Abstract
Ulcerative colitis (UC) is one of the primary inflammatory bowel diseases (IBDs) and causes a serious threat to human public health around the world. Currently, there are no proven safe and effective treatment options to treat UC. Fraxetin (Fxt) is a widely recognized antioxidant and anti-inflammatory legume derived from ash bark. In the present study, we investigated the protective effect and mechanism of Fxt on UC. Our results showed that Fxt significantly attenuated the body weight, colon length reduction, tissue damage, and disease activity index induced by dextran sodium sulphate (DSS). Moreover, the DSS-induced activation of the NF-κB pathway and NLRP3 inflammasomes was inhibited, and the inflammatory response was reduced. Fxt restored gut barrier function by increasing the number of goblet cells and the levels of tight junction proteins (ZO-1 and occludin). In addition, Fxt can alter the intestinal microbiota by enhancing the diversity of the microbiota, increasing the relative abundance of beneficial bacteria and inhibiting the growth of harmful bacteria. These results revealed that Fxt alleviates DSS-induced colitis by modulating the inflammatory response, enhancing epithelial barrier integrity and regulating the gut microbiota. This study may provide a scientific basis for the potential therapeutic effect of Fxt in the prevention of colitis and other related diseases.
Keywords: Fraxetin, Colitis, Intestinal barrier, TLR4/NF-κB signalling pathway, NLRP3
1. Introduction
Ulcerative colitis (UC) is a kind of chronic nonspecific inflammatory bowel disease [1]. It usually affects the large intestine or rectum and can result in diarrhoea, cramps, and rectal bleeding [2]. It is characterized by a destructive inflammatory response and disruption of epithelial barrier function in pathology [3]. Dietary intake, intestinal bacteria and hereditary factors all play an important role in UC [4,5]. The epithelial intestinal barrier can effectively prevent the permeation of pathogens [6]. Epithelial integrity is crucial in the treatment of UC. The integrity of the intestinal barrier is mainly maintained by tight junctions (TJs) [7], which mainly consist of zonula occludens-1 (ZO-1) and occludin proteins [8]. The structure and position of TJ proteins may influence TJ function and then affect the function of the intestinal barrier. Thus, the restoration of TJ proteins is an important target for UC. Accumulating evidence has shown that dysbiosis of the gut microbiota is associated with UC [9]. When the intestinal flora is disrupted, the gut microbiota may cause or contribute to diseases. Thus, it is potentially feasible to treat UC by modulating the gut microbiota, reinforcing intestinal barrier integrity and inhibiting inflammatory responses.
Although drugs such as amino salicylic acid, glucocorticoids, immunosuppressants, and biologics are used to treat UC, they often have unpleasant side effects such as fever, vomiting, and acute pancreatitis [10,11]. Thus, developing novel and safe drugs is currently in high demand. In recent years, increasing attention has been given to some natural products that exist in traditional Chinese medicines due to their high safety and few side effects. Fraxetin (Fxt) [12], a plant-derived coumarin, has potent anticancer, antioxidant, anti-osteoporosis, antibacterial, and neuroprotective properties with low toxicity [13,14]. Studies have demonstrated that Fxt activates the Toll-like receptor 4 (TLR4)/nuclear factor (NF)-κB pathway in chondrocytes to prevent IL-1β-induced death of chondrocytes and decrease the release of inflammatory mediators [15]. Fxt also prevents inflammation and hepatocyte death by altering the NF-κB, MAPK, and Bcl-2/Bax signalling pathways. Recent research has demonstrated that Fxt can reduce the development and metastasis of tumours in a variety of cancer types, including lung cancer, breast cancer, and osteosarcoma [16]. In addition, Fxt suppressed cell viability and induced apoptotic cell death in HT29 and HCT116 cells [17]. In this research, we sought to identify the effects of Fxt in mice with DSS-induced colitis and further elucidated its potential molecular mechanisms. Importantly, this study can further elucidate the underlying mechanism of UC and aid in the development of new treatment strategies.
2. Materials and methods
2.1. Materials
Dextran sulphate sodium (DSS, purity ≥98 %) was purchased from Meilun Biotechnology Co., Ltd, Dalian, Liaoning, China. Fxt (purity ≥98 %) was purchased from Chengdu Herbpurify Co., Ltd, Chengdu, Sichuan, China.
The BCA protein concentration assay kit and RIPA buffer were purchased from Beyotime, Shanghai, China. Electrochemical luminescence (ECL) was purchased from Vazyme, Nanjing, China. TNF-α, IL-1β, IL-6 and IL-10 ELISA kits were purchased from Nanjing JianCheng Institute of Biological Engineering, Nanjing, China. TLR4, MyD88, p–NF–κB p65, NF-κB p65, NLRP3, β-actin, and HRP goat anti-rabbit IgG were purchased from ABclonal Technology Co., Ltd., Wuhan, China. Occludin and ZO-1 were purchased from Cell Signaling Technology, Danvers, MA, USA. All other chemicals were of analytical grade.
2.2. Animal experiments
Male C57BL/6 mice 6–8 weeks of age weighing 20 ± 2 g were purchased from the Laboratory Animal Center, Huazhong Agricultural University, Wuhan, China. All animal experiments were performed in compliance with the relevant laws and approved by the Huazhong Agricultural University Animal Protection and Use Committee, Wuhan, China (Permit number: HZAUMO-2022-0210). The mice were well-fed and had free access to food and water to adapt for one week. One week later, the mice were randomly divided into six groups (n = 6): control group, DSS (2 %, w/v) group, Fxt (60 mg/kg) group and DSS + Fxt (10, 30, and 60 mg/kg) groups [[18], [19], [20]]. The entire process lasted 25 days. Fxt was gavaged from the first day of the study, and DSS (2 %, w/v) was added to drinking water on Days 18–25 to induce a mouse model of colitis (Fig. 1A).
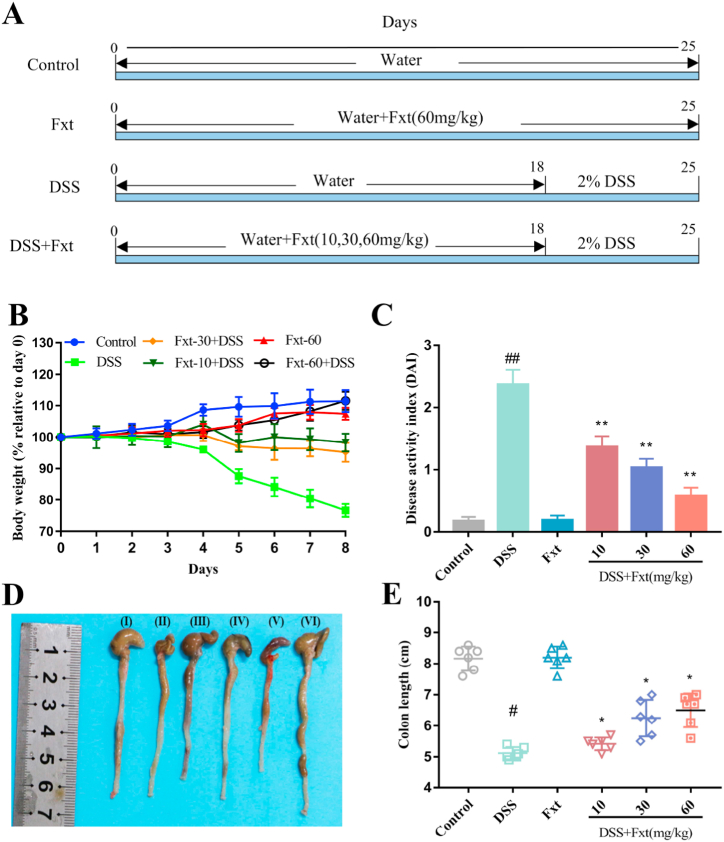
Fig. 1.
