methods, methods used for retinopathy grading, and study populations. Several studies compared the population with normal GTT with impaired glucose tolerance (IGT) and inferred that there was a lower prevalence of retinopathy in the normal GTT population (3.0%, IQR 0.3-7.4%) than prediabetes (6.7%, IQR 1.9-10.1%). According to this data, a greater retinopathy prevalence was found in prediabetic populations.
Keywords: diabetic retinopathy, impaired glucose tolerance, prevalence, impaired fasting glucose, prediabetes
Introduction and background
The definition of prediabetic is a higher blood glucose level that does not reach the threshold of type 2 diabetes mellitus [1-4]. All over the world, approximately 374 million people (7.3%) are shown to have prediabetic status, and most of the individuals are unaware of the diagnosis. The International Diabetes Federation predicts that the prevalence of prediabetes will rise to 8.3% among the global adult population by the year 2045. The World Health Organization (WHO) definition of prediabetes is as follows: (a) impaired fasting glucose (IFG) is said when fasting plasma glucose (FPG) is 6.1-6.9 mmol/L (110-125 mg/dL) and (b) impaired glucose tolerance (IGT) is said when two-hour plasma glucose is 7.8-11.0 mmol/L (140-200 mg/dL) after ingestion of 75 g of oral glucose or a combination of the two based on a two-hour oral glucose tolerance test (OGTT) [1]. The cut-off value for IGT used by the American Diabetes Association (ADA) is 140-200 mg/dL, and for IFG, it is 100-125 mg/dL, which is a lower cut-off value than IGT [4].
ADA included hemoglobin A1c (HbA1c) levels ranging from 5.7% to 6.4% along with the above criteria to define the prediabetic state. Current diagnostic levels for fasting and two-hour post-glucose load plasma glucose levels are based on the estimation of the presence of diabetic retinopathy (DR) in the literature available [5]. Several studies have evaluated the prevalence of prediabetes worldwide: WHO criteria in the ESTEBAN French survey and in an English national cohort state that the prevalence of DR was 9.9% and 11%, respectively, and according to ADA criteria in Luxembourg and South Korea, it was 25% and 23.9%, respectively [6]. The population with prediabetes has shown a greater risk of converting to type 2 diabetes mellitus in the future [7]. Every year, around 5-10% of people with prediabetes become diabetic, and according to current data, up to 70% of prediabetic states eventually develop diabetes mellitus [8,9].
In the Diabetes Prevention Program Outcomes Study, a random assignment was done for 20 years. Among 1,614 participants, 24% developed type 2 diabetes mellitus, and 14% (885) who had not developed diabetes were found to have DR. After complete analyses from the entire study duration of follow-up, as compared with the non-Hispanic White race, the American Indian race showed less association with DR, higher HbA1c, fasting, and two-hour plasma glucose levels during an OGTT, weight, and history of hypertension, dyslipidemia, and smoking were associated with DR more frequently [10].
According to the DETECT-2 study, DR was associated with an FPG of 6.5 mmol/l [10]. It also concluded that end-organ damage like DR and nephropathy can occur before the onset of type 2 diabetes mellitus [9]. It was observed that people with prediabetic conditions have an increased prevalence of microvascular disease, a greater rate of mortality, and a double coronary heart disease mortality rate as compared to people with normal glucose tolerance (NGT) [11,12]. It is seen that people with a prediabetic state and associated microvascular disease have a higher tendency to develop type 2 diabetes mellitus [13-16]. Throughout the world, DR prevalence in the diabetic population in the form of diabetic macular edema, proliferative DR, and sight-threatening DR including intragel hemorrhage and tractional retinal detachment is 7.0%, 34.6%, 6.8%, and 10.2%, respectively [17,18].
This is the reason why it is important to diagnose it early to save sight [9,19]. Nowadays, a commonly used method to screen the diabetic population for retinopathy is digital retinal photography, though it can vary from place to place [20]. Isolated retinopathies without diabetes and hypertension were found in the 2.6-8.6% range, which can be considered a prediabetic state [21,22]. The present study aimed to estimate and discuss the DR prevalence in the prediabetic population by conducting a systematic review of published literature using various databases as evidence and using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Review
Methods
Literature searches were done using the Web of Science, CINAHL, Google Scholar, Cochrane, EMBASE, and PubMed databases from inception to April 2023. Our search included the words prediabetes, DR, and risk factors. All searches were looked at for methodological quality and evidence.
Inclusion and Exclusion Criteria for Literature Search
The inclusion and exclusion criteria for the literature search encompassed various facets. These comprised study types, sampling methods, and specifically targeting the prediabetic population. The studies included those reporting the prevalence of conditions such as DR, vision-threatening DR, or clinically significant macular edema among individuals with prediabetes or IGT. Clear definitions of prediabetic states were delineated, considering specific fasting and post-glucose ingestion plasma glucose levels. These criteria, aligned with ADA guidelines, specified IFG and IGT thresholds. Additionally, HbA1c levels between 5.7% and 6.4% were incorporated to define the prediabetic state as per ADA guidelines. DR was characterized by retinal abnormalities observed through various diagnostic methods. Exclusion criteria involved studies conducted solely in clinical settings or hospitals, clinical trials, and duplicates, along with those lacking full-text articles.
Outcomes
Outcomes were defined as primary and secondary. The primary outcome included the evident retinal findings on fundus photographs that comprised the DR in the prediabetic population, as defined by the International Clinical Diabetic Retinopathy Severity Scale (ICDRSS) classification [24]. Features observed on retinal photography were (1) microaneurysms, (2) dot and blot hemorrhage, (3) hard exudates, (4) cotton-wool spots, (5) venous beading and looping, (6) intraretinal microvascular abnormalities, (7) new vessels at the optic disc or elsewhere, and (8) intragel or pre-retinal hemorrhage. Some studies reported additional data on imaging with fundus fluorescein angiography (FFA) and optical coherence tomography (OCT). Additional data such as weight, BMI, blood pressure, urine protein, etc. were also studied for diagnosing prediabetes and cardiovascular and metabolic syndrome [1-3,25,26].
