Summary
Background
Mucosal antibodies play a key role in the protection against SARS-CoV-2 infection in the upper respiratory tract, and potentially in limiting virus replication and therefore onward transmission. While systemic immunity to SARS-CoV-2 is well understood, we have a limited understanding about the antibodies present on the nasal mucosal surfaces.
Methods
In this study, we evaluated SARS-CoV-2 mucosal antibodies following previous infection, vaccination, or a combination of both. Paired nasal fluid and serum samples were collected from 143 individuals, which include convalescent, vaccinated, or breakthrough infections.
Findings
We detected a high correlation between IgG responses in serum and nasal fluids, which were higher in both compartments in vaccinated compared to convalescent participants. Contrary, nasal and systemic SARS-CoV-2 IgA responses were weakly correlated, indicating a compartmentalization between the local and systemic IgA responses. SARS-CoV-2 secretory component IgA (s-IgA) antibodies, present exclusively on mucosal surfaces, were detected in the nasal fluid only in a minority of vaccinated subjects and were significantly higher in previously infected individuals. Depletion of IgA antibodies in nasal fluids resulted in a tremendous reduction of neutralization activity against SARS-CoV-2, indicating that IgA is the crucial contributor to neutralization in the nasal mucosa. Neutralization against SARS-CoV-2 was higher in the mucosa of subjects with previous SARS-CoV-2 infections compared to vaccinated participants.
Interpretation
In summary, we demonstrate that currently available vaccines elicit strong systemic antibody responses, but SARS-CoV-2 infection generates higher titers of binding and neutralizing mucosal antibodies. Our results support the importance to develop SARS-CoV-2 vaccines that elicit mucosal antibodies.
Funding
The work was funded by the COVID-19 National Research Program 78 (grant number 198412) of the Swiss National Science Foundation.
Keywords: SARS-CoV-2, COVID-19, Mucosal immunity, Secretory IgA
Research in context.
Evidence before this study
SARS-CoV-2 primarily infects the upper respiratory tract, where mucosal antibodies play a key role in protection against infection. Previous studies suggested that these antibodies may significantly reduce RNA viral load in the upper respiratory tract leading to lower infectious virus shedding and decreased human to human transmission. While systemic SARS-CoV-2 antibody responses are well characterized, there is only a limited number of studies, which analysed mucosal antibody. We searched PubMed and MedRxiv for studies that investigated mucosal antibodies in response to SARS-CoV-2 vaccination or natural infection and were published before the 24th of March 2023. Search items included “SARS-CoV-2” “COVID-19” “convalescent” “vaccinated” “mucosal” “nasal” “antibody” “IgA” “secretory component IgA” and “neutralization”. Most of the studies analysed mucosal antibody responses in saliva samples and only a few studies examined nasal responses. These studies demonstrated that previous infection leads to significantly higher SARS-CoV-2- specific IgA and neutralizing antibodies in mucosal lining fluids compared to vaccination. Secretory component IgA (s-IgA), which is found exclusively on mucosal surfaces, was detected in saliva only in a minority of vaccinated subjects. No study measured s-IgA in responses to SARS-CoV-2 vaccination or infection in nasal fluids.
Added value of this study
We analysed mucosal and systemic SARS-CoV-2 antibody responses in 143 individuals, which include convalescent, vaccinated, and vaccine breakthrough infections (hybrid immunity). In this study we compared SARS-CoV-2-specific locally produced s-IgA antibodies in the nasal mucosa to functional neutralizing antibodies. We demonstrate that previous infection elicits significantly higher s-IgA responses, while vaccination leads to the s-IgA responses only in a minority of individuals and boosting by a 3rd vaccine dose does not improve these responses. We show that protection by neutralization against different SARS-CoV-2 strains was higher in previously infected individuals compared to those vaccinated only. Interestingly, neutralization of Omicron BA.5 strain was comparable in individuals with previously confirmed BA.1 or Delta SARS-CoV-2 infections. Furthermore, there is strong evidence that IgA substantially contributes to virus neutralization in the nasal mucosa.
Implications of all the available evidence
While currently available intramuscularly-administered vaccines provide robust systemic immune responses, they fail to elicit high titers of binding and neutralizing mucosal antibody responses. The results of our study provide a support for the development of intranasally-administered vaccines with the potential to reduce human-to-human transmission. This study contributes to a better understanding of SARS-CoV-2 mucosal antibody responses, which can be further used for the development of such mucosal vaccines.
Introduction
Less than one year after the emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19), highly effective COVID-19 vaccines were licensed.1,2 To date, more than 13 billion doses of COVID-19 vaccines have been administered worldwide, significantly reducing complications and hospitalizations.3 However, breakthrough infections have been reported even for SARS-CoV-2 variants that are antigenically similar to the vaccine strain and more frequently since the emergence of the Omicron variant with its efficient immune evasion properties.4,5 These results indicate that current vaccines can only temporarily and incompletely reduce the risk of upper respiratory tract (URT) infections.6,7 In contrast, studies in mice and rhesus macaques demonstrated that intranasal immunization leads to complete protection against SARS-CoV-2 infection of the URT, resulting in reduced onward transmission.8,9 In humans, the level of mucosal IgA antibodies, elicited mainly by previous SARS-CoV-2 infection, was associated with protection from infection with Omicron subvariants.10 However, whether these mucosal IgA antibodies are derived from systemic circulation or locally-produced as well as their functional properties have not been investigated in detail.
The URT harbours a distinct part of the immune system called the mucosa associated lymphatic tissue (MALT), where local plasma cells secrete multimeric IgA (mainly dimers but also trimers and tetramers), which is transported across the mucosal epithelium by the polymeric immunoglobulin receptor (pIgR). During this process the extracellular part of the pIgR, called the secretory component is bound to the multimeric IgA forming secretory IgA (s-IgA). The main function of s-IgA antibodies is neutralization.11 In particular, s-IgA dimers were shown to neutralize SARS-CoV-2 on average 15 times more potently than IgA monomers present in serum.12 Mucosal IgG is either locally produced by B cells in the lamina propria and transported across the epithelium by neonatal Fc receptors, or excreted as a transudate from serum together with monomeric IgA antibodies.13 While potent systemic IgA and IgG responses are shown to be induced by infection and vaccination, mucosal antibody responses are mainly induced by infection.14 Notably, some studies demonstrated that virus-specific mucosal IgA antibodies were found in some seronegative patients with COVID-19, suggesting a discrepancy between local and systemic antibody immune responses.15,16 In convalescent patients higher SARS-CoV-2-specific mucosal antibodies were associated with lower viral loads and more efficient symptom resolution17; these antibody responses remained detectable for at least 3 months post infection in saliva,18 and up to 9 months post infection in nasal secretions.17,19
Vaccination was shown to significantly reduce infectious viral loads in breakthrough infections with the Alpha variant, but the effect was weaker for breakthrough infections with Delta,20,21 and even less potent for the Omicron BA.1 variant, where the decrease of viral loads was shown only after boosting with a 3rd vaccine dose.22,23 However, it remains unclear whether this reduction is caused by mucosal or systemic immunity. Even though s-IgA responses were detectable in saliva of some of the vaccinated subjects,24 these responses were significantly lower in comparison to previously infected individuals.25,26 Additionally, most virus-specific antibody titers in saliva and their neutralizing capacity significantly decayed six months post vaccination.25 One study showed that mucosal antibody responses induced by vaccination were low or undetectable, but Delta breakthrough infections led to significant increases of antibody titers in saliva.27 According to one study, previous infection, but not vaccination, induced strong IgA responses and detectable neutralizing titers against Omicron subvariants in the nasal mucosa.28
In this study we characterized mucosal antibody responses in the URT of convalescent non vaccinated, vaccinated and subjects with vaccine breakthrough infections (hybrid immunity). We quantified and compared the levels of mucosal and systemic IgA and IgG responses in these groups as well as analyzed the levels of neutralizing antibodies in the nasal lining fluid (NLF).
