Abstract
Introduction
The clinical management of placenta accreta spectrum (PAS) depends on placental topography and vascular involvement. Our aim was to determine whether transabdominal and transvaginal ultrasound signs can predict PAS management.
Material and methods
We conducted a retrospective cohort study of consecutive prenatally suspected PAS cases in a single tertiary‐care PAS center between January 2021 and July 2022. When PAS was confirmed during surgery, abdominal and transvaginal ultrasound scans were analyzed in relation to PAS management. The preferred surgical approach of PAS was one‐step conservative surgery (OSCS). Massive blood loss and PAS topography in the lower bladder trigone necessitated cesarean hysterectomy. Transvaginal ultrasound‐diagnosed intracervical hypervascularity was split into three categories based on their quantity. Anatomically, the internal cervical os is located at the level of the bladder trigone and was used as landmark for upper and lower bladder trigone PAS.
Results
Ninety‐one women underwent OSCS and 35 women underwent cesarean hysterectomy (total 126 women with PAS). Abdominal and transvaginal ultrasound features differed significantly between women that underwent OSCS and cesarean hysterectomy: decreased myometrial thickness (<1 mm), 82.4% vs. 100%, p = 0.006; placental bulge, 51.6% vs. 94.3%, p < 0.001; bladder wall interruption, 62.6% vs. 97.1%, p < 0.001; abnormal placental lacunae, 75.8% vs. 100%, p < 0.001; hypervascularity (large lacunae feeding vessels, 57.8% vs. 94.6%, p < 0.001; parametrial hypervascularity, 15.4% vs. 60%, p < 0.001; the rail sign, 6.6% vs. 28.6%, p = 0.003; three‐dimensional Doppler intra‐placental hypervascularity, 81.3% vs. 100%, p < 0.001; intracervical hypervascularity 60.4% vs. 94.3%, p < 0.001); and cervical length 2.5 ± 0.94 vs. 2.2 ± 0.73, p = 0.038. Other ultrasound signs were not significantly different. The results of multivariable logistic regression showed placental bulge (odds ratio [OR] 9.3; 95% CI 1.9–44.3; p = 0.005), parametrial hypervascularity (OR 4.1; 95% CI 1.541–11.085; p = 0.005), and intracervical hypervascularity (OR 9.2; 95% CI 1.905–44.056; p = 0.006) were weak predictors of OSCS. Intracervical hypervascularity Grade 1 (vascularity <50% of cervical tissue) was more present in OSCS than higher gradings two and three (91% vs. 27.6% vs. 14.3%; p < 0.001).
Conclusions
Cesarean hysterectomy is associated with the PAS signs of placental bulge and Grade 2 and 3 intracervical hypervascularity. OSCS is associated with intracervical hypervascularity Grade 1 on transvaginal ultrasound. Prospective validation is required to formulate predictors for PAS management.
Keywords: hysterectomy, one‐step conservative surgery, placenta accreta spectrum, pregnancy, ultrasound
Pregnant women with a high risk of placenta accreta spectrum (PAS) should also have transvaginal ultrasound in combination with transabdominal ultrasound screening for advanced grading of PAS, which is seen as hypervascularity. This may predict the possibility of one‐step conservative surgery and grading of the intracervical hypervascularity may correlate with surgical management of PAS.
Abbreviations
- FIGO
the International Federation of Gynecology & Obstetrics
- OSCS
one‐step conservative surgery
- PAS
placenta accreta spectrum
- TVUS
transvaginal ultrasound
- US
ultrasound
Key message.
Pregnant women with a high risk of PAS should have transvaginal ultrasound in combination with transabdominal ultrasound to investigate lower PAS invasion. The assessment and grading of intracervical hypervascularity may be useful in the prediction of maternal outcomes and planning patient management.
1. INTRODUCTION
Placenta accreta spectrum (PAS) is one of the most challenging complications in obstetrics due to its difficult surgery and high morbidity. 1 The incidence of PAS has increased worldwide over the recent decades. 2 , 3 Management of PAS is still controversial and there is no clear recommendation for the optimal strategy for PAS surgery because this mainly depends on the surgeon's experience, antenatal staging, and multidisciplinary team planning. 4 , 5 The best‐known conservative surgery methods are one‐step conservative surgery (OSCS) and triple‐P surgery. 6 , 7 In OSCS, myometrial resection is performed (focal or diffuse) after intra‐surgical staging and surgical vascular control. OSCS can only be performed successfully if the placenta is located above the level of the bladder trigone and so depends on the PAS topography. 3 , 8 The difference with triple‐P surgery is the vascular control, for which triple‐P surgery uses interventional radiology to temporarily block the internal iliac artery with a balloon catheter. 7
Ultrasound is one of the most widely used diagnostic tools for staging of PAS because it is cheap, easy to use, and widely available. Although clinical staging of PAS by the grading system of FIGO (the International Federation of Gynecology & Obstetrics) may not correlate with the surgical outcome, it is important for epidemiological data. 1 , 9 , 10 Several ultrasound signs are standardized by FIGO and the Society for Maternal‐Fetal Medicine to reduce the level of diversity, which can lead to different perceptions between sonographers. 11 , 12 , 13 , 14 Three important principles in the ultrasound examination of PAS are the uteroplacental interface, abnormal lacunae, and the hypervascularity sign. 14 , 15 , 16 Ultrasound has a high sensitivity and specificity for PAS screening, especially in cases of multiple positive ultrasound signs, but there is a lack of correlation with the clinical treatment. 17 , 18 It is still not clear which of the ultrasound signs are best for determining the clinical treatment. Despite the fact that several ultrasound studies have attempted to correlate ultrasound signs with histopathology, 19 the relationship between ultrasound signs and the success of OSCS remains unknown. 18 The aim of this study was to determine which ultrasound signs could serve as good predictors for successful treatment of PAS by OSCS.