Study Selection and Data Collection
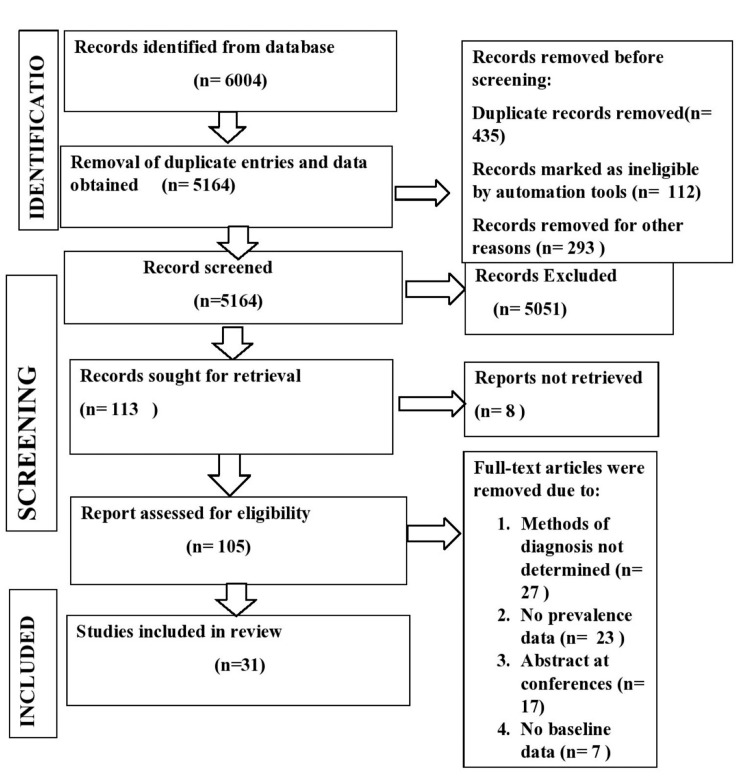
One of the reviewers (MS) screened titles and abstracts to see if the study matched the eligibility criteria or not independently. Disagreements on the studies were resolved by discussion. Articles fulfilling the criteria were selected for full-text assessment. All population-based studies were considered, and only prediabetic populations were considered in the whole study, where different groups were a part of the study. PRISMA guidelines were followed, and the study process is shown in Figure 1. The prevalence was also estimated for comorbid ocular conditions like cataracts and cardiovascular risk factors, e.g., raised blood pressure and metabolic syndrome.
Figure 1. PRISMA flowchart showing the study selection process.
Results
Characteristics of the Studies
From electronic database searches, all the duplicate entries were removed, 105 full-text articles were selected, and individuals (10,539) with prediabetes were included for final evaluation. Thirty-one studies were included after the screening. Population-based data were used in 26 studies (83.87%). The prediabetic population screened was 10,539. The estimated prevalence of retinopathy ranged between 0.3% and 20.9%, showing a median prevalence of 8.1%. The interquartile range (IQR) is 4.2-11%, with high variation in rates due to different screening methods used to diagnose and grading methods used for retinopathy and study populations. Several studies comparing the population with normal GTT with impaired GTT inferred that there was a lower prevalence of retinopathy in the normal GTT population (3.0%, IQR 0.3-7.4%). The estimated DR prevalence in prediabetes was found to be 6.7% (IQR 1.9-10.1%) which is higher than normal GTT. According to this data, a greater DR prevalence was found in the prediabetic population than in the previous systematic reviews. Of the prediabetic population studied, 27 studies reported age data. Male predominance was observed among the 31 studies that reported gender data, wherein gender ratios ranged from 17.7% to 69.2% male. Details of the studies included in the review are shown in Table 1.
Table 1. Characteristics of the studies included in the review.
- data not reported, * prediabetes group defined by HbA1c criteria only, $ average value including other study groups (NGT, type 2 diabetes mellitus)
ChS: cohort study, CSS: cross-sectional study, HBS: hospital-based study, RCT: randomized-controlled trial, PBS: population-based study, IFG: impaired fasting glucose, IGT: impaired glucose tolerance, NGT: normal glucose tolerance, HAm: Hispanic, NHBp: non-Hispanic Black, NHWp: non-Hispanic White
| Author | Country | Study period | Design | Study group(s) | Sample size (prediabetics) | Mean age ± SD or median age (range) | Male gender (%) | Race or ethnicity |
| White et al. (2022) [10] | USA | 16 years up to 2020 | PBS, ChS | IGT | 747 | 10.39 | 29.85 | NHW, American Indian |
| Nagi et al. (1997) [27] | USA | 1982-1990 | PBS, CSS | IGT | 288$ (incl NGT) | 45$ (15-93 incl NGT) | 51$ (incl NGT) | Pima Indians |
| Penman et al. (2015) [28] | USA | 2009-2012 | HBS, CSS | IGT | 266 | 65.7±9.6 | 40.2 | African American |
| Tyrberg et al. (2008) [29] | Sweden | - | RCT | IFG | 154 | - | 58.4 | - |
| Hanssen et al. (2020) [31] | Netherland | 2010-2013 | PBS, ChS | IFG or IGT | 478 | 61.6±7.6 | 54 | - |
| Guney et al. (2022) [32] | Turkey | Jan 20219 to Sep 2019 | PBS, CSS | IFG or IGT or combined IFG and IGT | 86 | - | - | Turkish |
| Chen et al. (2012) [33] | China | 2005-2007 | PBS, CSS | IGT | 110 | 50.33±12.2 | 40.9 | Chinese |
| Gabriel et al. (2020) [34] | Australia, Bulgaria, Austria, Kuwait, Poland, Serbia, Spain, Turkey | Ongoing | RCT | IFG or IGT | 809 | 58.5±7.6 | 41.9 | |
| Dowse et al. (1998) [35] | Mauritius | 1987-1992 | PBS, CSS | IGT | 165 | - | 45.2$ | Indians, Creoles, Chinese |
| Klein et al. (1991) [36] | USA | 1984-1987 | PBS, CSS | IGT | 418 | - | 39 | White |
| Collins et al. (1995) [37] | Samoa | 1978-1991 | PBS, CSS | IGT | 97 | - | 37.2 | Samoan |
| Rajalakshmi et al. (2022) [38] | India | 2019 | PBS, CSS | IFG or IGT or IFG with IGT | 192 | 48±13 | 55.2 | Indian |
| van Leiden et al. (2002) [39] | Netherland | 1989-1892 | PBS, CSS | IGT | 177 | 64.2±7.3 | 51 | Caucasians |
| Sundling et al. (2012) [40] | Norway | 2004-2005 | PBS, CSS | IGT | 38 | 57±15 | 36.8 | - |
| Lamparter et al. (2014) [41] | Germany | 2007-2008 | PBS, ChS | PD* | 1112 | 59.9±9.1 | 51.8 | - |
| Kawasaki et al. (2006) [42] | Japan | 2000-2002 | PBS, CSS | IGT | 303$ | 58.6$ | 42.3$ | Japanese |
| Arshid et al. (2022) [43] | India | 2020 to 2022 (2 years) | HBS, CSS | IFG or IGT | 200 | 56.7 | 53.5 | Indian |
| Pang et al. (2011) [44] | China | 1996-2007 | PBS, CSS | IGT | 865 | 62.3±10.8 | 42.9 | Chinese |
| Bower et al. (2013) [45] | USA | 2005-2008 | PBS, CSS | PD* | 636 | - | - | NHWp 41%, NHBp 30%, HAm 29% |
| Bhargava et al. (2014) [46] | Singapore | - | PBS, CSS | PD* | 829 | - | - | Indian |
| Munch et al. (2011) [48] | Denmark | 1991-2001 | PBS, CSS | IFG or IGT or IFG with IGT | 275 | - | - | - |
| Callaghan et al. (2020) [49] | USA | 2015 | HBS, CSS | IFG or IGT | 56 | 44.7±11.4 | 17.9 | White 78.8%, White 69.6%, White 87.8% |
| Hu et al. (2015) [50] | China | 2006 | PBS, CSS | IFG or IGT | 657 | 45.6±1.3 | 35 | Chinese |
| Tikellis et al. (2007) [51] | Australia | 1999-2000 | PBS, CSS | IFG or IGT | 960 | 58.9±13.5 | 43 | - |
| Hermans et al. (1998) [52] | Egypt | - | PBS, CSS | IGT | 103 | - | 41 | - |
| Dyck et al. (2012) [53] | USA | 2000-2005 | PBS, CSS | IFG or IGT or IFG with IGT | 174 | 64 (22-76) | 67.8 | - |
| Sokolowska et al. (2016) [54] | Poland | - | HBS, CSS | IFG or IGT | 61 | 58 | 33.3 | |
| Akhter et al. (2013) [55] | Bangladesh | - | PBS, CSS | IFG or IGT | 54 | - | 56 | Bangladeshi |
| Rajala et al. (1998) [56] | Finland | 1990-1992 | PBS, CSS | IGT | 204 | - | 43.9$ | - |
| Kumar et al. (2021) [57] | India | Feb 2019 to Aug 2020 | HBS, CSS | IFG or IGT | 100 | 55.5±8.7 | 58 | Indian |
| Shrestha et al. (2023) [58] | Nepal | Jan 2022 to April 2022 | PBS, CSS | IFG or IGT | 141 | 60.69±13.1 | - | Nepalis |
Studies were conducted in 26 different countries. Out of 31, eight studies were from the European continent, seven were from the United States of America (USA), 13 studies were from the Western Pacific (China, Japan, Singapore, India, Turkey, Bangladesh, Australia, Nepal, and Samoa), and one each in Africa (Mauritius) and the Eastern Mediterranean (Egypt). One of the studies included a total of nine countries [27]. Among the studies, 20 reported data on race or ethnicity, and three examined Pima Indians, African Americans, and non-White Hispanics with American Indians, all of whom are from the USA exclusively [10,27,28]. The majority of published data were from cross-sectional and population-based studies (28/31, 90.32%).
