Summary
Background
Diabetic foot ulcers (DFUs) are a common complication of diabetes, associated with important morbidity. Appropriate animal models of DFUs may improve drug development, and subsequently the success rate of clinical trials. However, while many models have been proposed, they are extremely heterogeneous, and no standard has emerged. We thus propose a systematic review with a network meta-analysis (NMA) to gather direct and indirect evidence, and compare the different mouse models of diabetes-related ulcers.
Methods
The systematic search was performed in Pubmed and Embase. The main outcomes were wound size measurement at days 3, 7, 11 and 15 (±1 day). The risk of bias and methodological quality of all included studies was assessed by using the Systematic Review Center for Laboratory animal Experimentation (SYRCLE) risk of bias tool. Meta-regressions were done on prespecified variables, including mouse strain, type of ulcer, sex, age, and use of a splint.
Findings
We included 295 studies. Among all models, only db/db, ob/ob, streptozotocin (STZ), and STZ + high fat diet mice showed a significantly delayed wound healing, compared with controls, at each time point. Age, sex and ulcer type had influence on wound healing, although not at all time points.
Interpretation
In conclusion, the db/db model is associated with the largest delay in wound healing The STZ model also exhibits significantly decreased wound healing. STZ + high fat diet and ob/ob mice may also be relevant models of diabetes-related ulcers, although the results rely on a more limited number of studies.
Funding
This work was funded by the Agence Nationale de la Recherche (grant ANR-18-CE17-0017).
Keywords: Network meta-analysis, Diabetes, Wound healing, Animal models, Diabetic foot ulcers
Research in context.
Evidence before this study
There is an extensive amount of experimental studies using animal models of diabetes-related ulcers, but few have compared the different models. We searched PubMed and Embase, with the terms “models, animal” OR “disease models, animal” AND “diabetes mellitus” AND “wound healing” AND “mice” from their inception until February 18th, 2021. In addition, we searched for systematic reviews on the topic (Pubmed with the search terms “models, animal” OR “disease models, animal” AND “diabetes mellitus” AND “wound healing”, applying the “systematic review” filter). We found 39 original articles including direct comparisons between a wide variety of different models, and only one meta-analysis providing estimates of the impact of different models on wound healing but without comparison between these models. Therefore, the amount of available articles (direct comparisons) but the lack of evidence for each comparison between models of diabetes-related ulcers justified this work.
Added value of this study
To address this question, we conducted a network meta-analysis combining direct and indirect comparisons. Based on 267 studies, this work compares the different mouse models of diabetes-related ulcers and provides new information on the relevance of these models. More specifically, this study suggests that only the db/db mice and STZ models were consistently associated with impaired wound healing. Finally, the use of splinting to inhibit early contraction reduces the impact of diabetes on wound healing, and may only be used on db/db mice.
Implications of all the available evidence
By providing comparisons of the effect of many experimental models on wound healing, this study may help researchers to use adequate preclinical models of diabetes-related ulcers. This may further contribute to standardizing practices, and make comparisons between interventions tested on different models easier.
Introduction
Diabetes is a chronic disease affecting nearly 537 million adults worldwide.1 Among its complications, diabetic foot ulcers (DFU) are associated with significant morbidity and represent a public health burden, diabetes being the leading cause of nontraumatic lower extremity amputations. Indeed, DFU are responsible for a lower limb loss every 30 s worldwide.1 Finally, long-term survival is poor in patients who have undergone major surgery: a recent, prevalent cohort study shows that more than 11% of patients who had major amputation died within 30 days.2
To date, there is no specific pharmacological treatment with demonstrated efficacy to enhance wound healing. The heterogeneity of the disease and its complex pathophysiology have made drug development particularly challenging in this field, with many failures at the clinical stage. Appropriate preclinical models may improve our understanding of the disease and help establishing the proof-of-concept for new drug candidates, and subsequently the success rate of clinical trials. Preclinical research on DFUs has mostly relied on murine models. Yet, a variety of models have been proposed, combining several features: first, the model of diabetes, based on specific genetic strains or the use of toxic agents or diabetogenic diet3,4; second, the type wound and the ulceration protocol5; finally, other variables like age, sex, duration of diabetes, use of splinting or wound covering also vary across the models.6, 7, 8
The multiplicity of combinations makes the comparisons between existing models difficult, and most direct comparisons rely on small sample sizes. This prevents from establishing standards. The objective of this study is to gather direct and indirect evidence through a systematic review with a network meta-analysis (NMA), in order to compare the different mouse models of diabetes-related ulcers.
Methods
The protocol for this systematic review and NMA was registered on PROSPERO (CRD42021286873, available at https://www.crd.york.ac.uk/PROSPERO) and was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement.9
Search methods and study selection
The systematic search was performed in Pubmed and Embase databases from their inception until February 18th, 2021. Search key words are available in Table S1. We also screened all references of included articles. After the initial search, one investigator screened the titles and the abstracts to identify duplicates. Then, two investigators (A.C. and C.C.) independently screened eligible articles, based on the abstract and title, or the full manuscript when needed. Any discrepancy was discussed with a third investigator (M.R.) to homogenize our search strategy and study selection.
