Abstract
Introduction
Fetal growth may be affected by both maternal polycystic ovary syndrome (PCOS) and metformin therapy. Here, we explore the effect of intrauterine metformin exposure on birth anthropometrics of infants born to women with PCOS. We also investigated whether the effect of metformin on birth anthropometrics is modified by maternal pre‐pregnancy body mass index, PCOS hyperandrogenic phenotype, serum androgen levels, preconception use of metformin and offspring sex. Additionally, we assessed newborn anthropometrics in relation to a national reference population.
Material and methods
Individual data from three randomized controlled triasl were pooled. The randomized controlled trials investigated the effects of metformin in pregnant women with PCOS. In all, 397 and 403 were randomized to the metformin and placebo groups, respectively. A Scandinavian growth reference was used to calculate sex and gestational age adjusted z‐scores. Linear regression models were used to estimate the effect of metformin on offspring z‐scores of head circumference, birth length, birthweight, placental weight, body mass index, ponderal index and birthweight:placental weight ratio. S‐testosterone, s‐androstenedione, and s‐sex‐hormone binding globulin from four timepoints in pregnancy were analyzed.
Results
Compared with the PCOS‐placebo group, newborns in the PCOS‐metformin group had larger head circumference (head circumference z‐score: mean difference = 0.25, 95% CI = 0.11– 0.40). This effect of metformin on head circumference z‐score was particularly observed among offspring of overweight/obese mothers and mothers with hyperandrogenic PCOS‐phenotype. We observed no difference in other anthropometric measures between the metformin and placebo groups or any clear interaction between maternal androgen levels and metformin. Newborns in the PCOS‐placebo group were shorter than in the reference population (birth length z‐score: mean = −0.04, 95% CI = –0.05 to −0.03), but head circumference and birthweight were similar.
Conclusions
Larger head circumference was observed at birth in metformin‐exposed offspring of mothers with PCOS. PCOS‐offspring were also shorter, with a similar birthweight to the reference population, indirectly indicating higher weight‐to‐height ratio at birth.
Keywords: anthropometry, birth length, birthweight, head circumference, metformin, polycystic ovary syndrome, pregnancy, prenatal exposure
We explored the effect of metformin vs placebo on newborn anthropometrics born to women with PCOS, and found that newborns exposed in utero to metformin compared with placebo had larger head circumference at birth.
Abbreviations
- BMI
body mass index
- d
mean difference
- CI
confidence interval
- GDM
gestational diabetes mellitus
- HC
head circumference
- ITT
intention‐to‐treat
- LGA
large for gestational age
- PCOS
polycystic ovary syndrome
- SGA
small for gestational age
- SHBG
sex hormone binding globulin
- WHO
World Health Organization
Key message.
Newborns exposed in utero to metformin compared with placebo had larger head circumference at birth. Further, newborns of mothers with PCOS had a similar birthweight, but were shorter than the reference population, an indirect indication of higher weight‐to‐height ratio.
1. INTRODUCTION
Polycystic ovary syndrome (PCOS) affects 10%–15% of women worldwide. 1 , 2 Women with PCOS have increased risk of pregnancy complications, such as miscarriage, gestational diabetes mellitus (GDM), preeclampsia and preterm delivery. 3 , 4 , 5 The combination of maternal PCOS, associated comorbidities and pregnancy complications may result in a suboptimal environment for the developing fetus with short‐ and long‐term consequences.
Either being born small for gestational age (SGA) or large for gestational age (LGA) is a risk factor for development of overweight and obesity later in life. 6 , 7 The influence of PCOS on fetal growth is somewhat uncertain, with previous studies variously reported a higher risk of both SGA 3 , 5 , 8 , 9 , 10 , 11 and LGA, 9 , 10 and others reporting no influence on birthweight when adjusted for gestational age. 12
Metformin has been used to improve pregnancy outcomes for women with GDM, obesity and PCOS. 13 , 14 Metformin freely passes through the placenta and therapeutic concentrations reach the fetus; however, metformin is not considered teratogenic. 15 , 16 , 17 Among women with obesity, metformin had no apparent effect on offspring birthweight and did not reduce the risk of being born LGA. 14 , 18 Furthermore, our own analyses of a smaller cohort suggested that infants born to mothers with PCOS had a larger head circumference when exposed to metformin. 19 High maternal body mass index (BMI) appeared to modify the effect of metformin, resulting in larger head circumference, whereas among normal‐weight mothers, the effect of metformin compared with placebo was a reduced birth length. 19 In the current study we extend these analyses to include data from our three randomized controlled trials, the Pilot study, the PregMet study and the PregMet2 study, which investigated the effects of metformin in women with PCOS on pregnancy complications. 20 , 21 , 22 In these three studies, we have shown that, compared with placebo, metformin reduced late miscarriages and preterm birth but had no effect on the incidence of GDM or preeclampsia, despite reduced gestational weight gain. 22 , 23
The aims of the current study were threefold: (1) estimate the effect of in utero metformin exposure on newborn anthropometrics compared with placebo in randomized controlled trials; (2) investigate whether the effect of metformin on birth anthropometrics is modified by maternal pre‐pregnancy BMI, PCOS hyperandrogenic phenotype, serum androgen levels, preconception use of metformin and offspring sex; (3) assess birth anthropometrics in PCOS‐offspring by comparing this with a reference population.
2. MATERIAL AND METHODS
The current study is a post hoc analysis of individually pooled data from three double‐blinded, placebo‐controlled, randomized trials: The Pilot study, the PregMet study and the PregMet2 study (Figure 1). 20 , 21 , 22 In total, 801 women with PCOS were included in these studies which compared metformin‐treatment with placebo from the first trimester and throughout pregnancy. Inclusion criteria were: diagnosis of PCOS according to the Rotterdam criteria, age between 18 and 45 years, and first trimester pregnancy with a single viable fetus. 24 PCOS phenotypes were categorized based on clinical history and review of patient files into the following groups: type A (hyperandrogenism [HA] + oligomenorrhea [OA] + polycystic ovaries [PCO]), type B (HA + OA), type C (HA + PCO) and type D (OA + PCO). 25 Hyperandrogenism was defined either clinically or biochemically. In the current analyses, PCOS androgen phenotypes were dichotomized as either hyperandrogenic (type A, B or C) or normo‐androgenic (type D). The participants received counseling on lifestyle and diet according to national guidelines. The studies have been described in more detail elsewhere. 20 , 21 , 22
FIGURE 1.