There was a wide range of sample sizes, varying between 38 and 1112. Twenty-two studies defined prediabetes according to WHO criteria and eight according to ADA criteria. Among them, two studies used non-standard WHO criteria, one could not define prediabetes, and one used non-standard IFG criteria (Table 2) [29].
Table 2. Prevalence of DR in the prediabetic population.
- data not reported, IFG: impaired fasting glucose, IGT: impaired glucose tolerance, NGT: normal glucose tolerance, PDP: prediabetic population, ADA: American Diabetes Association, CSMO: clinically significant macular oedema, DRDSS: Diabetic Retinopathy Disease Severity Scale, ICDRSS: International Clinical Diabetic Retinopathy Severity Scale, ETDRS: Early Treatment Diabetic Retinopathy Study, HE: hard exudate, PDR: proliferative diabetic retinopathy, WES-DR: Wisconsin Epidemiologic Study of Diabetic Retinopathy, WHO: World Health Organization, FFA: fundus fluorescein angiography
| Author | Study group(s) | Sample size | Definition of prediabetes | Definition of retinopathy | Method of diagnosis of retinopathy | No. of retinopathy (n ) | Prevalence of retinopathy (%) | Secondary outcome |
| White et al. (2022) [10] | IGT | 747 | WHO | ETDRS | Stereoscopic fundus photography | 127 | 15 | PDR: 0% |
| Nagi et al. (1997) [27] | IGT | 288 (incl NGT) | WHO | Modified Airlie-House | Mydriatic, imaging- 2-field, 45-degree digital | 8 | 12 | - |
| Penman et al. (2015) [28] | IGT | 266 | WHO | ETDRS ≥14 | Mydriatic, 7-field color digital | 25 | 9.4 | CSMO: 7.8% |
| Tyrberg et al. (2008) [29] | IFG | 154 | Nonstandard | Alternative WES-DR ≥21 | Mydriatic, 2-field, color film | 16 | 10.4 | - |
| Hanssen et al. (2020) [31] | IFG or IGT | 478 | WHO | ETDRS and ICDRSS | Color digital | - | 0.3 | - |
| Guney et al. (2022) [32] | IFG or IGT or combined IFG and IGT | 86 | WHO | ETDRS | on FFA findings | 16 | 18.6 | PDR: 0% |
| Chen et al. (2012) [33] | IGT | 110 | ADA | DRDSS | FFA using TOPCON TRC 50IX | 23 | 20.9 | PDR: 0% |
| Gabriel et al. (2020) [34] | IFG or IGT | 809 | WHO | ETDRS ≥14 | Mydriatic, 3-field color digital | 34 | 4.2 | - |
| Dowse et al. (1998) [35] | IGT | 165 | WHO | Airlie-House | 3-field, 45-degree digital | 15 | 9.1 | PDR: 0% |
| Klein et al. (1991) [36] | IGT | 418 | WHO | ≥15 | Dark-adapted, 1-field, 45-degree | 6 | 1.4 | - |
| Collins et al. (1995) [37] | IGT | 97 | WHO | Airlie-House | Mydriatic, 3-field, 45-degree | 7 | 7.2 | PDR:1% |
| Rajalakshmi et al. (2022) [38] | IFG or IGT or IGT with IFG | 192 | WHO | ICDR | Nonmydriatic ultrawide field fundus photography | 12 | 6.3 | PDR: 0% |
| Rajalakshmi et al. (2022) [38] | IGT | 177 | WHO | EURODIAB: ≥1 MA, Hg or hard exudate | Mydriatic, 2-field color digital | - | 11 | HE: 6% |
| Sundling et al. (2012) [40] | IGT | 38 | WHO | DRDSS: 5 stages | Nonmydriatic, imaging-1-field, 45-degree color digital | 1 | 2.9 | - |
| Lamparter et al. (2014) [41] | PD* | 1112 | ADA | ETDRS | Dark-adapted, 2-field | 75 | 8.1 | CSMO: 0.2% |
| Kawasaki et al. (2006) [42] | IGT | 303 | WHO | No standard, any MA, Hg, or exudate | Nonmydriatic, 1-field, 45-degree color digital | - | 14.1 | - |
| Arshid et al. (2022) [43] | IFG or IGT | 200 | WHO | ETDRS | - | 12 | 6 | PDR: 0% |
| Pang et al. (2011) [44] | IGT | 865 | ADA | DRDSS: G1-4 | Dark-adapted, imaging-1-field, 45-degree color digital | 22 | 2.5 | CSMO: 2.4% |
| Bower et al. (2013) [45] | PD* | 636 | WHO | ETDRS ≥14 | Nonmydriatic, 2-field, 45-degree color digital | - | 9.7 | - |
| Bhargava et al. (2014) [46] | PD* | 829 | ADA | ETDRS >14 | Mydriatic, 2-field | 55 | 6.6 | - |
| Munch et al. (2011) [48] | IFG or IGT or combined IFG and IGT | 58, 152.5, 64.6 | WHO | ETDRS ≥15 | Mydriatic 7 field 60 deg color digital | - | 11.7 8.4 9.5 | - |
| Callaghan et al. (2020) [49] | IFG or IGT | 56 | ADA | - | Nonmydriatic color digital | - | 1.9 | - |
| Hu et al. (2015) [50] | IFG or IGT | 657 | ADA | ETDRS | Dark-adapted, 1-field | 9 | 1.4 | - |
| Tikellis et al. (2007) [51] | IFG or IGT | 960 | WHO | Wisconsin | Nonmydriatic, 2-field, 45-degree color digital | 66 | 6.9 | - |
| Hermans et al. (1998) [52] | IGT | 103 | WHO | Wisconsin | Mydriatic digital imaging | - | 1.9 | - |
| Dyck et al. (2012) [53] | IFG or IGT or IFG with IGT | 118, 19, 37 | ADA and WHO | NSC: R0-R3 | 7-field color digital fundus imaging | - | IFG-4.3, IGT-9.9, IFG with IGT-8.7 | WHO cut-off for IFG (6.1 mmol/L) but also included abnormal A1c as per ADA |
| Sokolowska et al. (2016) [54] | IFG or IGT | 61 | - | - | Color digital | 6 | 9.8 | - |
| Akhter et al. (2013) [55] | IFG or IGT | 54 | WHO | ETDRS | Mydriatic, 3-field color | 7 | 13 | - |
| Rajala et al. (1998) [56] | IGT | 207 | WHO | University Hospital of Oulu classification: G1-4 | Dark-adapted, 1-field, 45-degree film | - | 2 | - |
| Kumar et al. (2021) [57] | IFG or IGT | 100 | ADA | ETDRS | - | 14 | 14 | PDR: 0% |
| Shrestha et al. (2023) [58 | IFG or IGT | 141 | WHO | ETDRS | Slitlamp biomicroscopy and indirect ophthalmoscopy | 8 | 5.67 | PDR: 0% |
Risk of Bias Assessment