Eligibility criteria were: english language; in vivo experiments in a mouse model of diabetes-related ulcers; with at least one model and its non-diabetic control, or two different models of diabetes-related ulcer; without any active pharmacological treatment (placebo or vehicle were not considered as treatments and were then eligible). We also excluded any combination of >1 model in the same group of animals (e.g., diabetes-related ulcer + model for another disease/condition).
Data extraction
The main outcomes were wound size measurement (or wound healing) at days 3, 7, 11 and 15 (±1 day). When possible, the data were initially transformed to be expressed as the percentage of the ulcer size at day 0. Pre-specified variables were also extracted: model of diabetes, mouse strain, weight, sex, age of the animals, type of ulcer (excisional vs pressure), wound location, wound size at day 0, method to measure the wound size, use of a splint, wound covered or not, and ulcer treated with a placebo/vehicle or not.
During a first extraction stage, data were extracted by two investigators (A.C. and C.C.), independently, and compared to make sure that data extraction was consistent between the two investigators. Any discrepancy was discussed with a third investigator (M.R.), until data extraction was deemed homogenous between the two investigators, which was reached after approximately ten percent of all articles were extracted. Data extraction for the rest of the articles was completed by one of these two investigators.
Assessment of methodological quality
The risk of bias and methodological quality of all included studies was assessed by using the Systematic Review Center for Laboratory animal Experimentation (SYRCLE) risk of bias tool.10 We scored the selection, performance, detection, attrition, and reporting biases as “Yes”, “No”, or “Unclear”.
The same approach than for data extraction was used to homogenize risk of bias and quality assessment, i.e., review by two investigators, independently, and discrepancies discussed with a third investigator until rating was deemed homogenous.
Statistical analysis
Since the data were expressed differently between studies (e.g., percentage of wound closure or wound healing, wound area in cm2 or in mm2, or wound diameter), they were all expressed as standardized mean differences (SMD), with their 95% confidence interval. In case of missing standard deviations (SD), they were calculated from the standard error of the mean, when available, or imputed using mean SD from studies expressing wound healing using the same units. Studies with extreme values (SMD <−10 or >10) were first checked for data accuracy and excluded from the analysis if there were no data extraction error. Normality and absence of skewness of SMD were checked by calculating the highest possible value minus the observed mean, divided by the SD. No ratio was inferior to two, suggesting no skewness.11
For each time point, we performed a frequentist network meta-analysis to assess the impact of the model on wound healing using the netmeta package. Given the heterogeneity in included studies in the meta-analyses, we used random-effects models. Convergence of the model was examined through trace plots and Gelman-Rubin shrink factor. We assessed network heterogeneity using τ and I2 statistics. To assess the transitivity assumption we examined the distribution of possible effect modifiers between mouse models including sex, age, weight and wound size. To assess the consistency of the network meta-analyses we compared the model fitting statistics between consistent and inconsistent models. We assessed the presence of network consistency using a global (design-by-treatment inconsistency model) and a local method (back-calculation method). P-scores based on the point estimates and standard errors of the frequentist NMA were used to rank the different models. The small study effect was assessed through visually inspecting comparison-adjusted funnel plots and Egger regression tests.
Additional analyses were conducted. First, to assess whether the background mouse strain had an impact on wound healing, we divided the wild-type group according to the strain and re-ran the NMA. We also performed several prespecified subgroup meta-regressions on animal age at wounding, sex, ulcer type, splint, wound size at baseline, wound covering, or use of topic moisture over the wound. Meta-regressions were performed using a Bayesian approach with Markov Chain Monte Carlo simulation (models with 4 chains and 100,000 iterated simulations, with an initial 10,000 iteration burn-in) with noninformative prior distributions, a normal likelihood, an identity link function and a shared treatment-interaction model.
The analyses were performed using R (version.3.6.0) and netmeta, meta, gemtc and BUGSnet packages.
Role of funders
The funder was not involved in study design, data collection, data analyses, interpretation, or writing of report.
Results
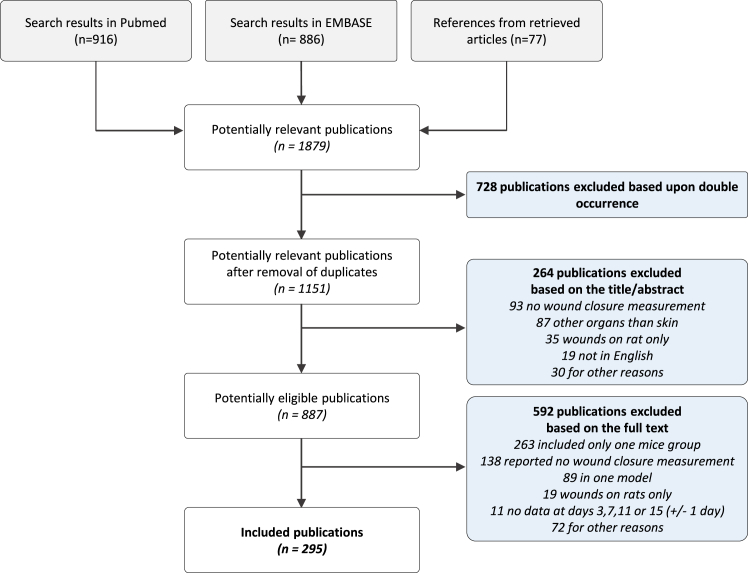
Our literature review yielded 1151 references from which we finally selected 295 studies (Fig. 1; Table S2) and 798 study arms. Among them, 661 arms (247 studies) reported data at day 3, 743 arms (276 studies) at day 7, 563 arms (207 studies) at day 11, and 446 arms (163 studies) at day 15. We identified 10 models of diabetic mice (Table 1), among which streptozotocin (STZ) and db/db were the most widely used.