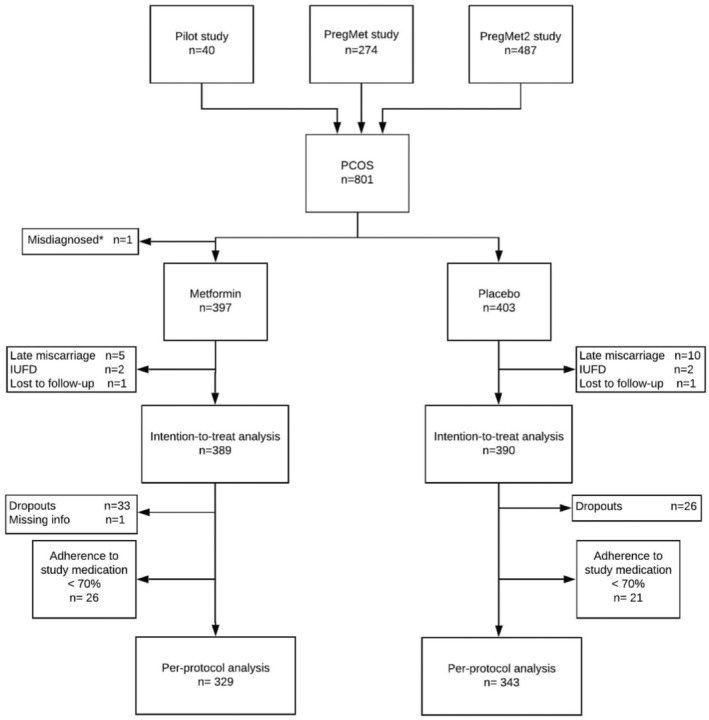
Flowchart on inclusion, randomization and exclusions.
2.1. Endpoints
Main endpoints of the present study are birth anthropometrics measured at delivery, including head circumference, birth length, birthweight and their respective z‐scores. Secondary endpoints are placental weight and the offspring proportionality scores: BMI, ponderal index and birthweight:placental weight ratio. The z‐score calculations for birth anthropometrics are based on Niklasson's continuous growth reference, and are adjusted for sex and gestational age. 26
2.2. Serum measurements
The included women provided serum samples after an overnight fasting at five time points in pregnancy, approximately in gestational week 10, 19, 28, 32 and 36. The serum samples were drawn from the antecubital vein in non‐heparinized tubes and stored at −80°C. All samples were stored from 3 to 11 years at −80°C after collection and analyzed for the androgens testosterone (T) and androstenedione (A4) with liquid chromatography–tandem mass spectrometry. Sex‐hormone binding globulin (SHBG) was determined by immunoassay on a Roche Cobas e602 System (Roche Diagnostics, Mannheim, Germany). All hormones were analyzed at the Department of Biochemistry, Lillebelt Hospital, Denmark. Free testosterone index was calculated as (T/SHBG) × 100.
2.3. Statistical analyses
Statistical analyses were conducted using STATA/MP16.1. The data were analyzed both according to the intention‐to‐treat (ITT) principle and per‐protocol. The ITT analyses include all participants where it was possible to evaluate the main endpoints. Per‐protocol analyses comprise participants with acceptable adherence to study medication, defined as >70% tablet intake. The metformin effect was estimated using linear regression analyses, both with and without adjustment for maternal pre‐pregnancy BMI. The inclusion of pre‐pregnancy BMI as a covariate was decided a priori due to this being an important prognostic factor for birth anthropometrics and in order to improve statistical efficiency. 27 The estimated mean differences between the metformin and placebo groups are presented for each birth anthropometric together with 95% confidence intervals (95% CI).
2.4. Subgroup analyses and treatment effect heterogeneity
Subgroup analyses were performed to assess the possible treatment effect heterogeneity due to maternal and infant characteristics: pre‐pregnancy BMI, PCOS phenotype, metformin‐use at conception, and offspring sex. For these analyses, maternal pre‐pregnancy BMI was divided into three categories from the World Health Organization (WHO) classification: Underweight/normal weight: BMI <25 kg/m2, overweight: BMI 25–29 kg/m2 ; obese: BMI ≥30 kg/m2. 28 Women were defined as having hyper‐ or normo‐androgenic PCOS phenotype as described above. Separate linear regression models were used to assess the effect of metformin within each subgroup and evidence for interaction between these subgroups and metformin. Interaction with pre‐pregnancy BMI was also assessed on a continuous scale.
Additionally, we assessed whether serum androgen levels were associated with the effect of metformin. We estimated the association between androgen levels and birth anthropometrics in the placebo group for each sample collection timepoint using linear regression, and the effect of metformin on androgen levels throughout pregnancy using mixed linear regression models. We then assessed the presence of an interaction between metformin and androgen levels on birth anthropometrics.
2.5. Ethics statement
All participants provided written informed consent before inclusion in the study. Ethics approval was obtained from the Regional Committee for Health Research Ethics of Central Norway. Pilot: REK 51–2000, date of approval: March 28, 2000. PregMet: REK 145–04, date of approval: September 6, 2004. PregMet2: REK 2011/1434, date of approval: October 6, 2011. Clinical Trial Registration: The original studies are registered at https://www.clinicaltrials.gov with the following ClinicalTrials.gov identifier numbers:
Protocol number 220800 (The Pilot study, post‐registered), NCT00159536 (The PregMet study), NCT01587378 (The PregMet2 study).
3. RESULTS
3.1. Participant characteristics
In total, 801 mother–infant dyads were enrolled in the three studies and the current intention‐to‐treat analysis includes 779 dyads, with 389 and 390 in the metformin and placebo groups, respectively. After excluding dropouts (n = 59) and non‐adherents (n = 47), the per‐protocol analyses included 672 dyads (Figure 1). Baseline characteristics for women included in the metformin and placebo groups were similar apart from mean maternal BMI, which was 28.9 kg/m2 in the metformin and 27.9 kg/m2 in the placebo group (Table 1, and Table S1 for the per‐protocol analyses).