Effect of Fxt on DSS-induced colitis symptoms in mice. (A) Schematic diagram of the experimental design. (B) Weight changes of the mice in each group. (C) Changes in the disease activity index (DAI) in mice. (D) Representative colon photographs. (E) Analysis of changes in colon length. All data are expressed as mean ± SEM (n = 6). #P < 0.05 and ##P < 0.01 vs Control group. *P < 0.05 and **P < 0.01vs DSS group.
Body weight change, stool consistency, gross bleeding and diet of mice were assessed daily. On Day 25, after all mice were anaesthetized and sacrificed, their colons were collected, partly stored at −80 °C and partly fixed with paraformaldehyde.
2.3. Disease activity index (DAI)
DAI, calculated as a composite of body weight loss, stool consistency and stool blood, was scored on alternate days to analyse the anti-inflammatory potential of Fxt. The Cooper method was slightly modified to quantify the score [21]. The clinical scoring method is shown in Table 1. DAI was defined as the sum of the scores of the above three parameters.
Table 1.
Calculated disease activity index (DAI) score.
| Score | Body weight loss | Stool consistency | Blood in the stool |
|---|---|---|---|
| 0 | 0 | Normal | negative |
| 1 | 1–5% | formed feces/easily adhere | weak positive |
| 2 | 5–10 % | semi-formed/soft feces | positive |
| 3 | 10–20 % | slurry stool/not adherent to the anus | strong positive |
| 4 | >20 % | Diarrhoea/adherence to the anus | gross bleeding |
2.4. H&E-stained sections and immunohistochemistry
Colon tissue samples were fixed in 4 % paraformaldehyde solution for 24 h. Then, the tissues were dehydrated, embedded, and sliced into 3 μm thick sections. To observe the histopathological changes between different groups, the colon tissues were stained with haematoxylin and eosin (HE) and observed and photographed under a microscope (Nikon, Japan). Histological scores were determined by assessing the extent of tissue damage in the samples, including the degree of intestinal epithelial cell injury and the degree of inflammatory infiltration. Briefly, the scoring criteria were summarized as follows: 0 (normal morphology and no inflammation), 1 (loss of goblet cell loss and mild inflammatory infiltration), 2 (large area of goblet cell loss and moderate inflammatory infiltration), 3 (crypt loss and extensive inflammatory infiltration of the mucosal muscular layer with mucosal oedema and thickening), and 4 (large area of crypt loss and extensive inflammatory infiltration of the submucosa layer) [22]. Immunohistochemistry staining was carried out according to the kit instructions. Three-micron-thick tissue sections were stained with antibodies against ZO-1 and Occludin.
2.5. Determination of inflammatory cytokines
The contents of tumour necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6) and interleukin-10 (IL-10) were measured by ELISA kits according to the instructions.
2.6. Detection of Myeloperoxidase (MPO) activity in colon tissue
Tissue MPO activity was measured as reported previously with some modifications [23]. A small portion of colon tissue was removed and rinsed with ice-cold, saline, blotted dry, weighed, and minced. Tissue segments weighing 100–200 mg were homogenized in potassium phosphate buffer (50 mg/mL, 50 mmol/L potassium phosphate, pH = 6.0) and centrifuged at 20000 g for 20 min. The supernatant was removed and HTAB buffer (Hexadecyltrimethylammonium Bromide: 5 g/L in 50 mmol/L potassium phosphate buffer) was added. The pellet was homogenized and centrifuged at 10000 g. The samples were sonicated, frozen in liquid nitrogen, thawed, and centrifuged at 10000 g for 10 min. This step was repeated twice. The level of MPO activity was determined from the total supernatant by adding 200 μL of O-dianisidine buffer (16.7 mg O-dianisidine dihydrochloride in 5 mmol/L phosphate buffer containing 0.005 % H2O2). The change in absorbance at 450 nm was determined every 30 s over a 3-min period after adding the O-dianisidine buffer by a Synergy HT microplate reader. Values are expressed as units of MPO activity per gram of tissue sample where 1 unit of MPO is defined as that which degrades 1 μmol of hydrogen peroxide per minute.
2.7. Western blot
Whole colon tissue was homogenized in 1 mL of ice-cold RIPA buffer containing 0.1 % phenyl methyl sulfonyl fluoride. The protein concentration was determined by a Pierce BCA protein assay kit. Western blotting was performed as previously described [24]. The dilution ratios of various antibodies were as follows: TLR4 antibody, 1:1000; MyD88 antibody, 1:1000; IκBα antibody, 1:800; NF-κB p65 antibody, 1:1000; p–NF–κB p65 antibody, 1:1000; NLRP3 antibody, 1:1000; caspase-1 antibody, 1:1000; IL-1β, 1:1000; β-actin antibody, 1:2000; and horseradish peroxidase-labelled secondary antibodies, 1:10000. The protein was coloured by electrochemical luminescence (ECL). The target protein relative expression was computed as the target protein gray value/β-actin gray value.
2.8. Sequencing of gut microbiota
The faecal samples were detected by 16S rRNA sequencing at Novogene Technology Co., Ltd., China. The sequences were combined into operational taxonomic units (OTUs) at 97 % similarity. The alpha diversity and β-diversity of each sample were estimated based on the OTU relative abundances. Based on the unweighted UniFrac distance matrix, nonmetric multidimensional scaling (NMDS) and principal coordinate analysis (PCoA) were used to evaluate the β-diversity of the bacterial community. Taxa abundances at the family and genus levels were statistically compared among groups. The differences in gut microbiota composition in different groups were analysed by linear discriminant analysis (LDA) effect size (LEfSe).
2.9. Statistical analysis
All data was represented as the mean ± SEM. Statistical analysis was performed using GraphPad Prism 7.0 software utilizing one-way ANOVA and Dunnett's multiple comparison test and considered significant if p values were <0.05.
