Abstract
Hard-surface contact primes the conidia of Colletotrichum gloeosporioides to respond to plant surface waxes and a fruit-ripening hormone, ethylene, to germinate and form the appressoria required for infection of the host. Our efforts to elucidate the molecular events in the early phase of the hard-surface contact found that EGTA (5 mM) and U73122 (16 nM), an inhibitor of phospholipase C, inhibited (50%) germination and appressorium formation. Measurements of calmodulin (CaM) transcripts with a CaM cDNA we cloned from C. gloeosporioides showed that CaM was induced by hard-surface contact maximally at 2 h and then declined; ethephon enhanced this induction. The CaM antagonist, compound 48/80, completely inhibited conidial germination and appressorium formation at a concentration of 3 μM, implying that CaM is involved in this process. A putative CaM kinase (CaMK) cDNA of C. gloeosporioides was cloned with transcripts from hard-surface-treated conidia. A selective inhibitor of CaMK, KN93 (20 μM), inhibited (50%) germination and appressorium formation, blocked melanization, and caused the formation of abnormal appressoria. Scytalone, an intermediate in melanin synthesis, reversed the inhibition of melanization but did not restore appressorium formation. The phosphorylation of 18- and 43-kDa proteins induced by hard-surface contact and ethephon was inhibited by the treatment with KN93. These results strongly suggest that hard-surface contact induces Ca2+-calmodulin signaling that primes the conidia to respond to host signals by germination and differentiation into appressoria.
Conidia of many plant-pathogenic fungi sense physical or chemical signals from the plant surface to trigger germination and differentiation into an infection structure called the appressorium that is required to successfully penetrate into the host plant (10, 30). In anthracnose fungi belonging to the genus Colletotrichum, several species have been reported to produce appressoria in response to physical signals related to the topography of the leaf surface (17, 20, 31). Chemical signals have also been suggested to induce appressorium formation (14, 25). Germination and appressorium formation by Colletotrichum gloeosporioides were found to be induced specifically by the surface wax of its host but not by other plant waxes (27). Recently ethylene, the host ripening hormone, was found to signal germination and appressorium formation and thus help this fungus to time its infection to coincide with the ripening of the host fruit (11).
The signal transduction pathways involved in the perception of the various signals leading to infection structure formation are unclear. The use of inhibitors of protein kinases and protein phosphatase suggested that protein phosphorylation is involved in the induction of appressorium formation by Colletotrichum (12). A calmodulin (CaM) antagonist inhibited both germination and appressorium formation in Colletotrichum trifolii (9), implying that Ca2+ and CaM could function in the infection process. In C. gloeosporioides the response to host wax and ethylene requires the contact of conidia with a hard surface for a 2-h period (12). This hard-surface contact may be a touch-like response, which has been found to induce CaM-like proteins in Arabidopsis thaliana (2). On the basis of these observations we postulate that the hard-surface contact which primes the conidia to respond to chemical signals might use the CaM and CaM kinase (CaMK) signaling pathway in this early phase of interaction with the host. Here we present evidence that strongly supports this hypothesis. We also present evidence that a selective inhibitor of CaMK inhibits the melanization of the appressorium and that scytalone, an intermediate in melanin synthesis, can partially overcome this inhibition, suggesting that the CaMK inhibitor affects melanin synthesis at a step prior to that involving scytalone.
MATERIALS AND METHODS
Materials.
C. gloeosporioides, isolated from avocados, was provided by Dov Prusky (Volcani Centre, Bet Dagan, Israel). Cultures were maintained at 25°C on potato dextrose agar (PDA). KN93, U73122, and compound 48/80 were obtained from Calbiochem (La Jolla, Calif.). Ethephon (2-chloroethylphosphonic acid), EGTA, and other general reagents were purchased from Sigma (St. Louis, Mo.). Scytalone was a generous gift from Yasuyuki Kubo at Kyoto Prefectural University, Kyoto, Japan.
Tests for inhibition of germination and appressorium formation by inhibitors.
Germination and appressorium formation were tested on a cover glass surface as described previously (11, 27). Briefly, conidia from cultures grown on PDA for 3 to 5 days were harvested and washed with ice-cold water twice. Conidia (103) were suspended in 100 μl of 10 μM ethephon, which is known to produce ethylene in water (12), and the suspension was applied to a cover glass surface exposed by a 1-cm-diameter hole in the papafilm that was attached to the cover glass. The spore suspension containing 10 μM ethephon was mixed with appropriate concentrations of CaMK inhibitor KN93, phospholipase C inhibitor U73122, CaM antagonist compound 48/80, or Ca2+ chelator EGTA. The cover glasses were placed on polystyrene petri plates, and the plates were wrapped with aluminum foil to prevent inhibitor degradation by light and incubated for 18 h in the dark at 24°C. Germination and appressorium formation were recorded as described previously (27). To determine at which stage the inhibitors affect germination and appressorium formation, the inhibitors were added after different time periods without inhibitors and germination and appressorium formation were assessed at the end of the total 18-h period. Without inhibitor, virtually all of the conidia (>98%) germinated and almost all of them (>95%) formed appressoria after the 18-h incubation that was used in all experiments. Percent germination includes all conidia that germinated including those which formed appressoria. Percent inhibition of germination and percent inhibition of appressorium formation are based on the values obtained without the inhibitor.
Melanin synthesis inhibition and partial recovery of melanin synthesis by adding scytalone.
Conidia (103) were suspended as described above in a mixture of 10 μM ethephon, 20 μM KN93, and 1 mM scytalone. Germination and appressorium formation were assessed as indicated above. Spore samples were stained with lactophenol cotton blue, and pictures were taken with a Zeiss microscope.
Protein phosphorylation.
