Abstract
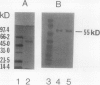
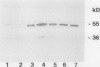
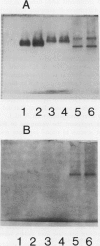
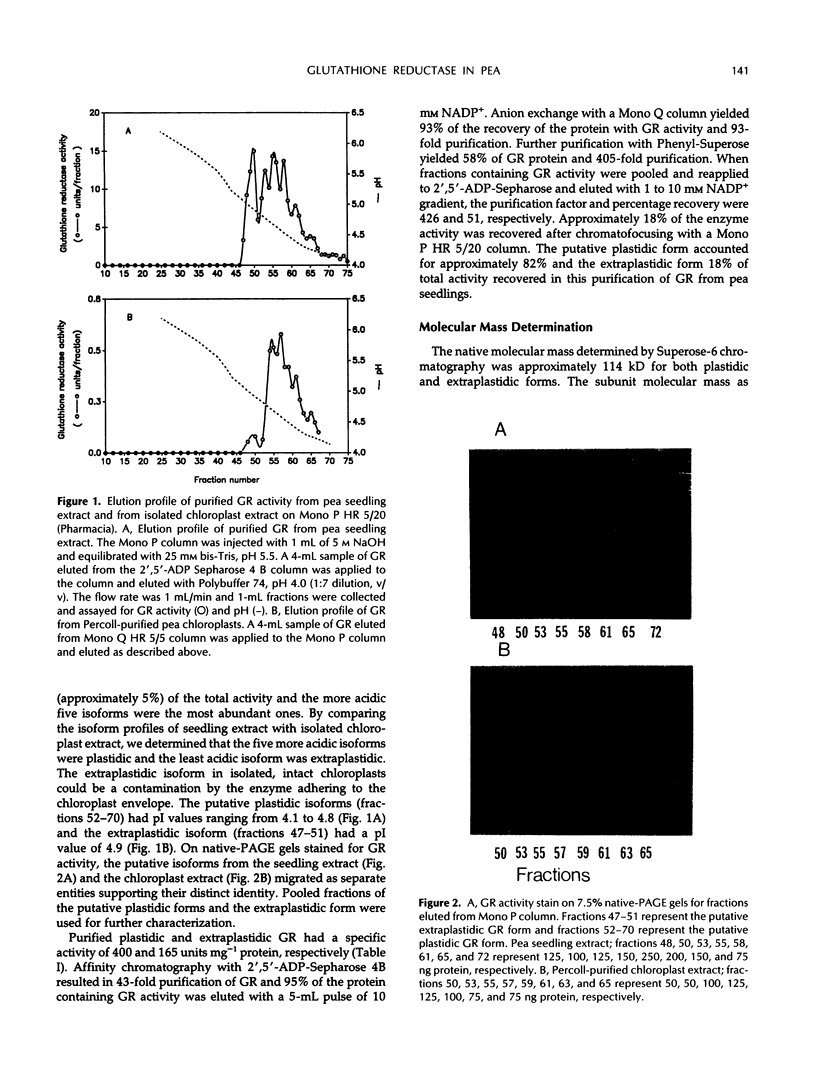
Glutathione reductase was purified from pea seedlings using a procedure that included 2′,5′-ADP Sepharose, fast protein liquid chromatography (FPLC)-anion exchange, and FPLC-hydrophobic interaction chromatography. The purified glutathione reductase was resolved into six isoforms by chromatofocusing. The isoform eluting with an isoelectric point of 4.9 accounted for 18% of the total activity. The five isoforms with isoelectric points between 4.1 and 4.8 accounted for 82% of the activity. Purified glutathione reductase from isolated, intact chloroplasts also resolved into six isoforms after chromatofocusing. The isoform eluting at pH 4.9 constituted a minor fraction of the total activity. By comparing the chromatofocusing profile of the seedling extract with that of the chloroplast extract, we inferred that the least acidic isoform was extraplastidic and that the five isoforms eluting from pH 4.1 to 4.8 were plastidic. Both the plastidic (five isoforms were pooled) and extraplastidic glutathione reductases had a native molecular mass of 114 kD. The plastidic glutathione reductase is a homodimer with a subunit molecular mass of 55 kD. Both glutathione reductases had optimum activity at pH 7.8. The Km for the oxidized form of glutathione (GSSG) was 56.0 and 33.8 μm for plastidic and extraplastidic glutathione reductase, respectively, at 25°C. The Km for NADPH was 4.8 and 4.0 μm for plastidic and extraplastidic isoforms, respectively. Antiserum raised against the plastidic glutathione reductase recognized a 55-kD polypeptide from purified antigen on western blots. In addition to the 55-kD polypeptide, another 36-kD polypeptide appeared on western blots of leaf crude extracts and the purified extraplastidic isoform. The lower molecular mass polypeptide might represent GSSG-independent enzyme activity observed on activity-staining gels of crude extracts or a protein that has an epitope similar to that in glutathione reductase. Fumigation with 75 nL L−1 ozone for 4 h on 2 consecutive days had no significant effect on glutathione reductase activity in peas (Pisum sativum L.). However, immunoblotting showed a greater level of glutathione reductase protein in extracts from ozone-fumigated plants compared with that in control plants at the time when the target concentration was first reached, approximately 40 min from the start of the fumigation, and 4 h on the first day of fumigation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. V., Hess J. L., Chevone B. I. Purification, characterization, and immunological properties for two isoforms of glutathione reductase from eastern white pine needles. Plant Physiol. 1990 Nov;94(3):1402–1409. doi: 10.1104/pp.94.3.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell G. P., Bell J. N., Cramer C. L., Schuch W., Lamb C. J., Dixon R. A. L-Phenylalanine ammonia-lyase from Phaseolus vulgaris. Characterisation and differential induction of multiple forms from elicitor-treated cell suspension cultures. Eur J Biochem. 1985 Jun 3;149(2):411–419. doi: 10.1111/j.1432-1033.1985.tb08941.x. [DOI] [PubMed] [Google Scholar]