3. Results
3.1. Fraxetin relieves disease symptoms in DSS-induced colitis mice
To explore the protective effects of Fxt on UC, 2 % DSS was used to induce colitis in mice. During the experiment, the mental state, activity, faecal traits and body weight of mice were observed daily to evaluate the DAI score. Body weight change and colon length loss are important features of the severity of intestinal inflammation in DSS-induced colitis mice. The results showed that the body weight of mice in the DSS group decreased from Day 4 compared to the other groups, while Fxt significantly decreased body weight loss (Fig. 1B). Correspondingly, the DAI score of the mice from the DSS group was significantly lower than that of the mice from the DSS + Fxt (10/30/60 mg/kg) group (P < 0.01) (Fig. 1C). The colon length of mice in the DSS group was significantly shorter than that in the control group, but the DSS + Fxt (10/30/60 mg/kg) group significantly improved the damage of DSS to the colon length of mice (P < 0.05) (Fig. 1D and E). Therefore, Fxt may attenuate the symptoms of DSS-induced colitis in mice.
3.2. Fraxetin reduces colonic histological changes in DSS-induced colitis mice
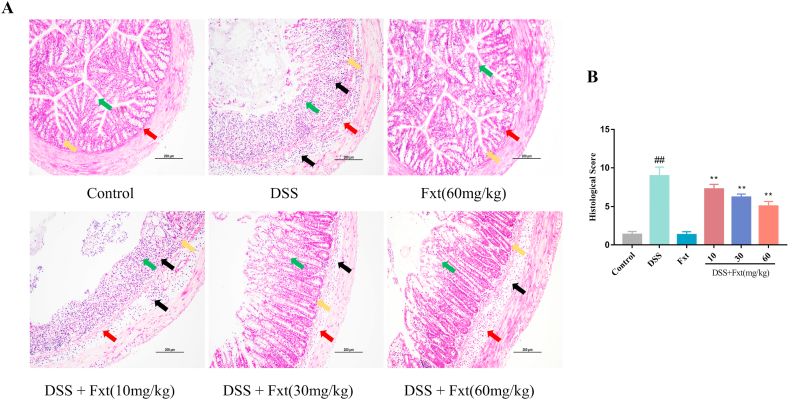
DSS can lead to chronic intestinal inflammation and further cause injury to the colonic mucosa. To evaluate the protective effect of Fxt on DSS-induced colitis, we performed histopathological analysis of colon tissues. As shown in the HE staining results (Fig. 2A), the colonic tissue structure in mice in the control group was normal, and there were no obvious changes. The colonic tissue of mice in the DSS group exhibited mucosal erosion and oedema (red arrow), inflammatory cell infiltration (black arrow), crypt loss (yellow arrow), and goblet cell depletion (green arrow). The pathological score in the DSS group was noticeably higher than that in the control group (P < 0.01), which was the same as the pathological results (Fig. 2B). Luckily, Fxt significantly reduced the pathological changes and the pathological score of colon tissue induced by DSS (P < 0.05). Thus, our results suggest that Fxt can reduce the severity of colon inflammation in DSS-induced colitis in mice.
Fig. 2.
Effect of Fxt administration on colonic pathological damage induced by DSS in mice. (A) Representative micrographs of H&E staining of the colonic tissue. (B) Pathological injury score of the colon tissues. All data are expressed as mean ± SEM (n = 6). ##P < 0.01 vs Control group. **P < 0.01 vs DSS group.
3.3. Fraxetin alleviates the secretion of inflammatory cytokines in DSS-induced colitis mice
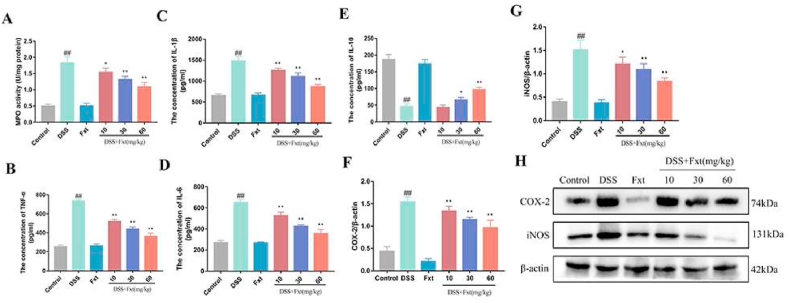
Neutrophils and macrophages play important roles in the inflammatory response and can secrete MPO, a highly oxidative enzyme that is a key mediator of the damaging innate immune response. To further explore the effects of Fxt on DSS-induced colitis, MPO activity in colon tissue was measured. The results showed that MPO activity was significantly increased in the DSS group compared with the control group (P < 0.01), and the DSS + Fxt (10/30/60 mg/kg) group showed significantly decreased MPO activity (P < 0.05) (Fig. 3A). We also analysed the protein levels of inflammatory cytokines, including TNF-α, IL-6, IL-1β and IL-10. As shown in Fig. 3B–E, the levels of proinflammatory cytokines (TNF-α, IL-6 and IL-1β) were significantly increased (P < 0.01) and that of the anti-inflammatory cytokine IL10 was suppressed in the colon tissue of the DSS control group (P < 0.05). However, Fxt significantly decreased the expression of pro-inflammatory cytokines while increasing anti-inflammatory cytokines. Simultaneously, it has been reported that iNOS and COX-2 play an important role in inflammation. The expression of COX-2 and iNOS was significantly increased in the DSS group, while Fxt pretreatment obviously inhibited the expression of iNOS and COX-2 (P < 0.05) (Fig. 3F–H). These results suggest that Fxt can effectively inhibit the inflammatory response in DSS-induced colitis mice.
Fig. 3.
Effect of Fxt administration on the levels of cytokine and enzymes in the colon tissues of mice with UC. (A–E) Protein levels of MPO, TNF-α, IL-1β, IL-6, and IL-10 in the colon tissues. (F–H) Protein levels of COX-2 and iNOS in the colon tissues. β-actin served as the loading control. All data are represented as mean ± SEM (n = 6). #P < 0.05 and ##P < 0.01vs Control group. *P < 0.05 and **P < 0.01 vs DSS-treated group.
3.4. Fraxetin protects against intestinal barrier dysfunction in DSS induced colitis mice
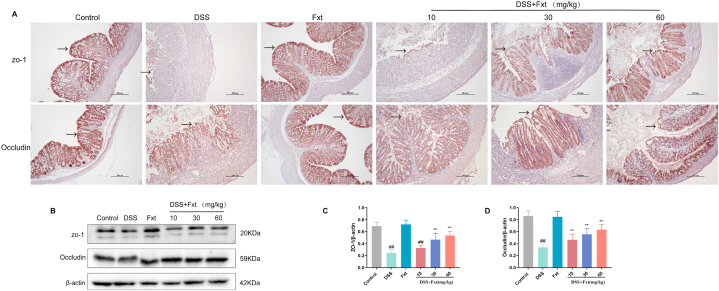
As the most important line of defence in the body, the intestinal barrier can effectively resist macromolecules and microorganisms entering the blood and organs. ZO-1 and Occludin are major tight junction proteins for intestinal mucosal barrier function (Fig. 4A). Therefore, we next measured the expression of the tight junction proteins ZO-1 and Occludin by immunohistochemistry and western blotting (Fig. 4B). The results showed that the expression of ZO-1 and Occludin was significantly decreased in the DSS group and that Fxt enhanced ZO-1 and occludin expression after DSS exposure (Fig. 4C and D). The results showed that Fxt attenuates intestinal inflammation and intestinal epithelial barrier damage induced by DSS in mice.