Methods
Study design and participants
The study was approved by the Cantonal Ethics Committee at the University Hospital of Geneva (CCER no. 2020-02323). Both sexes were recruited to the study and gender was self-reported. All study participants provided written informed consent. NLF and serum samples were collected during a single visit from all the participants from September 2021 to June 2022. The samples were collected from healthy volunteers with no symptoms of respiratory illness. All the sampling procedures were performed by trained healthcare professionals. The information about previous infections (date of infection confirmed by RT-PCR or rapid antigenic test) and vaccination (date of vaccination, number of vaccine doses and the vaccine manufacturer) was collected using a questionnaire during the visit.
NLF samples were collected using the Nasosorption™ FX·i nasal sampling device (Hunt Developments, UK) as previously described.29 Briefly, a synthetic absorptive matrix (SAM) strip was inserted in the nostril of the participant against the inferior turbinate. After pressing on the side of the nostril for 1 min, the SAM strip was removed and placed in the collection tube. The samples were stored at −80 °C before processing. Nasosorption devices were thawed on ice, the SAM was removed and placed in the collection tube with 300 μL of elution buffer (PBS/1% BSA). Following incubation for 10 min at room temperature, the SAM was placed on the spin-X filter Eppendorf tube and centrifuged at 16,000g for 10 min at 4 °C. Eluted liquid was aliquoted and kept at −80 °C until further use.
Immunoassays
Roche Elecsys anti-SARS-CoV-2 S RBD and Roche Elecsys anti-SARS-CoV-2 NP
Elecsys anti-SARS-CoV-2 S and anti-SARS-CoV-2 were used to determine the levels of SARS-CoV-2 specific receptor-binding domain (RBD) and nucleoprotein (NP) antibodies, respectively. Antibody measurements were performed on the cobas e801 analyser (Roche Diagnostics, Rotkreuz, Switzerland) in the clinical laboratory of the University Hospital of Geneva. For anti-RBD antibodies, results are reported as concentrations (U/mL) and positivity was determined by using the manufacturer’s cut-off >0.8 U/mL. For anti-NP antibodies, the positivity was determined with a cut-off index (COI), where COI ≥1.0 is defined as positive.
Recombinant proteins
Full-length trimerized SARS-CoV-2 spike (triS) was provided by the EPFL protein production facility. Nucleoprotein was obtained from Prospec Bio (Catalog #SARS-044).
Human total IgA ELISA
The levels of total IgA in NLF were measured by IgA Human Uncoated ELISA Kit (Catalog #88-50600, Invitrogen) following the manufacturer’s instructions. NLF samples were diluted to 1:1000 and 1:10,000 in assay buffer prior to measurement. The standard curve was generated from recombinant human IgA using four-parameter logistic (4PL) regression model. The relative IgA concentration (ng/mL) of test samples was determined according to the dynamic range of the standard curve by interpolating the concentration of the standards that correspond to the absorbance value.
To normalize all SARS-CoV-2 IgA and s-IgA levels in NLF samples to total IgA, a correction factor was applied to all the NLF samples. This factor was calculated for each sample by dividing the mean of total IgA for all samples by the total IgA measured in the sample which was used for SARS-CoV-2 specific IgA and s-IgA.
Human total IgG ELISA
The levels of total IgG in NLF were measured by IgG Human Uncoated ELISA Kit (Catalog #BMS2091, Invitrogen) following the manufacturer’s instructions. NLF samples were diluted to 1:100 in assay buffer prior to measurement. The total IgG concentration was determined in the same way as total IgA.
IgA and IgG anti-SARS-CoV-2 triS ELISA
Maxisorp plates (Thermo Fisher Scientific) were coated with SARS-CoV-2 full triS protein diluted in Phosphate-Buffered Saline (Thermo Fisher Scientific) at a concentration of 2 μg/mL. 50 μL of diluted antigen was added to each well and incubated overnight at 4 °C. To control for unspecific binding half of the plate was coated with PBS only. Following three washes with washing buffer (PBS/0.1% tween-20), plates were blocked for 1 h at 37 °C with assay buffer (PBS/1% BSA/0.1% tween-20). Human IgA SARS-CoV-2 S monoclonal antibodies (IgA1 AR222, Geneva Antibody Facility) were serially diluted in assay buffer (3-fold serial dilutions from 300 ng/mL to 0.41 ng/mL) and added to each plate to generate a relative IgA anti-S standard curve. Human IgG SARS-CoV-2 S monoclonal antibody (IgG1 AR222, Geneva Antibody Facility) (3-fold serial dilutions from 100 ng/mL to 4.6 pg/mL) was used to generate IgG anti-S standard curve. The specificity of human IgA and IgG SARS-CoV-2 S monoclonal antibodies was evaluated by testing pre-pandemic serum samples (48 for IgG and 56 for IgA) and SARS-CoV-2 reference samples obtained from convalescent individuals. For IgA anti-SARS-CoV-2 triS ELISA, 3-fold dilutions from 1:5 to 1:45 in assay buffer prepared for NLF and 1:100, 1:900, and 1:2700 dilutions in assay buffer prepared for serum samples. For IgG anti-SARS-CoV-2 triS ELISA, 9-fold dilutions were prepared for serum (1:100 to 1:24,300) and 3-fold dilutions were prepared for NLF (1:15 to 1:45). 50 μL of standard and sample dilutions in duplicates were added to coated and uncoated wells and incubated for 1 h at 37 °C. Plates were washed four times and 50 μL of Peroxidase AffiniPure Goat Anti-Human Serum IgA (Cat. #109-035-011, Jackson ImmunoResearch Labs, RRID:AB_2337592) for IgA ELISA or Peroxidase AffiniPure F(ab′)₂ Fragment Goat Anti-Human IgG (#109-036-098, Jackson ImmunoResearch Labs, RRID:AB_2337596) antibodies for IgG ELISA at a 1:5000 dilution were added to all wells. After 1 h of incubation at 37 °C and four washes and plates were developed with 3,3′,5,5′Tetramethylbenzidine (TMB) (Sigma-Aldrich) for 20 min in the dark. The reactions were stopped with 1N sulfuric acid. The developed plates were read at 450 nm wavelength. The absorbance values measured from uncoated wells were subtracted from values obtained from antigen-coated wells. The SARS-CoV-2 IgA standard curve was generated from the human IgA SARS-CoV-2 S mAb using a 4PL regression model. The relative anti-S IgA concentrations (ng/mL) of test samples were determined by interpolation of optical density values on a standard curve. The mean + 3SD of eleven negative samples was used to set a cut-off for anti-SARS-CoV-2 IgA and IgG in NLF. The mean + 3SD of 56 or 48 pre-pandemic samples was used to set a cut-off in serum for IgA anti-SARS-CoV-2 or IgG anti-SARS-CoV-2, respectively.