Protein phosphorylation was examined according to the methods described previously (12) with minor modifications. Spores (106 in 1 ml) were incubated for 3 h with 100 μCi of carrier-free disodium [32P]phosphate (Du Pont). One-half of the spore suspension (5 × 105 spores) was transferred to a Pyrex glass plate (150 mm in diameter) containing 20 ml of H2O and ethephon (10 μM) with or without KN93 (20 μM) for the time periods indicated in Fig. 8. At the end of incubation, conidia were harvested by scraping them off the plate with a rubber policeman and were recovered in a 2-ml microcentrifuge tube by centrifugation at 12,000 × g for 2 min. After being washed with water, conidia were broken in 400 μl of a buffer containing 10 mM Tris-HCl (pH 7.0), 1% β-mercaptoethanol, and 1% sodium dodecyl sulfate (SDS). Aliquots were assayed for radioactivity, and fractions containing 250,000 dpm of 32P were boiled for 5 min after a buffer (20% by volume) containing 60 mM Tris-HCl (pH 6.8), 25% glycerol, 2% SDS, 14.4 mM β-mercaptoethanol, and 0.1% bromophenol blue was added; the aliquots were then subjected to SDS–12% polyacrylamide gel electrophoresis. After the gel was dried, protein bands were analyzed either by a PhosphorImager (Molecular Dynamics) or by autoradiography.
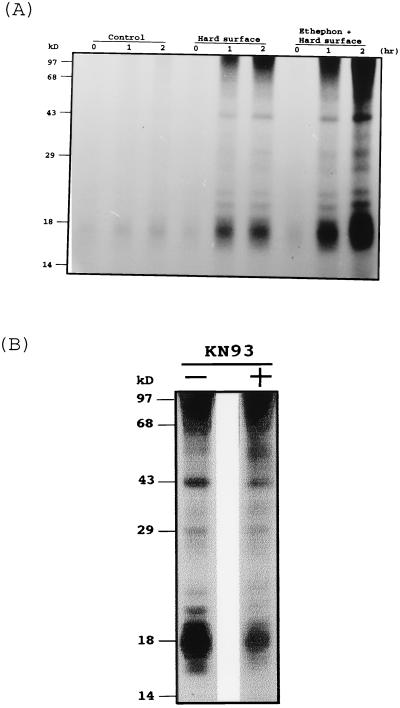
FIG. 8.
In vivo phosphorylation of proteins in C. gloeosporioides spores caused by exposure to a hard surface or to a hard surface and ethephon. (A) Spores were labeled with carrier-free inorganic [32P]phosphate for 3 h and then plated on Pyrex glass plates with or without ethephon (10 μM) for the indicated time. For the control lanes, conidia were incubated in a 2-ml tube for the indicated times. Proteins were subjected to SDS-polyacrylamide gel electrophoresis, and the gel was dried and subjected to autoradiography. (B) Spores were labeled and plated as described for panel A in 10 μM ethephon with or without KN93 (20 μM) for 2 h.
Isolation of total RNA for RNA blot analysis.
Conidia harvested from 3- to 4-day-old cultures were washed twice with ice-cold water, and 5 × 105 conidia suspended in 20 ml of water were placed in each of 30 Pyrex glass petri plates (150 mm in diameter) and incubated for different periods. At the end of incubation, conidia were harvested as described above and recovered by centrifugation at 12,000 × g for 10 min. The conidia were homogenized in 500 μl of a buffer (50 mM LiCl, 25 mM Tris-HCl (pH 7.6), 35 mM EGTA, 0.5% SDS)–500 μl of phenol-chloroform (50:50 [vol/vol]) with a Mini-bead beater (Biospec Products, Bartlesville, Okla.) for 5 min. After centrifugation at 12,000 × g for 5 min, the upper layer was transferred to a new tube and extracted with chloroform. RNA, precipitated by adding an equal volume of 4 M LiCl, was recovered by centrifugation at 12,000 × g for 10 min.
For RNA blot analysis total RNA (1 to 3 μg) was dissolved in a mixture containing 50% formamide, 16% formaldehyde, 20 mM MOPS [3-(N-morpholino)propanesulfonic acid], 5 mM sodium acetate, and 1 mM EDTA (pH 7.0) and incubated for 15 min at 65°C. Denatured RNA was subjected to electrophoresis on a 1.2% agarose gel containing 0.66 M formaldehyde and was blotted onto Nytran membranes (Schleicher & Schuell). Prehybridization and hybridization were performed at 65°C overnight in 6× SSPE (0.9 mM NaCl, 5 mM EDTA, 50 mM NaH2PO4, pH 7.4)–5× Denhardt’s solution (0.1% Ficoll, 0.1% polyvinylpyrrolidone, 0.1% bovine serum albumin)–0.1% SDS–100 μg of sheared salmon sperm DNA per ml. The 32P-labeled probe was prepared by random-primed labeling (Amersham). After hybridization the membranes were washed for 20 min at ambient temperature in 2× SSPE–0.1% SDS. Additional washing was carried out with 0.1× SSPE–0.1% SDS at 65°C for 20 min. The membranes were exposed to X-ray film at −80°C.
Cloning of a cDNA segment encoding CaM from C. gloeosporioides by RT-PCR.
RNA (30 μg) was transcribed by SuperScript II H− reverse transcriptase (RT) (BRL) with oligo(dT) to produce the first-strand cDNA in a 50-μl reaction mixture. After a 25-fold dilution of the first-strand reaction mixture with water, a 2-μl aliquot was used for PCR in a 50-μl reaction mixture with the following primers: sense primer, 5′-ATG GC(G/C) GAC TC(C/T) CT(G/T) AC(C/T) GAA G-3′; antisense primer, 5′-TTA (C/T)TT (C/T)TG CAT CAT (A/G)AG (C/T)TG G-3′. The PCR procedure consisted of an initial denaturation step at 94°C for 2.5 min and 40 cycles of the following steps: denaturation at 94°C for 30 s, annealing at 50°C for 30 s, and extension at 72°C for 30 s. A last elongation step was performed at 72°C for 15 min. The PCR product was analyzed on a 1% agarose gel and cloned into a pCR2.1 vector (Invitrogen), yielding pCM3.
