Sir,
It remains debated as to whether the quantification of tumor-infiltrating T lymphocytes (TILs) could help stratify outcome of follicular lymphoma (FL) patients, as suggested by pioneer gene expression profiling studies (1). Among T-cell subsets of particular interest, PD1+ T follicular helper (TFH) cells, FOXP3+ T regulatory cells (Tregs), and CD8+ cytotoxic T-cells were shown to correlate with outcome (2–5). However, some of these individual biomarkers have been studied before the introduction of rituximab and/or in FL patients with high clinical heterogeneity including early- and advanced-stage as well as untreated patients. Moreover, discrepant immunohistochemical (IHC) data have been presented for each marker (6) (for review), so that the reliability of this approach has been questioned (7).
In order to overcome the potential biases due to the heterogeneity of treatments that weakened the conclusion of previous studies, we had previously investigated TIL markers in a large series of FL samples from the PRIMA randomized trial, which represents, to our knowledge, the largest cohort of FL samples from rituximab-treated patients reported thus far (8). It has shown a better PFS in previously untreated patients in need of therapy who received 2 years of Rituximab (R) maintenance after immunochemotherapy induction vs observation (8). Pathological tumor material (FL grade 1, 2, or 3A) was processed as previously described (9). Briefly, immunostainings of CD3, CD4, CD8, PD1, ICOS, and FOXP3 were automatically quantified in tissue samples of 417, 287, 418, 406, 379, and 369 patients; respectively. The optimal IHC cut-off value had been calculated using the X-Tile software (10). The corresponding mRNA transcripts were quantified from frozen tissues of 148 patients using bulk RNAseq.
We could previously show that high CD3 counts and, to a lesser extent, high PD1 counts, were associated with better outcome after 5 years of follow-up (9). However, when a stringent statistical analysis was applied by dividing the whole cohort into a training and a validation set, none of the TIL markers showed significance in both subsets, suggesting that their prognostic value may be mitigated by the efficacy of Rituximab (9), a hypothesis also suggested by recent studies (11). Importantly, the prognostic impact of TILs with long-term follow up has never been extensively studied.
We report herein a 10 years follow-up (y-FU) update of TILs prognostic correlations in the PRIMA trial. Survival curves were constructed with the Kaplan-Meier method and compared with the log-rank test.
The present results after 10 y-FU show that the better progression free survival (PFS) associated with high CD3 counts at 5 y-FU (p=0.011) was still present and significant (p=0.029) (Supplementary Figure S1A). The increased rate of PFS events at 10 y-FU was 3.2% (n=5) and 7.9% (n=21) in the subgroups of pts with low CD3 counts (n=155) and with high CD3 counts (n=262), respectively, suggesting that the progression of the disease may be delayed in pts with high CD3 counts. In contrast, the favorable prognostic value of high PD1 counts at 5 y-FU (p=0.044) was no more observed at 10 y-FU (p=NS). The status of other TIL markers (CD4, CD8, ICOS, FOXP3), which were devoid of prognostic value at 5 y-FU, remained unchanged at 10 y-FU in the whole cohort.
When considering only R-maintenance arm pts (n=177), the favorable influence of high CD3 counts which had been observed at 5 y-FU (p=0.023) was still present at 10 y-FU (p= 0.030), but not in the observation/control arm (p= 0.31) (Figure 1A and 1B). Moreover, a better PFS associated to the R-maintenance treatment was only visible in pts with high CD3 counts (n=236; p=0.0036) but not in pts with low CD3 counts (n=140; p=0.28) (Figure 1C and 1D). Similarly, the R-maintenance treatment was associated to a better PFS for pts with high CD8 counts (n=305; p=0.0069) but not in pts with low CD8 (n=75; p=0.064) (Figure 2A and 2B). In contrast, pts with high FOXP3 counts (n=169) had no benefit of R-maintenance whereas pts with low FOXP3 counts (n=164) had a better PFS when treated by R-maintenance (p=0.016) (Figure 2C and 2D). These results appear almost similar to our previous 5 y-FU data. In fact, a better PFS in patients treated with R-maintenance compared to the control arm had been also observed at 5 y-FU in the subsets of pts with high CD3 (n=236; p=0.013), high CD8 (n=305; p=0.037) and low FOXP3 counts (n=164; p=0.037); whereas the 5 y-FU PFS did not differ between R-maintenance and control arm in pts with low CD3 (n=140; p=0.23) or high FOXP3 counts (n=169; p=0.112). One difference, however, between 5y-FU and 10 y-FU was that pts with low CD8 counts also benefited of R-maintenance therapy as compared to the control arm (n=75; p=0.023).