Fig. 1.
Flow diagram.
Table 1.
Baseline characteristics of animals by diabetic mouse model.
| Diabetes model | Number of arms | Proportion of studies including males only (%) | Age at wound creation (weeks) | Weight at wound creation (g) | Glycemia at wound creation (mg/dL) | Wound size (mm2) | Number of animals per arm |
|---|---|---|---|---|---|---|---|
| Akita | 1 | 100 | 11 | – | – | 19.6 | 35 |
| Alloxan | 8 | 87.5 | 9 (8.5; 9.5) | 26.9 (23.3; 30.4) | 464 (433; 495) | 78.5 (45.6; 78.5) | 8 (6; 8.5) |
| NONcNZO10/Ltj (NON/NZ) | 5 | 75 | 13 | – | – | 31.0 (31.0; 34.0) | 12 (8; 12) |
| Leprdb or db/db (−/−) | 165 | 64.2 | 10 (9; 12) | 41.0 (36.1; 46.8) | 398 (345; 438) | 28.3 (28.3; 50.3) | 6 (5; 10) |
| Db/db heterozygous (+/−) | 42 | 66.7 | 11 (10; 12) | 25.4 (21.4; 28.9) | 162 (153; 171) | 28.3 (28.3; 50.3) | 6.5 (5; 10) |
| High Fat Diet (HFD) | 7 | 80 | 23 (15; 27.5) | 45 (40; 47.1) | 216 (185; 265) | 28.3 (19.6; 28.3) | 5 (5; 5.8) |
| Lepob or ob/ob (−/−) | 9 | 85.7 | 10 (7.5; 11.5) | 45 (35; 48.5) | 423 (423; 423) | 19.6 (19.6; 28.3) | 12 (6; 14) |
| Other diabetic model | 8 | 57.1 | 12 (8.8; 13.5) | 38.9 (35.8; 41.9) | – | 20.4 (11.9; 33.8) | 7 (6; 14.5) |
| Streptozotocin (STZ) | 208 | 84 | 11 (9.5; 15) | 23.5 (22.5; 27.1) | 391 (346; 455) | 28.3 (19.6; 50.2) | 6.5 (5; 10) |
| Streptozotocin and High Fat Diet (STZ + HFD) | 17 | 93.8 | 12 (10; 19) | 29.1 (25.7; 30.6) | 403 (354; 427) | 28.3 (28.3; 44.4) | 6 (5; 6) |
| Wild type (+/+) | 348 | 75.7 | 10 (8; 12.8) | 26 (24; 27.5) | 126 (125; 170) | 28.3 (19.6; 50.3) | 6 (5; 10) |
Data are expressed as median (with 1st and 3rd quartiles) for continuous variables. –: Missing data.
Comparison of the different models on wound healing
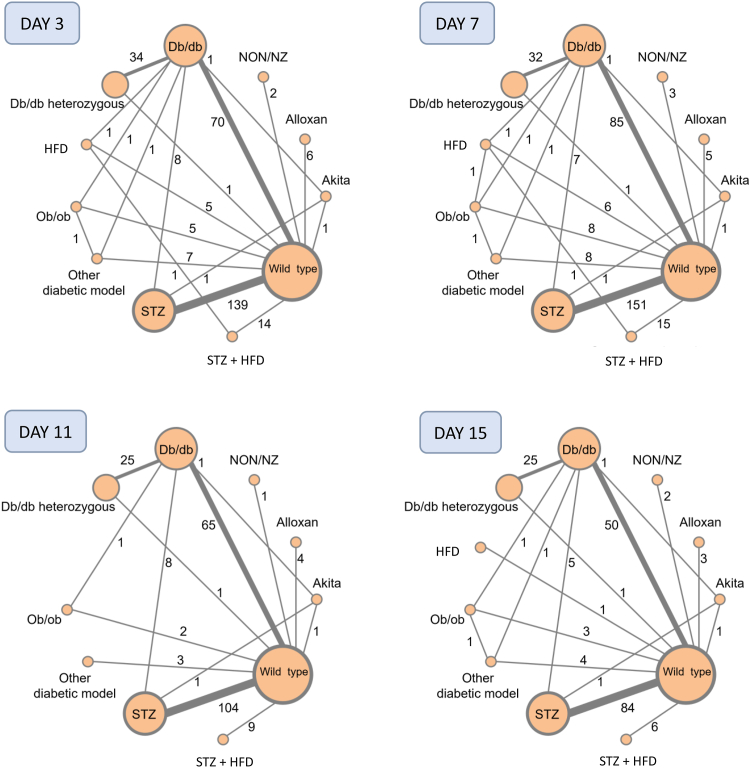
Graphical representations of the networks of direct comparisons between the different models at each time points are presented in Fig. 2. As expected, most models have direct comparisons with wild-type mice used as controls. The heterogeneity statistics (τ and I2), global Q scores for inconsistency and back calculation methods (local inconsistency) at each time point are presented in Tables S3 and S4.