IgA purification
IgA were purified from NLF samples using CaptureSelect™ IgA Affinity Matrix (Thermo, #194288005). Briefly, 90 μL of the matrix slurry diluted in PBS was loaded on the spin columns (Thermo, #69702) and centrifuged. 100 μL NLF was diluted 1:3 in PBS, loaded on a column and mixed on a horizontal shaker. Following 1 h incubation at room temperature, the columns were centrifuged and eluted liquid was collected and kept as unpurified fraction (here referred to as IgG Fraction). IgA-purified fraction was eluted with 400 μL of 0.1 M glycine (pH 3).
Multiplex immunoassay
For s-IgA analysis in NLF, a fluorescent-bead-based multiplex immunoassay was developed. Full-length His-tagged SARS-CoV-2 Spike and Nucleoprotein were each coupled to MagPlex carboxylated polystyrene microparticles using xMAP® Antibody Coupling Kit (Luminex) following manufacturer’s instructions.
Antigen-conjugated microspheres were resuspended at a concentration of 1 × 104/mL and incubated in assay buffer (PBS/1% BSA/0.1% tween-20) for 1 h at room temperature. A total of 4 NLF samples collected from a subject with previously confirmed SARS-CoV-2 infection were pooled together and used to create standard curves for SARS-CoV-2 triS and NP s-IgA. 3-fold serial dilutions were performed for NLF samples (1:3 to 1:81 in assay buffer). Diluted samples and standards were incubated with antigen-coupled microspheres for 2 h while shaking at 800 rpm. Following 3 washes with PBS/0.1% BSA/0.1% tween-20, mouse anti-Human IgA secretory component antibodies (Catalog #ABIN6155159, Antibodies online) was added to microspheres and incubated for 1 h at room temperature. Following three washes the data was acquired on Bio-Plex® MAGPIX™ Multiplex Reader. MFI was converted to arbitrary units (AU/mL) by interpolation from a log-4PL-parameter logistic standard curve (3000 AU/mL to 4.12 AU/mL). Samples with values of less than 12 AU/mL were considered negative and an arbitrary value of 6 AU/mL was assigned to these samples. The mean + 3SD of eleven negative samples was used to set a cut-off for NLF.
Viruses and cells
Vero E6 were kindly provided by Volker Thiel, Vero E6-TMPRSS cells were obtained from the National Institute for Biological Standards and Controls (Catalog #100978). Cells were cultured in complete DMEM GlutaMAX medium supplemented with 10% FBS, 1× non-essential amino acids and 1% antibiotics (penicillin–streptomycin) (all reagents from Gibco). Recent mycoplasma testing was performed. Commonly misidentified lines have not been used in this study.
All SARS-CoV-2 viruses used in this study were isolated from residual nasopharyngeal swabs collected from patients at the University Hospital of Geneva under general informed consent that allows the usage of anonymized left-over materials. All patient specimens from which isolates were obtained were fully sequenced. The SARS-CoV-2 B.1 variant was isolated and propagated on Vero-E6 cells. The Omicron-BA.5 variant was primarily isolated on Vero-TMPRSS cells, then transferred to Vero-E6 for generation of virus stock. All virus stocks were titrated on Vero E6-TMPRSS cells and fully sequenced. All infection experiments were performed under Biosafety Level 3 conditions.
Focus reduction neutralization assays
Serially diluted NLF and SARS-CoV-2 (50 focus-forming units) were combined in serum-free Opti-Pro medium (Gibco) and DMEM + 1% FBS (Corning Cellgro) and incubated at 37 °C for 1 h. The antibody-virus mixture was added to a monolayer of Vero E6-TMPRSS cells and incubated at 37 °C for 1 h. After 1 h at 37 °C, the media were removed, and pre-warmed medium mixed with 2.4% Avicel (DuPont) at a 1:1 ratio was overlaid. Plates were incubated at 37 °C for 24 h and then fixed and stained for SARS-CoV-2 nucleocapsid protein as described previously.22
The 50% reduction endpoint titers (FRNT50) were calculated by fitting a 4-PL logistics curve with variable slope to the number of foci of each NLF using GraphPad Prism version 9.1.0. If the extrapolation reached a titer below 0.5, the value of 0.5 was attributed to the sample.
Statistical analysis
Data collection was done using Excel 2019. There was no missing data for any participants. All statistical analyses were performed using R statistical software version 4.1.1 (Foundation for Statistical 185 Computing) and Prism version 9.3.1 (GraphPad). All IgA and IgG antibody titers were log10 transformed, and samples with no detectable antibodies were set to 1 ng/mL for the purpose of analysis. Differences between antibody titers between the different groups were analyzed by generalised linear model with Quasi-Poisson distribution. For groups containing excess of zero we used zero-inflated function of the R package pscl. p values were adjusted for multiple testing with the Benjamini–Hochberg method. An overview of all statistics can be found in Supplemental Table S2. Correlations between antibody titers were analyzed using Spearman’s rank correlation coefficient. For this test IgA and IgG antibody titers were log10 transformed, and samples with no detectable antibodies were set to 1 ng/mL for the purpose of analysis.
Role of funders
The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Results
Study design and participants
In this study we determined the quantity and quality of SARS-CoV-2-specific mucosal antibodies in individuals that have only been previously infected, vaccinated or, have hybrid immunity (breakthrough infection post-vaccination) and compared them to the systemic responses. A total of 143 adults were recruited between September 2021 and June 2022. Paired nasal lining fluid (NLF) and serum samples were collected during a single visit from all study participants. 11 participants, which had not been vaccinated and never tested positive for SARS-CoV-2, served as a negative control group. 29 and 25 participants had been vaccinated with 2 or 3 doses, respectively, but never tested positive for SARS-CoV-2. 21 participants had been tested positive for SARS-CoV-2 previously, but were not vaccinated. 31 and 26 participants tested positive after having received 1/2 or 3 doses of COVID-19 vaccine, respectively. All groups had a similar median age ranging between 28.5 and 39 years. Percentage of female participants was slightly higher in the negative, convalescent and vaccinated (2 doses) groups. All participants reported mild to moderated disease at the time of infection. We used days since the last immune response (DLIR) either elicited by infection or vaccination to compare the different groups. Median DLIR was 68 days for convalescent, 176 and 70 days for individuals vaccinated with 2 or 3 doses, respectively, and 44 or 47.5 days for vaccine breakthrough after 1/2 or 3 doses, respectively (Table 1). Sequence information to determine the infecting variant was only available for a minority of participants. Nevertheless, in case only one variant circulated at the time of the positive test, we assumed that this variant infected the participant. If more than one variant circulated at the time, we marked the presumed infecting variant as unknown. More details on the type of vaccinations received and the time between sampling, vaccination and infection are shown in Supplemental Table S1.