TABLE 1.
Baseline characteristics for women with polycystic ovary syndrome in the intention‐to‐treat analyses.
| Metformin (n = 389) | Placebo (n = 390) | |
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Age (years) | 29.7 (4.5) | 29.6 (4.2) |
| Height (cm) | 167.6 (5.8) | 167.2 (5.8) |
| Weight (kg) | 81.1 (18.4) | 77.9 (17.5) |
| BMI (kg/m2) | 28.9 (6.4) | 27.9 (6.1) |
| Systolic blood pressure (mm Hg) | 117 (11) | 114 (12) |
| Diastolic blood pressure (mm Hg) | 73 (9) | 71 (9) |
| n (%) | n (%) | |
| Parity | ||
| 0 | 227 (58) | 214 (55) |
| 1+ | 162 (42) | 176 (45) |
| Education a | ||
| Elementary school | 11 (4) | 10 (4) |
| High school | 76 (29) | 67 (26) |
| College | 99 (39) | 106 (42) |
| University | 68 (27) | 71 (28) |
| Work status b | ||
| Working | 327 (84) | 318 (82) |
| Other | 62 (16) | 69 (18) |
| Civil status c | ||
| Married/co‐habitant | 250 (98) | 248 (97) |
| Other | 4 (2) | 7 (3) |
| Ethnicity | ||
| White | 376 (97) | 368 (94) |
| Non‐white | 13 (3) | 22 (6) |
| Smokers d | 23 (6) | 21 (5) |
| PCOS phenotype e | ||
| A (HA + OA + PCO) | 226 (59) | 229 (59) |
| B (HA + OA) | 45 (12) | 56 (15) |
| C (HA + PCO) | 21 (5) | 14 (4) |
| D (OA + PCO) | 93 (24) | 87 (22) |
Abbreviations: BMI, body mass index; HA, hyperandrogenism; OA, oligomenorrhea; PCO, polycystic ovaries; PCOS, polycystic ovary syndrome; SD, standard deviation.
n = 254 (metformin); n = 254 (placebo).
n = 389 (metformin); n = 387 (placebo).
n = 254 (metformin); n = 255 (placebo).
n = 388 (metformin); n = 390 (placebo).
n = 385 (metformin); n = 386 (placebo).
3.2. Effect of intrauterine metformin exposure on birth anthropometrics
Infants born to mothers in the metformin group had a larger head circumference than did those in the placebo group with a mean difference (d) of 0.42 cm (95% CI 0.18–0.66 cm). Adjustment for maternal pre‐gestational BMI had little impact on the effect estimate (d = 0.40 cm, 95% CI 0.16–0.64 cm). Similarly, the head circumference z‐score was increased in the metformin compared with the placebo group (d = 0.25, 95% CI 0.11–0.40; adjusted: d = 0.23, 95% CI 0.08–0.38) (Table 2). Birth length, birthweight and placental weight did not differ significantly between the metformin and placebo groups, nor did the variables of proportionality, ie BMI, ponderal index and birthweight:placental weight ratio (Table 2). The per‐protocol analyses produced very similar findings (Table S2) and there was no clear difference between the proportion of infants born SGA and those born LGA (Table S3).
TABLE 2.
Intention‐to‐treat analyses of anthropometric characteristics of offspring born to women with PCOS exposed to metformin or placebo.
| Metformin | Placebo | Crude | Adjusted b | |||||
|---|---|---|---|---|---|---|---|---|
| Anthropometrics | n a | Mean (95% CI) | n a | Mean (95% CI) | Mean difference (95% CI) | P‐value | Mean difference (95% CI) | P‐value |
| Head circumference | ||||||||
| In cm | 387 | 35.5 (35.3–35.7) | 386 | 35.1 (34.9–35.3) | 0.42 (0.18–0.66) | 0.001 | 0.40 (0.16–0.64) | 0.001 |
| z‐score | 386 | 0.3 (0.1–0.4) | 386 | 0.0 (−0.1 to 0.1) | 0.25 (0.11–0.40) | 0.001 | 0.23 (0.08–0.38) | 0.002 |
| Birth length | ||||||||
| In cm | 383 | 50.2 (50.0–50.5) | 383 | 50.1 (49.8–50.4) | 0.12 (−0.23 to 0.48) | 0.495 | 0.12 (−0.24 to 0.48) | 0.506 |
| z‐score | 382 | −0.4 (−0.5 to −0.3) | 383 | −0.4 (−0.5 to −0.3) | 0.01 (−0.16 to 0.17) | 0.924 | −0.01 (−0.17 to 0.16) | 0.939 |
| Birthweight | ||||||||
| In grams | 389 | 3548 (3495–3602) | 390 | 3517 (3457–3577) | 31 (−49–111) | 0.445 | 25 (−56–105) | 0.549 |
| z‐score | 388 | −0.1 (−0.2 to 0.0) | 390 | −0.1 (−0.2 to 0.0) | 0.02 (−0.12 to 0.16) | 0.784 | 0.00 (−0.14 to 0.14) | 0.956 |
| Placental weight (grams) | 348 | 673 (651–695) | 338 | 662 (646–678) | 11 (−16 to 38) | 0.431 | 9 (−18 to 36) | 0.516 |
| BMI (kg/m2) | 383 | 14.0 (13.9–14.2) | 383 | 13.9 (13.8–14.1) | 0.1 (−0.1 to 0.3) | 0.310 | 0.1 (−0.1 to 0.3) | 0.427 |
| PI (grams ×100/cm3) | 383 | 2.8 (2.8–2.8) | 383 | 2.8 (2.8–2.8) | 0.0 (0.0–0.1) | 0.416 | 0.0 (0.0–0.0) | 0.566 |
| BWPW‐ratio (bw/pw) | 348 | 5.5 (5.4–0.5–6) | 338 | 5.5 (5.3–5.7) | 0.0 (−0.2 to 0.2) | 0.760 | 0.0 (−0.2 to 0.2) | 0.743 |
Note: Differences estimated using a two‐sample t‐test.