Fig. 2.
Networks of included models of diabetes at day 3, 7, 11 and 15 (±1 day). Node size is proportional to the number of arms using the model. Lines between the nodes indicate direct comparisons between two models. The thickness of the lines and the number on it indicate the number of direct comparisons. HFD: high fat diet; NON/NZ: NONcNZO10/LtJ; STZ: streptozotocin.
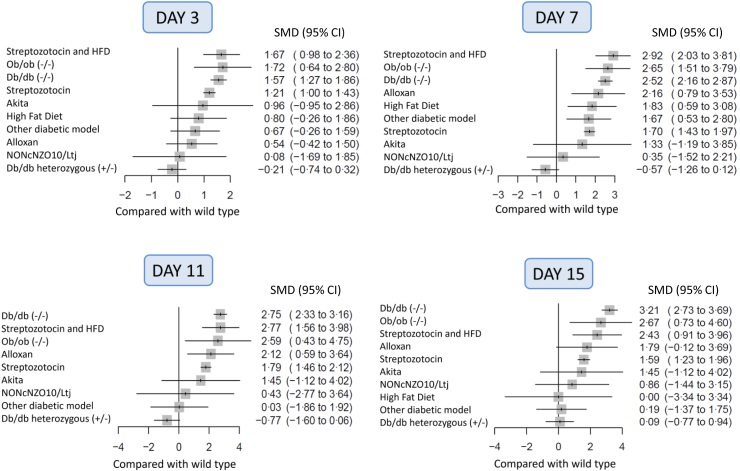
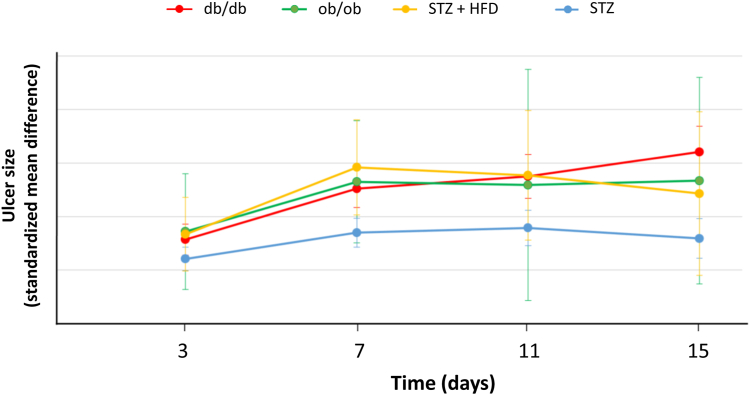
The results of the frequentist NMA are presented as forest plots representing the SMD of ulcer size between each model and wild-type mice (Fig. 3). Full results are also presented as league tables (Tables S4 and S5). Four models showed statistically delayed wound healing, compared with the wild-type, throughout follow-up: db/db, ob/ob, STZ and STZ + high fat diet (Fig. 4). Db/db mice show the most important delay in wound healing at every time point (based on the lower bound of the confidence interval and on P-scores, Table S6), and this difference reaches significance when compared to STZ mice at day 3, 7, 11, and 15 (Fig. 3, Fig. 4). The ranking of the different models at each time point using P-scores shows that db/db, ob/ob, and STZ + high fat diet models show the greatest impairment in wound healing (Table S6).
Fig. 3.
Forest plots of ulcer size between each diabetes model and wild-type at day 3, 7, 11 and 15 (±1 day). Data are expressed as standardized mean differences (SMD) with their 95% confidence intervals (95% CI).
Fig. 4.
Wound healing over time expressed as the standardized mean differences (with their 95% confidence intervals) of ulcer size between wild-type mice and the four main diabetic models. HFD: high fat diet; STZ: streptozotocin.
Meta-regressions and subgroup analyses
The results of meta-regressions and subgroup analyses are presented in Table 2. Among predefined covariates, none was significantly and consistently associated with wound healing over time. However, we observed a slower healing rate for excisional ulcers compared with pressure ulcers, which reached statistical significance at day 7 and 11. Similarly, male exhibited slower healing rate than female mice, the difference being statistically significant at day 7 and 11. Post hoc analysis on db/db mice shows consistent results, although the difference only reaches statistical significance at day 7. Meta-regressions also show a significant, negative relationship between age and wound healing, which reach statistical significance at day 11 and day 15 (Table 2). Finally, there was a trend towards a negative relationship between splinting and wound healing at day 3, suggesting that the difference in early wound healing between diabetic mice and controls is lower with a splint.
Table 2.
