Abstract
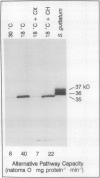
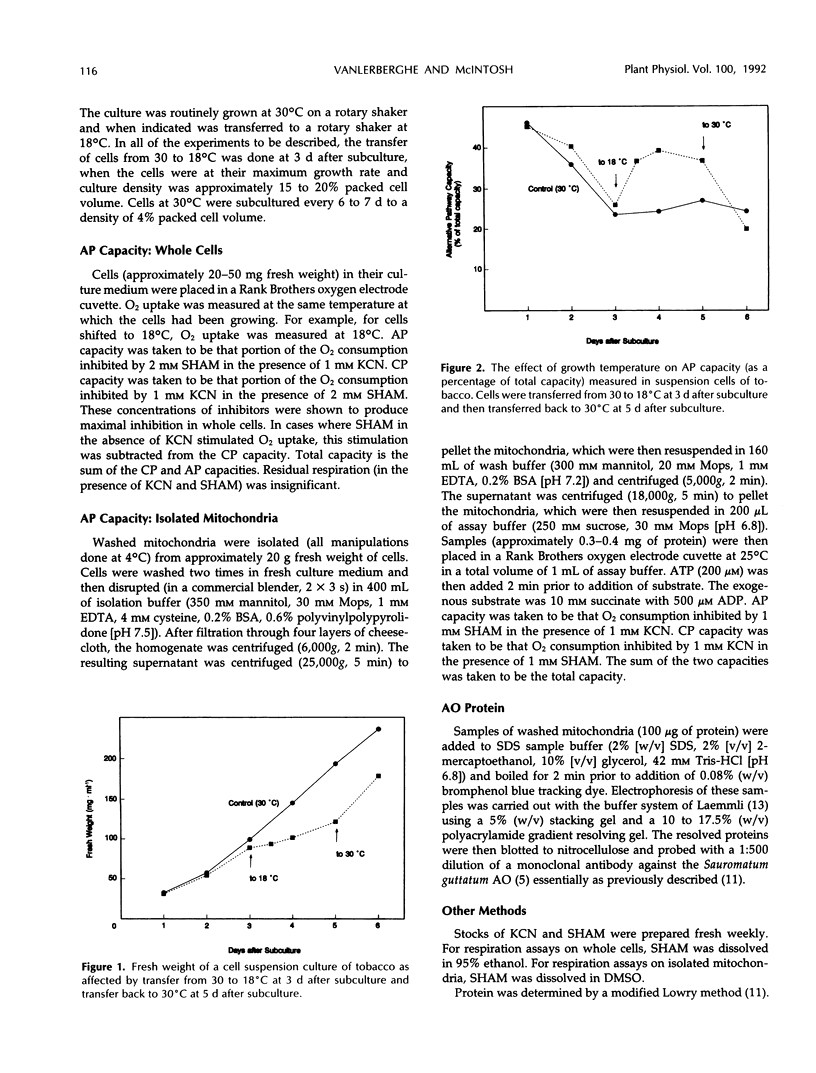
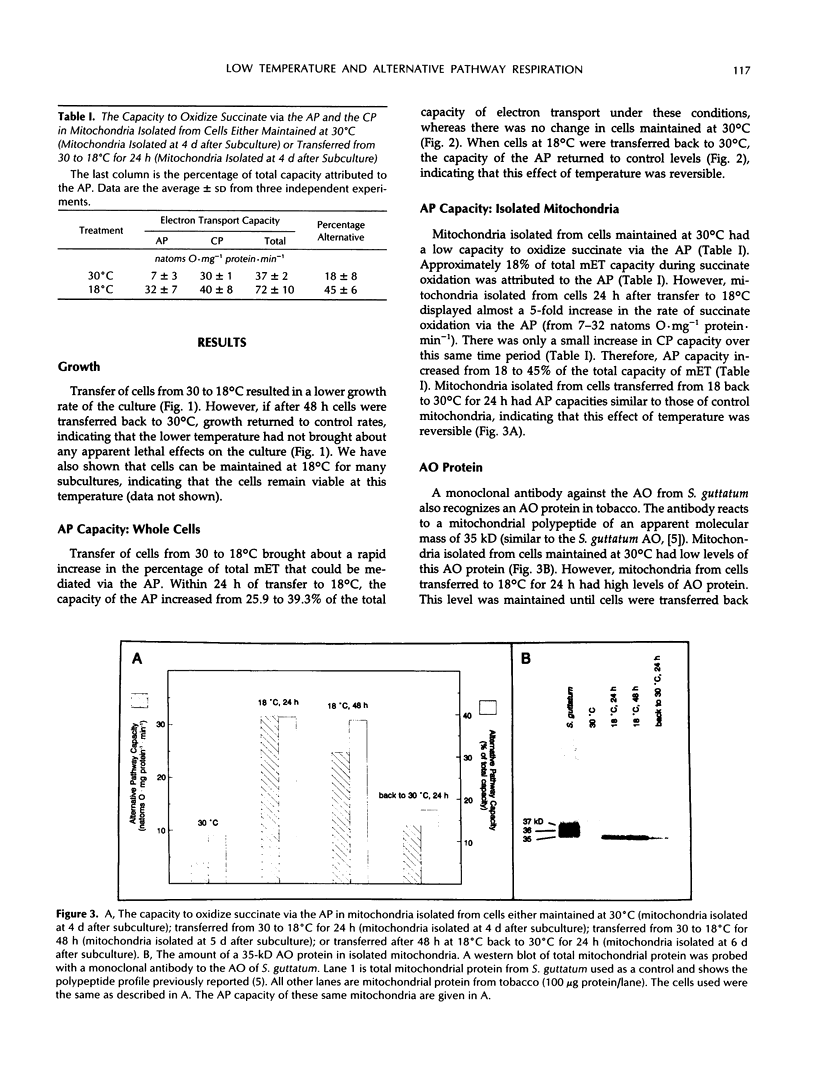
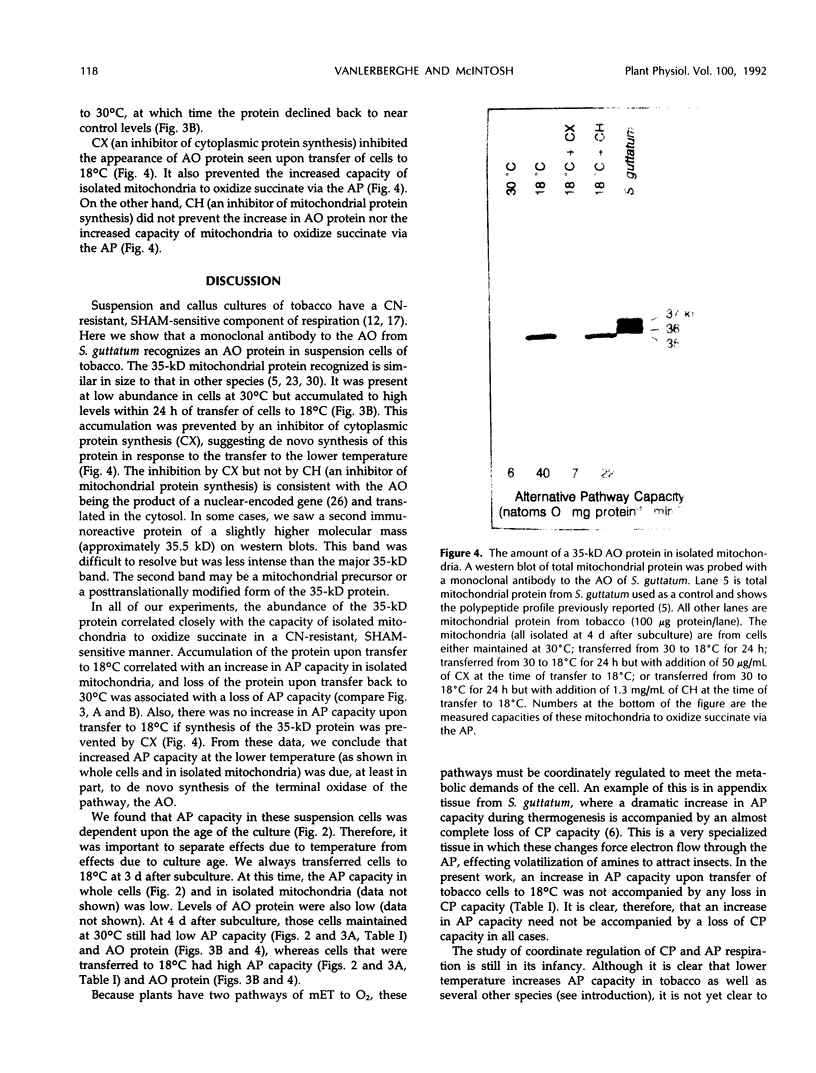
Suspension cells of NT1 tobacco (Nicotiana tabacum L. cv bright yellow) have been used to study the effect of growth temperature on the CN-resistant, salicylhydroxamic acid-sensitive alternative pathway of respiration. Mitochondria isolated from cells maintained at 30°C had a low capacity to oxidize succinate via the alternative pathway, whereas mitochondria isolated from cells 24 h after transfer to 18°C displayed, on average, a 5-fold increase in this capacity (from 7 to 32 nanoatoms oxygen per milligram protein per minute). This represented an increase in alternative pathway capacity from 18 to 45% of the total capacity of electron transport. This increased capacity was lost upon transfer of cells back to 30°C. A monoclonal antibody to the terminal oxidase of the alternative pathway (the alternative oxidase) from Sauromatum guttatum (T.E. Elthon, R.L. Nickels, L. McIntosh [1989] Plant Physiology 89: 1311-1317) recognized a 35-kilodalton mitochondrial protein in tobacco. There was an excellent correlation between the capacity of the alternative path in isolated tobacco mitochondria and the levels of this 35-kilodalton alternative oxidase protein. Cycloheximide could inhibit both the increased level of the 35-kilodalton alternative oxidase protein and the increased alternative pathway capacity normally seen upon transfer to 18°C. We conclude that transfer of tobacco cells to the lower temperature increases the capacity of the alternative pathway due, at least in part, to de novo synthesis of the 35-kilodalton alternative oxidase protein.