Abbreviations: BMI, body mass index; bw, birthweight; BWPW‐ratio, birthweight:placental weight‐ratio; CI, confidence interval; PI, ponderal index; pw, placental weight; PCOS, polycystic ovary syndrome; SD, standard deviation.
The numbers vary due to missing data.
Adjusted for maternal pre‐pregnancy BMI.
3.3. Maternal pre‐pregnancy BMI, metformin exposure and birth anthropometrics
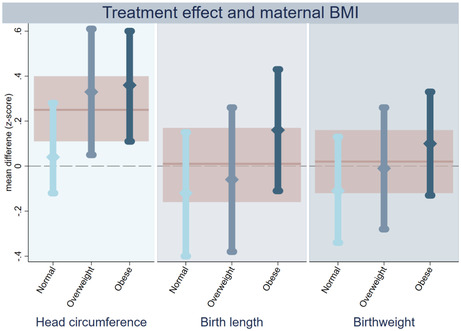
When assessing possible treatment effect heterogeneity based on maternal pre‐pregnancy BMI category, we observed that infants in the metformin group born to overweight and obese women had a larger head circumference and head circumference z‐score than did those in the placebo group (Figure 2, Table S4). There was no clear effect of metformin on head circumference of infants born to women with a normal pre‐pregnancy weight. However, formal assessment of the interactive effect between metformin and maternal BMI was not statistically significant (P = 0.075 and 0.135 for head circumference and head circumference z‐score, respectively). For both birth length and birthweight, the observed mean z‐score difference was higher in the metformin group when the mother was obese and lower if she had a normal pre‐pregnancy BMI (Figure 2). However, the estimated effect of metformin on birth length and birthweight within each BMI subgroup was inconclusive, and the interaction term was not statistically significant (Table S4). Also, when considering BMI as a continuous variable, we found no conclusive interactive effect between metformin and maternal BMI on z‐scores for head circumference, birth length or birthweight (Figure 3). The effect of metformin on placental weight and the proportionality scores did not substantially differ across the maternal BMI categories (Table S4).
FIGURE 2.
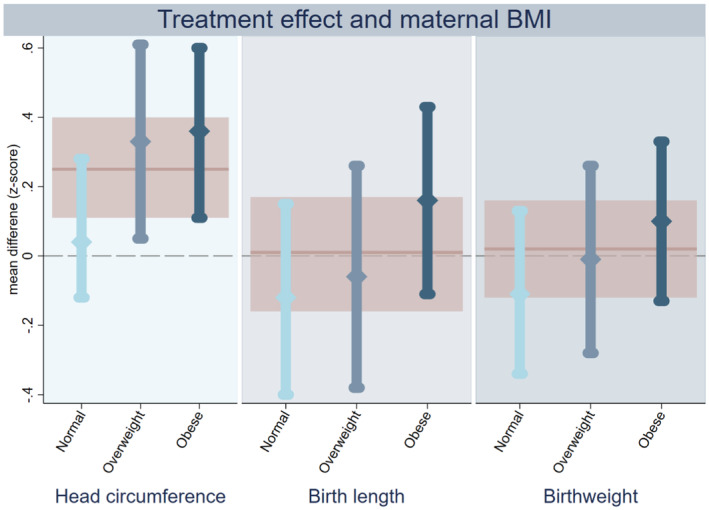
Intention‐to‐treat analyses on treatment effect of metformin on anthropometric z‐scores of offspring born to women with PCOS, stratified according to maternal pre‐pregnancy BMI category. Treatment effect of metformin stratified by maternal pre‐pregnancy BMI on head circumference (left panel), birth length (middle) and birthweight (right). The estimated effect of metformin among normal (light blue), overweight (mid‐blue) and obese women (dark blue) is shown as a mean different in z‐score with 95% confidence interval. The pink line and shaded area represent the estimated metformin effect in the whole group without stratification. PCOS = polycystic ovary syndrome.
FIGURE 3.
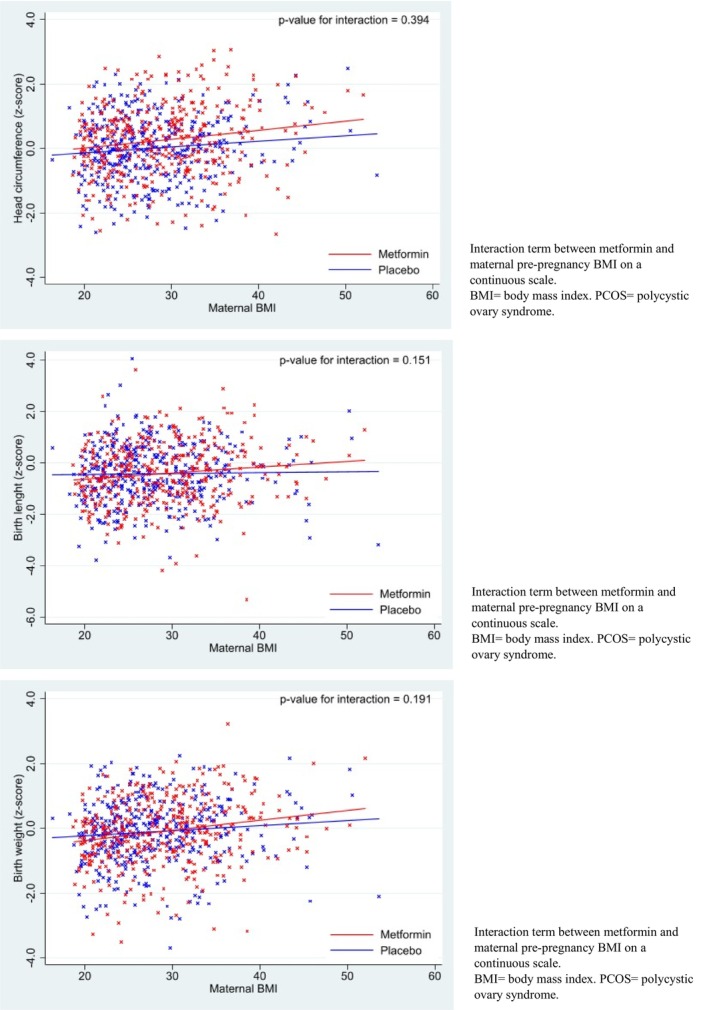
Intention‐to‐treat analyses on anthropometric z‐scores of offspring born to women with PCOS, stratified according to maternal BMI.