2. MATERIAL AND METHODS
This is a retrospective cohort study conducted during 18 months (January 2021 till July 2022) in a single tertiary‐care PAS center, Dr. Soetomo Academic General Hospital, in Indonesia. All women with a previous cesarean section and a placenta previa in the index pregnancy were identified as having risk factors for PAS and were screened by ultrasound. The PAS topography was analyzed and graded according to a recent publication. 8
Exclusion criteria were dehiscence of the uterine scar without abnormally adherent placenta, no signs of increased vascularity on the uteroplacental surface and uterine serosa during the surgery, and women in an unstable hemodynamic condition before surgery without adequate pre‐surgical ultrasound. Emergency PAS cases with vaginal bleeding or uterine rupture but in a stable hemodynamic condition were not excluded if ultrasound examinations could be performed.
The grading of PAS was confirmed after the surgery with pathology examination of the specimen using the FIGO classification. 20 FIGO Grade 1 implies that the placenta is abnormally adherent to the myometrium, making it impossible for the surgeon to separate the placenta with gentle traction, and the histopathology reveals the absence of decidua between villous tissue and myometrium, with placental villi attached directly to the superficial myometrium. FIGO Grade 2 indicates bluish and bulge in the uteroplacental interface with increased vascularity and placental villi within the myometrial muscle during histopathology examination. Grade 3A indicates that the placental tissue is seen to be invading through the surface of the uterus (serosa) and the histopathology shows villous tissue within or breaching the uterine serosa. FIGO Grade 3B is comparable to Grade 3A with the addition of bladder invasion, whereas Grade 3C indicates pelvic organ invasion.
2.1. Management of PAS
Management of PAS depends on the surgical grading of PAS. 8 , 21 , 22 PAS topography above the bladder trigone (supero‐anterior or upper parametrium PAS) was deemed suitable for OSCS. The most crucial step in OSCS is dissecting the bladder and placing multiple sutures in the colpouterine pedicles to control vaginal artery anastomosis before placental resection. 3 , 22 Because the colpouterine pedicles will be sutured to the healthy uterine corpus to control surgical bleeding, 6 these colpouterine sutures are an important step to success in OSCS.
The lower bladder trigone PAS topography (lower anterior or lower parametrium of PAS) was an indication for cesarean hysterectomy because damaged tissue of the lower uterine wall, including the cervix, makes uterine reconstructive surgery impossible due to a lack of healthy tissue and an increased risk of surgical bleeding. 6 In case of anterior lower uterine PAS topography (in the lower level of the bladder trigone) with massive fibrotic tissue between the placenta and bladder, or lower parametrium, hysterectomy was performed with a (temporary) aortic clamp to reduce the blood loss. 21
The surgical procedures were performed by two surgeons (one surgeon with expertise in PAS surgery and one of the Maternal–Fetal Medicine trainees who was participating in a PAS surgery fellowship at Dr. Soetomo Academic General Hospital). The surgeons were not informed about the ultrasound results.
Leaving the placenta in situ as a treatment option was not chosen in this study period because long‐term follow up of women is not possible in our setting. 23
Emergency surgery for PAS was performed in cases of major antepartum bleeding 24 and/or uterine rupture and/or hematuria before surgery.
Unexpected bleeding, resulting in intra‐surgical massive blood loss, could arise as massive vaginal bleeding or after accidental damage to vessels. This situation needed internal manual aortic compression to control blood loss in order to continue the surgery.
2.2. Ultrasound diagnosis of PAS
Before the surgery, a high suspicion of PAS was raised when more than three ultrasound signs were suggestive for PAS. 17 All ultrasound reports were reviewed by an expert in ultrasonographic PAS diagnosis (RAA and NIC) using the results of transabdominal gray‐scale ultrasound (Figure 1A), Doppler ultrasound (Figure 1B), and transvaginal ultrasound. 12 , 25 , 26 , 27 , 28 , 29 , 30 The expert was blinded to the surgical outcome.
FIGURE 1.
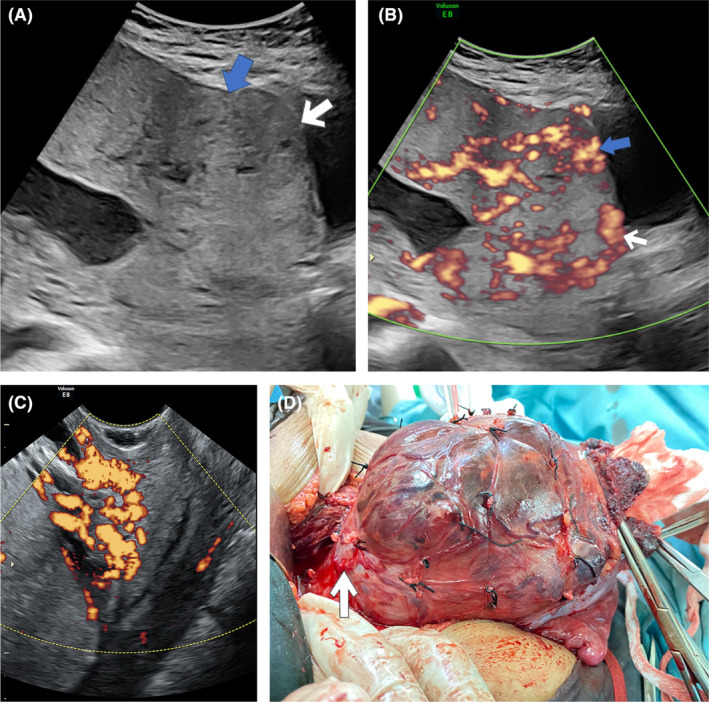
(A) Transabdominal ultrasound: loss of clear zone (blue arrow) following the placental bulge (white arrow) that suggests placenta accreta spectrum in gray‐scale ultrasound. (B) Doppler ultrasound: bridging vessels (blue arrow) and subplacental hypervascularity in the lower uterine segment (white arrow) in addition to gray‐scale ultrasound. (C) Transvaginal ultrasound: tortuous hypervascularized anechoic spaces <50% of cervical tissue that measured from internal cervical ostium to external cervix. (D) The colpouterine vessels after bladder dissection (black arrow).