Fig. 4.
Effect of Fxt on DSS-induced destruction of colonic barrier integrity in mice. (A) Immunohistochemistry pictures of ZO-1 and occludin in the colon tissues. (B) Representative bands of Western blot analysis of ZO-1 and occludin in colon tissues. (C–D) Results of the gray-scale analysis of the corresponding proteins. β-actin served as the loading control Scale bar: 100 μm. All data are represented as mean ± SEM (n = 6). ##P < 0.01 vs control group. **P < 0.01 vs DSS group.
3.5. Fraxetin affected the TLR4/NF-κB signalling pathway in DSS-induced colitis mice
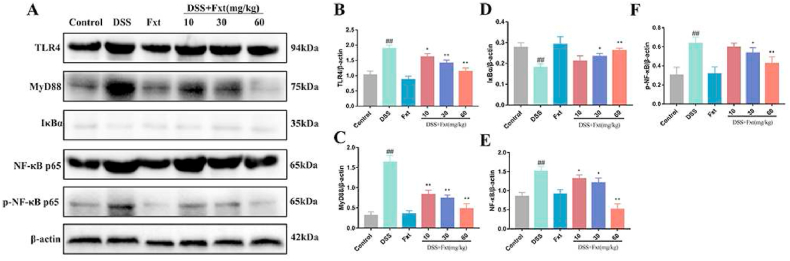
The TLR4/NF-κB signalling pathway plays an important role in inflammatory responses. We examined whether Fxt alleviated inflammatory responses by inhibiting the activation of the TLR4/NF-κB signalling pathway. The results demonstrated that the protein expression levels of TLR4, MyD88, p65 and p-p65 in the DSS group were significantly increased (P < 0.05), and IκBα was degraded (Fig. 5A–F). However, the expression of TLR4 and its downstream proteins was significantly decreased in the DSS + Fxt (10/30/60 mg/kg) group (P < 0.05) (Fig. 5A).
Fig. 5.
Effect of Fxt administration on DSS-induced activation of NF-κB signalings in mice. (A) Protein levels of TLR4, MyD88, IκB, p65 and p-p65 in the colon tissues. (B–F) Results of the gray-scale analysis of the corresponding protein bands. β-actin served as the loading control. All data are represented as mean ± SEM (n = 6). #P < 0.05 and ##P < 0.01vs Control group. *P < 0.05 and **P < 0.01 vs DSS-treated group.
3.6. Fraxetin suppressed NLRP3 inflammasome activation in DSS-induced colitis mice
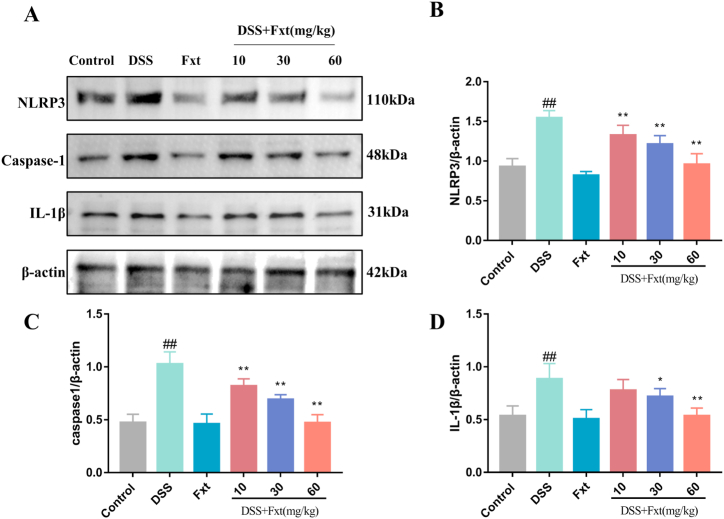
The NLRP3 inflammasome is an important intracellular multiprotein complex formed by NLRP3, ASC and caspase‐1. We therefore investigated the role of the NLRP3 inflammasome in colitis. The results showed that the protein expression levels of NLRP3, caspase-1 and IL-1β were significantly increased in the DSS group compared to the control group (P < 0.01) (Fig. 6). However, these proteins were inhibited in the DSS + Fxt (10/30/60 mg/kg) group (P < 0.05)
Fig. 6.
Effect of Fxt administration on DSS-induced activation of NLRP3 signalings in mice. (A) Protein levels of NLRP3, caspasein the colon tissue-1 and IL-1β. (B–D) Results of the gray-scale analysis of the corresponding protein bands. β-actin served as the loading control. All data are represented as mean ± SEM (n = 6). #P < 0.05 and ##P < 0.01vs Control group. *P < 0.05 and **P < 0.01 vs DSS-treated group.
3.7. Fraxetin regulates the structure and composition of gut microbiota
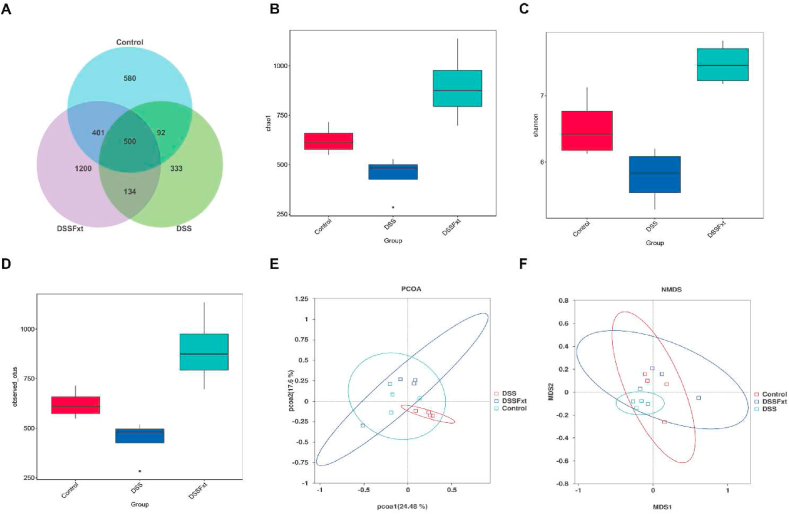
Intestinal bacteria play a key role in maintaining intestinal homeostasis. To clarify whether the beneficial effect of Fxt on colitis is related to the regulation of intestinal flora, we collected mouse feces and performed 16S rDNA sequencing analysis. The Venn diagram showed that the number of unique OTUs in the control group, DSS group and DSS + Fxt group was 580, 333 and 1200, respectively (Fig. 7A). To evaluate the effect of Fxt on gut microbiota richness and diversity, α-diversity was determined. The Chao index reflects the microbial community richness (Fig. 7B), while the Shannon index reflects the species diversity (Fig. 7C). The results showed that Fxt exhibited a microbiota with significantly higher diversity relative to that of the DSS group (Fig. 7D). To assess the difference in intestinal microbial communities, β-diversity was calculated. The PCoA and NMDS results revealed that the microbial community structure and composition were significantly different between the DSS group and the control group (Fig. 7E and F). These results show that Fxt regulates the gut microbiota.