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azcón-Bieto J., Lambers H., Day D. A. Effect of photosynthesis and carbohydrate status on respiratory rates and the involvement of the alternative pathway in leaf respiration. Plant Physiol. 1983 Jul;72(3):598–603. doi: 10.1104/pp.72.3.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elthon T. E., Nickels R. L., McIntosh L. Monoclonal antibodies to the alternative oxidase of higher plant mitochondria. Plant Physiol. 1989 Apr;89(4):1311–1317. doi: 10.1104/pp.89.4.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elthon T. E., Stewart C. R., McCoy C. A., Bonner W. D. Alternative Respiratory Path Capacity in Plant Mitochondria: Effect of Growth Temperature, the Electrochemical Gradient, and Assay pH. Plant Physiol. 1986 Feb;80(2):378–383. doi: 10.1104/pp.80.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiser C., McIntosh L. Alternative Oxidase of Potato Is an Integral Membrane Protein Synthesized de Novo during Aging of Tuber Slices. Plant Physiol. 1990 May;93(1):312–318. doi: 10.1104/pp.93.1.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn M. E., Mertz D. Cyanide-Resistant Respiration in Suspension Cultured Cells of Nicotiana glutinosa L. Plant Physiol. 1982 Jun;69(6):1439–1443. doi: 10.1104/pp.69.6.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leopold A. C., Musgrave M. E. Respiratory changes with chilling injury of soybeans. Plant Physiol. 1979 Nov;64(5):702–705. doi: 10.1104/pp.64.5.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H. G., Lü C. S. A Comparative Study of CN-Resistant Respiration in Different Cultures of Tobacco Callus. Plant Physiol. 1984 Jul;75(3):876–878. doi: 10.1104/pp.75.3.