The risk of bias assessment was done by assessing individual items that are deemed to reflect the methodological risk of bias by using the modified McMaster Critical Appraisal Tool. Points were scored on individual items to be assessed for risk. The majority of studies (22/31, 70.96%) showed a "low" risk of DR, while the remaining nine showed a "moderate" risk. Most studies (10 studies) did not show proper randomization of the study population. Few studies lacked pharmacological mydriasis (14 studies), raising questions of validity and reliability. There was clinical and statistical heterogeneity (94%) and a lot of inequality in study populations, the degree of fields used to capture retinal photographs, the use of different retinopathy classifications, the use of mydriatic agents or nonmydriatic fundus pictures, and diagnostic criteria for prediabetes [30]. In studies where quantitative data availability was found, median prevalence and ranges were calculated. In the absence of quantitative data, we opted for a narrative analysis. Details are shown in Table 2.
Primary Outcome
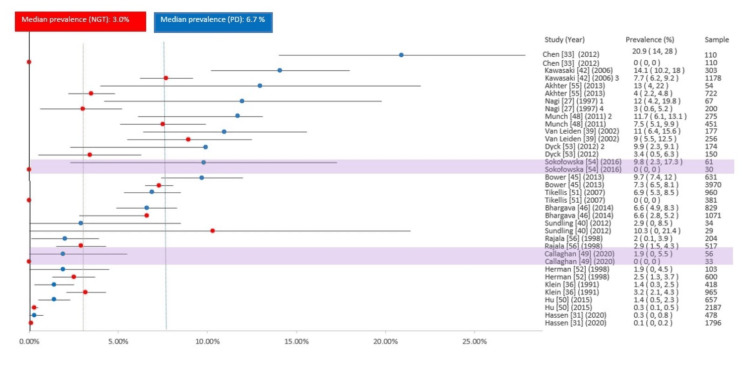
The estimated prevalence of retinopathy ranged between 0.3% and 20.9% (median 8.1%, IQR 4.2-11%) [31-33]. However, the estimation of prevalence varied widely. A study from the Netherlands showed 0.3% (n=478), whereas a study from Turkey showed a prevalence of 18.6% (n=86) [31,32], and Chen et al. showed 20.9% [33]. The median sample size for prediabetes was 165 (range 38-1112). The prevalence of DR in the studies included is shown in Table 2 and Figure 2.
Figure 2. Forest plot of the prevalence of retinopathy in the prediabetic population from the included studies.
∗ Prediabetes group size estimated from the reported retinopathy prevalence and the number of affected individuals.
∗∗ Aggregate prevalence estimates presented for impaired fasting glucose (IFG), impaired glucose tolerance (IGT), and combined IFG and IGT (IFG-IGT).
All studies are population-based, except three hospital-based studies (lavender highlights) and two randomized-controlled trials (yellow highlights).
CI: confidence interval
Subgroup Analyses of Primary Outcome
Wherever we found a lack of quantitative data subgroups, a narrative analysis was done. The median estimated retinopathy was 8.1% (0.3-20.9%). In the USA, the value was 9.4% (1.4-15%), while the median value for Europe was 8.1% (0.3-14.0%) [31,34]. In Asia, the reported value was 6.3% (1.4-20.9%). Only a study from the African continent showed 9.1% [35]. According to the data available, the USA (10.28%) estimated the mean highest prevalence of DR in prediabetics, followed by Asia (9.6%) (Table 2).
Demographic Features
Only three of the studies had age-specific data on the prevalence of prediabetics. Most of the studies found that the maximum number of participants in the study fell between 50 and 70 years of age. Klein et al. and Herman et al. observed a higher DR prevalence in people aged more than 50 years compared to those aged below 45 [36,52]. All studies showed a higher prevalence in males [18]. A higher prevalence of retinopathy was found in females only in one study [36]. In two of the studies, female participants were more frequent [10,34]. Two studies compared ethnicity with respect to DR prevalence, with a higher prevalence in Hispanic White people (10.0%) and non-Hispanic Black people (11.6%) [35,10]. The highest prevalence of DR (20.9%) was found in Turkey and China [32,33].
Prevalence of DR in Subtypes of Prediabetes
A median prevalence of 8.5% (1.9-18.6%) for IGT was reported by 15 studies, and a median DR prevalence in individuals with IFG was 10.4% (4.3-15%) concluded in five studies. Among the studies, one study opted for the upper limit of IFG as <6.1 mmol/l, which differed from ADA and WHO criteria (<7.0 mmol/l). Four studies observed DR prevalence combined with IFG/IGT, with a median prevalence of 8.5% (0.3-15%) for DR in prediabetics. Prevalence estimates for retinopathy among participants with IFG or IGT, with a median prevalence of 6.8% (1.4-18.6%), were reported by 12 studies (Table 2).
Grade of Retinopathy
Different studies used different types of classification to grade retinopathy. Some studies utilized the Early Treatment Diabetic Retinopathy Study (ETDRS) classification, while others employed the international classification of DR. Penman et al. reported the prevalence of retinopathy by ETDRS grade [28], involving 266 participants with IGT. The ETDRS grades revealed 15, 20, and 35 grades of retinopathy, with frequencies of 12 (4.5%), 9 (3.4%), and 4 (1.5%), respectively. In a study conducted by Collins et al. (n=97), a single case of advanced DR was reported in a Samoan population [37]; however, a study conducted by Dowse et al. (n=165) did not find a single case of proliferative DR where a mixed population of Chinese, Indian, and Creole were participants [35]. Rajalakshmi et al. reported DR in 12 (6.3%) participants with nine (4.7%) mild non-proliferative DR (NPDR) and three (1.6%) with moderate NPDR. None reported severe, sight-threatening DR [38].