3.4. Maternal phenotype, metformin exposure and birth anthropometrics
Offspring of women with PCOS hyperandrogenic phenotype were born with larger head circumference when exposed to metformin in utero compared with placebo also after adjusting for maternal BMI (head circumference z‐score: d = 0.31, 95% CI 0.14–0.48) (Figure 4, Table S5). In contrast, the observed effect of metformin on head circumference at birth in normo‐androgenic mothers was negligible (head circumference z‐score: adjusted d = −0.04, 95% CI −0.34 to 0.27) and there was some indication that hyperandrogenic phenotype status may modify this effect (P‐value for interaction: P = 0.061 and 0.047 in crude and adjusted analyses, respectively).
FIGURE 4.
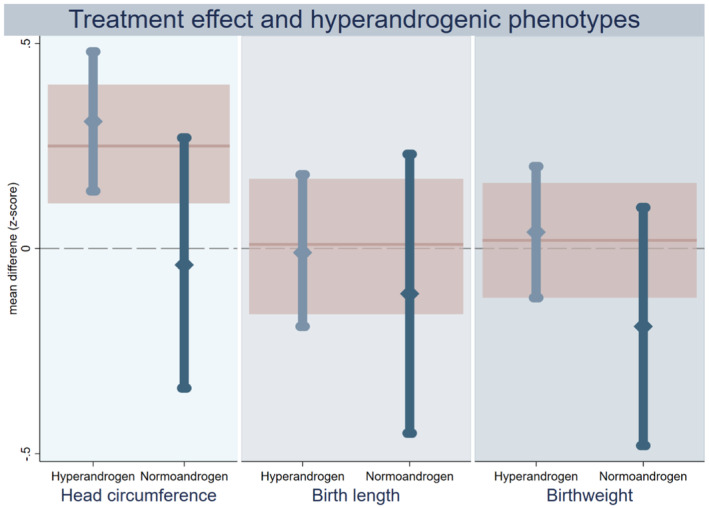
Intention‐to‐treat analyses on treatment effect of metformin on anthropometric z‐scores of offspring born to women with PCOS, stratified according to maternal androgen phenotype. Treatment effect of metformin stratified by maternal androgenic phenotype on head circumference (left panel), birth length (middle) and birthweight (right). The estimated effect of metformin among hyperandrogen (mid‐blue) and normoandrogen (dark blue) is shown as a mean different in z‐score with 95% confidence interval. The pink line and shaded area represent the estimated metformin effect in the whole group without stratification. PCOS = polycystic ovary syndrome.
3.5. Maternal androgen levels, metformin exposure and birth anthropometrics
Since the effect of metformin on head circumference appeared to be clearest in the PCOS hyperandrogenic phenotype, we further sought to determine whether higher serum androgen levels were also associated with a greater effect of metformin. Consistent with previous reports from women with PCOS, we found that levels of testosterone, androstenedione and SHBG gradually increase throughout pregnancy, and the average free testosterone index decreases (Table S6). We did not find evidence of a consistent association between any of the androgen levels throughout pregnancy and head circumference, birth length or birthweight within the placebo group (Table S7). When considering the effect of metformin on androgen levels, the metformin group was observed to have lower levels of testosterone and lower free testosterone index during the second and third trimesters (Figure S1, Table S8). However, there was a high degree of variation between participants and insufficient evidence to conclude that this difference was due to metformin.
The possibility of an interactive effect between metformin and androgen levels was assessed for each birth anthropometric and each pregnancy timepoint. Although the P‐value for interaction indicated some evidence of interaction effect between metformin and specific androgens at specific timepoints on specific anthropometrics, there were no substantial or consistent interactive effects observed (Figure S2 for head circumference and Table S9 also for birth length and birthweight).
3.6. Subgroup analyses on offspring sex and metformin use at conception
Stratification by use of metformin at conception (Table S10) and infant sex (Table S11) did not suggest any statistically significant treatment effect heterogeneity.
3.7. Birth anthropometrics in the PCOS‐placebo group compared with growth reference
Compared with the growth reference, newborns of PCOS mothers in the placebo group were shorter (birth length z‐score: d = −0.4, 95% CI −0.5 to −0.3), yet had only a slightly lower birthweight and a similar head circumference (Table 2). In this placebo group, 11.3% (95% CI 8.5–14.8%) of the infants were born SGA and 7.4% (95% CI 5.2–10.5%) LGA (Table S3).
4. DISCUSSION
The main findings of this study are that newborns exposed to metformin during pregnancy had larger head circumference than those born to mothers with PCOS receiving placebo treatment, and that this effect was predominantly seen among overweight/obese women and those with hyperandrogenic phenotype. Further, we found that babies born to women with PCOS are shorter than the reference population.