Cloning of a cDNA segment encoding CaMK by RT-PCR.
After the first-strand cDNA was synthesized with SuperScript II H− RT (BRL) as described above, the resulting cDNA was used as a template to amplify the kinase with the following degenerate primers: sense primers, 5′-GA(C/T) TT(A/G) AA(A/G) CC(G/A/T/C) GA(A/G) AA-3′ and 5′-GA(C/T) CT(G/A/T/C) AA(A/G) CC(G/A/T/C) GA(A/G) AA-3′ based on amino acid sequence DLKPEN, conserved domain VI of serine/threonine protein kinase; antisense primers, 5′-CC (A/G/T)AT (A/G)CT CCA (A/G/T)AT (A/G)TC-3′ and 5′-CC (A/G/T)AT (G/A/T/C)GA CCA (A/G/T)AT (A/G)TC-3′, based on the amino acid sequence of conserved domain IX, DIWSIG. The conditions for PCR were the following: an initial denaturation step at 94°C for 2.5 min; 40 cycles of 30 s at 94°C, 30 s at 47°C, and 30 s at 72°C; and a last elongation step at 72°C for 15 min. PCR products were cloned into a pCR2.1 vector (Invitrogen). Clones were sequenced with an automated sequencer from Applied Biosystems (Foster City, Calif.). The protein homology search was conducted with a BLAST program from the National Center for Biotechnology Information (1). The one that had the greatest homology to CaMK was used as a probe for screening a cDNA library.
Isolation of a CaMK cDNA clone.
After the first-strand cDNA was synthesized from 60 μg of total RNA with SuperScript II H− RT (BRL), the second-strand cDNA was synthesized with a cDNA synthesis kit (Promega). After remnant RNA was digested with RNase A, cDNA was purified with a Geneclean kit (BIO 101), ligated with an EcoRI adapter, and used to construct a library in a λZAPII vector (Stratagene). The library was screened with the PCR fragment from the above step as the probe, and the insert from a positive clone was excised following the procedure provided by Stratagene to yield pBluescript plasmid pCgCMK, which was completely sequenced and analyzed as indicated above.
Nucleotide sequence accession numbers.
The sequences obtained during this study have been assigned GenBank accession no. AF034963 and AF034964.
RESULTS
Induction of CaM in conidia of C. gloeosporioides by hard-surface treatment and by ethephon.
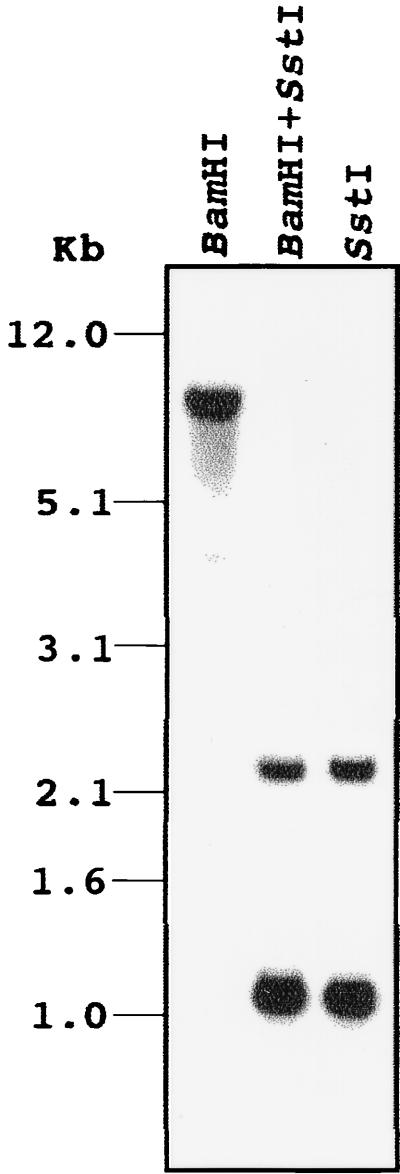
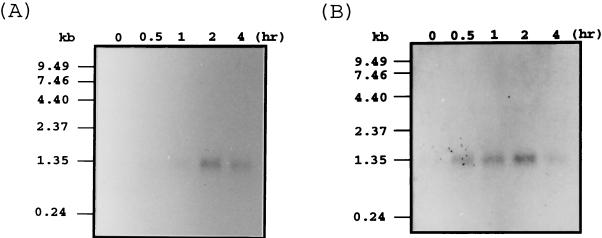
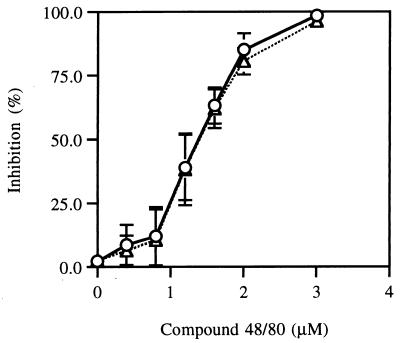
Previous experiments showed that induction of appressorium formation by host signals such as wax and ethylene required prior hard-surface contact of the conidia and that protein phosphorylation was involved in this process (12, 15). Conidia incubated on a soft surface such as 2% agar or agarose did not produce appressoria regardless of the presence of ethylene, although they germinated under this condition (data not shown), and a hydrophobic soft surface, petroleum jelly, did not allow appressorium formation (21). We reasoned that the hard-surface contact may be a touch-like stimulus, which has been shown to elevate CaM mRNA levels in Arabidopsis thaliana (2). To test whether hard-surface treatment of fungal conidia induces CaM synthesis, we first cloned CaM from C. gloeosporioides by PCR with primers designed from the CaM of other filamentous fungi such as Neurospora crassa and Aspergillus oryzae (23, 34). This approach yielded a single product that was sequenced to reveal an open reading frame (ORF) of 450 bp encoding 149 amino acids, the sequence of which was identical to those of CaM from N. crassa and C. trifolii (9a). At the nucleotide level the CaM cDNA from C. gloeosporioides showed 87 and 88% identity with those of N. crassa and C. trifolii, respectively. To analyze the genomic organization of the Colletorichum cam gene, genomic Southern blot analysis was performed with the cDNA as the probe (Fig. 1). BamHI digestion yielded a single band, whereas SstI digestion with or without BamHI showed two bands as expected from the presence of an internal SstI site in the cDNA. These results indicate that CaM of C. gloeosporioides exists as single copy, as found for other fungi (23, 28). RNA blot analysis