Prediabetics and Comorbid Ocular Pathology
Clinically significant macular edema was reported in very few studies. A 6% DR prevalence was reported by van Leiden et al. (n=165), which included only NPDR cases among participants with IGT [39]. In a study by Sundling et al. (n=38), one participant (2.6%) reported having hypertensive retinopathy among individuals with IGT [40]. In IGT, NGT, and diabetic populations, there was no statistical difference in the prevalence rate of age-related macular degeneration or glaucoma. Cataract and pseudophakia status were hardly reported; hence, the prevalence of cataracts could not be measured reliably in prediabetics [41].
Comorbid Risk Factors
The included studies showed an association with hypertension, showing a prevalence of 61% (31-71%) for hypertension in prediabetes. In one study, microalbuminuria was found in 11% of prediabetics, which is a sign of nephropathy [42-44].
Diagnosis of Prediabetes
Twenty-two studies showed median prevalence that used WHO criteria (9.6%, 0.3-18.6%), whereas eight studies that used ADA criteria showed prevalence estimates (2.5%, 1.4-8.6%). HbA1c-related data were available in 14 studies, whereas data from 14 studies was available for FPG and 15 studies for OGTT. Three of the studies only used HbA1c as criteria for the diagnosis of prediabetes, wherein the median prevalence was 8.1% (6.6-9.7%) (Table 2) [41,45,46].
Methods Used to Diagnose DR
This data, collected from multiple studies, is based on retinal images captured using various degrees of field, ranging from one- to seven-field fundus imaging. In studies conducted using mydriatic drugs, the highest median DR prevalence (10.9%, 1.9-20%) was found. Studies performed without mydriatic drops showed a prevalence of 6.3% (1.9-14%). Five studies that had done imaging after dark adaptation obtained physiological mydriasis and observed a lower prevalence rate of 2.0% (1.4-8.1%). The rest of the study didn’t provide sufficient information.
Secondary Outcomes
In the current review, no study showed any microvascular abnormalities that were not defined as primary outcomes (features of DR). Only three studies observed the presence of maculopathy and its prevalence in the prediabetic population. The prevalence rate of CSMO was found to be 0.2% and 2.4% by Lamparter et al. (n=922) and Pang et al. (n=865), respectively [41,44,47]. Most of the studies have no maculopathy, and retinopathy was restricted to a mild form only [28,38].
Multivariate analysis
Tables 1-2 show the summary of all the study groups. Exploratory post hoc comparisons were performed to observe if the DR prevalence in the prediabetic population is greater than that of NGT. Among the 31 studies, 18 also reported the DR prevalence in NGT. In this review of the included studies, the median observed DR prevalence in prediabetic populations was 8.1% (IQR 4.2-12%). The prevalence of NGT observed was 3.0% (IQR 0.3-7.4%), which is summarized in Figure 3. There was a wide variation in DR prevalence and sample sizes in NGT, ranging from 0.1% to 10.3% and 29 to 3970 participants, respectively. In this review, 14 studies out of 18 (77.7%) observed a higher DR prevalence rate in prediabetic populations than NGT.
Figure 3. Forest plot of the prevalence of retinopathy in the prediabetic population and NGT from included studies reporting data for both groups.
Normal glucose tolerance (NGT) prevalence estimates in red and prediabetes prevalence estimates in blue.
1 Prediabetes group size estimated from reported retinopathy prevalence and number of affected individuals.
2 Impaired fasting glucose (IFG), impaired glucose tolerance (IGT), and combined IFG and IGT (IFG/IGT) retinopathy prevalence estimates aggregated with total prediabetes group size used for 95% confidence interval (CI) estimation.
3 NGT group size estimated from the total study sample minus the reported prediabetes population.
4 Prediabetes group size estimated from reported retinopathy prevalence and number of affected individuals. All studies are population-based, except two hospital-based studies (lavender highlights).
Discussion
We observed the median DR prevalence in the prediabetic population to be 8.1% (range 0.3-20.9%) in this systematic review, of which 22 studies (70.96%) reported a prevalence of ≥5% in the prediabetic population, which is higher than the review done by Kirthi et al. [16]. This may be due to recent Asian studies, which observed a higher prevalence rate [32,39]. However, the range was wide from 0.3% to 20.9%. This might be due to physiological and pharmacological mydriasis as well as the differences in the field observed in different studies. Despite variations, multivariate analysis revealed a higher median prevalence of DR (6.7%) in prediabetes within the same studies compared to NGT (3.0%). There was a low prevalence of moderate retinopathy and CSMO in prediabetic populations, which may be due to insufficient information on the grade of retinopathy.
Demographic Risk Factors
Important risk factors for DR include ethnicity, age, disease duration, and hyperglycemia status [17]. The mean ages of prediabetic participants were matched to those reported in diabetic population studies. Only two studies reported a higher rate of DR prevalence in females, despite reports showing higher retinopathy prevalence in males in diabetes or prediabetes conditions [10,23,47]. One study reported data on ethnicity and observed an excess DR prevalence among non-Hispanic Black individuals, similar to the diabetic population [17], and others compared non-Hispanic Whites with American Indians.
HbA1c, IFG, and IGT Comparisons
Retinopathy prevalence rates were observed differently for participants with IFG, IGT, and combined IFG and IGT subgroups, of which four studies published separate data for all subgroups, though many had combined IFG and IGT participants [38]. Individuals where combined IFG and IGT data were available were shown to have a higher risk of conversion p to type 2 diabetes mellitus (15-19%) as compared to only IFG (6-9%) or IGT (4-6%). However, in view of DR, things are not similar [59]. Most of the studies showed more DR prevalence in IGT than in the IFG subgroup [59]. Pathological mechanisms varied in IFG and IGT based on the insulin resistance that originated, which was predominantly hepatic and muscular, respectively [8,9]. This explanation justifies the variations in retinopathy prevalence in prediabetics. HbA1c is an indicator of chronic glycemia, whereas the OGTT is a single-time point measurement. DETECT-2 demonstrated a relationship between HbA1c and OGTT similar to OGTT [60-63]. Single HbA1c, when used as a diagnostic criterion (5.7-6.4%) showed similar annualized incidence rates of diabetes as in IFG and IGT participants (7%) [9].
The Severity of Retinopathy
Early-stage retinopathy was found predominantly using ETDRS grades, and microaneurysms were the most common lesion observed [61]. Among the studies, only two with 262 individuals observed the prevalence of advanced DR in the prediabetic population [62]. In a study conducted by Rajalakshmi et al., the DR prevalence was 6.3% (n=12). Nine (4.7%) individuals reported mild NPDR and three (1.6%) had moderate NPDR. None of them had severe DR [38].
Methods of Retinal Imaging
Pharmacological mydriasis gives more field visibility for DR screening than using non-mydriatic fundus cameras (3.7% compared to 19.7%, respectively) [63]. Our review showed greater median DR prevalence in studies wherein mydriatic agents were used compared to studies with no dilatation or physiological dilatation of the pupil. The extent of retinal fields for imaging used was also different among studies, which may have reflected on DR prevalence [64]. The correlation of seven-field fundus imaging was well observed with a clinical examination done by an ophthalmologist [65].