The transabdominal ultrasound investigations were performed according to a previously established method (Supporting Information Table S1). 12 , 14 , 15 , 16 , 28 During transvaginal ultrasound, the internal cervical ostium is located at the level of the bladder trigone and is a landmark for upper and lower bladder trigone PAS. 31 The cervical length and intracervical hypervascularity—defined as multiple tortuous anechoic spaces within the cervix, appearing as hypervascular with color Doppler—were analyzed. 27 The latter marker may correlate with the increased vascularity from the colpouterine pedicles of the uterus, which represent the anastomosis of the vaginal and uterine arteries. The number of intracervical lacunae was classified in three levels:
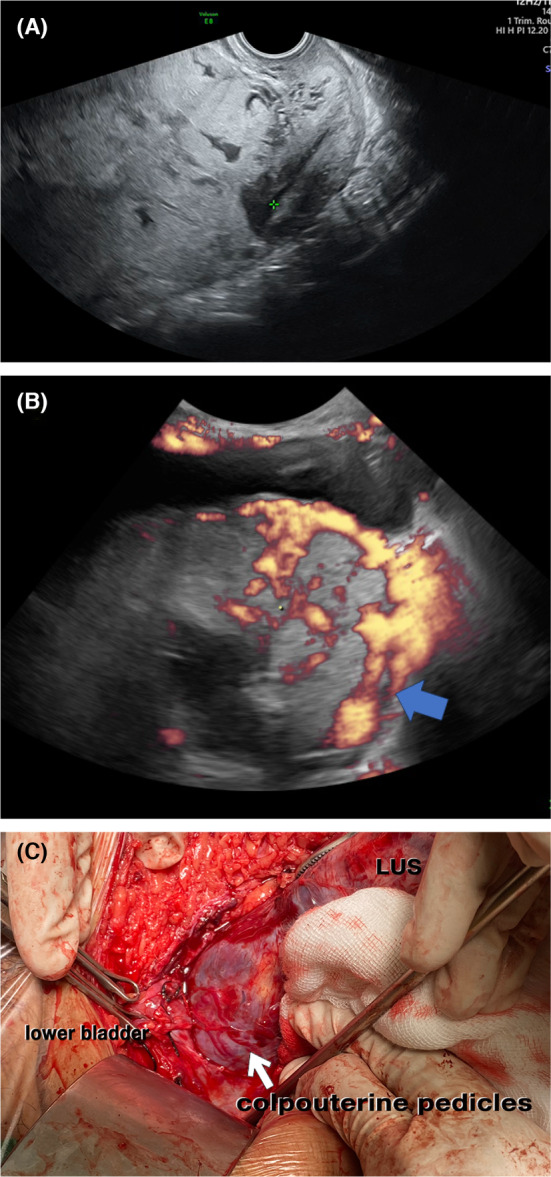
Grade 1: Tortuous hypervascularized anechoic spaces <50% of cervical tissue that measured from internal cervical ostium to external cervix (the vascular anastomoses of the cervix <50%) (Figure 1C).
Grade 2: Tortuous hypervascularized anechoic spaces >50% of cervical tissue that measured from internal cervical ostium to external cervix (the vascular anastomoses of the cervix >50%) (Figure 2A).
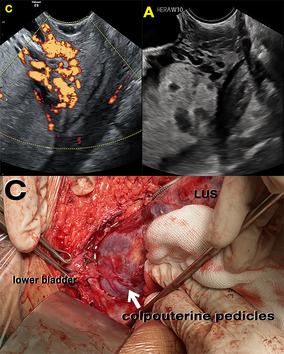
Grade 3: multiple hypoechoic images of the cervix >50% of cervical tissue (the vascular anastomoses of the cervix >50%) with loss of clear zone between placental and cervical tissue (Figure 3A).
FIGURE 2.
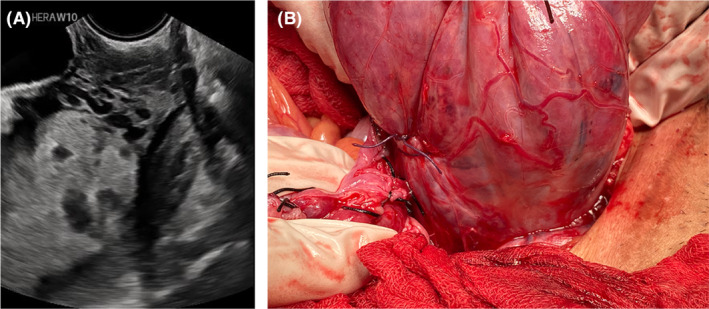
(A) Transvaginal ultrasound: tortuous hypervascularized anechoic spaces along the cervical tissue. (B) Colpouterus hypervascularized below the placenta accreta spectrum.
FIGURE 3.
(A) Transvaginal ultrasound: multiple hypoechoic images of the cervix >50% of cervical tissue with loss of clear zone between placental and cervical tissue. (B) Transabdominal ultrasound: complex, irregular arrangement of vessels, exhibiting tortuous courses and varying calibers in the parametrial region (transverse probe position). (C) Placental tissue was seen in the colpouterine area (below the lower uterine segment (LUS) with hypervascularization (arrow) after bladder dissection, which may indicate that the placenta was abnormally adherent in the cervical tissue.
All ultrasound examinations were performed less than 48 hours before surgery.
All transabdominal and transvaginal ultrasound (TVUS) signs were correlated with the outcome of PAS surgery. A second analysis compared the grading of the intracervical hypervascularity with the surgical results.
2.3. Statistical analyses
Statistical analysis was performed using the Statistical Package for Social Science (SPSS), version 29. The Kolmogorov–Smirnov test was used to assess the normality of the distribution of the data. The two‐group analysis (OSCS vs. cesarean hysterectomy), independent t test, Mann–Whitney U test, and chi‐squared test were performed for the various maternal outcome variables and ultrasound signs. Three groups of cervical grading, analysis of variance, and the Kruskal–Wallis test were used for normally and non‐normally distributed data, respectively. A binary logistic regression analysis was performed to explore the most important ultrasound sign associated with cesarean hysterectomy in PAS.
2.4. Ethics statement
Ethical approval was obtained from the Ethics Committee in Health Research of the Dr. Soetomo Academic General Hospital (number 1169/LOE/301.4.2/XII/2022 – December 19, 2022).