Fig. 7.
Effect of Fxt administration on the diversity and structure of intestinal flora in mice with colitis induced by DSS. (A) Wayne diagram shows common and unique OTUs between groups; (B) Chao1 index analysis; (C) Shannon index analysis; (D) Observed_species index analysis; (E) NMDS analysis of intestinal flora based on Bray Curtis distance. (F) principal coordinate analysis of intestinal flora based on the weighted UniFrac distance.
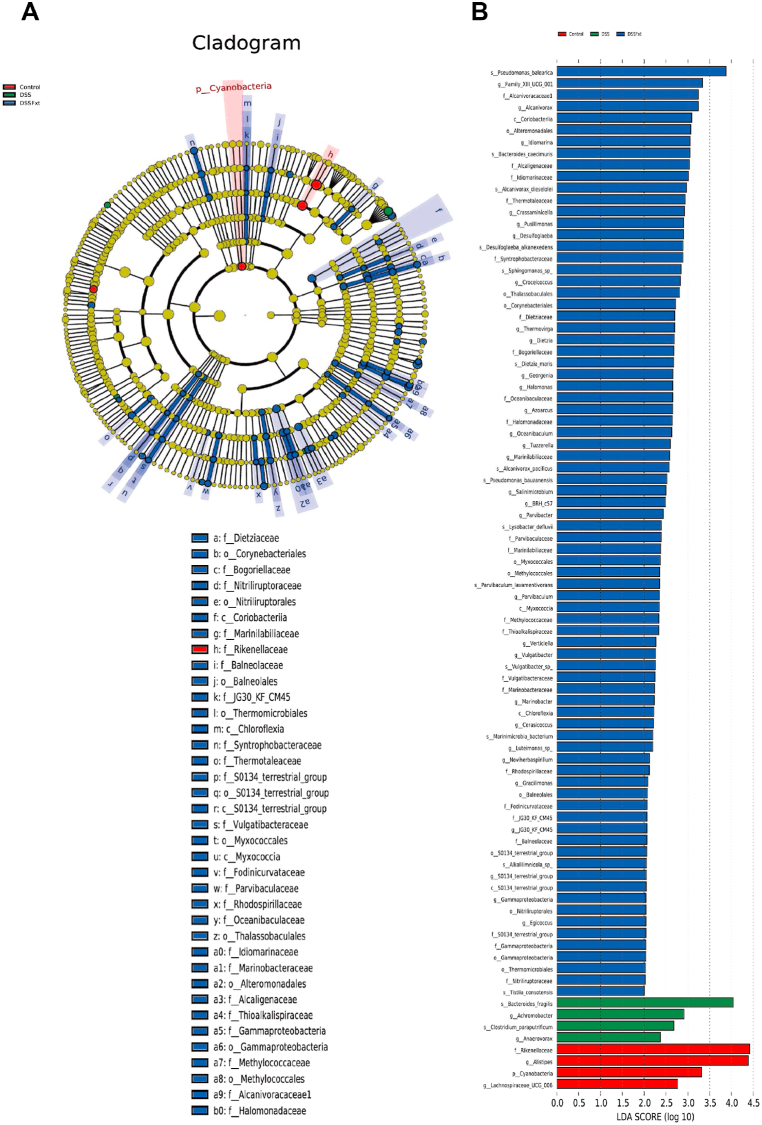
Linear discriminant analysis (LDA) effect size (LEfSe) analysis was performed to demonstrate the effects of Fxt on the gut microbiota composition. The results suggest that the predominant flora in DSS group mice were Bacteroides, Achromobacter and Clostridium. However, Pseudomonas, Alcaniroracaceae and Coriobacteriia were the key species of intestinal flora in the DSS + Fxt group (Fig. 8A and B). To further elucidate the effect of Fxt on the gut microbiota composition, the gut microbiota was analysed at the family and genus levels.
Fig. 8.
Effect of Fxt administration on the composition of intestinal flora in DSS-treated mice. (A) Evolutionary cladistic diagram of LEfSe analysis; (B) LDA of LEfSe analysis (LDA >3, P < 0.05).
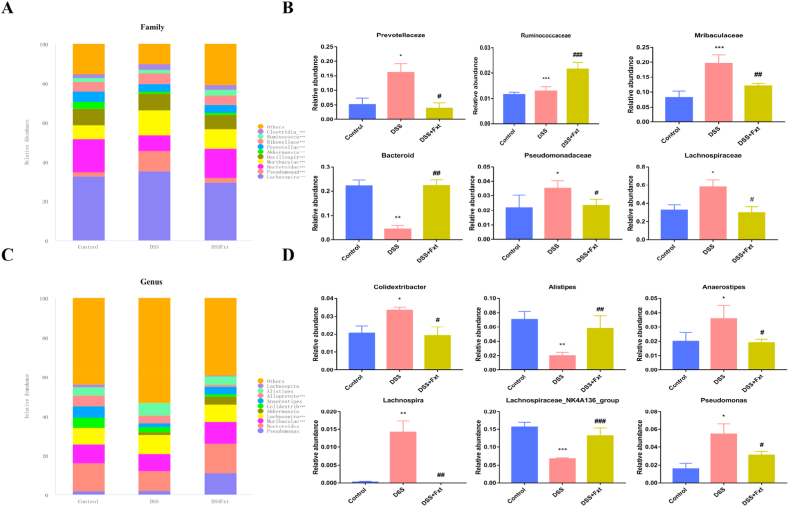
At the family level, the relative abundances of Prevotellaceae, Muribaculaceae, Pseudomonadaceae and Lachnospiraceae were increased in the DSS group, while Fxt decreased the relative abundances of these flora (Fig. 9A and B). At the genus level, Fxt significantly decreased the relative abundance of Colidextribacter, Anaerostipes, Lachnospira and Pseudomonas compared to the control group (Fig. 9C and D).
Fig. 9.
Effect of Fxt administration on the relative abundance of specific intestinal bacteria in mice with colitis induced by DSS. (A) The relative abundance of intestinal flora in each group at the family level; (B) Difference in the relative abundance of specific intestinal bacteria at the family level between groups; (C) Relative abundance of intestinal flora at the genus level in each group; (D) Difference of relative abundance of specific intestinal bacteria at the genus level between groups. All data are expressed as mean ± SEM (n = 6). *P < 0.05, **P < 0.01 and ***P < 0.001 vs Control group. #P < 0.05, ##P < 0.01 and ###P < 0.001 vs DSS-treated group.
4. Discussion
UC is a disease characterized by intestinal barrier damage and intestinal inflammation, mainly manifested as abdominal pain, diarrhoea or stool bleeding [25]. DSS-induced colitis in mice is highly similar to the symptoms of UC patients and is often used as a model for studying UC. In this study, successful establishment of a mouse model of colitis was accompanied by weight loss, colon shortening and DAI score increase. As expected, we found that Fxt improved these performances.