Comorbid Ocular and Metabolic Disease
Limited data is available on ocular and metabolic disorders. Insufficient data on the lens status was published (phakic or pseudo-phakic). Very few studies mentioned the cataract status [38]. Studies showed that in prediabetes hypertension, components of metabolic syndrome like dyslipidemia and BMI were higher compared to NGT. Also, serum uric acid levels, estimated glomerular filtration rate (eGFR), and antihyperlipidemic drugs were associated with DR [32]. Control of blood glucose levels and retinal damage are well correlated. Hypertension was found to be one of the important risk factors for retinopathy [66-68]. Various studies in human and animal models postulated that retinal vascular endothelial dysfunction and chronic inflammatory processes are common etiopathogeneses for both hypertensive and DR [21,69]. In prediabetes, it was observed that there was low-density lipoprotein and raised triglycerides, leading to dyslipidemia [70,71]. Several studies have reported associations between microalbuminuria and DR in prediabetics [57,72]. The association of retinopathy with dyslipidemia is varied, and there are reports published on the associations of hypercholesterolemia and retinal lesions (hard exudates) and also between hypertriglyceridemia- and diabetes-induced retinopathy [33,58,73,74].
Limitations of the current review
Among 31 studies, only eight (25.8%) included participants above 500, and six studies (19.3%) had participants less than 100. Studies with fewer participants carry a risk of bias in estimating the prevalence and have less reliability. Diagnostic criteria for prediabetes differed among the studies, and data on IFG, IGT, and IFG and IGT of the same individuals were reported by only two studies. There was higher clinical heterogeneity in diagnostic criteria and statistical variabilities found in study design and methods, so a meta-analysis was limited.
Conclusions
DR prevalence was found to be higher in the prediabetic population (median 8.1%) as compared to the non-diabetic population. This review indicates a sizable number of people with prediabetes have end organ damage that is below the threshold level of glycemia for diabetes. There is an increase in the conversion rate of prediabetes to diabetes by around 10% annually, and this review shows evident early involvement of organs like the eye and renal resulting in DR and nephropathy, respectively, which needs greater monitoring to avoid end-organ damage in prediabetes. Risk factors like hypertension and components of metabolic syndrome like dyslipidemia and body BMI were higher in prediabetics compared to NGT. Also, serum uric acid levels, eGFR, and antihyperlipidemic drugs were associated with DR. Control of blood glucose levels and retinal damage are well correlated. Hypertension was found to be one of the important risk factors for retinopathy, along with prediabetes. Monitoring of these associated risk factors is also important.
Acknowledgments
We are grateful to Dr. Rekha Khandelwal, Professor in the Department of Ophthalmology at NKP Salwe Medical College, Nagpur, for her appraisal of the search strategies performed in this systematic review.
The authors have declared that no competing interests exist.
References
- 1.Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Alberti KG, Zimmet PZ. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 2.Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Alberti KG, Eckel RH, Grundy SM, et al. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 3.2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care. 2018;41:0–27. doi: 10.2337/dc18-S002. [DOI] [PubMed] [Google Scholar]
- 4.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33 Suppl 1:0–9. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 6.Prevalence of prediabetes and undiagnosed type 2 diabetes in France: results from the national survey ESTEBAN, 2014-2016. Lailler G, Piffaretti C, Fuentes S, Nabe HD, Oleko A, Cosson E, Fosse-Edorh S. Diabetes Res Clin Pract. 2020;165:108252. doi: 10.1016/j.diabres.2020.108252. [DOI] [PubMed] [Google Scholar]
- 7.Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. Tuomilehto J, Lindström J, Eriksson JG, et al. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 8.Impaired fasting glucose and impaired glucose tolerance: implications for care. Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, Zinman B. Diabetes Care. 2007;30:753–759. doi: 10.2337/dc07-9920. [DOI] [PubMed] [Google Scholar]
- 9.Prediabetes: a high-risk state for diabetes development. Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Lancet. 2012;379:2279–2290. doi: 10.1016/S0140-6736(12)60283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Risk factors for the development of retinopathy in prediabetes and type 2 diabetes: the Diabetes Prevention Program experience. White NH, Pan Q, Knowler WC, et al. Diabetes Care. 2022;45:2653–2661. doi: 10.2337/dc22-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Tabák AG, Jokela M, Akbaraly TN, Brunner EJ, Kivimäki M, Witte DR. Lancet. 2009;373:2215–2221. doi: 10.1016/S0140-6736(09)60619-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Risk of cardiovascular disease and death in individuals with prediabetes defined by different criteria: the Whitehall II study. Vistisen D, Witte DR, Brunner EJ, Kivimäki M, Tabák A, Jørgensen ME, Færch K. Diabetes Care. 2018;41:899–906. doi: 10.2337/dc17-2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corneal confocal microscopy identifies small-fiber neuropathy in subjects with impaired glucose tolerance who develop type 2 diabetes. Azmi S, Ferdousi M, Petropoulos IN, et al. Diabetes Care. 2015;38:1502–1508. doi: 10.2337/dc14-2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peripheral neuropathy and nerve dysfunction in individuals at high risk for type 2 diabetes: the PROMISE cohort. Lee CC, Perkins BA, Kayaniyil S, et al. Diabetes Care. 2015;38:793–800. doi: 10.2337/dc14-2585. [DOI] [PubMed] [Google Scholar]
- 15.Pathophysiology-based subphenotyping of individuals at elevated risk for type 2 diabetes. Wagner R, Heni M, Tabák AG, et al. Nat Med. 2021;27:49–57. doi: 10.1038/s41591-020-1116-9. [DOI] [PubMed] [Google Scholar]
- 16.The prevalence of retinopathy in prediabetes: a systematic review. Kirthi V, Nderitu P, Alam U, et al. Surv Ophthalmol. 2022;67:1332–1345. doi: 10.1016/j.survophthal.2022.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Global prevalence and major risk factors of diabetic retinopathy. Yau JW, Rogers SL, Kawasaki R, et al. Diabetes Care. 2012;35:556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Male-female differences in diabetic retinopathy? Ozawa GY, Bearse MA Jr, Adams AJ. Curr Eye Res. 2015;40:234–246. doi: 10.3109/02713683.2014.958500. [DOI] [PubMed] [Google Scholar]
- 19.People With Diabetes Can Prevent Vision Loss. [ Aug; 2023 ];https://www.nei.nih.gov/sites/default/files/2019- 06/diabetes-prevent-vision-loss.pdf 2020 2019:6. [Google Scholar]