The individually pooled data from all three trials give us an adequate sample size to make reasonably precise estimates of the effect of metformin on birth anthropometrics. As such, we have sufficient numbers to detect both the effect of metformin on head circumference and head circumference z‐score when given to women with PCOS during pregnancy, and to be fairly confident that metformin does not have a clinically significant effect on other anthropometric measures at birth. The finding that in utero exposure to metformin appears to result in a larger head circumference is consistent with our previous study based on the PregMet study alone, where the increase could be traced back to ultrasonographic measurements of biparietal diameter at gestational week 32. 19 Existing literature provides limited and diverging evidence as to whether metformin influences offspring head circumference. In line with our own findings, two trials performed by Garcia et al. showed that metformin treatment during pregnancy resulted in larger head circumference and higher weight of the brain at birth compared with controls in a porcine model of intrauterine growth restriction. 29 , 30 Head circumference in children is strongly correlated with brain volume, and at a population level larger brain volume is associated both with better cognitive function and autism spectrum disorder. 31 , 32 Head circumference at birth is also a reliable measure of brain weight and fetal brain growth, and within the normal range of head circumference, a Danish study showed that larger head circumference at birth was associated with a decreased risk of intellectual disability. 33 , 34 Even so, it has also been shown that small differences in head circumference may not be associated with differences in IQ. 35 Despite such discrepancies in the evidence, we believe that the observed difference in head circumference seen in our study is interesting from a mechanistic and biological perspective. In a follow‐up study of children from the Pilot and PregMet studies (n = 99) at age 6–15 years, we found no difference in mean full‐scale IQ in those exposed to metformin (100.0 [SD 12.3]) and those exposed to placebo (100.9 [SD 10.1]). However, the sample size was small, and the confidence intervals wide, such that we cannot exclude the possibility that metformin may positively or negatively influence offspring IQ. 35 We intend to follow up the offspring of the PregMet2 regarding neurocognitive function.
The biologic mechanisms for our findings are not clear, and animal studies may suggest that metformin can mimic a catabolic state despite adequate nourishment in the fetus. 15 In a murine model, in utero metformin exposure resulted in a lower birthweight in offspring and a body composition similar to models of maternal malnourishment in pregnancy. 36 These findings may further substantiate the results in the studies by Garcia et al. that found larger offspring head circumference when using intrauterine growth restriction porcine models. 29 , 30 These signs of apparent malnourishment could be explained by one of the mechanisms of metformin: a cascade reaction that activates adenosine monophosphate‐activated protein kinase which in turn inhibits gluconeogenesis and promotes a “starving”, catabolic state despite adequate nourishment. 37 Further, one might speculate that the reduced access to adenosine triphosphate (ATP) due to metformin mediates a form of “brain sparing effect” 38 as a reaction to the experienced starvation‐like environment for the fetus, 37 which may be a possible explanation for the increased head circumference.
To the best of our knowledge, this is the first study to investigate the interactive effect of metformin and maternal BMI on the head circumference of infants at birth. When evaluating the subgroups according to BMI categories and PCOS phenotype, the effect of metformin on head circumference was particularly apparent in overweight and obese mothers and those with hyperandrogenic phenotype. Conversely, metformin was observed to have minimal effect on head circumference in infants born to normal‐weight women and those with normo‐androgenic phenotype. However, there remains a degree of statistical uncertainty around the effect of metformin on head circumference in each of these subgroups such that the presence of an interactive effect between metformin and both pre‐pregnancy BMI and PCOS phenotype should be interpreted cautiously. In particular, the normo‐androgenic phenotype group had few participants and we cannot exclude the possibility that metformin also has an effect on head circumference in these women. Infants of overweight/obese mothers have a risk of high birthweight and being large for gestational age. 39 A meta‐analysis investigated the effect of metformin in pregnant, obese, non‐diabetic women and found no effect of metformin on birthweight compared with placebo. 40 Metformin is widely used in pregnancy and is recommended as first line treatment along with insulin for gestational diabetes in several countries. It is of outmost importance to explore the metformin‐effect on offspring anthropometrics in GDM pregnancies, as PCOS and GDM overlap.
Although PCOS androgen phenotype may modify the effect of metformin, we did not see any clear evidence that maternal androgen levels during pregnancy influence the effect of metformin on head circumference, birth length or birthweight. Similarly, maternal androgen levels were not consistently associated with either birth anthropometrics or metformin use, which is consistent with the previously published results on a smaller group. 41 Evidence on how maternal androgens affect fetal anthropometry is scarce, especially concerning the impact on head circumference. A study by Huang et al. suggests that maternal androgens may influence the programming of intrauterine growth, by reporting associations between high maternal androgen levels and low offspring BMI z‐score at birth. 42 This is not easily interpreted using our findings, since head circumference was not an outcome in their study. Further investigations on maternal androgens and birth anthropometrics could be of clinical interest.
An interesting observation is that offspring born to mothers with PCOS are born shorter than the reference population, with slightly lower birthweight and similar head circumference. The average BMI for the PCOS mothers in this study was 28.4 kg/m2, whereas it was 24 kg/m2 in the Niklasson's reference population. There is no indication that women in the PCOS group were shorter than the reference population, since maternal height was on average. Considering the known positive correlation between pre‐pregnancy BMI and offspring birthweight, one would expect higher birthweight z‐scores in the PCOS offspring than in the reference population, 43 also because around 25% of these mothers had GDM according to WHO 1999 criteria and 40% according to new WHO 2003 criteria. In contrast, we observed that infants born to PCOS mothers were shorter and nonsignificantly lighter, which may indicate that PCOS has a restrictive effect on both birthweight and birth length, with a greater negative impact on birth length, indirectly indicating fatter infants. A potential confounder is the possibility that the reference population also contains women with PCOS. If so, our results are at least not overestimated. Our findings are in accordance with former reports on similar or lower birthweight in PCOS offspring compared with controls. 3 , 5 , 8 , 9 , 10 , 11
The strengths of this study in addition to its size and randomized, placebo‐controlled, double‐blinded design are the relative narrow confidence intervals for the effect of metformin on birth length, birthweight, placental weight and measures of proportionality, indicating that a clinically significant difference is unlikely. Additionally, the use of anthropometric z‐scores which are adjusted for both offspring sex and gestational age increases the accuracy of the effect estimates in this study. Baseline characteristics were comparable, except for weight and BMI. To account for this limitation, we analyzed the data with adjustment for BMI. Another possible limitation is the predominance of white women among the participants; the results may not be directly applicable to all ethnicities. An additional limitation of the current study is that it is a post hoc analysis which includes multiple statistical comparisons. Since this is an exploratory analysis of correlated birth anthropometric measures, we opted not undertake adjustment for multiple comparisons. Nonetheless, for the same reasons, we have interpreted the result cautiously and focused on findings which are consistent. Particularly the subgroup results should be interpreted with caution and need to be repeated in future studies.
