Abstract
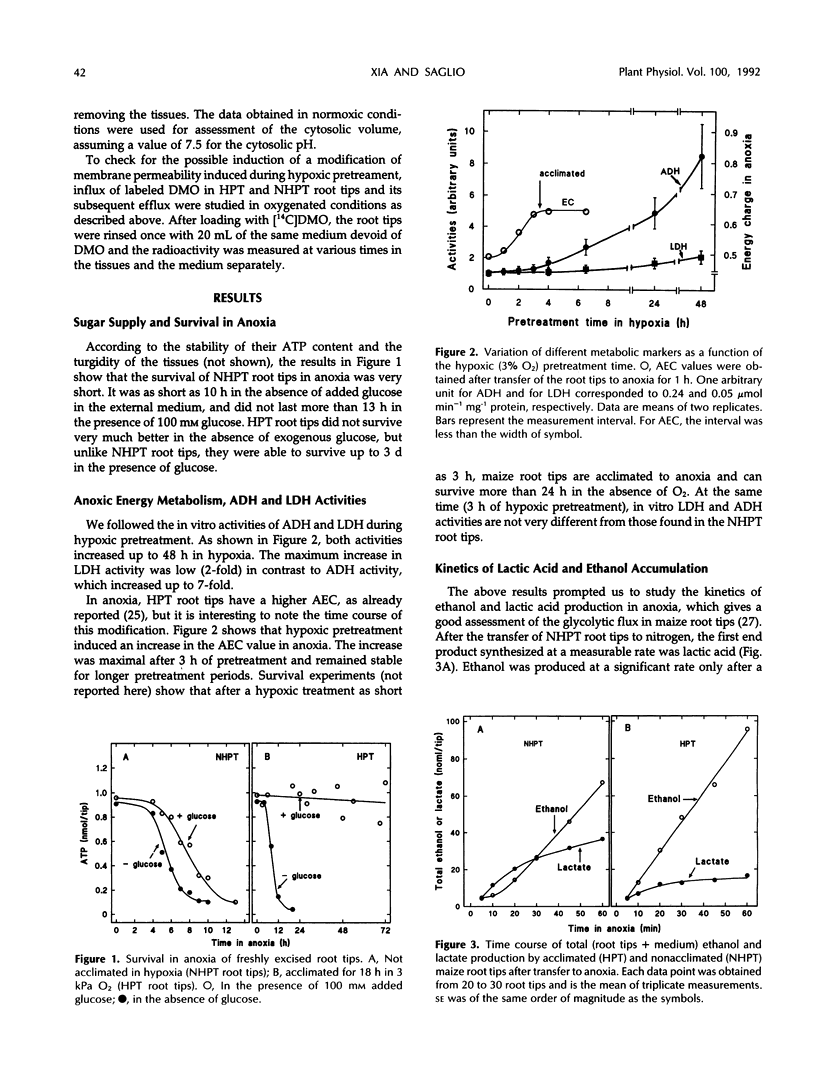
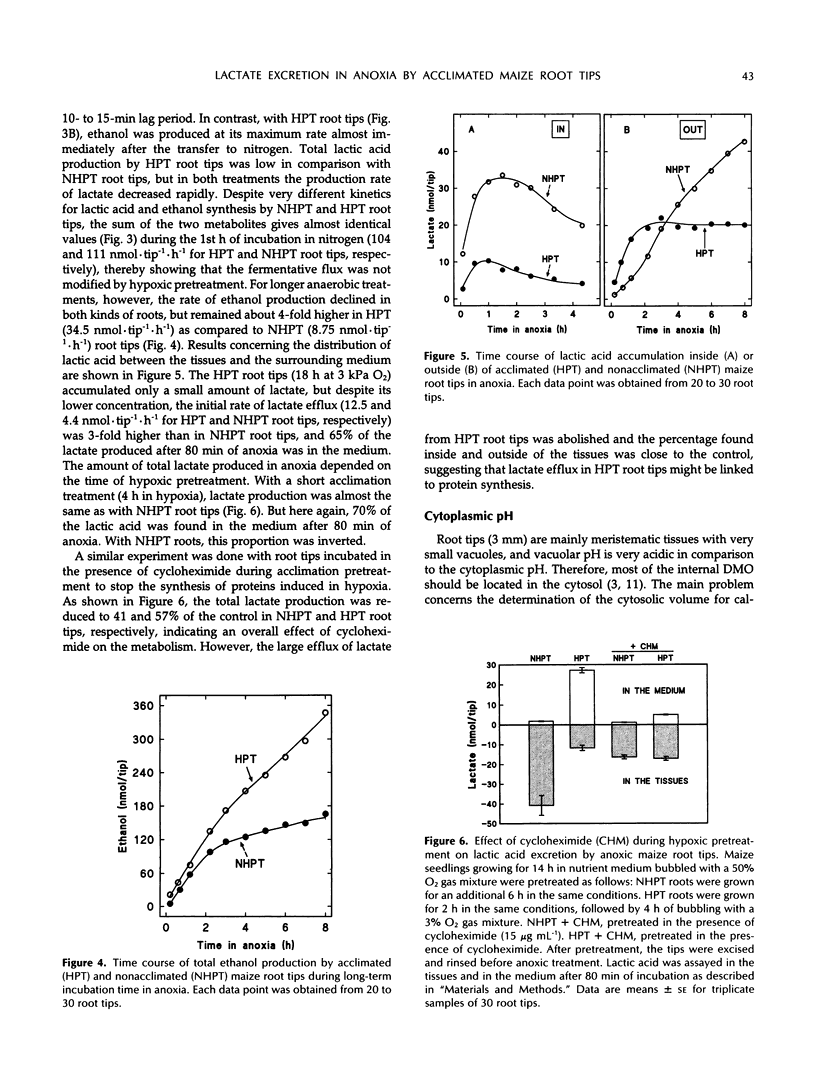
Hypoxic pretreatment (3 kPa oxygen) of maize (Zea mays L.) root tips improved their survival time in a subsequent anoxic incubation from 10 h to more than 3 d, provided that glucose was added to the medium to sustain metabolism. The glycolytic flux (lactate + ethanol) was the same in both pretreated and untreated root tips during the 1st h after transfer to anoxia. It was only after 2 h that it declined sharply in untreated tips, but was sustained in pretreated ones. Right after the transition from normoxia to anoxia of untreated root tips, the only fermentative product detected was lactic acid, which accumulated in a 7:1 proportion after 30 min in tissue and medium, respectively. It took 10 min before ethanol could be detected and 20 min for it to be produced at its maximum rate at the expense of lactate production, which slowed down. In contrast, in hypoxically pretreated root tips, ethanol was produced at a maximum rate right after the transfer to anoxia. Concurrently, low amounts of lactic acid were produced that accumulated in a 1:1 proportion after 30 min in tissue and medium, respectively. This large efflux of lactic acid could account for the higher cytoplasmic pH values always found in pretreated tissues. The presence of cycloheximide during pretreatment abolished this difference, suggesting that the greater efficiency of lactate efflux was linked to protein synthesis. The role of lactate in cytosolic pH regulation and in sensitivity to anoxia is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Goodenough-Trepagnier C., Tarry E., Prather P. Derivation of an efficient nonvocal communication system. Hum Factors. 1982 Apr;24(2):163–172. doi: 10.1177/001872088202400202. [DOI] [PubMed] [Google Scholar]