- 20.Diabetic Retinopathy Screening: a short guide. [ Feb; 2022 ]. 2021. https://apps.who.int/iris/bitstream/handle/10665/336660/9789289055321-eng.pdf https://apps.who.int/iris/bitstream/handle/10665/336660/9789289055321-eng.pdf
- 21.Retinal vascular changes in pre-diabetes and prehypertension: new findings and their research and clinical implications. Nguyen TT, Wang JJ, Wong TY. Diabetes Care. 2007;30:2708–2715. doi: 10.2337/dc07-0732. [DOI] [PubMed] [Google Scholar]
- 22.The prevalence and risk factors of retinal microvascular abnormalities in older persons. Wong TY, Klein R, Sharrett AR, et al. Ophthalmology. 2003;110:658–666. doi: 10.1016/S0161-6420(02)01931-0. [DOI] [PubMed] [Google Scholar]
- 23.Sex differences in risk factors for retinopathy in non-diabetic men and women: the Tromsø Eye Study. Bertelsen G, Peto T, Lindekleiv H, et al. Acta Ophthalmol. 2014;92:316–322. doi: 10.1111/aos.12199. [DOI] [PubMed] [Google Scholar]
- 24.Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Wilkinson CP, Ferris FL, Klein RE, et al. Ophthalmology. 2003;110:1677–1682. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 25.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. https://pubmed.ncbi.nlm.nih.gov/12485966/ Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 26.European guidelines for obesity management in adults. Yumuk V, Tsigos C, Fried M, Schindler K, Busetto L, Micic D, Toplak H. Obes Facts. 2015;8:402–424. doi: 10.1159/000442721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diabetic retinopathy assessed by fundus photography in Pima Indians with impaired glucose tolerance and NIDDM. Nagi DK, Pettitt DJ, Bennett PH, Klein R, Knowler WC. Diabet Med. 1997;14:449–456. doi: 10.1002/(SICI)1096-9136(199706)14:6<449::AID-DIA367>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 28.P-selectin plasma levels and genetic variant associated with diabetic retinopathy in African Americans. Penman A, Hoadley S, Wilson JG, Taylor HA, Chen CJ, Sobrin L. Am J Ophthalmol. 2015;159:1152–1160. doi: 10.1016/j.ajo.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Retinopathy in subjects with impaired fasting glucose: the NANSY-Eye baseline report. Tyrberg M, Melander A, Lövestam-Adrian M, Lindblad U. Diabetes Obes Metab. 2008;10:646–651. doi: 10.1111/j.1463-1326.2007.00759.x. [DOI] [PubMed] [Google Scholar]
- 30.Measuring inconsistency in meta-analyses. Higgins JP, Thompson SG, Deeks JJ, Altman DG. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fasting and post-oral-glucose-load levels of methylglyoxal are associated with microvascular, but not macrovascular, disease in individuals with and without (pre)diabetes: the Maastricht Study. Hanssen NM, Scheijen JL, Houben AJ, et al. Diabetes Metab. 2021;47:101148. doi: 10.1016/j.diabet.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 32.The prevalence and risk factors of retinopathy and nephropathy in prediabetic population. Guney SC, Cay Y, Yildirim Simsir I, Kabaroglu C, Afrashi F, Saygili LF. Int J Diabetes Metab. 2022;43:1–8. [Google Scholar]
- 33.Prevalence and risk factors of diabetic retinopathy in Chongqing pre-diabetes patients. Chen X, Zhao Y, Zhou Z, Zhang X, Li Q, Bai L, Zhang M. Eye (Lond) 2012;26:816–820. doi: 10.1038/eye.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Early prevention of diabetes microvascular complications in people with hyperglycaemia in Europe. ePREDICE randomized trial. Study protocol, recruitment and selected baseline data. Gabriel R, Boukichou Abdelkader N, Acosta T, et al. PLoS One. 2020;15:0. doi: 10.1371/journal.pone.0231196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prevalence and risk factors for diabetic retinopathy in the multiethnic population of Mauritius. Dowse GK, Humphrey AR, Collins VR, et al. Am J Epidemiol. 1998;147:448–457. doi: 10.1093/oxfordjournals.aje.a009470. [DOI] [PubMed] [Google Scholar]
- 36.Visual impairment and retinopathy in people with normal glucose tolerance, impaired glucose tolerance, and newly diagnosed NIDDM. Klein R, Barrett-Connor EL, Blunt BA, Wingard DL. Diabetes Care. 1991;14:914–918. doi: 10.2337/diacare.14.10.914. [DOI] [PubMed] [Google Scholar]
- 37.High prevalence of diabetic retinopathy and nephropathy in Polynesians of Western Samoa. Collins VR, Dowse GK, Plehwe WE, Imo TT, Toelupe PM, Taylor HR, Zimmet PZ. Diabetes Care. 1995;18:1140–1149. doi: 10.2337/diacare.18.8.1140. [DOI] [PubMed] [Google Scholar]
- 38.Prevalence and risk factors for diabetic retinopathy in prediabetes in Asian Indians. Rajalakshmi R, UmaSankari G, Sivaprasad S, et al. J Diabetes Complications. 2022;36:108131. doi: 10.1016/j.jdiacomp.2022.108131. [DOI] [PubMed] [Google Scholar]
- 39.Blood pressure, lipids, and obesity are associated with retinopathy: the hoorn study. van Leiden HA, Dekker JM, Moll AC, et al. Diabetes Care. 2002;25:1320–1325. doi: 10.2337/diacare.25.8.1320. [DOI] [PubMed] [Google Scholar]
- 40.Retinopathy and visual impairment in diabetes, impaired glucose tolerance and normal glucose tolerance: the Nord-Trøndelag Health Study (the HUNT study) Sundling V, Platou CG, Jansson RW, Bertelsen G, Wøllo E, Gulbrandsen P. Acta Ophthalmol. 2012;90:237–243. doi: 10.1111/j.1755-3768.2010.01998.x. [DOI] [PubMed] [Google Scholar]
- 41.Prevalence and associations of diabetic retinopathy in a large cohort of prediabetic subjects: the Gutenberg Health Study. Lamparter J, Raum P, Pfeiffer N, et al. J Diabetes Complications. 2014;28:482–487. doi: 10.1016/j.jdiacomp.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 42.Cardiovascular risk factors and retinal microvascular signs in an adult Japanese population: the Funagata Study. Kawasaki R, Wang JJ, Rochtchina E, et al. Ophthalmology. 2006;113:1378–1384. doi: 10.1016/j.ophtha.2006.02.052. [DOI] [PubMed] [Google Scholar]
- 43.The prevalence of diabetic retinopathy in prediabetes: a prospective observational study in Kashmir. Arshid Arshid, Arshid Arshid, Adfar Adfar, Adfar I. Int J Inf Res Rev. 2022;9:10. [Google Scholar]
- 44.Determination of diabetic retinopathy prevalence and associated risk factors in Chinese diabetic and pre-diabetic subjects: Shanghai diabetic complications study. Pang C, Jia L, Jiang S, et al. Diabetes Metab Res Rev. 2012;28:276–283. doi: 10.1002/dmrr.1307. [DOI] [PubMed] [Google Scholar]
- 45.No ethnic differences in the association of glycated hemoglobin with retinopathy: the national health and nutrition examination survey 2005-2008. Bower JK, Brancati FL, Selvin E. Diabetes Care. 2013;36:569–573. doi: 10.2337/dc12-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prevalence and risk factors for retinopathy in persons without diabetes: the Singapore Indian Eye Study. Bhargava M, Cheung CY, Sabanayagam C, et al. Acta Ophthalmol. 2014;92:0–9. doi: 10.1111/aos.12446. [DOI] [PubMed] [Google Scholar]