Table 1.
Participants characteristics.
| Group | Negative | Vaccinated (2 doses) | Vaccinated (3 doses) | Convalescent | Hybrid immunity (1 or 2 doses) | Hybrid immunity (3 doses) |
|---|---|---|---|---|---|---|
| Group description | Non-vaccinated, never tested positive, NP negative, RBD negative | Never tested positive, anti-NP negative RBD positive | Never tested positive, NP negative, anti-RBD positive | Non-vaccinated, tested positive, NP positive, RBD positive | Infection after 1 or 2 doses of vaccine, NP positive, RBD positive | Infection after 3 doses of vaccine, NP positive, RBD positive |
| Number of patients | 11 | 29 | 25 | 21 | 31 | 26 |
| Age: median (range) | 28.5 (24–46) | 33 (24–59) | 37.5 (24–76) | 32 (23–64) | 39 (25–59) | 37.5 (23–55) |
| Sex: females (%) | 8 (72.7%) | 20 (69%) | 13 (52%) | 16 (76.2%) | 16 (51.6%) | 15 (57.7%) |
| Sex: males (%) | 3 (27.3%) | 9 (31%) | 12 (48%) | 5 (23.8%) | 15 (48.4%) | 11 (42.3%) |
| No of participants with >1 infection | na | na | na | 2 | 8 | 3 |
| Days since last immune response (DLIR), days, median (IQR) | na | 176 (159.3–210.5) | 70 (49–123) | 68 (37.25–376.75) | 44 (29–54) | 47.5 (37.75–58.25) |
| Statistical differences of means for DLIR between the groups (determined by Kruskal–Wallis test) | ||||||
| Vaccinated (2 doses) | na | na | p < 0.001 | p = 0.021 | p < 0.001 | p < 0.001 |
| Vaccinated (3 doses) | na | na | >0.999 | p = 0.1752 | p = 0.678 | |
| Convalescent | na | na | p = 0.049 | p = 0.211 | ||
| Hybrid Immunity (1 or 2 doses) | na | na | p > 0.999 | |||
| Hybrid Immunity (3 doses) | na | na | ||||
| Vaccinated (2 doses) | na | |||||
| No. of subjects with unknown date of infection | na | na | na | 3 | – | – |
To ensure that participants were assigned to the correct group, we tested serum samples for the presence of SARS-CoV-2-specific anti-NP and RBD antibodies using the Roche Elecsys N and S assays. Highest anti-RBD serum responses were detected in participants vaccinated with 3 doses and with hybrid immunity, irrespective of the number of vaccine doses received. Participants vaccinated with 2 doses or convalescent individuals had lower anti-RBD titers, while no anti-RBD antibodies were detected in the negative group (Supplemental Figure S1a). Anti-NP responses were only detected in convalescent individuals or participants with hybrid immunity, but not in negative or vaccinated participants confirming the absence of previous infection in these groups (Supplemental Figure S1b). Therefore, we used samples from the negative cohort to determine the assay background and define the cut-offs of positivity for antibody responses in NLF. Since IgA concentrations in NLF might vary between individuals and between samplings we measured the level of total IgA. We found similar levels of total IgA in the NLF of most groups compared to the negative control group while total IgA levels in vaccinated subjects were slightly but significantly higher (vaccinated with 3 doses) or lower (vaccinated with 2 doses) (Supplemental Figure S1c). Therefore, to avoid a possible sampling bias, we normalized the levels of SARS-CoV-2-specific IgA and s-IgA antibodies in NLF.
Distinct nasal and systemic SARS-CoV-2 IgA responses
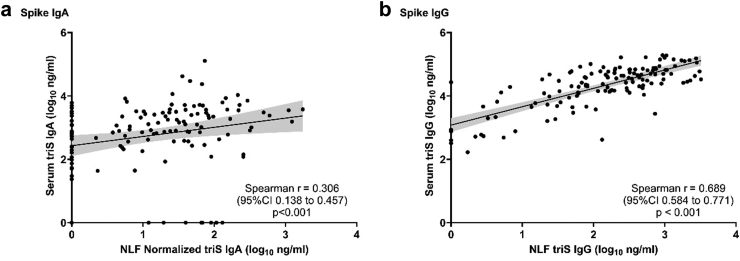
First, we investigated whether there are differences between antibodies present at the nasal mucosa and those circulating the blood. Therefore, we measured the levels of IgA and IgG antibodies responses to trimeric spike (triS) protein of SARS-CoV-2 (strain: Wuhan-HU-1) in serum and NLF using an ELISA. We detected only a low correlation between levels of anti-triS IgA antibodies in serum and NLF (r = 0.306, p < 0.001, Spearman's rank), but a high correlation between anti-triS IgG responses in serum and NLF (r = 0.689, p < 0.001, Spearman's rank) (Fig. 1a and b). These findings indicate that while SARS-CoV-2 IgG mucosal and systemic responses are highly comparable, there is a compartmentalization between IgA responses in mucosa and serum.
Fig. 1.
Correlation of SARS-CoV-2 IgA and IgG antibody responses in NLF and serum. Correlation between anti-triS serum and NLF IgA (a) and IgG (b) antibody titers (log10 ng/mL) of samples collected from convalescent, vaccinated and subjects with hybrid immunity. NLF triS IgA titers normalized to the levels of total IgA from the same sample. Spearman rank correlation coefficient and p values are shown.
SARS-CoV-2 mucosal antibodies in response to infection and vaccination
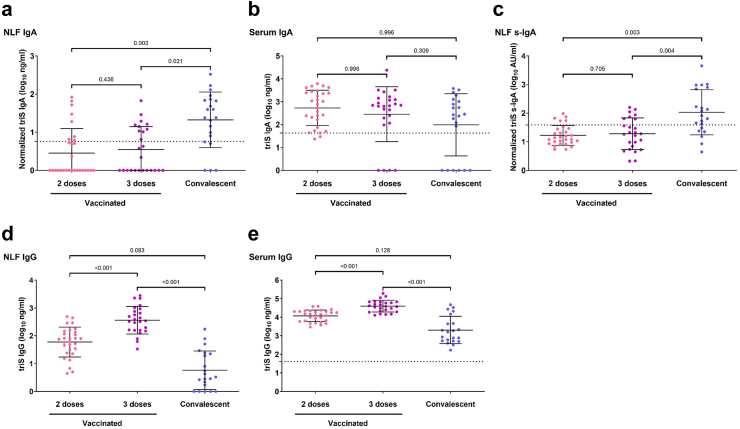
Next, we investigated the level of mucosal antibodies induced by vaccination or infection by analyzing anti-triS IgA responses in NLF of vaccinated and convalescent subjects. In most of vaccinated subjects, NLF IgA responses were below the cut-off of positivity (21 out of 29 double-vaccinated and 15 of 25 in triple-vaccinated below the cut-off) and there was no difference if participants had received 2 or 3 doses despite the higher median DLIR in double-vaccinated individuals (176 vs 70 days). In contrast, positive IgA responses were detected in NLF of most of the convalescent individuals (16 out of 21 above cut-off), and they were significantly higher in comparison to vaccinated subjects (Fig. 2a). Conversely, anti-triS IgA responses were moderately higher in serum of vaccinated subjects that had received 2 or 3 doses compared to the convalescent group, but the difference was not significant (Fig. 2b).