3. RESULTS
In 149 women the suspicion of PAS was reached antenatally by TVUS. In 23 women during surgery, dehiscence of the uterine scar without abnormally adherent placenta was present and these cases were excluded from this study. A total of 126 women with presurgically diagnosed PAS had this diagnosis confirmed during surgery and were included in the analysis (Figure 4). General characteristics and pregnancy outcomes are described in Table 1. None of the women had a history of uterine surgery. Eight pregnant women had a low‐lying placenta, one woman had an anterior placenta, and one woman had lateral placenta previa, all in the OSCS group. Most women had complete placenta previa.
FIGURE 4.
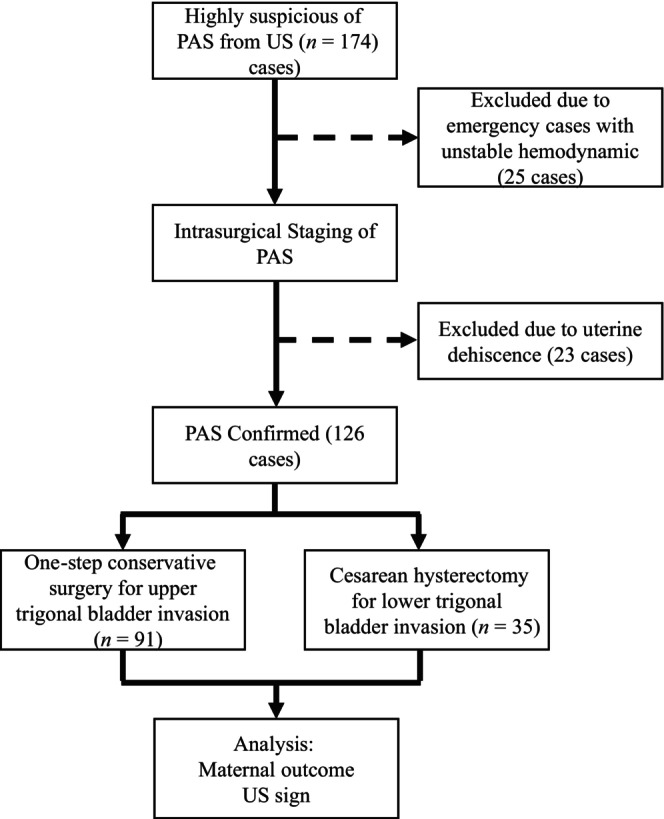
Enrolment data based on the medical record, surgical report, ultrasound reports, pathological report. PAS, placenta accreta spectrum; US, ultrasound.
TABLE 1.
Maternal demographics and outcome between one‐step conservative surgery and cesarean hysterectomy.
| OSCS (N = 91) | Cesarean hysterectomy (N = 35) | p | |
|---|---|---|---|
| Maternal demographics | |||
| Maternal age (years) a | 33 (22–46) | 33 (22–42) | 0.847 |
| Gravida b | 3 (2–6) | 3 (2–7) | 0.919 |
| Number of CS b | 2 (1–3) | 2 (1–3) | 0.427 |
| Group of CS c | |||
| CS 1× | 31 (34.1%) | 14 (40%) | 0.406 |
| CS 2× | 51 (56%) | 19 (54.3%) | |
| CS ≥3× | 9 (9.9%) | 2 (5.7%) | |
| GA surgery (weeks) b | 36 (29–39) | 36 (27–40) | 0.384 |
| Emergency surgery c | 14 (15.4%) | 6 (17.1%) | 1000 |
| Maternal outcome | |||
| Blood loss (mL) b | 2358 (±1225) | 3086 (±1720) | 0.028 |
| Blood transfusion (units) b | 2 (0–6) | 3 (0–9) | 0.011 |
| Aortic compression c | 9 (9.9%) | 19 (54.3%) | <0.001 |
| Unexpected bleeding c | 7 (7.7%) | 7 (20%) | 0.098 |
| Complications c | 9 (9.9%) | 4 (11.4%) | 1000 |
| FIGO grading PAS c | |||
| Grade 1 | 16 (17.6%) | 0 | <0.001 |
| Grade 2 | 54 (59.3%) | 7 (20%) | |
| Grade 3A | 15 (16.5%) | 10 (28.6%) | |
| Grade 3B‐C | 6 (6.6%) | 18 (51.4%) | |
| PAS topography c | |||
| Type 1 | 78 (85.7%) | 0 | <0.001 |
| Type 2 | 0 | 2 (5.7%) | |
| Type 3 | 13 (14.3%) | 19 (54.3%) | |
| Type 4 | 0 | 13 (37.1%) | |
| Type 5 | 0 | 1 (2.9%) |
Independent t test.
Mann–Whitney U test.
Chi‐squared test.
Abbreviations: CS, cesarean section; GA, gestational age; OSCS, one‐step conservative surgery; PAS, placenta accreta spectrum.
In addition, emergency surgery was performed in 15 women with major vaginal bleeding, one woman with hematuria, and four women with an uterine rupture. The emergency surgery had worsened outcomes in this study but was not related to cesarean hysterectomy. Surgical complications included seven bladder injuries, two intra‐abdominal abscesses after emergency surgery, two uterine atony after OSCS (which needed additional uterine compression sutures), and two cases of disseminated intra‐coagulation due to massive bleeding before surgery. There were no maternal deaths (Table 1).
Most ultrasound signs showed differences between the OSCS and cesarean hysterectomy groups (Table 2). PAS topography was mostly type one (above the bladder trigone) in the OSCS group (85.7%), but in the cesarean hysterectomy group it was mostly type three (lower bladder trigone) and type four (type three with fibrotic tissue between placenta and bladder).
TABLE 2.