of total RNA from conidia resting on a hard surface in the absence (Fig. 2A) or presence (Fig. 2B) of ethephon showed that the CaM cDNA hybridized to a single major RNA band of approximately 1,300 bp. This analysis showed that the CaM transcript level reached a maximum after 2 h of contact with the hard surface and then declined. Quantitation by a phosphorimager showed that the amount of RNA increased 2-fold after 30 min and 11-fold after 2 h of contact with the hard surface. Ethephon treatment of the conidia resting on the hard surface enhanced the level of CaM transcripts. The amount of RNA increased 8-fold after 30 min and 13-fold after 2 h in the presence of ethephon. To test whether a CaM antagonist has an inhibitory effect on germination and appressorium formation, compound 48/80 was used (32). Compound 48/80 completely inhibited both germination and appressorium formation at 3 μM (Fig. 3).
FIG. 1.
Southern blot analysis of CaM. A gel blot of 10 μg of restricted genomic DNA from C. gloeosporioides was probed with a 32P-labeled CaM cDNA.
FIG. 2.
RNA blot analysis of CaM. Three micrograms of total RNA from hard-surface-treated conidia (A) and 1 μg of total RNAs from hard-surface- and ethephon-treated conidia (B) were subjected to Northern blot analysis.
FIG. 3.
Inhibition of germination and appressorium formation by compound 48/80, a CaM antagonist. One thousand conidia were incubated in water containing 10 μM ethephon and the indicated concentrations of compound 48/80 on glass plates. Germination and appressorium formation were measured as described in Materials and Methods. The averages and standard deviations of the percent inhibitions of germination (▵) and appressorium formation (○) from three experiments are shown.
Cloning of CaMK from C. gloeosporioides.
The involvement of CaM in the early steps in conidium germination and appressorium formation suggested the possibility that CaMK may be also involved in this process. To test this possibility, we examined whether CaMK transcripts are present in the hard-surface-treated conidia. We used an RT-PCR approach to isolate a DNA segment encoding CaMK. Degenerate oligonucleotides coding for conserved sequence DLKPEN from domain VI and conserved sequence DIWSIG from domain IX of fungal CaMKs (18, 26) were used for RT-PCR with RNA extracted from hard-surface-treated conidia. This approach yielded a 190-bp product whose size is consistent with the segment coding for known CaMKs (data not shown). When this fragment was cloned and eight clones were sequenced, one clone showed a high level of homology in the regions from domain VI through domain IX of CaMKs. This cDNA segment was used to screen a cDNA library prepared from hard-surface-treated conidia. The resulting putative CaMK-encoding clone, designated pCgCMK, was 1,741 bp long and contained a single ORF that would encode a protein of 420 amino acids with an estimated molecular mass of 47 kDa. The deduced amino acid sequence of CgCMK is shown in Fig. 4. It has nine putative phosphorylation sites. Alignment of CgCMK to optimize homology with the predicted amino acid sequences of CaMKs from Aspergillus, Saccharomyces cerevisiae, and entomopathological fungus Metarrhizium anisopliae revealed that CgCMK had 31, 28, 28, and 32% identity with ACMPK, YCMKI, YCMKII, and MaCMK, respectively (Fig. 4). CgCMK contains the CaM binding domain (boxed in Fig. 4) and the 11 conserved kinase domains.
FIG. 4.
Alignment of the predicted amino acid sequences of CgCMK and other fungal CaMKs: ACMPK from Aspergillus nidulans (18), YCMK1 and YCMK2 from Saccharomyces cerevisiae (26), and MaCMK from Metarrhizium anisopliae (16a). The sequences in the box represent the putative CaM-binding domains of CgCMK, YCMK1, YCMK2, and MaCMK. Solid circles above the CgCMK sequence indicate potential autophosphorylation sites containing the KRXXST consensus phosphorylation site for CaMKs.
Inhibition of germination and appressorium formation by selective inhibitor of CaMK KN93.