Fig. 2.
Mucosal and serum antibody responses in subjects with previous infection compared to vaccinated only. (a and b) Anti-triS IgA (log10 ng/mL) measured in NLF (a) and serum (b) of vaccinated with 2 or 3 doses, or convalescent. (c) Anti-triS s-IgA (log10 AU/mL) antibody titers in NLF of vaccinated with 2 or 3 doses, or convalescent. (d and e) Anti-triS IgG (log10 ng/mL) measured in NLF (d) and serum (e) of vaccinated with 2 or 3 doses, or convalescent. A generalized linear model was used to determine differences of means, p values are shown above brackets. The mean + 3SD of 11 negative samples was used to set a cut-off for anti-triS IgA, s-IgA and IgG in NLF. The mean + 3SD of 56 pre-pandemic serum samples was used to set a cut-off for anti-triS IgA in serum samples. The mean + 3SD of 48 pre-pandemic serum samples was used to set a cut-off for anti-triS IgG in serum samples.
To exclude that the observed differences in IgA are biased by exudate of serum IgA, we analyzed the levels of locally-produced anti-triS s-IgA in NLF. Notably, we observed a moderate to strong, significant correlation between anti-triS IgA and anti-triS s-IgA responses in NLF (r = 0.592, p < 0.001, Spearman's rank) (Supplemental Figure S2) indicating that the majority of IgA measured in NLF is locally produced s-IgA. Among most of the subjects who received two or three vaccine doses, the detected levels of Spike-specific s-IgA responses were either below the cut-off of positivity or they were very low (24 out of 29 double-vaccinated and 18 of 25 in triple-vaccinated below the cut-off), whereas previous infection elicited significantly higher anti-triS s-IgA responses in comparison to vaccination (15 out of 21 above cut-off) (Fig. 2c). Five samples from vaccinated individuals negative for triS IgA were however positive for s-IgA, possibly indicating the differences of sensitivity between the assays. Similar to anti-triS IgA responses, there also was no significant difference of anti-triS s-IgA between vaccinated subjects which received two or three vaccine doses, indicating that a 3rd vaccine dose does not boost local s-IgA responses.
We then analyzed the influence of vaccination and infection on mucosal and systemic IgG responses. We detected significantly higher anti-triS IgG responses in NLF of subjects who received 3 vaccine doses compared to subjects who received 2 vaccine doses and the convalescent group (Fig. 2d). A similar pattern was observed in serum anti-triS IgG responses, confirming the highly similar profiles of SARS-CoV-2 IgG responses in nasal mucosa and serum (Fig. 2e).
Influence of hybrid immunity on mucosal antibody responses
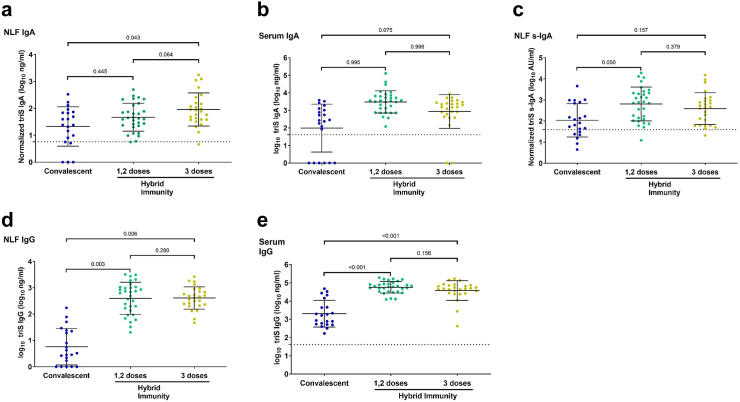
To evaluate the impact of hybrid immunity on mucosal antibody responses, we compared antibody titers between convalescent participants and vaccinated participants which were subsequently infected. We detected moderately higher anti-triS IgA responses in NLF of individuals with hybrid immunity in comparison to convalescent subjects, however these differences were significantly higher only in subjects that have received three vaccine doses (Fig. 3a). Serum anti-triS IgA responses were moderately higher in subjects with hybrid immunity after in comparison to convalescent subjects, but the observed differences were not significant (Fig. 3b). To assess the influence of hybrid immunity on the locally-generated immune responses, we determined the levels of anti-triS s-IgA in these groups. Moderately elevated levels of anti-triS s-IgA were detected in individuals with hybrid immunity compared to convalescent subjects, however these were only significant in subjects that received 1/2 vaccine doses (Fig. 3c). These results suggest that vaccination before infection has only a moderate impact on locally-generated immune responses.
Fig. 3.
Mucosal and serum antibody responses in convalescent compared to subjects with hybrid immunity. (a and b) Anti-triS IgA (log10 ng/mL) measured in NLF (a) and serum (b) of convalescent or subjects with hybrid immunity. (c) Anti-triS s-IgA (log10 AU/mL) antibody titers in NLF of convalescent or subjects with hybrid immunity. (d and e) Anti-triS IgG measured in NLF (d) and serum (e) of convalescent or subjects with hybrid immunity. A generalized linear model was used to determine differences of means, p values are shown above brackets. The mean + 3SD of 11 negative samples was used to set a cut-off for anti-triS IgA, s-IgA and IgG in NLF. The mean + 3SD of 56 pre-pandemic serum samples was used to set a cut-off for anti-triS IgA in serum samples. The mean + 3SD of 48 pre-pandemic serum samples was used to set a cut-off for anti-triS IgG in serum samples.
We then evaluated the impact of hybrid immunity on local and systemic IgG responses. Anti-triS IgG responses in NLF were significantly higher in vaccine breakthroughs in comparison to convalescent participants (Fig. 3d). Similar to NLF, anti-triS IgG serum responses were also significantly elevated in breakthroughs in comparison to convalescent participants (Fig. 3e). These results suggest that while vaccination boosts local IgG responses, it has rather limited impact on local IgA responses.