- Hanson A. D., Jacobsen J. V. Control of lactate dehydrogenase, lactate glycolysis, and alpha-amylase by o(2) deficit in barley aleurone layers. Plant Physiol. 1984 Jul;75(3):566–572. doi: 10.1104/pp.75.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman N. E., Bent A. F., Hanson A. D. Induction of lactate dehydrogenase isozymes by oxygen deficit in barley root tissue. Plant Physiol. 1986 Nov;82(3):658–663. doi: 10.1104/pp.82.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J., Cobb B. G., Drew M. C. Hypoxic Induction of Anoxia Tolerance in Root Tips of Zea mays. Plant Physiol. 1989 Nov;91(3):837–841. doi: 10.1104/pp.91.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegus F., Cattaruzza L., Chersi A., Fronza G. Differences in the Anaerobic Lactate-Succinate Production and in the Changes of Cell Sap pH for Plants with High and Low Resistance to Anoxia. Plant Physiol. 1989 May;90(1):29–32. doi: 10.1104/pp.90.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegus F., Cattaruzza L., Mattana M., Beffagna N., Ragg E. Response to Anoxia in Rice and Wheat Seedlings: Changes in the pH of Intracellular Compartments, Glucose-6-Phosphate Level, and Metabolic Rate. Plant Physiol. 1991 Mar;95(3):760–767. doi: 10.1104/pp.95.3.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocquot B., Ricard B., Pradet A. Rice embryos can express heat-shock genes under anoxia. Biochimie. 1987 Jun-Jul;69(6-7):677–681. doi: 10.1016/0300-9084(87)90188-x. [DOI] [PubMed] [Google Scholar]
- Perata P., Alpi A. Ethanol-induced injuries to carrot cells : the role of acetaldehyde. Plant Physiol. 1991 Mar;95(3):748–752. doi: 10.1104/pp.95.3.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricard B., Rivoal J., Spiteri A., Pradet A. Anaerobic stress induces the transcription and translation of sucrose synthase in rice. Plant Physiol. 1991 Mar;95(3):669–674. doi: 10.1104/pp.95.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. K., Callis J., Jardetzky O., Walbot V., Freeling M. Cytoplasmic acidosis as a determinant of flooding intolerance in plants. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6029–6033. doi: 10.1073/pnas.81.19.6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. K., Callis J., Wemmer D., Walbot V., Jardetzky O. Mechanisms of cytoplasmic pH regulation in hypoxic maize root tips and its role in survival under hypoxia. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3379–3383. doi: 10.1073/pnas.81.11.