Results of meta-regressions and subgroup analyses for each time point.
| Covariate | D3 |
D7 |
D11 |
D15 |
||||
|---|---|---|---|---|---|---|---|---|
| Estimate (95% CrI) | N | Estimate (95% CrI) | N | Estimate (95% CrI) | N | Estimate (95% CrI) | N | |
| Sex (female vs male) | 0.61 (−0.11, 1.33) | 210 | 1.00 (0.20, 1.80) | 231 | 1.26 (0.32, 2.22) | 163 | 1.14 (−0.32, 2.60) | 132 |
| Ulcer type (pressure vs excisional) | 1.63 (−0.03, 3.29) | 280 | 3.15 (1.60, 4.70) | 308 | 2.59 (0.95, 4.27) | 214 | 1.66 (−0.61, 3.99) | 178 |
| Wound size at baseline (mm2) | 0.06 (−0.38, 0.50) | 266 | 0.20 (−0.28, 0.69) | 289 | 0.44 (−0.24, 1.12) | 201 | 0.46 (−0.48, 1.40) | 157 |
| Covering (not covered vs covered) | −0.19 (−0.63, 0.24) | 288 | −0.00 (−0.53, 0.53) | 318 | 0.10 (−0.59, 0.78) | 220 | 0.20 (−0.65, 1.07) | 183 |
| Topic moisture (no topic vs topic) | −0.28 (−0.69, 0.14) | 284 | −0.09 (−0.60, 0.41) | 314 | −0.00 (−0.66, 0.65) | 217 | 0.24 (−0.58, 1.07) | 180 |
| Age at wounding (weeks) | −0.08 (−0.49, 0.32) | 204 | −0.44 (−0.94, 0.06) | 230 | −0.92 (−1.54, −0.30) | 152 | −1.04 (−1.77, −0.31) | 130 |
| Splint (no splint vs splint) | −0.44 (−1.01, 0.13) | 288 | −0.38 (−0.26, 1.02) | 317 | −0.03 (−0.93, 0.88) | 220 | 1.11 (0.17, 2.06) | 183 |
Data are expressed as the standardized mean difference of the percentage of wound area at baseline, with their 95% credibility interval. The further from zero the estimate, the higher the between-group difference in wound healing. Statistically significant results appear in bold.
Comparisons of mouse strains
To assess whether the strain influenced wound healing among non-diabetic controls, we split the latter according to mouse strain (Balb_c, CD1, FVB_N, ICR mice, NODSCID, NODShiLt, SCID, SV129, SWR_J, or C57Bl6_J) in a sensitivity analysis. Networks are available as Figure S1. Overall, there was no significant difference among the different strains, except a lower healing rate for NODSCID mice vs C57 at days 7 and 11, and for Balb_c and CD1 at day 15. The full results of this NMA are available as Tables S7 and S8.
Small study effect and risk of bias
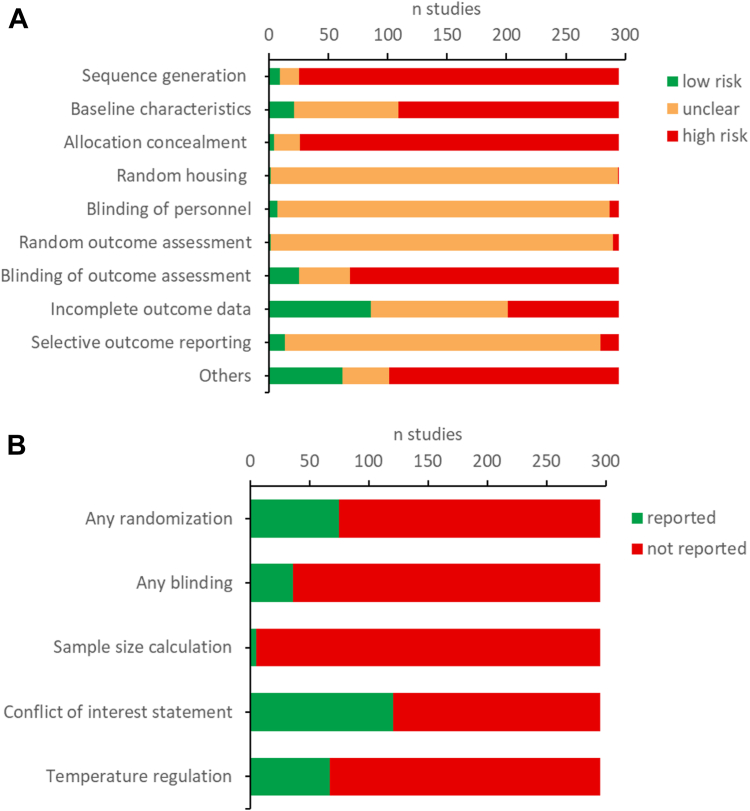
Small study effect was investigated through comparison-adjusted funnel plots (Figure S2). Visual inspection of forest plots and Egger regression tests highlighted systematic deviations in the funnel plot asymmetry for all time points. Moreover, a non-negligeable number of small studies displayed very large effect size (SMD >5), notably for later timepoints. Thus, this small study effect is probably due to a combination of publication bias and methodological flaws in small studies. Fig. 5 shows the results of the SYRCLE RoB and an additional indicator. Many items present a risk of bias. In addition, some items are missing for all articles.
Fig. 5.
Risk of bias assessment using the SYRCLE tool (A) and additional indicators (B).
Discussion
This systematic review with a network meta-analysis combines direct and indirect evidence to compare different mouse models of diabetes-related ulcers. The results show that db/db mice, ob/ob mice, and the STZ + high fat diet model exhibit the most important delay in wound healing. The STZ model also exhibits delayed wound healing, although to a lesser extent.