We also examined if anti-NP s-IgA responses are induced by infection in NLF and compared them to anti-triS antibody titers. Remarkably, there was only a weak correlation between anti-triS and anti-NP s-IgA mucosal responses in individuals with previous infection (r = 0.16; p = 0.16, Spearman's rank) (Supplemental Figure S3a). Anti-NP s-IgA responses were not detected in vaccinated individuals (Supplemental Figure S3b). In the majority of subjects with previous infection anti-NP s-IgA responses in NLF were below the cut-off of positivity (17 out of 21 in convalescent, 24 out of 31 in subjects with hybrid immunity after 1/2 doses and 17 of 26 in subjects with hybrid immunity after 3 doses and) and there was no significant difference between convalescent subjects and vaccine breakthroughs (Supplemental Figure S3b), suggesting that anti-NP s-IgA responses are either produced at very low levels or wane quickly.
Neutralization capacity of mucosal antibodies
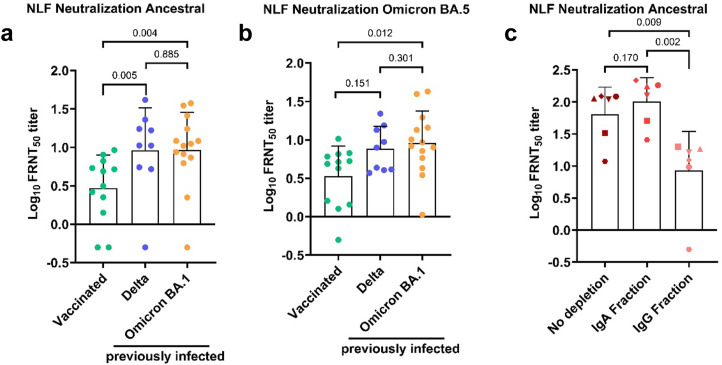
Last, we asked how efficient mucosal antibodies neutralize the ancestral SARS-CoV-2 strain and the Omicron BA.5 variant. Therefore, we performed a focus reduction neutralization test (FRNT) using NLF from a subset of participants. Since NLFs are not sterile and might contain compounds reducing viral replication, we first investigated if there is any neutralizing activity in NLFs collected from participants of the negative group. No neutralization was observed for ten negative samples, confirming that our assay is specifically measuring antibody-dependent neutralization. Next, we compared neutralizing antibody titers in participants vaccinated with three vaccine doses to subjects with hybrid immunity infected with the Delta or Omicron BA.1 variant after vaccination. Notably, there was no significant difference in DLIR between Delta and Omicron BA.1 breakthrough infections (median time post infection: 50 days (IQR, 41.5–63) for Delta and 39.5 days (IQR, 34–62.5) for Omicron BA.1 infections). Individuals with hybrid immunity had significantly higher neutralizing antibody titers against the ancestral SARS-CoV-2 strain in NLF compared to vaccinated individuals independently of the infecting variant (Fig. 4a). Next, we wanted to assess whether individuals with Delta and Omicron BA.1 derived hybrid immunity were better protected against infection with Omicron BA.5 compared to vaccinated subjects. We observed significantly higher neutralizing antibody titers against Omicron BA.5 in subjects that were previously infected with Omicron BA.1 compared to vaccinated only (Fig. 4b). Since neutralization is measured isotype independent in our assay, we wanted to determine the contribution of nasal IgA and IgG isotypes to SARS-CoV-2 neutralization. To this end, we purified IgA antibodies from the NLFs collected from subjects with hybrid immunity with elevated anti-triS s-IgA and IgG antibody titers. Measuring total IgA and total IgG antibodies in purified IgA fraction and the remaining fraction (here referred to as IgG fraction) demonstrated absent or very low IgA antibodies in IgG fraction and IgG antibodies in IgA fraction (Supplemental Figure S4). Antibody titers against the ancestral strain were similar in the IgA and unpurified fractions, but significantly lower in the IgG fraction (Fig. 4c), indicating that nasal IgA is the main contributor to neutralization in the mucosa.
Fig. 4.
Neutralizing antibody responses in the nasal mucosae of vaccinated and subjects with hybrid immunity. (a) Each dot represents the neutralizing titer (FRNT50) of an individual NLF sample against SARS-CoV-2 ancestral (a) and Omicron BA.5 variant (b). (c) IgA antibodies were purified from the NLFs, each symbol represents the same subject. Neutralizing titers against ancestral SARS-CoV-2 measured from different fractions are displayed. The generalized linear model was used to determine differences of means, p values are shown above brackets.
Discussion
In this study, we investigated mucosal antibody responses following COVID-19 vaccination, infection with SARS-CoV-2 or infection in vaccinated individuals, i.e. hybrid immunity. We demonstrated that IgA antibodies, which are present only on mucosal surfaces and not in systemic circulation, are the main contributor to neutralization in the mucosa. Additionally, we have shown that s-IgA responses are significantly elevated in subjects with previous infection, whereas vaccination-induced s-IgA responses are rarely detected and only at a very low level. Moreover, we found significantly higher neutralization titers against the ancestral SARS-CoV-2 strain in individuals with hybrid immunity compared to vaccinated subjects, providing evidence that previous infection elicits higher titers of functional mucosal antibodies. Interestingly, infection with Omicron BA.1 or Delta led to a similar neutralization capacity against Omicron BA.5 despite the smaller antigenic distance between BA.1 and BA.5, suggesting that the infecting variant is less important for protection. Contrary, in a study by Malato et al. a higher protection against BA.5 infection was found in individuals previously infected with BA.1/2 compared to Delta. However, in this study the difference in protection from Omicron BA.5 infection by previous infection with BA.1/2 or Delta was small and potential increased waning of immunity in Delta infected participants due to the longer time span was not accounted for.30
Our results show that mucosal and systemic IgG responses are highly similar, whereas mucosal IgA responses are compartmentalized from systemic responses. This is consistent with previous studies that show the discordance of local and systemic SARS-CoV-2 antibody responses.15,16,31 In convalescent subjects, neutralization activity poorly correlated between nasopharyngeal and blood samples.16,31 Interestingly, potent antibody responses in nasal fluids were found in some seronegative participants,15,16 indicating that in some cases SARS-CoV-2 infections lead only to a generation of local but not systemic immune responses.