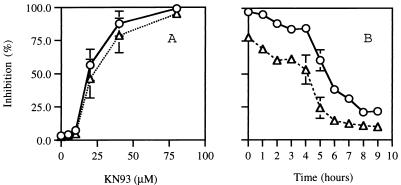
To determine whether CaMK is involved in germination and appressorium formation, we tested the effects of selective inhibitor KN93 (22) on germination and appressorium formation induced by ethephon on a hard surface. Germination and appressorium formation were severely inhibited by KN93 (Fig. 5). The inhibition was detected at around 10 μM KN93; at 20 μM KN93 50% inhibition of germination and appressorium formation was observed, and at 80 μM nearly complete inhibition was observed (Fig. 5A). Previous observation of appressorium formation induced by ethephon showed that the C. gloeosporioides conidia began to germinate within 3 to 5 h and that the tip of the germ tube began to swell within 5 to 9 h (11). A mature appressorium with a dark brown cell wall appeared after 10 to 12 h. To test at which stage the inhibitor interferes with germination and appressorium formation, KN93 was added to the solution into which conidia were placed at various periods after the incubation started (Fig. 5B). KN93 inhibited more than 50% of the germination when it was added within 4 h of the beginning of incubation with ethephon on a hard surface. Appressorium formation was more severely inhibited: more than 80% of appressorium formation was inhibited by the same treatment. The addition of KN93 after 6 h had very little effect on germination and appressorium formation. These results suggest that CaMK affects germination and appressorium formation in the early phase of hard-surface contact.
FIG. 5.
Relationship between the inhibition of germination and appressorium formation and the time of addition of KN93 into water containing 10 μM ethephon and 103 conidia of C. gloeosporioides on glass plates. (A) KN93 (at indicated concentrations) was added to glass plates at the beginning of the experiment. Germination and appressorium formation were measured after 18 h of incubation, and percentages of germination (▵) and appressorium formation (○) inhibition from three experiments are shown. (B) KN93 (40 μM final concentration) was added to the culture fluid on glass plates after the indicated periods without inhibitor, and percentages of germination (▵) and appressorium formation (○) inhibition from two experiments are shown.
Effect of KN93 on melanization of the appressorium and appressorial morphology.
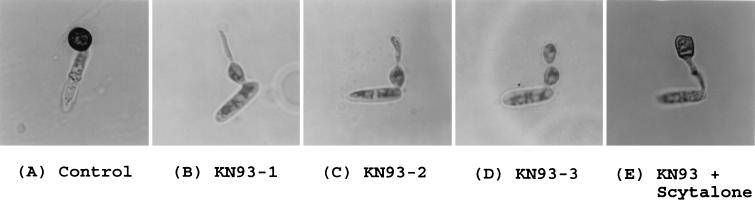
Microscopic examination of the appressoria formed in the presence of KN93 showed that this compound not only decreased the frequency of appressorium formation but also affected the structure of the appressoria that were formed. Without KN93, conidia of C. gleoesporioides produced highly melanized black appressoria (Fig. 6A). However, at around 20 μM KN93 less than 50% of the conidia produced appressorium-like structures and these structures showed much less melanization. Under such conditions the appressorium-like structures showed unusual morphological features including not-well-rounded shapes, long germ tube-like structures, and additional appressorium-like structures arising from the original appressorium, as illustrated in Fig. 6B, C, and D. In all of these cases the lack of melanization was obvious. To determine whether the inhibition of appressorium formation and melanization by KN93 involved steps prior to the formation of a key intermediate in melanin synthesis, scytalone, we tested whether exogenous scytalone, which is known to be incorporated into melanin (19), could reverse the effect of KN93. When conidia were incubated in 1 mM scytalone–20 μM KN93–10 μM ethephon there was no measurable effect on the degree of inhibition of appressorium formation. However, the appressorium-like structures formed in the presence of scytalone showed melanization even when the appressoria were not well rounded (Fig. 6E) and no secondary growth from the appressoria was found. These observations suggested that CaMK inhibited melanization at some step(s) prior to the formation of scytalone.
FIG. 6.
Effect of KN93 treatment on the morphology of the appressorium and the recovery of melanization by scytalone. (A) Spores were incubated in 10 μM ethephon on glass plates for 18 h; (B, C, and D) spores were incubated in 10 μM ethephon and 20 μM KN93; (E) spores were incubated in 10 μM ethephon, 20 μM KN93, and 1 mM scytalone.
Involvement of Ca2+ in germination and appressorium formation.
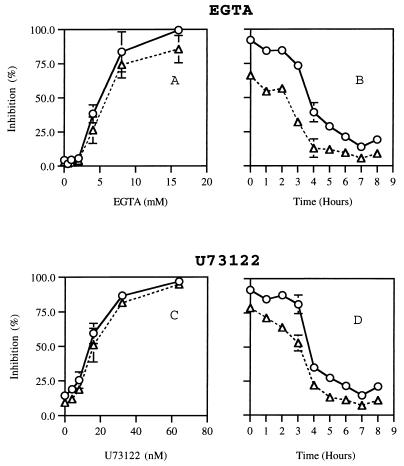
CaMK involvement in germination and appressorium formation is expected to involve Ca2+ (4). To test whether Ca2+ was involved in germination and appressorium formation, the effect of specific Ca2+ chelator EGTA was tested (Fig. 7A). The inhibition of germination and appressorium formation by EGTA began at around 2 mM and reached 50% at 5 mM, with severe inhibition at 16 mM. The cytosolic Ca2+ concentration can be elevated by a release from intracellular stores via receptor-generated second messengers (3). One common mechanism is the release of internal Ca2+ by IP3 generated by phospholipase C. To test for the possible involvement of this mechanism in germination and appressorium formation, the effect of U73122, an inhibitor of phospholipase C (35), on germination and appressorium formation was tested. This inhibitor was found to be highly effective in inhibiting germination and appressorium formation, with complete inhibition at 64 nM (Fig. 7C). To test at which stage EGTA and U73122 interfere with germination and appressorium formation, inhibitors were added to the solution in which conidia were placed after various periods of incubation on the hard surface with ethephon (Fig. 7B and D). The addition of EGTA within 2 h of hard-surface contact caused 84 ± 4% inhibition of appressorium formation and 56 ± 2% inhibition of germination. The addition of EGTA during subsequent periods caused much less inhibition; in all cases appressorium formation was more severely inhibited than germination (Fig. 7B). U73122 addition during the early period of hard-surface contact (2 to 3 h) caused severe inhibition of appressorium formation and less inhibition of germination (Fig. 7D). These results suggest that Ca2+ is involved in the early phase of germination and appressorium formation.