Intestinal barrier integrity is essential for maintaining intestinal health [26,27]. The colon of DSS-treated mice showed destruction of epithelial architecture with crypt architecture disarray, inflammatory cell infiltration in the mucosa and submucosa, and epithelial and goblet cell loss. Fxt improved these pathological changes and became more pronounced at higher doses. There are many factors that affect the integrity of the intestinal barrier, and intestinal membrane tight junction proteins are important factors. Intestinal membrane tight junction proteins mainly consist of intracellular scaffolding protein (ZO-1) [28] and transmembrane proteins (Occludin, Claudin-1, Claudin-3) [29], which constitute a mechanical barrier against mucosal tissue damage [30]. The results of this study showed that the expression of ZO-1 and Occludin in colon tissue of DSS group mice decreased significantly, suggesting that DSS induced intestinal barrier damage. The expression of ZO-1 and Occludin in colon tissue of mice treated with Fxt significantly increased, suggesting that Fxt may interfere with the intestinal barrier damage caused by DSS.
MPO is a lysosomal protein released to neutrophil phagosomes during threshing [31] and a peroxidase existing in neutrophils [23]. It is a biological marker of acute intestinal inflammation and oxidative stress in experimental UC [32,33]. Therefore, it can be used as a specific indicator of intestinal mucosal damage by detecting the activity of MPO [33]. The results showed that Fxt significantly decreased the activity of MPO in colon tissue.
Furthermore, some studies have focused on the inflammation-related signalling pathways associated with the mechanism of UC [34]. The NLRP3 inflammasome consists of the NLRP3 receptor, ASC adaptor and caspase-1 effector, which cleave pro-IL-1β into IL-1β to exacerbate inflammation [35,36]. NLRP3 inflammasome activation promotes DSS-induced colitis in mice [37,38]. In this study, Fxt intervention significantly inhibited the expression of NLRP3 and caspase-1 in colitis mice, and the expression of IL-1β was also significantly reduced. High expression of IL-1β in colitis may stimulate activation of the NF-κB inflammatory pathway. The TLR4/NF-κB signalling pathway is involved in intestinal barrier dysfunction [39]. TLR4 is a Toll-like receptor that plays an important role in the recognition of pathogenic microorganisms and the control of acquired immune responses in natural immunity [40]. Under normal physiological conditions, a small amount of them can be detected in human intestinal mucosa, while a large amount of them is expressed in intestinal mucosal epithelial cells of UC patients [41]. NF-κB is a nuclear transcription factor that exists as a p50/p65 subunit heterodimeric complex and plays an important role in regulating the expression of inflammatory transmitters [42]. In this study, we found that NF-κB-p65 expression was increased and NF-κB-p65 phosphorylation was significantly higher in the colonic tissues of mice in the DSS group, while NF-κB-p65 phosphorylation was significantly lower in the Fxt intervention group. In response to external stimuli, IκB undergoes phosphorylation and ubiquitination and is then degraded by the proteasome, which rapidly enters the nucleus from NF-κB, binds to DNA [43,44], regulates various inflammatory responses, and aggravates the intestinal inflammatory response. In the present study, IκB protein expression and IκB phosphorylation were elevated in the DSS group. Fxt significantly decreased IκB protein expression and inhibited IκB phosphorylation. TNF-α, IL-1β, and IL-6 are important mediators in the inflammatory process, and the expression of TNF-α, IL-1β, and IL-6 in the DSS group was found to be increased in this study; Fxt intervention significantly reduced their expression levels.
An increasing body of evidence has indicated that the gut microbiota is associated with the progression of UC. The destruction of the intestinal flora structure leads to an immune response and intestinal barrier dysfunction. In the present study, the diversity of the gut microbiota was assessed. We found that the bacterial abundance was reduced in the DSS group compared to the control group. Fxt can restore the diversity of the intestinal flora.
The abundance of Pseudomonadaceae, Muribaculaceae, Prevotellaceae and Lachnospiraceae was significantly increased in DSS group mice compared with DSS group mice. Lachnospiraceae and Prevotellaceae can produce short-chain fatty acids (SCFAs). SCFAs are one of the main substances of intestinal bacterial metabolites. Abnormal metabolism of SCFAs contributes to intestinal bacterial imbalance [45]. The relative abundance of Lachnospiraceae was higher in UC and may cause DSS-induced colitis. Fxt administration markedly increased the relative abundance of Bacteroidetes compared to that in DSS group mice. Bacteroides are gram-negative, nonspore-forming, strictly anaerobic bacteria [46]. Previous studies have shown that Bacteroidetes are the dominant flora, accounting for more than 25 % of the total intestinal flora [47]. They can colonize the animal intestine and form a symbiotic relationship with the host [48]. Bacteroides are also considered prebiotics [45]. Thus, Fxt improved the gut microbiota disorder caused by DSS-induced colitis in mice.
5. Conclusions
In conclusion, our experiments showed that Fxt could significantly alleviate the symptoms, pathological damage and immune responses in DSS-induced colitis in mice by protecting the intestinal mucosa and inhibiting the TLR4/NF-κB and NLRP3 signalling pathways and could be used as a candidate drug for the treatment of UC.
Funding
This work was supported by the Fundamental Research Funds for the Central Universities (project no. 140422008).
Data Availability Statement
Bacterial DNA sequencing data were uploaded to the NCBI as PRJNA955678.
Additional information
No additional information is available for this paper.