Ultrasound signs between OSCS vs. cesarean hysterectomy of PAS.
| One‐step conservative surgery (OSCS) (N = 91) | Cesarean hysterectomy (N = 35) | p | |
|---|---|---|---|
| Transabdominal ultrasound a , b | |||
| Uteroplacental interface | |||
| Loss of clear zone | 91 (100%) | 35 (100%) | |
| Myometrial thickness | 75 (82.4%) | 35 (100%) | 0.006 |
| Placental bulge | 47 (51.6%) | 33 (14.3%) | <0.001 |
| Focal exophytic mass | 4 (4.4%) | 4 (11.4%) | 0.297 |
| Abnormal lacunae | 69 (75.8%) | 35 (100%) | <0.001 |
| Bladder wall interruption | 57 (62.6%) | 34 (97.1%) | <0.001 |
| 2D Doppler | |||
| Uterovesical/subplacental hypervascularity | 82 (90.1%) | 35 (100%) | 0.061 |
| Large lacunae feeding vessel | 52 (57.8%) | 35 (94.6%) | <0.001 |
| Bridging vessels | 85 (93.4%) | 35 (100%) | 0.185 |
| Parametrial hypervascularity | 14 (15.4%) | 21 (60%) | <0.001 |
| The “rail sign” | 6 (6.6%) | 10 (28.6%) | 0.003 |
| 3D Doppler intra‐placental hypervascularity | 74 (81.3%) | 35 (100%) | 0.003 |
| Transvaginal ultrasound | |||
| Cervical length (cm) a , b | 2.5 (±0.94) | 2.2 (±0.73) | 0.038 |
| Cervical funneling a , b | 31 (34.1%) | 10 (28.6%) | 0.706 |
| Intracervical hypervascularity a , b | 55 (60.4%) | 33 (94.3%) | <0.001 |
Mann–Whitney U test.
Chi‐squared test.
Abbreviations: 2D, two‐dimensional; 3D, three‐dimensional; OSCS, one‐step conservative surgery; PAS, placenta accreta spectrum.
According to the multivariate logistic regression analysis of all pregnant women (n = 126), three ultrasound signs proved to be predictive for cesarean hysterectomy: placental bulge (odds ratio [OR] 9.3; 95% confidence interval [CI] 1961–44 288; p = 0.005), increased parametrial hypervascularity (OR 4.1; 95% CI 1541–11 085; p = 0.005) (Figure 3B) and intracervical hypervascularity (OR 9.2; 95% CI 1905–44 056; p = 0.006). The results of the logistic regression analysis in case of planned surgery (n = 106) show that the presence of a placental bulge (OR 24; 95% CI 3085–193 399; p = 0.002) and intracervical hypervascularity (OR 10; 95% CI 2146–47 934; p = 0.003) are important ultrasound signs to predict cesarean hysterectomy.
In the majority of cases, intracervical hypervascularity (n = 88, 70%) was observed. In the OSCS group there was no difference in OSCS success between the absence of intracervical hypervascularity and intracervical hypervascularity Grade 1 (94.7% vs. 88.5%; p = 0.510).
Transvaginal ultrasound grading of intracervical hypervascularity shows that intracervical hypervascularity <50% (Grade 1) was correlated with successful OSCS (Table 3). Grades 2 and 3 were highly correlated with cesarean hysterectomy. There was no incidence of vaginal bleeding following transvaginal ultrasound in planned surgery, indicating the necessity for TVUS to provide additional information for surgical strategies before intra‐surgical staging of PAS. The difference between planned and emergency surgery is described in the Supporting Information (Tables S2 and S3); a diffuse invasion with advanced grading of PAS is more likely to suggest cesarean hysterectomy in line with parametrial hypervascularity in Doppler ultrasound.
TABLE 3.
Transvaginal ultrasound grading of intracervical hypervascularity and maternal outcome.
| Intracervical hypervascularity Grade 1 (N = 90) | Intracervical hypervascularity Grade 2 (N = 29) | Intracervical hypervascularity Grade 3 (N = 7) | p | |
|---|---|---|---|---|
| Maternal outcome overall | ||||
| GA surgery a | 36 (27–39) | 36 (31–40) | 35 (31–38) | 0.619 |
| Type of surgery b | ||||
| Uterine conservative–resective surgery | 82 (91.1%) | 8 (27.6%) | 1 (14.3%) | <0.001 |
| Cesarean hysterectomy | 8 (8.9%) | 21 (72.4%) | 6 (85.7%) | |
| FIGO grading PAS b | ||||
| Grade 1 | 14 (15.6%) | 2 (6.9%) | 0 | <0.001 |
| Grade 2 | 52 (57.8%) | 8 (27.6%) | 1 (14.3%) | |
| Grade 3A | 17 (18.9%) | 7 (24.1%) | 1 (14.3%) | |
| Grade 3B‐C | 7 (7.8%) | 12 (41.4%) | 5 (71.4%) | |
| PAS topography b | ||||
| Type 1 | 75 (83.3%) | 3 (10.3%) | 0 | <0.001 |
| Type 2 | 1 (1.1%) | 0 | 1 (14.3%) | |
| Type 3 | 13 (14.4%) | 17 (58.6%) | 2 (28.6%) | |
| Type 4 | 1 (1.1%) | 8 (27.6%) | 4 (57.1%) | |
| Type 5 | 0 | 1 (3.4%) | 0 | |
| Blood loss (mL) a | 2274 (±1171) | 3056 (±1646) | 4171 (±1756) | 0.001 |
| Blood transfusion (bag) a | 2 (0–8) | 3 (0–9) | 5 (2–6) | 0.003 |
| Aortic control b | 10 (11.1%) | 13 (44.8%) | 5 (71.4%) | <0.001 |
| Emergency surgery b | 15 (16.7%) | 1 (3.4%) | 4 (57.1%) | 0.004 |
| Unexpected bleeding b | 6 (6.7%) | 5 (17.2%) | 3 (42.9%) | 0.007 |
| Cervical length (cm) a | 2.5 (±0.96) | 2.2 (±0.61) | 1.8 (±0.54) | 0.018 |
Kruskal–Wallis.
Chi‐squared test.
Abbreviations: GA, gestational age; PAS, placenta accreta spectrum.
4. DISCUSSION
The present study shows that the presence of a placental bulge, increased parametrial hypervascularity, and intracervical hypervascularity in TVUS is associated with cesarean hysterectomy in women with PAS. The findings of this study were collected from a large series of pregnant women with PAS managed in one tertiary referral hospital in East Java. Dr. Soetomo Academic General Hospital is a tertiary hospital and the biggest PAS center in Indonesia, with an incidence of PAS of 12% of all deliveries per year.