Unlike the db/db model, the ob/ob model does not show a significantly greater impairment in wound healing than STZ mice. Interestingly, this model targets the same metabolic pathway than the db/db model. While the latter presents leptin receptor deficiency, ob/ob mice are leptin deficient.4 However, despite these similarities, the two phenotypes are different regarding glucose metabolism. In addition, db/db mice show a higher inflammatory tone in the subcutaneous adipose tissue.12 Yet, the results for ob/ob mice rely on a limited number of studies, with little statistical power.
Another model of obesity associated with diabetes, i.e., the high fat diet model, did not show significantly delayed wound healing compared to wild-type mice. When combined to STZ however, the results show a significant impairment in healing. Nonetheless, these results should be interpreted with caution considering the limited number of studies.
Among non-obese mouse-models, the STZ model, typically considered as a model of type 1 diabetes, has been widely used over the past decades. It is the only one showing impaired wound healing at all time points, despite heterogeneity in the protocol to induce of hyperglycaemia. The main two protocols are either repeated, low doses, or a single high dose. However, these protocols are not standardized and we observed many differences in STZ dose regimens.
Pre-specified meta-regressions included the exploration of the effect of splinting. Indeed, this splinting is commonly performed to reduce the contraction of the wound. This contraction phenomenon is mediated by the panniculus carnosus, which consists of a thin layer of muscle present in the subcutaneous tissue of rodents.13 This phenomenon is much more pronounced in rodent than in human skin. Indeed, mainly because of morphological differences, older studies suggested that the contraction was responsible for approximately eighty percent of initial wound closure in excisional wounds.14 Even if more robust techniques for studying this mechanism have emerged, this phenomenon still plays an important part in the scarring process.15 By reducing contraction, mostly using a silicone donut,8 splinting enhances wound closure mostly through re-epithelialization (and not contraction), a mechanism that mimics what is observed in humans. However, despite the considerable number of studies included in this meta-analysis, our results only show that splint use is associated with a non-significant trend towards a decrease in the wound healing difference between diabetic and control mice at day 3, Although the reduced contraction in db/db mice compared to wild-type has been reported,16,17 our results suggest that this effect might be smaller than expected.
We did not observe a consistent association between the difference in wound healing vs controls and the age of animals, but the association was significant at days 11 and 15. This decreased between-group difference at the later stages of healing might be due to the fact that older wild-type mice exhibit impaired wound healing compared to younger ones,18 and that this difference might be smaller in diabetic mice. Yet, this finding is not consistent with previous reports showing a worsening of neuropathies with age in different models of diabetes.19, 20, 21 A major limitation to the interpretation of this finding is that we mixed genetic models with chemically-induced models, and for the latter the age of mice at diabetes induction might be a confounder. While the duration of diabetes would be more useful, it is inconsistently reported in studies. Indeed, the delay between induction of diabetes hyperglycaemia and ulceration is often reported for STZ or high fat models, but scarcely for genetic models, because there is no induction. Although the literature provides approximations on the onset of hyperglycaemia in these models (e.g., between 4 and 8 months of life in db/db mice4), this was not accurate enough to be used in our meta-regressions. Similarly, glycaemia at baseline was reported in only 64 out of 295 studies (i.e., 78% of missing data), which we have considered to be insufficient to conduct a meta-regression. Other covariates of interest are frequently missing in studies, such as the quantification of neuropathy, skin microvascular function, arterial pressure, or insulin resistance. The use of insulin in STZ or alloxan-induced diabetes models, which limits weight loss, was also scarcely reported (in only 20 out of 216 study arms). This underlines the need for more systematic reporting of covariates of interest in experimental study articles. There was a strong effect of the wound model although it only reached statistical significance at day 7 and day 11, suggesting that excisional lesions induce more severe wounds than pressure ulcers. This is consistent with previous findings suggesting that pressure wounds are more superficial, and usually graded as stage I (nonblanchable redness of a localized area) or II (partial thickness loss of dermis).22,23 Similarly, there was an inconsistent effect of sex, with a greater impairment in males from day 7. This difference could be due to a protective role of female hormones. A beneficial effect of oestrogen on skin healing has already been described in humans and rats.24,25
The question of the control group is another key point to consider when designing an experimental study. Our sensitivity analysis separating the wild type group by strain did not reveal any major difference in wound healing duration, suggesting that any wild-type strain can be used as a control. However, wound healing was impaired for NODSCID mice vs C57, which is consistent with the characteristic of this strain, described as a model of prediabetes26 and immunodeficiency.27 In addition, we tested wound healing in db± mice, which is often used as a control for db/db (db−/−) mice. Although db ± mice have metabolic impairment, there is no significant difference in wound healing vs other control strains (i.e., wild-type).
A previous systematic review and meta-analysis has assessed the effect of diabetes on wound healing in mice.28 It suggests that wound healing is impaired for hardly all models and at all time points, while we observe a significant effect only for two models. This discrepancy might be explained by the fact that this meta-analysis only included direct comparisons, and thus relies on fewer studies (77 vs 295 in this report). A strength of our work is to gather direct and indirect evidence to rank the different models.