Figure 1: Correlation between CD3 immunostaining and survival at 10 years follow-up.
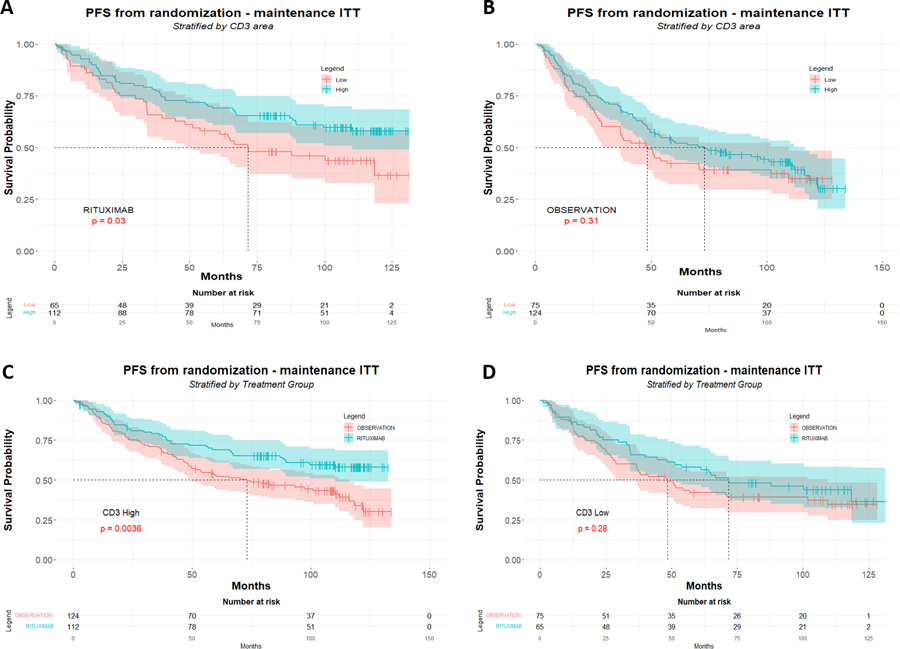
Ten years follow-up of PFS (months) according to CD3 IHC scores in the R-maintenance arm (A) and in the control (R-chemo without maintenance) arm (B) of the PRIMA trial. High amounts of CD3 (>30%) are significantly associated with PFS in the R-maintenance arm (A), but not in control arm (B). Panel C shows PFS in the subgroup of patients with high CD3 counts (>30%), whereas panel D shows PFS in the subgroup of patients with low counts (≤30%). There is a dramatic benefit of R-maintenance in CD3-high patients (C) when compared to CD3-low pts (D).
Figure 2: Correlation between CD8 or FOXP3 immunostaining and survival at 10 years follow-up.
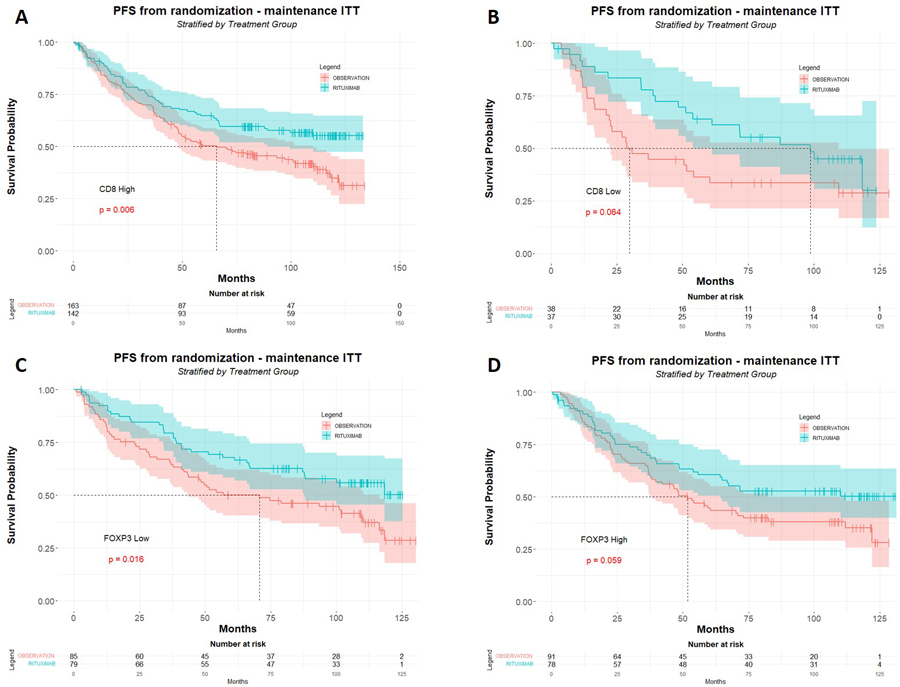
Progression free survival (months) at 10 years follow-up in the subgroups of patients in the subgroups of patients with high (A) and low CD8 counts (B). Patients with high amounts of CD8+ T-cells (>7%) display a significant benefit of the R-maintenance regimen (A), whereas this benefit shows a just trend in patients with low amounts of CD8+ T-cells (B). Patients with low amounts of FOXP3+ Tregs (≤4%) display a significant benefit of the R-maintenance regimen (C), whereas this benefit shows a just trend in pts with high amounts of FOXP3+ Tregs (D).