CRediT authorship contribution statement
Xiuxiu Sun: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing - original draft, Writing - review & editing. Xinxin Jin: Data curation, Formal analysis, Writing - original draft, Writing - review & editing. Lumeng Wang: Investigation, Writing - review & editing. Zhengdan Lin: Formal analysis, Writing - review & editing. Helong Feng: Methodology, Writing - review & editing. Cunlin Zhan: Methodology, Writing - review & editing. Xi Liu: Methodology, Writing - review & editing. Guofu Cheng: Funding acquisition, Resources, Validation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Li X., Lv H., Shi F., Song J., Zhang Z. The potential therapeutic effects of hydroxypropyl cellulose on acute murine colitis induced by DSS. Carbohydr. Polym. 2022;289 doi: 10.1016/j.carbpol.2022.119430. [DOI] [PubMed] [Google Scholar]
- 2.Jeengar M.K., Shrivastava S., Nair K., Singareddy S.R., Putcha U.K. Improvement of bioavailability and anti-inflammatory potential of curcumin in combination with emu oil. Inflammation. 2014;37:2139–2155. doi: 10.1007/s10753-014-9948-4. [DOI] [PubMed] [Google Scholar]
- 3.Lee A.S., Sung M.J., Kim W., Jung Y.J. COMP-angiopoietin-1 ameliorates inflammation-induced lymphangiogenesis in dextran sulfate sodium (DSS)-induced colitis model. J. Mol. Med. (Berl.) 2018;96:459–467. doi: 10.1007/s00109-018-1633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bersuder E., Terciolo C., Lechevrel M., Martin E., Quesnelle C., Freund J.N., Reimund J.M. Mesalazine initiates an anti-oncogenic beta-catenin/MUCDHL negative feed-back loop in colon cancer cells by cell-specific mechanisms. Biomed. Pharmacother. 2022;146 doi: 10.1016/j.biopha.2021.112543. [DOI] [PubMed] [Google Scholar]
- 5.Axelrad J.E., Lichtiger S., Yajnik V. Inflammatory bowel disease and cancer: the role of inflammation, immunosuppression, and cancer treatment. World J. Gastroenterol. 2016;22:4794–4801. doi: 10.3748/wjg.v22.i20.4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Z.G., Ying X.G., Gao P., Wang C.L., Wang Y.F., Yu X.W., Chen J., Wang B., Luo H.Y. Anti-inflammatory activity of a peptide from skipjack (Katsuwonus pelamis) Mar. Drugs. 2019;17:582. doi: 10.3390/md17100582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang K., Ding Y., Xu C., Hao M., Li H., Ding L. Cldn-7 deficiency promotes experimental colitis and associated carcinogenesis by regulating intestinal epithelial integrity. OncoImmunology. 2021;10 doi: 10.1080/2162402X.2021.1923910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ren Z., Huo Y., Zhang Q., Chen S., Lv H., Peng L., Wei H. Protective effect of lactiplantibacillus plantarum 1201 combined with galactooligosaccharide on carbon tetrachloride-induced acute liver injury in mice. Nutrients. 2021;13:4441. doi: 10.3390/nu13124441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang C., Sung J., Long D., Alghoul Z., Merlin D. Prevention of ulcerative colitis by autologous metabolite transfer from colitogenic microbiota treated with lipid nanoparticles encapsulating an anti-inflammatory drug candidate. Pharmaceutics. 2022;14:1233. doi: 10.3390/pharmaceutics14061233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long S.R., Liu R.D., Kumar D.V., Wang Z.Q., Su C.W. Immune protection of a helminth protein in the DSS-induced colitis model in mice. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.664998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hutchings H.A., Alrubiay L., Watkins A., Cheung W.Y., Seagrove A.C., Williams J.G. Validation of the Crohn's and Ulcerative Colitis questionnaire in patients with acute severe ulcerative colitis. United European Gastroenterol J. 2017;5:571–578. doi: 10.1177/2050640616671627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao C.N., Yao Z.L., Yang D., Ke J., Wu Q.L., Li J.K., Zhou X.D. Chemical constituents from fraxinus hupehensis and their antifungal and herbicidal activities. Biomolecules. 2020;10:74. doi: 10.3390/biom10010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miao Z., Zhang L., Gu M., Huang J., Wang X., Yan J., Xu Y., Wang L. Preparation of fraxetin long circulating liposome and its anti-enteritis effect. AAPS PharmSciTech. 2021;22 doi: 10.1208/s12249-021-01940-z. [DOI] [PubMed] [Google Scholar]
- 14.Lee W., Song G., Bae H. Suppressive effect of fraxetin on adipogenesis and reactive oxygen species production in 3T3-L1 cells by regulating MAPK signaling pathways. Antioxidants. 2022;11:1893. doi: 10.3390/antiox11101893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Q., Zhuang D., Feng W., Ma B., Qin L., Jin L. Fraxetin inhibits interleukin-1beta-induced apoptosis, inflammation, and matrix degradation in chondrocytes and protects rat cartilage in vivo. Saudi Pharmaceut. J. 2020;28:1499–1506. doi: 10.1016/j.jsps.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu G., Liu Z., Yan Y., Wang H. Effect of fraxetin on proliferation and apoptosis in breast cancer cells. Oncol. Lett. 2017;14:7374–7378. doi: 10.3892/ol.2017.7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee M., Yang C., Park S., Song G., Lim W. Fraxetin induces cell death in colon cancer cells via mitochondria dysfunction and enhances therapeutic effects in 5-fluorouracil resistant cells. J. Cell. Biochem. 2022;123:469–480. doi: 10.1002/jcb.30187. [DOI] [PubMed] [Google Scholar]
- 18.Hsieh Y.-H., Hung T.-W., Chen Y.-S., Huang Y.-N., Chiou H.-L., Lee C.-C., Tsai J.-P. In vitro and in vivo antifibrotic effects of fraxetin on renal interstitial fibrosis via the ERK signaling pathway. Toxins. 2021;13:474. doi: 10.3390/toxins13070474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H., Xiao B., Hao Z., Sun Z. Simultaneous determination of fraxin and its metabolite, fraxetin, in rat plasma by liquid chromatography-tandem mass spectrometry and its application in a pharmacokinetic study. Journal of Chromatography B 1017-1018. 2016:70–74. doi: 10.1016/j.jchromb.2016.02.030. [DOI] [PubMed] [Google Scholar]
- 20.Yin Y., Wang L., Chen G., You H. Effect of fraxetin on oxidative damage caused by isoproterenol-induced myocardial infarction in rats. Appl. Biochem. Biotechnol. 2022;194:5666–5679. doi: 10.1007/s12010-022-04019-y. [DOI] [PubMed] [Google Scholar]
- 21.Li R., Kim M.-H., Sandhu A.K., Gao C., Gu L. Muscadine grape (vitis rotundifolia) or wine phytochemicals reduce intestinal inflammation in mice with dextran sulfate sodium-induced colitis. J. Agric. Food Chem. 2017;65:769–776. doi: 10.1021/acs.jafc.6b03806. [DOI] [PubMed] [Google Scholar]
- 22.Ding A., Wen X. Dandelion root extract protects NCM460 colonic cells and relieves experimental mouse colitis. J. Nat. Med. 2018;72:857–866. doi: 10.1007/s11418-018-1217-7. [DOI] [PubMed] [Google Scholar]
- 23.Yin Y., Ye L., Niu Z., Fang W. Anti-inflammatory effects of Vicenin-2 on dextran sulfate sodium-induced colitis in mice. Drug Dev. Res. 2019;80:546–555. doi: 10.1002/ddr.21529. [DOI] [PubMed] [Google Scholar]