FIG. 7.
Effect of a calcium chelator and a phospholipase C inhibitor on germination and appressorium formation induced by ethephon on a hard surface. (A and C) The effect of concentration of the inhibitor on germination (▵) and appressorium formation (○); (B and D) the effect of the addition of 15 mM EGTA (B) and 40 nM U73122 (D) after the indicated periods of time without the inhibitor on germination (▵) and appressorium formation (○). Experiments were performed as indicated in Materials and Methods, and the standard deviation is shown for each data point.
Inhibition of protein phosphorylation by CaMK inhibitor in the early phase of germination and appressorium formation.
To test for the possible involvement of phosphorylation in the early phase of induction of germination and appressorium formation, in vivo protein phosphorylation caused either by hard-surface treatment or by hard-surface and ethephon treatment of conidia was examined. Conidia placed in 2-ml tubes rotating at 7 rpm were incubated with carrier-free disodium [32P]phosphate for 3 h to achieve 32P-labeling of the nucleotide pools. After this 3-h treatment, conidia were placed on a Pyrex glass plate with or without ethephon, and the protein phosphorylation pattern was monitored for a 2-h period. As shown in Fig. 8A, protein phosphorylation was apparently increased by hard-surface treatment compared to that of a control in which the conidia remained in the rotating tube; the protein phosphorylation was further enhanced by ethephon treatment. Two proteins of 18 and 43 kDa were more heavily labeled as a result of hard-surface and ethephon treatment (Fig. 8A). The phosphorylation of these proteins increased over a treatment period of up to 2 h.
Quantitation of the phosphorylation shown in Fig. 8B by a PhosphorImager showed that the application of KN93 to conidia at the time of their transfer to the Petri dish decreased the labeling of 18- and 43-kDa proteins by 50 and 30%, respectively. These data indicated that KN93 was an inhibitor of the fungal kinases involved in germination and appressorium formation induced by hard-surface and ethephon treatment.
DISCUSSION
Hard-surface contact is essential for the conidia of many fungal pathogens to germinate and form appressoria (13, 31). In C. gloeosporioides this differentiation process was found to be induced by host surface wax and ethylene. However, the response to these chemical signals required prior hard-surface treatment for a 2-h period (12). Conidia treated with a soft surface such as 2% agar or agarose exhibited only germination without appressorium formation regardless of the presence of ethylene (data not shown). The molecular events triggered in the conidia by the hard-surface contact are not understood. Since this contact may be a touch-like stimulus that is known to induce CaM-like proteins in Arabidopsis thaliana, we tested whether CaM is involved in signaling the conidial contact of the hard surface that is required for germination and appressorium formation in fungi. We cloned the C. gloeosporioides CaM and found it to be identical to CaM in other fungi (9a, 23). Transcript level measurements showed that during the contact with the hard surface CaM transcripts were induced to a maximum level after 2 h of contact, and ethylene enhanced CaM induction.
If CaM is involved in germination and appressorium induction, CaMK might be involved in this process. We cloned a putative cDNA for CaMK with transcripts from hard-surface-treated conidia of C. gloeosporioides and found it to be homologous to those encoding two other fungal CaMKs and yeast CaMKs so far cloned (16a, 18, 26). No CaMK gene from phytopathogenic fungi has been cloned heretofore. In support of our identification of the cDNA cloned from C. gloeosporioides as that encoding CaMK, the deduced amino acid sequence contains the CaM binding domain and the 11 conserved kinase domains. The involvement of CaMK in germination and appressorium formation was strongly suggested by the inhibition of germination and appressorium formation at a low concentration of a selective inhibitor of CaMK. The microscopic examination clearly showed that KN93 inhibited not only appressorium formation but also melanization. Lack of melanization was associated with development of unusual structures such as long germ tubes, the swelling of these secondary germ tubes, and the formation of appressorium-like structures from the original appressorium. Such structures were very similar to those observed with C. gloeosporioides conidia treated with protein phosphatase inhibitor calyculin A (12) and with the melanin-deficient albino mutants of Colletotrichum lagenarium (19) and Magnaporthe grisea (6). When the melanization was restored by the addition of scytalone, even in the presence of KN93, the appressorium that was formed did not develop the unusual secondary structures from the original appressorium-like structures. The recovery of melanization by the albino mutants with exogenous scytalone also prevented the development of the unusual structures in C. lagenarium (19). Present and previous reports (19) suggest that the melanized walls normally constitute the limiting structural barrier of the appressorium and that in the absence of such a structural constraint the appressorium continues to produce protrusions such as long germ tube-like and appressorium-like structures. Which step(s) in scytalone synthesis is inhibited by KN93 is not known. In any case, since scytalone could not reverse the inhibition of appressorium formation caused by KN93, it is likely that KN93 inhibited not only melanin synthesis but also other biochemical reactions involved in appressorium formation.
Ca2+ has been reported to be necessary for germination and appressorium formation (8). Our results with EGTA suggest that Ca2+ is necessary for germination and appressorium formation in C. gloeosporioides, and this observation is consistent with the suggested involvement of CaM and CaMK in this process as in other fungi such as Penicillium notatum (29) and C. trifolii (9). The strong inhibition of germination and appressorium formation by phospholipase C inhibitor U73122 suggests that release of internal Ca2+ by IP3 may be involved in this differentiation process. The inhibition of germination and appressorium formation by U73122 could also indicate that the involvement of the other product of phospholipase C, diacylglycerol, in the activation of protein kinase C might play a role in germination and appressorium formation. Although protein kinase C has not been shown to be involved in germination and appressorium formation in this fungus, such a possibility cannot be ruled out.