We demonstrated that currently available intramuscularly-administered vaccines have a limited impact on SARS-CoV-2 specific mucosal responses. These results go in line with another study showing that vaccination efficiently boosts nasal IgG responses whereas nasal IgA responses are only transiently increased, and rapidly decline after vaccination.19 In another study vaccination did not generate detectable neutralizing mucosal antibodies and only breakthrough infections in vaccinated subjects resulted in measurable neutralization.28 To date, the presence of SARS-CoV-2 s-IgA responses were detected in saliva and breastmilk of some individuals upon vaccination.32 However, the levels of s-IgA antibodies in most subjects, who received mRNA vaccines was very low or below the detection limit.26 According to one study, anti-triS s-IgA responses were detected in saliva in 30% of subjects after two doses of mRNA vaccines, and the detected levels were significantly lower compared to convalescent patients.25 It remains unknown how mucosal s-IgA responses can be induced upon intramuscular vaccination. SARS-CoV-2 spike protein was detected in plasma after mRNA vaccination, and the clearance of this antigen correlated with the production of IgA antibodies, indicating that this antigen could reach the MALT to further induce mucosal antibody responses.33 Alternatively, after vaccination antigen diffuses to the regional draining lymph nodes, where it is taken up by local antigen-presenting cells, that can further migrate to the MALT and activate B cells that generate s-IgA antibodies.34
Here we show that IgA is an important contributor to neutralization in the NLF. Similarly, it has been demonstrated that IgA antibodies in nasal secretions are most strongly correlated with SARS-CoV-2 neutralization.35 Another study has shown that depletion of IgA from nasal wash samples lead to a reduction of the neutralization capacity.31 One study demonstrated that higher levels of mucosal IgA but not IgG antibodies correlated with lower levels of viral replication and lower risk of infection with Omicron.36 Furthermore, increased disease severity and mortality was identified among patients with IgA deficiency.37 However, further studies which would evaluate the role of pre-existing immunity on SARS-CoV-2 shedding and infection rates are currently missing. A recent study evaluated the effect of different vaccine administration routes on viral transmission in hamsters. Intranasally-administered adenovirus-vector vaccine or infection lead to significantly lower cumulative shedding or airborne transmission in comparison to intramuscular vaccine administration.38 Indeed, mucosally-administered vaccines are proposed as a strategy to reduce onward transmission. In our study, we detected the highest local and systemic antibody responses in subjects with hybrid immunity. Heterologous vaccination strategies that mimic hybrid immunity by giving a systemic prime through intramuscular vaccination followed by a mucosal boost, by intranasal vaccination, were shown to induce robust T and B cell immunity in the respiratory mucosa.39,40 It is currently unknown how long mucosal antibodies titres induced by mucosal vaccination remain sufficiently elevated to protect from SARS-CoV-2 infection. A recent study demonstrated that protection mediated by mucosal IgA antibodies lasted at least for 8 months following SARS-CoV-2 infection.10
This study has some limitations. All participants were sampled only at a single time point, therefore we compared antibody responses between different individuals rather than following the same individuals to compare changes of antibody responses over time or after subsequent immune reactions. Moreover, the sampling time points were not identical for all the participants within each group. However, with the exception of double-vaccinated participants there was no difference in the median DLIR between groups and all groups have similar age and sex distribution validating our conclusions. Most of the study participants were young adults, which might present more potent immune responses in comparison to older subjects, therefore these results cannot be extrapolated across age groups. Furthermore, all of the participants received mRNA vaccines, while mucosal antibody responses to other types of COVID-19 vaccines, mainly used in the low- and middle-income countries, have not yet been investigated. Lastly, we had no pre-pandemic NLFs in our control group, however we used the samples of seronegative individuals with no history of SARS-CoV-2 infection of vaccination.
While serum neutralization titers are highly predictive of immune protection from COVID-19 disease, correlates of protection from infection and transmission are not well defined. In this study we have shown that prior infection leads to more robust mucosal binding and neutralizing antibody responses, with IgA seemingly to play a crucial role. Therefore, the development of vaccines that elicit strong and lasting mucosal antibody responses would be vital to curtail infectious shedding and further transmission.
Contributors
O.P., I.E. and B.M. conceptualized the study. O.P., K.H.F., Ma.B. and Me.B. conducted the recruitment of participants. O.P., Ma.B., K.H.F. and Me.B. collected the clinical samples. O.P., K.A., Me.B., and P.S. performed the laboratory experiments. O.P., N.H., I.E. and B.M. analyzed and interpreted the data. I.E. and B.M. supervised the work. O.P., I.E. and B.M. wrote the manuscript. All authors read and approved the final version of the manuscript. O.P. and B.M. verified the underlying data.
Data sharing statement
All data are available in the main text or the Supplementary Materials.
Declaration of interests
BM declares grants from Moderna, outside the scope of the submitted work. IE declares grants and speakers fees from Moderna, outside the scope of the submitted work. The remaining authors declare that they have no competing interests.
Acknowledgements
We thank Sophie Coudurier-Boeuf and Isabelle Arm-Vernez for their excellent technical help. We thank Pauline Vetter, Morgann Duverger, Rachel Goldstein and Christiane Eberhardt for their help with clinical sample collection. We thank Florence Pojer, Kelvin Lau, and David Hacker from the EPFL Protein Production Facility for providing us with the purified SARS-CoV-2 spike protein. We thank the staff of the laboratory of virology at the HUG for support. We are grateful for the patients who were willing to donate their samples and agree to participate in our research.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2023.104893.
Appendix A. Supplementary data
References
- 1.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feikin D.R., Higdon M.M., Abu-Raddad L.J., et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399(10328):924–944. doi: 10.1016/S0140-6736(22)00152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bekliz M., Adea K., Vetter P., et al. Neutralization capacity of antibodies elicited through homologous or heterologous infection or vaccination against SARS-CoV-2 VOCs. Nat Commun. 2022;13(1):3840. doi: 10.1038/s41467-022-31556-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carreño J.M., Alshammary H., Tcheou J., et al. Activity of convalescent and vaccine serum against SARS-CoV-2 Omicron. Nature. 2022;602(7898):682–688. doi: 10.1038/s41586-022-04399-5. [DOI] [PubMed] [Google Scholar]