Yet, our meta-analysis also shows several limitations. First, the network approach involves several assumptions such as the transitivity hypothesis, which is difficult to verify considering the large heterogeneity in methods. In addition, experimental studies usually include a limited number of animals, thus leading to a small study effect. Local and global inconsistency were important in the meta-analysis due to the large variability of pre-clinical results in the field, and possible unmeasured confounding factors impacting wound healing in mouse models. Such effect is visible on funnel plots, which show asymmetry, suggesting a possible publication bias. In addition, meta-regressions rely on assumptions (including linearity for quantitative predictors) that are difficult to assess considering the multiplicity of direct and indirect comparisons. We thus only checked linearity of predictors by visually inspecting the meta-regression plots in pairwise meta-analyses. Finally, SYRCLE quality assessment revealed that in most cases, there is a lack of quality and/or insufficient reporting in study design. Although this is common in meta-analyses of preclinical studies, it stresses the need for standardized reporting and more robust approaches such as randomization and blinding.29
More generally, although mouse models have been extensively used due to low cost, their tendency to produce reproducible models of chronic hyperglycaemia, large use of gene-editing technologies (such as the db/db model), and availability of detection methods (i.e., antibody kits),30 their relevance to human pathophysiology remains a key issue. For instance, mice usually heal faster that humans, and through different mechanisms, with wound contraction predominating over re-epithelialization.31 A unique model cannot capture all underlying causes of healing defects in patients, but models might be useful to better understand specific pathways underlying the disease (e.g., the relationship between hyperglycaemia, microvascular dysfunction and neuropathy, and eventually healing defect). Also, they have been successfully used to screen drugs or devices, such as dressings, stem cell therapy, or growth factors.30 Yet, these are isolated examples and the positive predictive value of animal models (i.e., the probability that a positive result in animals will also be positive in humans) is virtually impossible to establish.32
In conclusion, this systematic review and network meta-analysis compares different murine models of ulcers related to diabetes. We show that db/db mice is the model associated with the largest delay in wound healing compared to wild-type mice. The STZ model also exhibits significantly decreased wound healing. STZ + high fat diet and ob/ob mice may also be relevant models of diabetes-related ulcers, although the results rely on a more limited number of studies.
Contributors
A.C and C.C. did investigation, data curation, visualization, and wrote the original draft. J-L.C and D.S-R were involved in methodology and reviewed the manuscript. C.K was involved in methodology and data curation, did formal analysis, wrote the original draft, and reviewed the manuscript. M.R conceptualized the study, obtained the funding, did supervision and validation, wrote the original draft, and reviewed the manuscript. All authors read and approved the final version of the manuscript.
M.R. an C.K are the guarantors of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Data sharing statement
The dataset generated and analysed during the current study, as well as the source code used for analysis, are available in the OSF repository (https://osf.io/bxmw3/).
Declaration of interests
The authors declare no conflict of interest.
Acknowledgements
This work was funded by the Agence Nationale de la Recherche (grant ANR-18-CE17-0017).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2023.104856.
Appendix A. Supplementary data
References
- 1.International Diabetes Federation . 10th ed. 2021. IDF diabetes atlas. [Google Scholar]
- 2.Gurney J.K., Stanley J., Rumball-Smith J., York S., Sarfati D. Postoperative death after lower-limb amputation in a national prevalent cohort of patients with diabetes. Diabetes Care. 2018;41:1204–1211. doi: 10.2337/dc17-2557. [DOI] [PubMed] [Google Scholar]
- 3.Katsuda Y., Ohta T., Shinohara M., Bin T., Yamada T. Diabetic mouse models. OJAS. 2013;3:334–342. [Google Scholar]
- 4.King A.J. The use of animal models in diabetes research: animal models of diabetes. Br J Pharmacol. 2012;166:877–894. doi: 10.1111/j.1476-5381.2012.01911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong V.W., Sorkin M., Glotzbach J.P., Longaker M.T., Gurtner G.C. Surgical approaches to create murine models of human wound healing. J Biomed Biotechnol. 2011;2011:1–8. doi: 10.1155/2011/969618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Radenković M., Stojanović M., Prostran M. Experimental diabetes induced by alloxan and streptozotocin: the current state of the art. J Pharmacol Toxicol Methods. 2016;78:13–31. doi: 10.1016/j.vascn.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi K., Kojima R., Ito M. Strain differences in the diabetogenic activity of streptozotocin in mice. Biol Pharm Bull. 2006;29:1110–1119. doi: 10.1248/bpb.29.1110. [DOI] [PubMed] [Google Scholar]
- 8.Galiano R.D., Michaels J., Dobryansky M., Levine J.P., Gurtner G.C. Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen. 2004;12:485–492. doi: 10.1111/j.1067-1927.2004.12404.x. [DOI] [PubMed] [Google Scholar]