5. CONCLUSION
Infants born to women with PCOS had a larger average head circumference after in utero exposure to metformin compared with placebo. The effect of metformin on head circumference was most pronounced among offspring of overweight and obese mothers, and mothers with hyperandrogenic phenotype. Further studies are needed to confirm whether maternal pre‐pregnancy BMI or hyperandrogenism truly modify the effect of metformin on head circumference. We also found that, compared with the reference population, infants born to PCOS mothers in the placebo group were shorter at birth, with slightly lower birthweight but similar head circumference.
AUTHOR CONTRIBUTIONS
GØN conceptualized and designed the study, carried out the initial analyses, interpreted data, wrote the draft, and reviewed and revised the draft. MRS conceptualized and designed the study, carried out the formal/further analyses, wrote the draft, interpreted data and reviewed and revised the draft. LGEH conceptualized and designed the study and supervised the initial analyses and reviewed and revised the draft. TSL designed the data collection instruments, collected data and reviewed the draft for important intellectual content. RØ interpreted data and critically reviewed the draft for important intellectual content. LMTS shared androgen data and critically reviewed the draft for important intellectual content. MSA critically interpreted data and reviewed the draft for important intellectual content. PBJ calculated the z‐score data and reviewed and revised the draft. EV conceptualized and designed the study, designed the data collection instruments, coordinated and supervised data collection, interpreted data and reviewed and revised the draft and supervised the team. All authors approved the final article as submitted and have agreed to be accountable for all aspects of the work.
FUNDING INFORMATION
This work was supported by The Research Council of Norway, Novo Nordisk Foundation, St. Olav's University Hospital and the Norwegian University of Science and Technology. Weifa AS supplied metformin free of charge for the Pilot study.
CONFLICT OF INTEREST STATEMENT
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
Supporting information
Table S1.
Nilsen GØ, Simpson MR, Hanem LGE, et al. Anthropometrics of neonates born to mothers with PCOS with metformin or placebo exposure in utero. Acta Obstet Gynecol Scand. 2024;103:176‐187. doi: 10.1111/aogs.14637
Guro Ørndal Nilsen and Melanie Rae Simpson contributed equally as first authors.
REFERENCES
- 1. Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta‐analysis. Hum Reprod. 2016;31:2841‐2855. [DOI] [PubMed] [Google Scholar]
- 2. March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25:544‐551. [DOI] [PubMed] [Google Scholar]
- 3. Qin JZ, Pang LH, Li MJ, Fan XJ, Huang RD, Chen HY. Obstetric complications in women with polycystic ovary syndrome: a systematic review and meta‐analysis. Reprod Biol Endocrinol. 2013;11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Franks S. Polycystic ovary syndrome. N Engl J Med. 1995;333:853‐861. [DOI] [PubMed] [Google Scholar]
- 5. Kjerulff LE, Sanchez‐Ramos L, Duffy D. Pregnancy outcomes in women with polycystic ovary syndrome: a metaanalysis. Am J Obstet Gynecol. 2011;204(558):e1‐e6. [DOI] [PubMed] [Google Scholar]
- 6. Glavin K, Roelants M, Strand BH, et al. Important periods of weight development in childhood: a population‐based longitudinal study. BMC Public Health. 2014;14:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ibáñez L, Ong K, Dunger DB, de Zegher F. Early development of adiposity and insulin resistance after catch‐up weight gain in small‐for‐gestational‐age children. J Clin Endocrinol Metab. 2006;91:2153‐2158. [DOI] [PubMed] [Google Scholar]
- 8. Boomsma CM, Eijkemans MJ, Hughes EG, Visser GH, Fauser BC, Macklon NS. A meta‐analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update. 2006;12:673‐683. [DOI] [PubMed] [Google Scholar]
- 9. Bahri Khomami M, Joham AE, Boyle JA, et al. The role of maternal obesity in infant outcomes in polycystic ovary syndrome—a systematic review, meta‐analysis, and meta‐regression. Obes Rev. 2019;20:842‐858. [DOI] [PubMed] [Google Scholar]
- 10. Yu HF, Chen HS, Rao DP, Gong J. Association between polycystic ovary syndrome and the risk of pregnancy complications: a PRISMA‐compliant systematic review and meta‐analysis. Medicine. 2016;95:e4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fornes R, Simin J, Nguyen MH, et al. Pregnancy, perinatal and childhood outcomes in women with and without polycystic ovary syndrome and metformin during pregnancy: a nationwide population‐based study. Reprod Biol Endocrinol. 2022;20:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Finnbogadóttir SK, Glintborg D, Jensen TK, Kyhl HB, Nohr EA, Andersen M. Insulin resistance in pregnant women with and without polycystic ovary syndrome, and measures of body composition in offspring at birth and three years of age. Acta Obstet Gynecol Scand. 2017;96:1307‐1314. [DOI] [PubMed] [Google Scholar]
- 13. Balsells M, García‐Patterson A, Solà I, Roqué M, Gich I, Corcoy R. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta‐analysis. BMJ. 2015;350:h102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chiswick C, Reynolds RM, Denison F, et al. Effect of metformin on maternal and fetal outcomes in obese pregnant women (EMPOWaR): a randomised, double‐blind, placebo‐controlled trial. Lancet Diabetes Endocrinol. 2015;3:778‐786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vanky E, Zahlsen K, Spigset O, Carlsen SM. Placental passage of metformin in women with polycystic ovary syndrome. Fertil Steril. 2005;83:1575‐1578. [DOI] [PubMed] [Google Scholar]
- 16. Nguyen L, Chan SY, Teo AKK. Metformin from mother to unborn child ‐ are there unwarranted effects? EBioMedicine. 2018;35:394‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cassina M, Donà M, Di Gianantonio E, Litta P, Clementi M. First‐trimester exposure to metformin and risk of birth defects: a systematic review and meta‐analysis. Hum Reprod Update. 2014;20:656‐669. [DOI] [PubMed] [Google Scholar]