The diagnosis and treatment of PAS is still controversial, particularly ultrasound signs were not suited for selecting surgical strategies until recently. 18 In this study, 62% of PAS topography was above the bladder trigone, and 72% of PAS were managed using OSCS. The first uterine reconstruction study for PAS found OSCS only suitable for PAS above the bladder trigone. This correlates with the site of the previous cesarean section. 6 , 8
Other ultrasound studies of PAS show sonopathology of PAS, implying that the authors examined ultrasound signs in conjunction with the placental pathology. 32 This study's inclusion criteria were highly suspicious of invasive PAS because the ultrasonography signs of loss of clear zone, uterovesical–subplacental hypervascularity, and bridging arteries were not different (p > 0.05). 32 The difference in pre‐surgical ultrasound signs between OSCS and cesarean hysterectomy in our study may represent an imbalance in sample distribution regarding the FIGO histological classification and the PAS topography classification between the OSCS and cesarean hysterectomy groups. Cesarean hysterectomy is an indication of advanced grading of PAS, with diffuse PAS invasion, lower PAS topography (Figure 2B), and lower massive vascular anastomosis during surgery, requiring aortic control such as manual compression, a clamp, or a balloon in several conditions such as massive fibrotic tissue between placenta and bladder, 21 as in this study (9.9% vs. 54.3%; p < 0.001 for all PAS and 9.1% vs. 5.7%; p < 0.001 for planned surgery). As seen in the hysterectomy group, the abnormal uteroplacental interface, abnormal lacunae, and larger hypervascularity are associated with the ultrasound sign for advanced grading of PAS due to uterine remodeling. 32 The cervical sign of the transvaginal ultrasound where the advanced grade of PAS is correlated with a shorter cervix in this study is supported by another study that mentions that a short cervix has more intra‐surgical complexity. 33 The shortening of the cervix may represent the imbalance of angiogenic and anti‐angiogenic factors of PAS 34 that make the remodeling of cervical tissue more hypervascularized and lacking in healthy colpouterine tissue during surgery (Figure 3C).
A previous study showed that lower uterine hypervascularity correlates with more complex surgery and blood loss 30 and intracervical hypervascularity is independently associated with major postpartum hemorrhage, cesarean hysterectomy, and placenta percreta. 27 Intracervical hypervascularity indicates uterovaginal artery pedicles and cervical grading determines the success of OSCS. Placental bulge, especially in the lower part of the uterus, and hypervascular ultrasound findings may indicate diffuse placental invasion. 35 The placental bulge (with or without parametrial hypervascularity) and intracervical hypervascularity, in addition to other ultrasound signs of PAS, are signs that highly predict a cesarean hysterectomy. These signs describe the uterine S1–S2 sector for uterine–pelvic vascular anastomosis, of which the S2 sector has a more complex vascular network than the S1. 36
In our study, 70% of PAS had intracervical hypervascularity, implying that TVUS should be performed routinely in all PAS cases, especially before planned surgery. In the study by diPasquo et al., ultrasound signs of PAS were compared with placenta previa, together with the use of balloon tamponade for vascular control. 22 In this study, where OSCS uses triple‐vessel anastomosis vascular control 3 , 22 it could be a different approach for vascular control. The colpouterine pedicles sutured have an important role in OSCS, where the vaginal artery anastomosis represents intracervical hypervascularity from the ultrasound sign (Figure 1D). 3 , 22 This is probably related to hypervascularity from branches of the vaginal arteries. As hypervascularity is common in PAS, our grading of this hypervascularity can guide the surgeon to choose between OSCS and hysterectomy or predict adverse outcomes like unexpected bleeding during surgery.
We recommended three grades, following the previous study on intracervical hypervascularity. 27 OSCS was performed mostly with intracervical hypervascularity grade 1 (91% vs. 27.6% vs. 14.3%; p < 0.001) whereas higher grades required a cesarean hysterectomy. More severe intracervical hypervascularity like grade 3 can be difficult to insert multiple sutures of colpouterine pedicles, which makes it difficult to control bleeding and impossible to reconstruct the uterus due to a lack of healthy tissue. 8 The location of the previous incision in the cesarean section, where the lower incision makes a niche close to the cervical tissue and leads the implantation to the niche and changes the cervical tissue to become more vascularized, may affect the different cervical involvement. 37 , 38 , 39 , 40 Emergency surgery and unexpected bleeding of PAS are more common in women with Grade 3 intracervical hypervascularity, indicating that TVUS may be helpful in emergency PAS because of the high risk of unexpected vaginal bleeding during surgery.
Presurgical cervical grading should be used as standard PAS screening. This study also suggests that ultrasound provides important additional information in the choice of surgical strategies, although a larger multicenter and prospective study will be needed in the future.
The major limitation of this study is that it is a retrospective study and multiple surgeons performing PAS surgery, especially OSCS, may have influenced the outcome. On the other hand, the results were obtained in one single tertiary‐care center with a large number of referrals for PAS. The inclusion of this study is limited to women with PAS, which means the statistical analysis for the sensitivity or the specificity of PAS diagnosis could not be performed.
The strength of this study is that the surgery was performed by two surgeons, the surgeon who had experience in PAS surgery for more than 5 years with a high volume of PAS surgery, and the Maternal–Fetal Medicine trainee. This illustrates that in addition to being performed by PAS experts, the OSCS can also be performed by practicing clinicians, and the ultrasound signs may provide helpful insights into surgical strategies. In all circumstances, a comprehensive analysis was conducted for the ultrasound, and the PAS topographical types were observed in all cases.
5. CONCLUSION
A placental bulge with or without parametrial hypervascularity and intracervical hypervascularity covering more than 50% of the cervical tissue, as additional ultrasound markers, are associated with cesarean hysterectomy. OSCS is associated with intracervical hypervascularity Grade 1 on TVUS. Prospective validation is required to formulate predictors for PAS management.