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. K., Chang K., Webster C., Callis J., Walbot V. Dependence of Ethanolic Fermentation, Cytoplasmic pH Regulation, and Viability on the Activity of Alcohol Dehydrogenase in Hypoxic Maize Root Tips. Plant Physiol. 1989 Apr;89(4):1275–1278. doi: 10.1104/pp.89.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs M. M., Freeling M., Okimoto R. The anaerobic proteins of maize. Cell. 1980 Jul;20(3):761–767. doi: 10.1016/0092-8674(80)90322-0. [DOI] [PubMed] [Google Scholar]
- Saglio P. H., Drew M. C., Pradet A. Metabolic Acclimation to Anoxia Induced by Low (2-4 kPa Partial Pressure) Oxygen Pretreatment (Hypoxia) in Root Tips of Zea mays. Plant Physiol. 1988 Jan;86(1):61–66. doi: 10.1104/pp.86.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saglio P. H., Rancillac M., Bruzan F., Pradet A. Critical oxygen pressure for growth and respiration of excised and intact roots. Plant Physiol. 1984 Sep;76(1):151–154. doi: 10.1104/pp.76.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saglio P. H., Raymond P., Pradet A. Metabolic Activity and Energy Charge of Excised Maize Root Tips under Anoxia: CONTROL BY SOLUBLE SUGARS. Plant Physiol. 1980 Dec;66(6):1053–1057. doi: 10.1104/pp.66.6.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Ges V., Roby C., Bligny R., Pradet A., Douce R. Kinetic studies of the variations of cytoplasmic pH, nucleotide triphosphates (31P-NMR) and lactate during normoxic and anoxic transitions in maize root tips. Eur J Biochem. 1991 Sep 1;200(2):477–482. doi: 10.1111/j.1432-1033.1991.tb16207.x. [DOI] [PubMed] [Google Scholar]
- Wilson R. E., Keng P. C., Sutherland R. M. Drug resistance in Chinese hamster ovary cells during recovery from severe hypoxia. J Natl Cancer Inst. 1989 Aug 16;81(16):1235–1240. doi: 10.1093/jnci/81.16.1235. [DOI] [PubMed] [Google Scholar]
- Xia J. H., Saglio P. H Efflux and Hexose Transport under Imposed Energy Status in Maize Root Tips. Plant Physiol. 1990 Jun;93(2):453–459. doi: 10.1104/pp.93.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]