The initial hard-surface contact, that presumably primes the conidia to respond to chemical signals, induces protein phosphorylation as shown by the enhanced 32P incorporation. Such labeling could result from different kinases. In the present experiments the inhibition of the phosphorylation of 18- and 43-kDa proteins by KN93 strongly suggests that CaMK is involved in this enhanced phosphorylation. Ethylene enhanced the phosphorylation of these proteins. Previous experiments had indicated that host signals (wax and ethylene) enhanced the phosphorylation of 29- and 43-kDa proteins (12). The hard-surface contact and the chemical signals may trigger phosphorylation of the same components of the signal transduction pathway, while some components of this pathway may be unique to the specific signal. The differential inhibition of phosphorylation by kinase inhibitors had already indicated that induction of germination and appressorium formation by wax and ethylene involves unique and shared phosphorylation pathways (12). The present results indicate the involvement of another kinase during the very early phase of germination and appressorium induction by hard-surface contact. CaMK has been shown to affect nuclear division during cell cycle and hyphal growth in Aspergillus (7). Recent results have provided evidence for the involvement of cyclic AMP-dependent protein kinase (24) as well as adenyl cyclase (5) and mitogen-activated protein kinase in appressorium formation by M. grisea (33).
The molecular events triggered by hard-surface contact of the conidia represent the earliest communication between a fungus and its host. Since such a prior contact appears to be necessary for the chemical signals from the host to trigger germination and appressorium formation, the contact-triggered events play a crucial role in host-pathogen interaction. The CaM and CaMK involvement in the early signal transduction probably triggers the expression of a set of genes whose expression may prime the conidia to respond to the host’s chemical signals. We have recently isolated a set of these early genes, including one that encodes a ubiquitin-conjugating enzyme (21). The response to the chemical signals involves the induction of another set of genes. Several genes induced by the chemical signals have been previously cloned (16), and disruption of one of them was found to allow the formation of normal-looking appressoria with drastically decreased virulence. Such early processes may offer new targets for intervention in the host-pathogen interaction to protect plants.
ACKNOWLEDGMENTS
We are indebted to Yasuyuki Kubo for generously providing us with scytalone and to Linda Rogers and Zhimei Liu for helpful comments and technical assistance.
This work was supported in part by National Science Foundation grant no. IBN-9318554. Y.-K. Kim was supported by a doctoral fellowship from the Korean Ministry of Education.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Braam J, Davis R W. Rain-, wind-, and touch-induced expression of calmodulin and calmodulin-related genes in Arabidopsis. Cell. 1990;60:357–364. doi: 10.1016/0092-8674(90)90587-5. [DOI] [PubMed] [Google Scholar]
- 3.Braun A P, Schulman H. The multifunctional calcium/calmodulin-dependent protein kinase: from form to function. Annu Rev Physiol. 1995;57:417–445. doi: 10.1146/annurev.ph.57.030195.002221. [DOI] [PubMed] [Google Scholar]
- 4.Bush D S. Calcium regulation in plant cells and its role in signaling. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:95–122. [Google Scholar]
- 5.Choi W, Dean R A. The adenylate cyclase gene MAC1 of Magnaporthe grisea controls appressorium formation and other aspects of growth and development. Plant Cell. 1997;9:1973–1983. doi: 10.1105/tpc.9.11.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chumley F G, Valent B. Genetic analysis of melanin-deficient, nonpathogenic mutants of Magnaporthe grisea. Mol Plant-Microbe Interact. 1990;3:135–143. [Google Scholar]
- 7.Dayton J S, Sumi M, Nanthakumar N N, Means A R. Expression of a constitutively active Ca2+/calmodulin-dependent kinase in Aspergillus nidulans spores prevents germination and entry into the cell cycle. J Biol Chem. 1997;272:3223–3230. doi: 10.1074/jbc.272.6.3223. [DOI] [PubMed] [Google Scholar]
- 8.Dean R A. Signal pathways and appressorium morphogenesis. Annu Rev Phytopathol. 1997;35:211–234. doi: 10.1146/annurev.phyto.35.1.211. [DOI] [PubMed] [Google Scholar]
- 9.Dickman, M. B., T. L. Buhr, V. Warwar, G. M. Truesdell, and C. X. Huang. 1995. Molecular signals during the early stages of alfalfa anthracnose. Can. J. Bot. 73(Suppl. 1):S1169–S1177.
- 9a.Dickman, M. B., et al. GenBank accession no. U15993.
- 10.Emmet R W, Parberry D G. Appressoria. Annu Rev Phytopathol. 1975;13:147–167. [Google Scholar]
- 11.Flaishman M A, Kolattukudy P E. Timing of fungal invasion using host’s ripening hormone as a signal. Proc Natl Acad Sci USA. 1994;91:6579–6583. doi: 10.1073/pnas.91.14.6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flaishman M A, Hwang C-H, Kolattukudy P E. Involvement of protein phosphorylation in the induction of appressorium formation in Colletotrichum gloeosporioides by its host surface wax and ethylene. Physiol Mol Plant Pathol. 1995;47:103–117. [Google Scholar]
- 13.Freytag S, Bruscaglioni L, Gold R E, Mendgen K. Basidiospores of rust fungi (Uromyces species) differentiate infection structures in vitro. Exp Mycol. 1988;12:275–283. [Google Scholar]
- 14.Grover G K. Participation of host exudate chemicals in appressorium formation by Colletotrichum piperatum. In: Preece T F, Dickinson C H, editors. Ecology of leaf surface microorganisms. London, United Kingdom: Academic Press; 1971. pp. 509–518. [Google Scholar]
- 15.Hwang C-S, Kolattukudy P E. Isolation and characterization of genes expressed uniquely during appressorium formation by Colletotrichum gloeosporioides conidia induced by the host surface wax. Mol Gen Genet. 1995;247:282–294. doi: 10.1007/BF00293196. [DOI] [PubMed] [Google Scholar]
- 16.Hwang C-S, Flaishman M A, Kolattukudy P E. Cloning of a gene expressed during appressorium formation by Colletotrichum gloeosporioides and a marked decrease in virulence by disruption of this gene. Plant Cell. 1995;7:183–193. doi: 10.1105/tpc.7.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16a.Joshi, L., et al. GenBank accession no. U28358.