AUTHOR CONTRIBUTIONS
RAA: writing the manuscript, study design, the acquisition, analysis, and interpretation of data, drafting the manuscript. JJD: writing the manuscript, study design, revising manuscript critically for important intellectual content, final approval of the version to be published. HVB: revising manuscript critically for important intellectual content, agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. NIC: analysis, acquisition, and interpretation of data. GAR: acquisition and interpretation of data, revising manuscript critically for important intellectual content. EGD: revising manuscript critically for important intellectual content, final approval of the version to be published.
CONFLICT OF INTEREST STATEMENT
None.
Supporting information
Table S1.
Table S2.
Table S3.
ACKNOWLEDGMENTS
We thank Maternal‐Fetal Medicine Fellows in Dr. Soetomo Academic General Hospital‐Universitas Airlangga (Dr. Dharma Banjarnahor, Dr. Ario Danianto, Dr. Yusra Septivera, and Dr. Robert Ridwan) who contributed as the surgical team in this study.
Aryananda RA, Duvekot JJ, Van Beekhuizen HJ, Cininta NI, Ariani G, Dachlan EG. Transabdominal and transvaginal ultrasound findings help to guide the clinical management of placenta accreta spectrum cases. Acta Obstet Gynecol Scand. 2024;103:93‐102. doi: 10.1111/aogs.14715
REFERENCES
- 1. Jauniaux E, Grønbeck L, Bunce C, Langhoff‐Roos J, Collins SL. Epidemiology of placenta previa accreta: a systematic review and meta‐analysis. BMJ Open. 2019;9:e031193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aryananda RA. Resurgence of placenta accreta in Indonesia. Majalah Obstetri & Ginekologi. 2018;26(3):98. [Google Scholar]
- 3. Aryananda RA, Aditiawarman A, Gumilar KE, et al. Uterine conservative–resective surgery for selected placenta accreta spectrum cases: surgical–vascular control methods. Acta Obstet Gynecol Scand. 2022;101:639‐648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sentilhes L, Kayem G, Chandraharan E, Palacios‐Jaraquemada J, Jauniaux E. Panel for the FPAD and MEC. FIGO consensus guidelines on placenta accreta spectrum disorders: conservative management. Int J Gynaecol Obstet. 2018;140:291‐298. [DOI] [PubMed] [Google Scholar]
- 5. Allen L, Jauniaux E, Hobson S, Papillon‐Smith J, Belfort MA. Panel for the FPAD and MEC. FIGO consensus guidelines on placenta accreta spectrum disorders: nonconservative surgical management. Int J Gynaecol Obstet. 2018;140(3):281‐290. [DOI] [PubMed] [Google Scholar]
- 6. Bhide A, Sebire N, Abuhamad A, Acharya G, Silver R. Morbidly adherent placenta: the need for standardization. Ultrasound Obstet Gynecol. 2017;49:559‐563. [DOI] [PubMed] [Google Scholar]
- 7. Collins SL, Ashcroft A, Braun T, et al. Proposal for standardized ultrasound descriptors of abnormally invasive placenta (AIP). Ultrasound Obstet Gynecol. 2016;47:271‐275. [DOI] [PubMed] [Google Scholar]
- 8. Zosmer N, Jauniaux E, Bunce C, Panaiotova J, Shaikh H, Nicholaides KH. Interobserver agreement on standardized ultrasound and histopathologic signs for the prenatal diagnosis of placenta accreta spectrum disorders. Int J Gynaecol Obstet. 2018;140(3):326‐331. [DOI] [PubMed] [Google Scholar]
- 9. Adu‐Bredu TK, Rijken MJ, Nieto‐Calvache AJ, et al. A simple guide to ultrasound screening for placenta accreta spectrum for improving detection and optimizing management in resource limited settings. Int J Gynaecol Obstet. 2023;160:732‐741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cali G, Forlani F, Timor‐Trisch I, et al. Diagnostic accuracy of ultrasound in detecting the depth of invasion in women at risk of abnormally invasive placenta: a prospective longitudinal study. Acta Obstet Gynecol Scand. 2018;97:1219‐1227. [DOI] [PubMed] [Google Scholar]
- 11. D'Antonio F, Palacios‐Jaraquemada J, Timor‐Trisch I, Cali G. Placenta accreta spectrum disorders: Prenatal diagnosis still lacks clinical correlation. Acta Obstet Gynecol Scand. 2018;97:773‐775. [DOI] [PubMed] [Google Scholar]
- 12. Pagani G, Cali G, Acharya G, et al. Diagnostic accuracy of ultrasound in detecting the severity of abnormally invasive placentation: a systematic review and meta‐analysis. Acta Obstet Gynecol Scand. 2018;97:25‐37. [DOI] [PubMed] [Google Scholar]
- 13. Palacios‐Jaraquemada JM, Fiorillo A, Hamer J, Martinez M, Bruno C. Placenta accreta spectrum: a hysterectomy can be prevented in almost 80% of cases using a resective‐reconstructive technique. J Matern Fetal Neonatal Med. 2022;35:275‐282. [DOI] [PubMed] [Google Scholar]
- 14. Jauniaux E, Ayres‐de‐Campos D, Langhoff‐Roos J, Fox KA, Collins S, Panel FPAD and MEC . FIGO classification for the clinical diagnosis of placenta accreta spectrum disorders. Int J Gynaecol Obstet. 2019;146:20‐24. [DOI] [PubMed] [Google Scholar]
- 15. Nieto‐Calvache AJ, Palacios‐Jaraquemada JM, Aryananda RA, et al. How to identify patients who require aortic vascular control in placenta accreta spectrum disorders? Am J Obstet Gynecol MFM. 2022;4:100498. [DOI] [PubMed] [Google Scholar]
- 16. Nieto‐Calvache AJ, Palacios‐Jaraquemada JM, Aryananda R, et al. How to perform one‐step conservative surgery for placenta accreta spectrum move by move. Am J Obstet Gynecol MFM. 2023;5:100802. [DOI] [PubMed] [Google Scholar]