- 24.Han L., Meng M., Guo M., Cheng D., Shi L., Wang X., Wang C. Immunomodulatory activity of a water-soluble polysaccharide obtained from highland barley on immunosuppressive mice models. Food Funct. 2019;10:304–314. doi: 10.1039/c8fo01991f. [DOI] [PubMed] [Google Scholar]
- 25.Yang W., Huang Z., Xiong H., Wang J., Zhang H., Guo F., Wang C. Rice protein peptides alleviate dextran sulfate sodium-induced colitis via the keap1-nrf2 signaling pathway and regulating gut microbiota. J. Agric. Food Chem. 2022;70:12469–12483. doi: 10.1021/acs.jafc.2c04862. [DOI] [PubMed] [Google Scholar]
- 26.Gou H.Z., Zhang Y.L., Ren L.F., Li Z.J., Zhang L. How do intestinal probiotics restore the intestinal barrier? Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.929346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Y., Li J., Ding W., Ruan Z., Zhang L. Enhanced intestinal barriers by puerarin in combination with tryptophan. J. Agric. Food Chem. 2021;69:15575–15584. doi: 10.1021/acs.jafc.1c05830. [DOI] [PubMed] [Google Scholar]
- 28.Alvarez C.S., Badia J., Bosch M., Gimenez R., Baldoma L. Outer membrane vesicles and soluble factors released by probiotic Escherichia coli nissle 1917 and commensal ECOR63 enhance barrier function by regulating expression of tight junction proteins in intestinal epithelial cells. Front. Microbiol. 2016;7:1981. doi: 10.3389/fmicb.2016.01981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Che D., Zhao B., Fan Y., Han R., Zhang C., Qin G., Adams S. Eleutheroside B increase tight junction proteins and anti-inflammatory cytokines expression in intestinal porcine jejunum epithelial cells (IPEC-J2) J. Anim. Physiol. Anim. Nutr. 2019;103:1174–1184. doi: 10.1111/jpn.13087. [DOI] [PubMed] [Google Scholar]
- 30.Jiang R., Du X., Brink L., Lonnerdal B. The role of orally ingested milk fat globule membrane on intestinal barrier functions evaluated with a suckling rat pup supplementation model and a human enterocyte model. J. Nutr. Biochem. 2022;108 doi: 10.1016/j.jnutbio.2022.109084. [DOI] [PubMed] [Google Scholar]
- 31.Emília H., Izabela B., Jana Š., Ladislav S., Anna C., Alojz B. Anti-inflammatory potential of Lactobacillus plantarum LS/07 in acute colitis in rats. Acta Vet. 2018;68:55–64. [Google Scholar]
- 32.Piechota-Polanczyk A., Zielinska M., Piekielny D., Fichna J. The influence of lipoic acid on caveolin-1-regulated antioxidative enzymes in the mouse model of acute ulcerative colitis. Biomed. Pharmacother. 2016;84:470–475. doi: 10.1016/j.biopha.2016.09.066. [DOI] [PubMed] [Google Scholar]
- 33.Marin M., Gimeno C., Giner R.M., Rios J.L., Manez S., Recio M.A.C. Influence of dimerization of apocynin on its effects in experimental colitis. J. Agric. Food Chem. 2017;65:4083–4091. doi: 10.1021/acs.jafc.7b00872. [DOI] [PubMed] [Google Scholar]
- 34.Hernandez-Chirlaque C., Aranda C.J., Ocon B., Capitan-Canadas F., Ortega-Gonzalez M., Carrero J.J., Suarez M.D. Germ-free and antibiotic-treated mice are highly susceptible to epithelial injury in DSS colitis. J Crohns Colitis. 2016;10:1324–1335. doi: 10.1093/ecco-jcc/jjw096. [DOI] [PubMed] [Google Scholar]
- 35.Ren G., Zhang X., Xiao Y., Zhang W., Wang Y., Ma W., Wang X., Song P., Lai L., Chen H., Zhan Y., Zhang J., Yu M., Ge C., Li C., Yin R., Yang X. ABRO1 promotes NLRP3 inflammasome activation through regulation of NLRP3 deubiquitination. EMBO J. 2019;38 doi: 10.15252/embj.2018100376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cai B., Zhao J., Zhang Y., Liu Y., Ma C., Yi F., Zheng Y., Zhang L., Chen T., Liu H., Liu B., Gao C. USP5 attenuates NLRP3 inflammasome activation by promoting autophagic degradation of NLRP3. Autophagy. 2021;18:990–1004. doi: 10.1080/15548627.2021.1965426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li X., Wu X., Wang Q., Xu W., Zhao Q. Sanguinarine ameliorates DSS induced ulcerative colitis by inhibiting NLRP3 inflammasome activation and modulating intestinal microbiota in C57BL/6 mice. Phytomedicine. 2022;106 doi: 10.1016/j.phymed.2022.154394. [DOI] [PubMed] [Google Scholar]
- 38.Lv Q., Xing Y., Liu J., Dong D., Liu Y., Qiao H., Zhang Y. Lonicerin targets EZH2 to alleviate ulcerative colitis by autophagy-mediated NLRP3 inflammasome inactivation. Acta Pharm. Sin. B. 2021;11:2880–2899. doi: 10.1016/j.apsb.2021.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fukata M., Chen A., Klepper A., Krishnareddy S., Vamadevan S.A. Cox-2 is regulated by toll-like receptor-4 (TLR4) signaling and is important for proliferation and apoptosis in response to intestinal mucosal injury. Gastroenterology. 2006;131:862–877. doi: 10.1053/j.gastro.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romerio A., Peri F. Increasing the chemical variety of small-molecule-based TLR4 modulators: an overview. Front. Immunol. 2020;11:1210. doi: 10.3389/fimmu.2020.01210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meng Y., Xu Y., Chang C., Qiu Z., Hu J., Wu Y. Extraction, characterization and anti-inflammatory activities of an inulin-type fructan from Codonopsis pilosula. Int. J. Biol. Macromol. 2020;163:1677–1686. doi: 10.1016/j.ijbiomac.2020.09.117. [DOI] [PubMed] [Google Scholar]
- 42.Poma P. NF-kappaB and disease. Int. J. Mol. Sci. 2020;21:9181. doi: 10.3390/ijms21239181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peng L., Gao X., Nie L., Xie J., Dai T., Shi C. Astragalin attenuates dextran sulfate sodium (DSS)-Induced acute experimental colitis by alleviating gut microbiota dysbiosis and inhibiting NF-kappaB activation in mice. Front. Immunol. 2020;11:2058. doi: 10.3389/fimmu.2020.02058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma J., Yin G., Lu Z., Xie P., Zhou H., Liu J., Yu L. Casticin prevents DSS induced ulcerative colitis in mice through inhibitions of NF-kappaB pathway and ROS signaling. Phytother Res. 2018;32:1770–1783. doi: 10.1002/ptr.6108. [DOI] [PubMed] [Google Scholar]
- 45.Mazmanian S.K., Round J.L., Kasper D.L. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 46.Ochoa-Reparaz J., Mielcarz D.W., Wang Y., Begum-Haque S., Dasgupta S., Kasper D.L., Kasper L.H. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 2010;3:487–495. doi: 10.1038/mi.2010.29. [DOI] [PubMed] [Google Scholar]
- 47.Faith J.J., Guruge J.L., Charbonneau M., Subramanian S., Seedorf H., Goodman A.L., Clemente J.C., Knight R., Heath A.C., Leibel R.L., Rosenbaum M., Gordon J.I. The long-term stability of the human gut microbiota. Science. 2013;341 doi: 10.1126/science.1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deng H., Li Z., Tan Y., Guo Z., Liu Y., Wang Y., Yuan Y., Yang R., Bi Y., Bai Y., Zhi F. A novel strain of Bacteroides fragilis enhances phagocytosis and polarises M1 macrophages. Sci. Rep. 2016;6 doi: 10.1038/srep29401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Bacterial DNA sequencing data were uploaded to the NCBI as PRJNA955678.