- 17.Kolattukudy P E, Rogers L M, Li D, Hwang C-S, Flaishman M A. Surface signaling in pathogenesis. Proc Natl Acad Sci USA. 1995;92:4080–4087. doi: 10.1073/pnas.92.10.4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kornstein L B, Gaiso M L, Hammell R L, Bartelt D C. Cloning and sequence determination of a cDNA encoding Aspergillus nidulans calmodulin-dependent multifunctional protein kinase. Gene. 1992;113:75–82. doi: 10.1016/0378-1119(92)90671-b. [DOI] [PubMed] [Google Scholar]
- 19.Kubo Y, Suzuki K, Furusawa I, Yamamoto M. Scytalone as a natural intermediate of melanin biosynthesis in appressoria of Colletotrichum lagenarium. Exp Mycol. 1983;7:208–215. [Google Scholar]
- 20.Lapp M S, Skoropad W P. Location of appressoria of Colletotrichum graminicola on natural and artificial barley leaf surfaces. Trans Br Mycol Soc. 1978;70:225–228. [Google Scholar]
- 21.Liu, Z.-M., and P. E. Kolattukudy. Identification of a gene product induced by hard-surface contact of Colletotrichum gloeosporioides conidia as a ubiquitin-conjugating enzyme by yeast complementation. J. Bacteriol. 180:3592–3597. [DOI] [PMC free article] [PubMed]
- 22.Mamiya N, Goldenring J R, Tsunoda Y, Modlin I M, Yasui K, Usuda N, Ishikawa T, Natsume A, Hidaka H. Inhibition of acid secretion in gastric parietal cells by the Ca2+/calmodulin-dependent kinase II inhibitor KN93. Biochem Biophys Res Commun. 1993;195:608–615. doi: 10.1006/bbrc.1993.2089. [DOI] [PubMed] [Google Scholar]
- 23.Melnick M B, Melnick C, Lee M, Woodward D O. Structure and sequence of the calmodulin gene from Neurospora crassa. Biochim Biophys Acta. 1993;1171:334–336. doi: 10.1016/0167-4781(93)90079-s. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell T K, Dean R A. The cAMP-dependent protein kinase catalytic subunit is required for appressorium formation and pathogenicity by the rice blast pathogen Magnaporthe grisea. Plant Cell. 1995;7:1869–1878. doi: 10.1105/tpc.7.11.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parberry D G, Blakeman J P. Effect of substances associated with leaf surfaces on appressorium formation by Colletotrichum acutatum. Trans Br Mycol Soc. 1978;70:7–19. [Google Scholar]
- 26.Pausch M H, Kaim D, Kunisawa R, Admon A, Thorner J. Multiple Ca2+/calmodulin-dependent kinase genes in a unicellular eukaryote. EMBO J. 1991;10:1511–1522. doi: 10.1002/j.1460-2075.1991.tb07671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Podila G K, Rogers L M, Kolattukudy P E. Chemical signals from avocado surface wax trigger germination and appressorium formation in Colletotrichum gloeosporioides. Plant Physiol. 1993;103:267–272. doi: 10.1104/pp.103.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasmunssen C D, Means R L, Lu K P, May G S, Means A R. Characterization and expression of the unique calmodulin gene of Aspergillus nidulans. J Biol Chem. 1990;265:13767–13775. [PubMed] [Google Scholar]
- 29.Roufogalis B D. Specificity of trifluoperazine and related phenothiazines for calcium binding protein. In: Chung W Y, editor. Calcium and cell function. Vol. 3. New York: Academic Press; 1982. pp. 130–159. [Google Scholar]
- 30.Staples R C, Hoch H C. Infection structures—form and function. Exp Mycol. 1987;11:163–169. [Google Scholar]
- 31.Staples R C, Macko V. Formation of infection structures as a recognition response in fungi. Exp Mycol. 1980;4:2–16. [Google Scholar]
- 32.St. Leger R J, Butt T M, Roberts D W, Staples R C. Production in vitro of appressoria by the entomopathogenic fungus Metarrhizium anisopliae. Exp Mycol. 1989;13:274–288. [Google Scholar]
- 33.Xu J-R, Hamer J E. MAP kinase and cAMP signaling regulate infection structure formation and pathogenic growth in the rice blast fungus Magnaporthe grisea. Genes Dev. 1996;10:2696–2706. doi: 10.1101/gad.10.21.2696. [DOI] [PubMed] [Google Scholar]
- 34.Yasui K, Kitamoto K, Gomi K, Kumagai C, Ohya Y, Tamura G. Cloning and nucleotide sequence of the calmodulin-encoding gene (cmdA) from Aspergillus oryzae. Biosci Biotechnol Biochem. 1995;59:1444–1449. doi: 10.1271/bbb.59.1444. [DOI] [PubMed] [Google Scholar]
- 35.Yule D, Williams J A. U73122 inhibits Ca2+ oscillations in response to cholecystokinin and carbachol but not to JMV-180 in rat pancreatic acinar cells. J Biol Chem. 1992;267:13830–13835. [PubMed] [Google Scholar]