- 17. Palacios Jaraquemada JM, Pesaresi M, Nassif JC, Hermosid S. Anterior placenta percreta: surgical approach, hemostasis and uterine repair. Acta Obstet Gynecol Scand. 2004;83:738‐744. [DOI] [PubMed] [Google Scholar]
- 18. Sentilhes L, Seco A, Azria E, et al. Conservative management or cesarean hysterectomy for placenta accreta spectrum: the PACCRETA prospective study. Am J Obstet Gynecol. 2022;226:839.e1‐839.e24. [DOI] [PubMed] [Google Scholar]
- 19. Oyelese Y, Ananth C v. Placental Abruption. Obstet Gynecol. 2006;108:1005‐1016. [DOI] [PubMed] [Google Scholar]
- 20. Aryananda RA, Akbar A, Wardhana MP, et al. New three‐dimensional/four‐dimensional volume rendering imaging software for detecting the abnormally invasive placenta. J Clin Ultrasound. 2019;47:9‐13. [DOI] [PubMed] [Google Scholar]
- 21. Alfirevic Z, Tang AW, Collins SL, Robson SC, Palacios‐Jaraquemada J. Pro forma for ultrasound reporting in suspected abnormally invasive placenta (AIP): an international consensus. Ultrasound Obstet Gynecol. 2016;47:276‐278. [DOI] [PubMed] [Google Scholar]
- 22. di Pasquo E, Ghi T, Calì G, et al. Intracervical lakes as sonographic marker of placenta accreta spectrum disorder in patients with placenta previa or low‐lying placenta. Ultrasound Obstet Gynecol. 2020;55:460‐466. [DOI] [PubMed] [Google Scholar]
- 23. Shih JC, Kang J, Tsai SJ, Lee JK, Liu KL, Huang KY. The “rail sign” : an ultrasound finding in placenta accreta spectrum indicating deep villous invasion and adverse outcomes. Am J Obstet Gynecol. 2021;225:292.e1‐292.e17. [DOI] [PubMed] [Google Scholar]
- 24. Shih JC, Palacios Jaraquemada JMP, Su YN, et al. Role of three‐dimensional power Doppler in the antenatal diagnosis of placenta accreta: comparison with gray‐scale and color Doppler techniques. Ultrasound Obstet Gynecol. 2009;33:193‐203. [DOI] [PubMed] [Google Scholar]
- 25. Cali G, Forlani F, Lees C, et al. Prenatal ultrasound staging system for placenta accreta spectrum disorders. Ultrasound Obstet Gynecol. 2019;53:752‐760. [DOI] [PubMed] [Google Scholar]
- 26. Cali G, D'Antonio F, Forlani F, Timor‐Tritsch IE, Palacios‐Jaraquemada JM. Ultrasound detection of bladder‐Uterovaginal anastomoses in morbidly adherent placenta. Fetal Diagn Ther. 2017;41:239‐240. [DOI] [PubMed] [Google Scholar]
- 27. Palacios Jaraquemada JM, Garcia Monaco R, Barbosa NE, Ferle L, Iriarte H, Conesa HA. Lower uterine blood supply: extrauterine anastomotic system and its application in surgical devascularization techniques. Acta Obstet Gynecol Scand. 2007;86:228‐234. [DOI] [PubMed] [Google Scholar]
- 28. Jauniaux E, Zosmer N, Subramanian D, Shaikh H, Burton GJ. Ultrasound‐histopathologic features of the utero‐placental interface in placenta accreta spectrum. Placenta. 2020;97:58‐64. [DOI] [PubMed] [Google Scholar]
- 29. Polat M, Kahramanoglu I, Senol T, Ozkaya E, Karateke A. Shorter the cervix, more difficult the placenta percreta operations. J Matern Fetal Neonatal Med. 2016;29:2327‐2331. [DOI] [PubMed] [Google Scholar]
- 30. Bartels HC, Postle JD, Downey P, Brennan DJ. Placenta Accreta Spectrum: a review of pathology, molecular biology, and biomarkers. Rebelo I, ed. Dis Markers. 2018;2018:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jauniaux E, Collins S, Burton GJ. Placenta accreta spectrum: pathophysiology and evidence‐based anatomy for prenatal ultrasound imaging. Am J Obstet Gynecol. 2018;218:75‐87. [DOI] [PubMed] [Google Scholar]
- 32. Vervoort AJMW, Uittenbogaard LB, Hehenkamp WJK, Brölmann HAM, Mol BWJ, Huirne JAF. Why do niches develop in caesarean uterine scars? Hypotheses on the aetiology of niche development. Hum Reprod Open. 2015;30:2695‐2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med. 2012;18:1754‐1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Timor‐Tritsch IE, Monteagudo A, Cali G, et al. Cesarean scar pregnancy is a precursor of morbidly adherent placenta. Ultrasound Obstet Gynecol. 2014;44:346‐353. [DOI] [PubMed] [Google Scholar]
- 35. Zosmer N, Fuller J, Shaikh H, Johns J, Ross JA. Natural history of early first‐trimester pregnancies implanted in cesarean scars. Ultrasound Obstet Gynecol. 2015;46:367‐375. [DOI] [PubMed] [Google Scholar]
- 36. Palacios Jaraquemada JM, Garcia Monaco R, Barbosa NE, Ferle L, Iriarte H, Conesa HA. Lower uterine blood supply: extrauterine anastomotic system and its application in surgical devascularization techniques. Acta Obstet Gynecol Scand. 2007;86(2):228‐234. [DOI] [PubMed] [Google Scholar]
- 37. Vervoort AJMW, Uittenbogaard LB, Hehenkamp WJK, Brölmann HAM, Mol BWJ, Huirne JAF. Why do niches develop in Caesarean uterine scars? Hypotheses on the aetiology of niche development. Human Reproduct. 2015;30(12):2695‐2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med. 2012;18(12):1754‐1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Timor‐Tritsch IE, Monteagudo A, Cali G, et al. Cesarean scar pregnancy is a precursor of morbidly adherent placenta. Ultrasound Obstet Gynecol. 2014;44(3):346‐353. [DOI] [PubMed] [Google Scholar]
- 40. Zosmer N, Fuller J, Shaikh H, Johns J, Ross JA. Natural history of early first‐trimester pregnancies implanted in Cesarean scars. Ultrasound Obstet Gynecol. 2015;46(3):367‐375. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Table S2.
Table S3.