RNAseq analysis in the 148 available patients showed that the significant correlation between high levels of CD3 transcripts and better PFS which had been found at 5 y-FU (p=0.001), was still present to a much lesser extent at 10 y-FU (p=0.01) (Supplementary Figure S1B). Of note, the significant correlation between high levels of CD8 transcripts and better PFS previously seen at 5 y-FU (p = 0.037), remains significant at 10 y-FU (p=0.021). In a way similar to CD8 IHC, the R-maintenance treatment at 10 y-FU showed a trend to improve PFS in pts with high level of CD8 transcripts (n=68; p=0.057) but not in pts with low CD8 (n=66; p=0.12), as compared to the observation arm.
These data showing a persistent prognostic value at 10 y-FU of broad markers of T-cell infiltration like CD3 IHC counts and CD3 mRNA expression is in keeping with other reports, suggesting that the global amount of T-cell infiltration comprising multiple components of antitumor immunity was correlated with better outcome of FL patients in the rituximab era (11–14). The observation that the favorable effect of high IHC CD3 counts in the whole cohort was specifically related to the R-maintenance treatment arm is consistent with previous pioneer data showing that T cells play a key role in the efficacy of rituximab (15).
Furthermore, our data suggest a pronounced and lasting benefit of rituximab maintenance among FL patients with low number of T-reg FOXP3+ cells and high numbers of CD8+ cytotoxic cells, respectively. This may be related to rituximab-induced death of lymphoma cells, resulting in a release of tumor antigens triggering a lymphoma specific immune response (16). High CD8 counts and/or low FOXP3 may reflect a microenvironment capable of enhancing R-effects, leading to greater tumor killing, and generation of stronger T-cell responses in case of prolonged rituximab exposure.
In conclusion, this 10 y-FU update suggests that TILs markers that CD3, FOXP3 and CD8 IHC counts have predictive value by identifying patients with better sensitivity to R-maintenance. Our data confirm the influence of therapy on prognostic FL biomarkers, including those derived from the TME (17). They also highlight the lack of robustness of any single prognostic TIL IHC marker when used without considering the type of treatment regimen received. Since the vast majority of our patients were treated with R-CHOP as an induction regimen, our findings may not apply to other regimens. Such a treatment-specific approach will be probably necessary for the development of TME-derived FL prognostic biomarkers in the future.
Supplementary Material
ACKNOWLEDGMENTS
We thank pathologists and clinicians from the LYSA and the LYSA pathology technicians for their assistance in pathological reviewing.
Footnotes
CONFLICTS OF INTEREST
The authors are nothing to declare.
PATIENT CONSENT STATEMENT
The study was performed with approval of an Institutional review board and written informed consent was obtained from all participants at the time of enrollment in PRIMA trial (ClinicalTrials.gov identifier: NCT00140582).
DATA AVAILABILITY STATEMENT
The raw data supporting the results of this Letter to the editor will be made available upon reasonable request.