- Burke J. J., Hatfield J. L. Plant Morphological and Biochemical Responses to Field Water Deficits: III. Effect of Foliage Temperature on the Potential Activity of Glutathione Reductase. Plant Physiol. 1987 Sep;85(1):100–103. doi: 10.1104/pp.85.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell J. P., Mullet J. E. Pea chloroplast glutathione reductase: purification and characterization. Plant Physiol. 1986 Oct;82(2):351–356. doi: 10.1104/pp.82.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faye L., Mouatassim B., Ghorbel A. Cell Wall and Cytoplasmic Isozymes of Radish beta-Fructosidase Have Different N-Linked Oligosaccharides. Plant Physiol. 1986 Jan;80(1):27–33. doi: 10.1104/pp.80.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble P. E., Burke J. J. Effect of water stress on the chloroplast antioxidant system: I. Alterations in glutathione reductase activity. Plant Physiol. 1984 Nov;76(3):615–621. doi: 10.1104/pp.76.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunert K. J., Cresswell C. F., Schmidt A., Mullineaux P. M., Foyer C. H. Variations in the activity of glutathione reductase and the cellular glutathione content in relation to sensitivity to methylviologen in Escherichia coli. Arch Biochem Biophys. 1990 Nov 1;282(2):233–238. doi: 10.1016/0003-9861(90)90110-k. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MAPSON L. W., ISHERWOOD F. A. Glutathione reductase from germinated peas. Biochem J. 1963 Jan;86:173–191. doi: 10.1042/bj0860173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madamanchi N. R., Alscher R. G. Metabolic bases for differences in sensitivity of two pea cultivars to sulfur dioxide. Plant Physiol. 1991 Sep;97(1):88–93. doi: 10.1104/pp.97.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matringe M., Scalla R. Studies on the mode of action of acifluorfen-methyl in nonchlorophyllous soybean cells : accumulation of tetrapyrroles. Plant Physiol. 1988 Feb;86(2):619–622. doi: 10.1104/pp.86.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaedle M. Chloroplast glutathione reductase. Plant Physiol. 1977 May;59(5):1011–1012. doi: 10.1104/pp.59.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelle S., Chetelat R. T., Dorais M., Deverna J. W., Bennett A. B. Sink Metabolism in Tomato Fruit : IV. Genetic and Biochemical Analysis of Sucrose Accumulation. Plant Physiol. 1991 Apr;95(4):1026–1035. doi: 10.1104/pp.95.4.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]