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T. Y., Markhart A. H. Temperature Effects on Mitochondrial Respiration in Phaseolus acutifolius A. Gray and Phaseolus vulgaris L. Plant Physiol. 1990 Sep;94(1):54–58. doi: 10.1104/pp.94.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordentlich A., Linzer R. A., Raskin I. Alternative respiration and heat evolution in plants. Plant Physiol. 1991 Dec;97(4):1545–1550. doi: 10.1104/pp.97.4.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads D. M., McIntosh L. Isolation and characterization of a cDNA clone encoding an alternative oxidase protein of Sauromatum guttatum (Schott). Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2122–2126. doi: 10.1073/pnas.88.6.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. R., Martin B. A., Reding L., Cerwick S. Respiration and Alternative Oxidase in Corn Seedling Tissues during Germination at Different Temperatures. Plant Physiol. 1990 Mar;92(3):755–760. doi: 10.1104/pp.92.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. R., Martin B. A., Reding L., Cerwick S. Seedling growth, mitochondrial characteristics, and alternative respiratory capacity of corn genotypes differing in cold tolerance. Plant Physiol. 1990 Mar;92(3):761–766. doi: 10.1104/pp.92.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weger H. G., Guy R. D., Turpin D. H. Cytochrome and alternative pathway respiration in green algae : measurements using inhibitors and o(2) discrimination. Plant Physiol. 1990 May;93(1):356–360. doi: 10.1104/pp.93.1.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ap Rees T., Burrell M. M., Entwistle T. G., Hammond J. B., Kirk D., Kruger N. J. Effects of low temperature on the respiratory metabolism of carbohydrates by plants. Symp Soc Exp Biol. 1988;42:377–393. [PubMed] [Google Scholar]