REFERENCES
- 1.Dave SS, Wright G, Tan B, Rosenwald A, Gascoyne RD, Chan WC, et al. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N Engl J Med 2004;351(21):2159–69. [DOI] [PubMed] [Google Scholar]
- 2.Smeltzer JP, Jones JM, Ziesmer SC, Grote DM, Xiu B, Ristow KM, et al. Pattern of CD14+ follicular dendritic cells and PD1+ T cells independently predicts time to transformation in follicular lymphoma. Clin Cancer Res 2014;20(11):2862–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson LS, Mansfield JR, Lloyd R, Oguejiofor K, Salih Z, Menasce LP, et al. Automated prognostic pattern detection shows favourable diffuse pattern of FOXP3(+) Tregs in follicular lymphoma. Br J Cancer 2015;113(8):1197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolen CR, McCord R, Huet S, Frampton GM, Bourgon R, Jardin F, et al. Mutation load and an effector T-cell gene signature may distinguish immunologically distinct and clinically relevant lymphoma subsets. Blood Adv 2017;1(22):1884–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laurent C, Müller S, Do C, Al-Saati T, Allart S, Larocca LM, et al. Distribution, function, and prognostic value of cytotoxic T lymphocytes in follicular lymphoma: a 3-D tissue-imaging study. Blood 2011;118(20):5371–9. [DOI] [PubMed] [Google Scholar]
- 6.de Jong D, Fest T. The microenvironment in follicular lymphoma. Best Pract Res Clin Haematol 2011;24(2):135–46. [DOI] [PubMed] [Google Scholar]
- 7.Sander B, de Jong D, Rosenwald A, Xie W, Balagué O, Calaminici M, et al. The reliability of immunohistochemical analysis of the tumor microenvironment in follicular lymphoma: a validation study from the Lunenburg Lymphoma Biomarker Consortium. Haematologica 2014;99(4):715–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salles G, Seymour JF, Offner F, López-Guillermo A, Belada D, Xerri L, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet 2011;377(9759):42–51. [DOI] [PubMed] [Google Scholar]
- 9.Xerri L, Huet S, Venstrom JM, Szafer-Glusman E, Fabiani B, Canioni D, et al. Rituximab treatment circumvents the prognostic impact of tumor-infiltrating T-cells in follicular lymphoma patients. Hum Pathol 2017;64:128–36. [DOI] [PubMed] [Google Scholar]
- 10.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 2004;10(21):7252–9. [DOI] [PubMed] [Google Scholar]
- 11.Tsakiroglou AM, Astley S, Dave M, Fergie M, Harkness E, Rosenberg A, et al. Immune infiltrate diversity confers a good prognosis in follicular lymphoma. Cancer Immunol Immunother 2021;70(12):3573–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wahlin BE, Sundström C, Holte H, Hagberg H, Erlanson M, Nilsson-Ehle H, et al. T cells in tumors and blood predict outcome in follicular lymphoma treated with rituximab. Clin Cancer Res 2011;17(12):4136–44. [DOI] [PubMed] [Google Scholar]
- 13.Tobin JWD, Keane C, Gunawardana J, Mollee P, Birch S, Hoang T, et al. Progression of Disease Within 24 Months in Follicular Lymphoma Is Associated With Reduced Intratumoral Immune Infiltration. J Clin Oncol 2019;37(34):3300–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rai S, Inoue H, Sakai K, Hanamoto H, Matsuda M, Maeda Y, et al. Decreased expression of T-cell-associated immune markers predicts poor prognosis in patients with follicular lymphoma. Cancer Sci 2022;113(2):660–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gluck WL, Hurst D, Yuen A, Levine AM, Dayton MA, Gockerman JP, et al. Phase I studies of interleukin (IL)-2 and rituximab in B-cell non-hodgkin’s lymphoma: IL-2 mediated natural killer cell expansion correlations with clinical response. Clin Cancer Res 2004;10(7):2253–64. [DOI] [PubMed] [Google Scholar]
- 16.Hilchey SP, Hyrien O, Mosmann TR, Livingstone AM, Friedberg JW, Young F, et al. Rituximab immunotherapy results in the induction of a lymphoma idiotype-specific T-cell response in patients with follicular lymphoma: support for a « vaccinal effect » of rituximab. Blood 2009;113(16):3809–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bolen CR, Mattiello F, Herold M, Hiddemann W, Huet S, Klapper W, et al. Treatment dependence of prognostic gene expression signatures in de novo follicular lymphoma. Blood 2021;137(19):2704–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the results of this Letter to the editor will be made available upon reasonable request.