- 6.Powell A.A., Kirsebom F., Stowe J., et al. Protection against symptomatic infection with Delta (B.1.617.2) and Omicron (B.1.1.529) BA.1 and BA.2 SARS-CoV-2 variants after previous infection and vaccination in adolescents in England, August, 2021-March, 2022: a national, observational, test-negative, case-control study. Lancet Infect Dis. 2022;23(4):435–444. doi: 10.1016/S1473-3099(22)00729-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nielsen K.F., Moustsen-Helms I.R., Schelde A.B., et al. Vaccine effectiveness against SARS-CoV-2 reinfection during periods of alpha, Delta, or Omicron dominance: a Danish nationwide study. PLoS Med. 2022;19(11) doi: 10.1371/journal.pmed.1004037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hassan A.O., Kafai N.M., Dmitriev I.P., et al. A single-dose intranasal ChAd vaccine protects upper and lower respiratory tracts against SARS-CoV-2. Cell. 2020;183(1):169–184.e13. doi: 10.1016/j.cell.2020.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Afkhami S., D'Agostino M.R., Zhang A., et al. Respiratory mucosal delivery of next-generation COVID-19 vaccine provides robust protection against both ancestral and variant strains of SARS-CoV-2. Cell. 2022;185(5):896–915.e19. doi: 10.1016/j.cell.2022.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marking U., Bladh O., Havervall S., et al. 7-month duration of SARS-CoV-2 mucosal immunoglobulin-A responses and protection. Lancet Infect Dis. 2023;23(2):150–152. doi: 10.1016/S1473-3099(22)00834-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mostaghimi D., Valdez C.N., Larson H.T., Kalinich C.C., Iwasaki A. Prevention of host-to-host transmission by SARS-CoV-2 vaccines. Lancet Infect Dis. 2021;22(2):e52–e58. doi: 10.1016/S1473-3099(21)00472-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z., Lorenzi J.C.C., Muecksch F., et al. Enhanced SARS-CoV-2 neutralization by dimeric IgA. Sci Transl Med. 2021;13(577) doi: 10.1126/scitranslmed.abf1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwasaki A. Exploiting mucosal immunity for antiviral vaccines. Annu Rev Immunol. 2016;34:575–608. doi: 10.1146/annurev-immunol-032414-112315. [DOI] [PubMed] [Google Scholar]
- 14.Russell M.W., Mestecky J. Mucosal immunity: the missing link in comprehending SARS-CoV-2 infection and transmission. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.957107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cervia C., Nilsson J., Zurbuchen Y., et al. Systemic and mucosal antibody responses specific to SARS-CoV-2 during mild versus severe COVID-19. J Allergy Clin Immunol. 2021;147(2):545–557.e9. doi: 10.1016/j.jaci.2020.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith N., Goncalves P., Charbit B., et al. Distinct systemic and mucosal immune responses during acute SARS-CoV-2 infection. Nat Immunol. 2021;22(11):1428–1439. doi: 10.1038/s41590-021-01028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fröberg J., Gillard J., Philipsen R., et al. SARS-CoV-2 mucosal antibody development and persistence and their relation to viral load and COVID-19 symptoms. Nat Commun. 2021;12(1):5621. doi: 10.1038/s41467-021-25949-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isho B., Abe K.T., Zuo M., et al. Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients. Sci Immunol. 2020;5(52) doi: 10.1126/sciimmunol.abe5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liew F., Talwar S., Cross A., et al. SARS-CoV-2-specific nasal IgA wanes 9 months after hospitalisation with COVID-19 and is not induced by subsequent vaccination. EBioMedicine. 2023;87 doi: 10.1016/j.ebiom.2022.104402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pouwels K.B., Pritchard E., Matthews P.C., et al. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat Med. 2021;27(12):2127–2135. doi: 10.1038/s41591-021-01548-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singanayagam A., Hakki S., Dunning J., et al. Community transmission and viral load kinetics of the SARS-CoV-2 Delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study. Lancet Infect Dis. 2021;22(2):183–195. doi: 10.1016/S1473-3099(21)00648-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puhach O., Adea K., Hulo N., et al. Infectious viral load in unvaccinated and vaccinated individuals infected with ancestral, Delta or Omicron SARS-CoV-2. Nat Med. 2022;28(7):1491–1500. doi: 10.1038/s41591-022-01816-0. [DOI] [PubMed] [Google Scholar]
- 23.Woodbridge Y., Amit S., Huppert A., Kopelman N.M. Viral load dynamics of SARS-CoV-2 Delta and Omicron variants following multiple vaccine doses and previous infection. Nat Commun. 2022;13(1):6706. doi: 10.1038/s41467-022-33096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ketas T.J., Chaturbhuj D., Portillo V.M.C., et al. Antibody responses to SARS-CoV-2 mRNA vaccines are detectable in saliva. Pathog Immun. 2021;6(1):116–134. doi: 10.20411/pai.v6i1.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheikh-Mohamed S., Isho B., Chao G.Y.C., et al. Systemic and mucosal IgA responses are variably induced in response to SARS-CoV-2 mRNA vaccination and are associated with protection against subsequent infection. Mucosal Immunol. 2022;15(5):799–808. doi: 10.1038/s41385-022-00511-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sano K., Bhavsar D., Singh G., et al. SARS-CoV-2 vaccination induces mucosal antibody responses in previously infected individuals. Nat Commun. 2022;13(1):5135. doi: 10.1038/s41467-022-32389-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collier A.Y., Brown C.M., McMahan K.A., et al. Characterization of immune responses in fully vaccinated individuals after breakthrough infection with the SARS-CoV-2 delta variant. Sci Transl Med. 2022;14(641) doi: 10.1126/scitranslmed.abn6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Planas D., Staropoli I., Porot F., et al. Duration of BA.5 neutralization in sera and nasal swabs from SARS-CoV-2 vaccinated individuals, with or without omicron breakthrough infection. Med. 2022;3(12):838–847.e3. doi: 10.1016/j.medj.2022.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thwaites R.S., Jarvis H.C., Singh N., et al. Absorption of nasal and bronchial fluids: precision sampling of the human respiratory mucosa and laboratory processing of samples. J Vis Exp. 2018;131 doi: 10.3791/56413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malato J., Ribeiro R.M., Leite P.P., et al. Risk of BA.5 infection among persons exposed to previous SARS-CoV-2 variants. N Engl J Med. 2022;387:953–954. doi: 10.1056/NEJMc2209479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Butler S.E., Crowley A.R., Natarajan H., et al. Distinct features and functions of systemic and mucosal humoral immunity among SARS-CoV-2 convalescent individuals. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.618685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonçalves J., Juliano A.M., Charepe N., et al. Secretory IgA and T cells targeting SARS-CoV-2 spike protein are transferred to the breastmilk upon mRNA vaccination. Cell Rep Med. 2021;2(12) doi: 10.1016/j.xcrm.2021.100468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogata A.F., Cheng C.A., Desjardins M., et al. Circulating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine antigen detected in the plasma of mRNA-1273 vaccine recipients. Clin Infect Dis. 2022;74(4):715–718. doi: 10.1093/cid/ciab465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su F., Patel G.B., Hu S., Chen W. Induction of mucosal immunity through systemic immunization: phantom or reality? Hum Vaccin Immunother. 2016;12(4):1070–1079. doi: 10.1080/21645515.2015.1114195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright P.F., Prevost-Reilly A.C., Natarajan H., et al. Longitudinal systemic and mucosal immune responses to SARS-CoV-2 infection. J Infect Dis. 2022;226(7):1204–1214. doi: 10.1093/infdis/jiac065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Havervall S., Marking U., Svensson J., et al. Anti-spike mucosal IgA protection against SARS-CoV-2 Omicron infection. N Engl J Med. 2022;387(14):1333–1336. doi: 10.1056/NEJMc2209651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyts I., Bucciol G., Quinti I., et al. Coronavirus disease 2019 in patients with inborn errors of immunity: an international study. J Allergy Clin Immunol. 2021;147(2):520–531. doi: 10.1016/j.jaci.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Port J.R., Yinda C.K., Riopelle J.C., et al. Infection- or AZD1222 vaccine-mediated immunity reduces SARS-CoV-2 transmission but increases Omicron competitiveness in hamsters. Nat Commun. 2023;14(1):6592. doi: 10.1038/s41467-023-42346-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lapuente D., Fuchs J., Willar J., et al. Protective mucosal immunity against SARS-CoV-2 after heterologous systemic prime-mucosal boost immunization. Nat Commun. 2021;12(1):6871. doi: 10.1038/s41467-021-27063-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mao T., Israelow B., Peña-Hernández M.A., et al. Unadjuvanted intranasal spike vaccine elicits protective mucosal immunity against sarbecoviruses. Science. 2022;378(6622) doi: 10.1126/science.abo2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.