- 47.Prevalence of diabetic retinopathy in India stratified by known and undiagnosed diabetes, urban-rural locations, and socioeconomic indices: results from the SMART India population-based cross-sectional screening study. Raman R, Vasconcelos JC, Rajalakshmi R, et al. Lancet Glob Health. 2022;10:1764–1773. doi: 10.1016/S2214-109X(22)00411-9. [DOI] [PubMed] [Google Scholar]
- 48.Microvascular retinopathy in subjects without diabetes: the Inter99 Eye Study. Munch IC, Kessel L, Borch-Johnsen K, Glümer C, Lund-Andersen H, Larsen M. Acta Ophthalmol. 2012;90:613–619. doi: 10.1111/j.1755-3768.2011.2148.x. [DOI] [PubMed] [Google Scholar]
- 49.The prevalence and determinants of cognitive deficits and traditional diabetic complications in the severely obese. Callaghan BC, Reynolds EL, Banerjee M, et al. Diabetes Care. 2020;43:683–690. doi: 10.2337/dc19-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prevalence and risk factors of diabetes and diabetic retinopathy in Liaoning province, China: a population-based cross-sectional study. Hu Y, Teng W, Liu L, et al. PLoS One. 2015;10:0. doi: 10.1371/journal.pone.0121477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.The relationship of retinal vascular calibre to diabetes and retinopathy: the Australian Diabetes, Obesity and Lifestyle (AusDiab) study. Tikellis G, Wang JJ, Tapp R, et al. Diabetologia. 2007;50:2263–2271. doi: 10.1007/s00125-007-0822-x. [DOI] [PubMed] [Google Scholar]
- 52.Estimated glomerular filtration rate and urinary albumin excretion are independently associated with greater arterial stiffness: the Hoorn Study. Hermans MM, Henry R, Dekker JM, et al. J Am Soc Nephrol. 2007;18:1942–1952. doi: 10.1681/ASN.2006111217. [DOI] [PubMed] [Google Scholar]
- 53.Impaired glycemia and diabetic polyneuropathy: the OC IG Survey. Dyck PJ, Clark VM, Overland CJ, et al. Diabetes Care. 2012;35:584–591. doi: 10.2337/dc11-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prevalence of ocular abnormalities in prediabetic patients. Sokolowska-Oracz A, Litwinczuk-Hajduk J, Piatkiewicz P. https://pubmed.ncbi.nlm.nih.gov/29715403/ Klin Oczna. 2016;118:23–28. [PubMed] [Google Scholar]
- 55.Prevalence and associated risk indicators of retinopathy in a rural Bangladeshi population with and without diabetes. Akhter A, Fatema K, Ahmed SF, Afroz A, Ali L, Hussain A. Ophthalmic Epidemiol. 2013;20:220–227. doi: 10.3109/09286586.2013.809770. [DOI] [PubMed] [Google Scholar]
- 56.Prevalence of retinopathy in people with diabetes, impaired glucose tolerance, and normal glucose tolerance. Rajala U, Laakso M, Qiao Q, Keinänen-Kiukaanniemi S. Diabetes Care. 1998;21:1664–1669. doi: 10.2337/diacare.21.10.1664. [DOI] [PubMed] [Google Scholar]
- 57.A study of prevalence of microalbuminuria and retinopathy in pre-diabetic patients presenting to a tertiary care centre. Kumar MR, Kumar BA, Alapati SD. Eur J Mol Clin Med. 2021;8:1–6. [Google Scholar]
- 58.Diabetic retinopathy among patients with prediabetes attending the outpatient department of Ophthalmology in a tertiary eye care centre: a descriptive cross-sectional study. Shrestha A, Suwal R, Adhikari S, Shrestha N, Shrestha B, Khatri B. JNMA J Nepal Med Assoc. 2023;61:351–354. doi: 10.31729/jnma.8118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Relation between fasting glucose and retinopathy for diagnosis of diabetes: three population-based cross-sectional studies. Wong TY, Liew G, Tapp RJ, et al. Lancet. 2008;371:736–743. doi: 10.1016/S0140-6736(08)60343-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Glycemic thresholds for diabetes-specific retinopathy: implications for diagnostic criteria for diabetes. Colagiuri S, Lee CM, Wong TY, Balkau B, Shaw JE, Borch-Johnsen K. Diabetes Care. 2011;34:145–150. doi: 10.2337/dc10-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.The prevalence of retinopathy in impaired glucose tolerance and recent-onset diabetes in the Diabetes Prevention Program. Diabet Med. 2007;24:137–144. doi: 10.1111/j.1464-5491.2007.02043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Duration of diabetes and prediabetes during adulthood and subclinical atherosclerosis and cardiac dysfunction in middle age: the CARDIA study. Reis JP, Allen NB, Bancks MP, et al. Diabetes Care. 2018;41:731–738. doi: 10.2337/dc17-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Comparison of two reference standards in validating two field mydriatic digital photography as a method of screening for diabetic retinopathy. Scanlon PH, Malhotra R, Greenwood RH, Aldington SJ, Foy C, Flatman M, Downes S. Br J Ophthalmol. 2003;87:1258–1263. doi: 10.1136/bjo.87.10.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Are seven standard photographic fields necessary for classification of diabetic retinopathy? Moss SE, Meuer SM, Klein R, Hubbard LD, Brothers RJ, Klein BE. https://pubmed.ncbi.nlm.nih.gov/2656572/ Invest Ophthalmol Vis Sci. 1989;30:823–828. [PubMed] [Google Scholar]
- 65.The English national screening programme for diabetic retinopathy 2003-2016. Scanlon PH. Acta Diabetol. 2017;54:515–525. doi: 10.1007/s00592-017-0974-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. Nathan DM, Genuth S, Lachin J, et al. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 67.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. https://pubmed.ncbi.nlm.nih.gov/9742976/ Lancet Lond Engl. 1998;352:837–853. [PubMed] [Google Scholar]
- 68.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. https://pubmed.ncbi.nlm.nih.gov/9732337/ BMJ. 1998;317:713. [PMC free article] [PubMed] [Google Scholar]
- 69.Serum markers of endothelial dysfunction and inflammation increase in hypertension with prediabetes mellitus. Huang Z, Chen C, Li S, Kong F, Shan P, Huang W. Genet Test Mol Biomarkers. 2016;20:322–327. doi: 10.1089/gtmb.2015.0255. [DOI] [PubMed] [Google Scholar]
- 70.Serum lipid and hsCRP levels in prediabetes--impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) Chakarova N, Tankova T, Atanassova I, Dakovska L. Diabetes Res Clin Pract. 2009;86:56–60. doi: 10.1016/j.diabres.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 71.Lipid profile in prediabetes. Kansal S, Kamble TK. https://pubmed.ncbi.nlm.nih.gov/27731552/ J Assoc Physicians India. 2016;64:18–21. [PubMed] [Google Scholar]
- 72.Diagnostic thresholds for diabetes: the association of retinopathy and albuminuria with glycaemia. Tapp RJ, Zimmet PZ, Harper CA, et al. Diabetes Res Clin Pract. 2006;73:315–321. doi: 10.1016/j.diabres.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 73.Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy. Early Treatment Diabetic Retinopathy Study (ETDRS) Report 22. Chew EY, Klein ML, Ferris FL 3rd, et al. Arch Ophthalmol. 1996;114:1079–1084. doi: 10.1001/archopht.1996.01100140281004. [DOI] [PubMed] [Google Scholar]
- 74.Lipids and Diabetic Retinopathy. Modjtahedi BS, Bose N, Papakostas TD, Morse L, Vavvas DG, Kishan AU. Semin Ophthalmol. 2016;31:10–18. doi: 10.3109/08820538.2015.1114869. [DOI] [PubMed] [Google Scholar]