- 18. Syngelaki A, Nicolaides KH, Balani J, et al. Metformin versus placebo in obese pregnant women without diabetes mellitus. N Engl J Med. 2016;374:434‐443. [DOI] [PubMed] [Google Scholar]
- 19. Hjorth‐Hansen A, Salvesen Ø, Engen Hanem LG, et al. Fetal growth and birth anthropometrics in metformin‐exposed offspring born to mothers with PCOS. J Clin Endocrinol Metab. 2018;103:740‐747. [DOI] [PubMed] [Google Scholar]
- 20. Vanky E, Salvesen KA, Heimstad R, Fougner KJ, Romundstad P, Carlsen SM. Metformin reduces pregnancy complications without affecting androgen levels in pregnant polycystic ovary syndrome women: results of a randomized study. Hum Reprod. 2004;19:1734‐1740. [DOI] [PubMed] [Google Scholar]
- 21. Vanky E, Stridsklev S, Heimstad R, et al. Metformin versus placebo from first trimester to delivery in polycystic ovary syndrome: a randomized, controlled multicenter study. J Clin Endocrinol Metab. 2010;95:E448‐E455. [DOI] [PubMed] [Google Scholar]
- 22. Løvvik TS, Carlsen SM, Salvesen Ø, et al. Use of metformin to treat pregnant women with polycystic ovary syndrome (PregMet2): a randomised, double‐blind, placebo‐controlled trial. Lancet Diabetes Endocrinol. 2019;7:256‐266. [DOI] [PubMed] [Google Scholar]
- 23. Glueck CJ, Wang P, Kobayashi S, Phillips H, Sieve‐Smith L. Metformin therapy throughout pregnancy reduces the development of gestational diabetes in women with polycystic ovary syndrome. Fertil Steril. 2002;77:520‐525. [DOI] [PubMed] [Google Scholar]
- 24. Rotterdam ESHRE/ASRM‐Sponsored PCOS consensus workshop group . Revised 2003 consensus on diagnostic criteria and long‐term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19:41‐47. [DOI] [PubMed] [Google Scholar]
- 25. Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova‐Jordan L, Azziz R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril. 2016;106:6‐15. [DOI] [PubMed] [Google Scholar]
- 26. Niklasson A, Albertsson‐Wikland K. Continuous growth reference from 24th week of gestation to 24 months by gender. BMC Pediatr. 2008;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Holmberg MJ, Andersen LW. Adjustment for baseline characteristics in randomized clinical trials. JAMA. 2022;328:2155‐2156. [DOI] [PubMed] [Google Scholar]
- 28. Physical status: the use and interpretation of anthropometry. Report of a WHO expert committee. World Health Organ Tech Rep Ser. 1995;854:1‐452. [PubMed] [Google Scholar]
- 29. Garcia‐Contreras C, Vazquez‐Gomez M, Pesantez‐Pacheco JL, et al. The effects of maternal metformin treatment on late prenatal and early postnatal development of the offspring are modulated by sex. Pharmaceuticals (Basel). 2020;13:363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Garcia‐Contreras C, Vazquez‐Gomez M, Pesantez‐Pacheco JL, et al. Maternal metformin treatment improves developmental and metabolic traits of IUGR fetuses. Biomolecules. 2019;9:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pangelinan MM, Zhang G, VanMeter JW, Clark JE, Hatfield BD, Haufler AJ. Beyond age and gender: relationships between cortical and subcortical brain volume and cognitive‐motor abilities in school‐age children. Neuroimage. 2011;54:3093‐3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lukito S, Norman L, Carlisi C, et al. Comparative meta‐analyses of brain structural and functional abnormalities during cognitive control in attention‐deficit/hyperactivity disorder and autism spectrum disorder. Psychol Med. 2020;50:894‐919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aagaard K, Matthiesen NB, Bach CC, Larsen RT, Henriksen TB. Head circumference at birth and intellectual disability: a nationwide cohort study. Pediatr Res. 2020;87:595‐601. [DOI] [PubMed] [Google Scholar]
- 34. Cooke RW, Lucas A, Yudkin PL, Pryse‐Davies J. Head circumference as an index of brain weight in the fetus and newborn. Early Hum Dev. 1977;1:145‐149. [DOI] [PubMed] [Google Scholar]
- 35. Greger HK, Hanem LGE, Østgård HF, Vanky E. Cognitive function in metformin exposed children, born to mothers with PCOS—follow‐up of an RCT. BMC Pediatr. 2020;20:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Salomäki H, Vähätalo LH, Laurila K, et al. Prenatal metformin exposure in mice programs the metabolic phenotype of the offspring during a high fat diet at adulthood. PloS One. 2013;8:e56594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: from mechanisms of action to therapies. Cell Metab. 2014;20:953‐966. [DOI] [PubMed] [Google Scholar]
- 38. Bauer R, Walter B, Brust P, Füchtner F, Zwiener U. Impact of asymmetric intrauterine growth restriction on organ function in newborn piglets. Eur J Obstet Gynecol Reprod Biol. 2003;110(Suppl 1):S40‐S49. [DOI] [PubMed] [Google Scholar]
- 39. Yu Z, Han S, Zhu J, Sun X, Ji C, Guo X. Pre‐pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta‐analysis. PloS One. 2013;8:e61627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Elmaraezy A, Abushouk AI, Emara A, Elshahat O, Ahmed H. Effect of metformin on maternal and neonatal outcomes in pregnant obese non‐diabetic women: a meta‐analysis. Int J Reprod Biomed. 2017;15:461‐470. [PMC free article] [PubMed] [Google Scholar]
- 41. Andræ F, Abbott D, Stridsklev S, et al. Sustained maternal Hyperandrogenism during PCOS pregnancy reduced by metformin in non‐obese women carrying a male fetus. J Clin Endocrinol Metab. 2020;105:3762‐3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Huang G, Aroner SA, Bay CP, et al. Sex‐dependent associations of maternal androgen levels with offspring BMI and weight trajectory from birth to early childhood. J Endocrinol Invest. 2021;44:851‐863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stamnes Koepp UM, Frost Andersen L, Dahl‐Joergensen K, Stigum H, Nass O, Nystad W. Maternal pre‐pregnant body mass index, maternal weight change and offspring birthweight. Acta Obstet Gynecol Scand. 2012;91:243‐249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.