- 9.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol. 2021;134:178–189. doi: 10.1016/j.jclinepi.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Hooijmans C.R., Rovers M.M., de Vries R.B.M., Leenaars M., Ritskes-Hoitinga M., Langendam M.W. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins J.P.T., Thomas J., Chandler J., et al. Cochrane; 2022. Cochrane handbook for systematic reviews of interventions.www.training.cochrane.org/handbook Available from: [Google Scholar]
- 12.Suriano F., Vieira-Silva S., Falony G., et al. Novel insights into the genetically obese (ob/ob) and diabetic (db/db) mice: two sides of the same coin. Microbiome. 2021;9:147. doi: 10.1186/s40168-021-01097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zomer H.D., Trentin A.G. Skin wound healing in humans and mice: challenges in translational research. J Dermatol Sci. 2018;90:3–12. doi: 10.1016/j.jdermsci.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Grillo H.C., Watts G.T., Gross J. Studies in wound healing: I. Contraction and the wound contents. Ann Surg. 1958;148:145–152. doi: 10.1097/00000658-195808000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L., Mirza R., Kwon Y., DiPietro L.A., Koh T.J. The murine excisional wound model: contraction revisited. Wound Repair Regen. 2015;23:874–877. doi: 10.1111/wrr.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X.T., McKeever C.C., Vonu P., Patterson C., Liu P.Y. Dynamic histological events and molecular changes in excisional wound healing of diabetic DB/DB mice. J Surg Res. 2019;238:186–197. doi: 10.1016/j.jss.2019.01.048. [DOI] [PubMed] [Google Scholar]
- 17.Park S.A., Teixeira L.B.C., Raghunathan V.K., et al. Full-thickness splinted skin wound healing models in db/db and heterozygous mice: implications for wound healing impairment. Wound Repair Regen. 2014;22:368–380. doi: 10.1111/wrr.12172. [DOI] [PubMed] [Google Scholar]
- 18.Bonham C.A., Rodrigues M., Galvez M., et al. Deferoxamine can prevent pressure ulcers and accelerate healing in aged mice. Wound Repair Regen. 2018;26:300–305. doi: 10.1111/wrr.12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng C., Singh V., Krishnan A., Kan M., Martinez J.A., Zochodne D.W. Loss of innervation and axon plasticity accompanies impaired diabetic wound healing. PLoS One. 2013;8 doi: 10.1371/journal.pone.0075877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drel V.R., Mashtalir N., Ilnytska O., et al. The leptin-deficient (ob/ob) mouse: a new animal model of peripheral neuropathy of type 2 diabetes and obesity. Diabetes. 2006;55:3335–3343. doi: 10.2337/db06-0885. [DOI] [PubMed] [Google Scholar]
- 21.Sima A.A., Robertson D.M. Peripheral neuropathy in mutant diabetic mouse [C57BL/Ks (db/db)] Acta Neuropathol. 1978;41:85–89. doi: 10.1007/BF00689757. [DOI] [PubMed] [Google Scholar]
- 22.Kwek M.S.Y., Thangaveloo M., Hui S.L.B., Madden L.E., Phillips A.R.J., Becker D.L. Characterisation of an ischemia reperfusion model for the formation of a stage I pressure ulcer in mouse skin. J Tissue Viability. 2021;30:352–362. doi: 10.1016/j.jtv.2021.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Demiot C., Sarrazy V., Javellaud J., et al. Erythropoietin restores C-fiber function and prevents pressure ulcer formation in diabetic mice. J Invest Dermatol. 2011;131:2316–2322. doi: 10.1038/jid.2011.211. [DOI] [PubMed] [Google Scholar]
- 24.Ashcroft G.S., Dodsworth J., van Boxtel E., et al. Estrogen accelerates cutaneous wound healing associated with an increase in TGF-beta1 levels. Nat Med. 1997;3:1209–1215. doi: 10.1038/nm1197-1209. [DOI] [PubMed] [Google Scholar]
- 25.Pirilä E., Parikka M., Ramamurthy N.S., et al. Chemically modified tetracycline (CMT-8) and estrogen promote wound healing in ovariectomized rats: effects on matrix metalloproteinase-2, membrane type 1 matrix metalloproteinase, and laminin-5 gamma2-chain. Wound Repair Regen. 2002;10:38–51. doi: 10.1046/j.1524-475x.2002.10605.x. [DOI] [PubMed] [Google Scholar]
- 26.Makino S., Kunimoto K., Muraoka Y., Mizushima Y., Katagiri K., Tochino Y. Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu. 1980;29:1–13. doi: 10.1538/expanim1978.29.1_1. [DOI] [PubMed] [Google Scholar]
- 27.Vladutiu A.O. The severe combined immunodeficient (SCID) mouse as a model for the study of autoimmune diseases. Clin Exp Immunol. 2008;93:1–8. doi: 10.1111/j.1365-2249.1993.tb06488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huynh P., Phie J., Krishna S.M., Golledge J. Systematic review and meta-analysis of mouse models of diabetes-associated ulcers. BMJ Open Diabetes Res Care. 2020;8 doi: 10.1136/bmjdrc-2019-000982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masson-Meyers D.S., Andrade T.A.M., Caetano G.F., et al. Experimental models and methods for cutaneous wound healing assessment. Int J Exp Pathol. 2020;101:21–37. doi: 10.1111/iep.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du Y., Wang J., Fan W., Huang R., Wang H., Liu G. Preclinical study of diabetic foot ulcers: from pathogenesis to vivo/vitro models and clinical therapeutic transformation. Int Wound J. 2023 doi: 10.1111/iwj.14311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanapalli B.K.R., Yele V., Singh M.K., Thaggikuppe Krishnamurthy P., Karri V.V.S.R. Preclinical models of diabetic wound healing: a critical review. Biomed Pharmacother. 2021;142 doi: 10.1016/j.biopha.2021.111946. [DOI] [PubMed] [Google Scholar]
- 32.Matthews R.A. Medical progress depends on animal models - doesn't it? J R Soc Med. 2008;101:95–98. doi: 10.1258/jrsm.2007.070164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.