Abstract
The integration of microalgae cultivation in anaerobic digestion (AD) plants can take advantage of relevant nutrients (ammonium and ortho-phosphate) and CO2 loads. The proposed scheme of microalgae integration in existing biogas plants aims at producing approximately 250 t·y−1 of microalgal biomass, targeting the biostimulants market that is currently under rapid expansion. A full-scale biorefinery was designed to treat 50 kt·y−1 of raw liquid digestate from AD and 0.45 kt·y−1 of CO2 from biogas upgrading, and 0.40 kt·y−1 of sugar-rich solid by-products from a local confectionery industry. An innovative three-stage cultivation process was designed, modelled, and verified, including: i) microalgae inoculation in tubular PBRs to select the desired algal strains, ii) microalgae cultivation in raceway ponds under greenhouses, and iii) heterotrophic microalgae cultivation in fermenters. A detailed economic assessment of the proposed biorefinery allowed to compute a biomass production cost of 2.8 ± 0.3 €·kg DW−1, that is compatible with current downstream process costs to produce biostimulants, suggesting that the proposed nutrient recovery route is feasible from the technical and economic perspective. Based on the case study analysis, a discussion of process, bioproducts and policy barriers that currently hinder the development of microalgae-based biorefineries is presented.
Keywords: Heterotrophic and autotrophic microalgae cultivation, Anaerobic digestion, Resource recovery, Biorefinery, Mathematical modelling, Techno-economic assessment
Highlights
-
•
Process design and modelling for an algal biorefinery integrated in a biogas plant.
-
•
Techno-economic assessment and detailed cost breakdown with sensitivity analysis.
-
•
Sustainable production of 252 t/y of algal biomass at the cost of 2.5–3.1 €/kg.
-
•
Bioremediation of 78 kt/y of digestate and 0.4 kt/y of industrial by-products.
-
•
Identification of relevant economic and technological bottlenecks for the process.
1. Introduction
In 2021, the European Union (EU) hosted 18,843 biogas plants and 1,067 biomethane plants, producing 18.4 billion cubic meters (bcm) of biogas and biomethane through anaerobic digestion (AD). The growth of biogas and biomethane production responds to various needs including sustainable waste recycling, climate protection, and renewable energy production. The market has been boosted by political and legal drivers, such as the 2030 Energy Strategy or the REPowerEU Plan, aimed at increasing the share of renewable energy and the energy security of EU, and foreseeing massive infrastructure investments [1,2]. The resulting generation of biogenic CO2 is an appealing resource for the food and drinks industry, and for many others [2]. Similarly, digestate production is estimated to grow in the next decades, and those countries with highest biogas production (i.e., Germany, UK, Italy, and France) will be the largest digestate producers within Europe [2].
Digestate composition is strongly influenced by the influent feedstock and by AD operational parameters, being challenging to identify its mean composition [3]. Based on a recent large survey [4] and according to a digestate production in the EU [2], relevant flows can be exploited for fertilizing purposes: 9.5 Mt·y−1 of dry matter (DM), 6.7 Mt·y−1 of organic matter (OM), 3.7 Mt·y−1 of total organic carbon (TOC), 0.8 Mt·y−1 of total nitrogen (TN), 0.4 Mt·y−1 of mineral nitrogen, 0.5 Mt·y−1 of K2O and 0.3 Mt·y−1 of P2O5. According to Eurostat, 10.3 Mt of synthetic nitrogen fertilizers were consumed in EU 28 in 2018 and nitrogen recovered from digestate could replace up to 7.8 % of Haber–Bosch-derived fertilizers. Similarly, P mined from digestate could replace 17 % of the 1.1 Mt of P consumed in 2018 [2]. For these reasons, several technologies for nutrient recovery from digestate have been developed and studied. Digestate is commonly addressed to widespread solid/liquid (S/L) separation technologies, resulting in nutrient fractionation facilitating further treatment, transport and industrial applications [5]: screw pressing, filtration and centrifugation, possibly in combination with electro-/physico-chemical coagulation [6]. When nutrient recovery is required, the liquid fraction of digestate (LFD) can be treated with various technologies at high technology readiness level (TRL > 6): ammonia stripping, membrane filtration, struvite precipitation, ion exchange, and vacuum evaporation [7]. Ammonia stripping/scrubbing allows the recovery of a marketable fertilizer, but high temperature and/or pH, and sulfuric acid as a reagent are required. Nonetheless, it is among the most used full-scale methods for N recovery [6,8]. Membrane filtration implies the physical retention of a retentate and the release of a purified liquid phase (permeate), with membrane fouling and lifespan being the main bottlenecks [6,9]. Struvite (NH4MgPO4·6H2O) precipitation is worldwide spread with several full-scale units allowing for N and P recovery in a solid fertilizer [5,7]. Ion exchange absorption is a selective process applied at the pilot-scale exploiting resins to remove charged ions from LFD [3]. Vacuum evaporation is a full-scale energy-intensive method operating at low pressure for fast evaporation and subsequent nutrient concentration that provides a commercial solid fertilizer (ammonium sulphate) [10]. Though these treatment technologies have been largely applied for nutrient removal, various obstacles remain to be elucidated: i) logistics requirements at the local application scale, ii) technical reliability/operability and product quality, iii) regulatory issues, such as the status of recovered products, involving the overall production/supply chain (farmers and agri-food industries), and local authorities, and iv) economic constraints related to the low value of non-renewable counterparts.
For these reasons, the search for innovative solutions led to consider the integration of microalgae and cyanobacteria cultivation in biogas plants. This can increase the efficiency and sustainability of the biogas production and digestate treatment processes [11,12] and provide additional revenues from the commercialization of microalgae-based bio-products [[13], [14], [15]]. Recent feasibility studies have confirmed that integrating microalgae in biogas plants holds a large potential to cut biomass production costs, especially when using wastewaters and industrial waste streams combined with the bioremediation of gaseous streams (e.g., biogas or combustion flue gases) [[16], [17], [18], [19]]. Recent studies conducted at the pilot-scale showed that microalgae cultivation can be successfully integrated in side-stream digestate treatment processes, reaching high Total Ammoniacal Nitrogen (TAN) and ortho-phosphate removal efficiencies and allowing for consistent cost/energy savings [[20], [21], [22], [23]]. In particular, the development of algae-bacteria consortia makes nutrient removal efficient, despite suboptimal weather conditions reducing the activity of microalgae [22,24]. In such scheme, microalgal benefit from mass and energy flows available in AD plants, including digestate nutrients and CO2 contained in the biogas, and waste heat generated during biogas combustion. The LFD is interesting for growing microalgae due to the low turbidity and high concentrations of TAN, ortho-phosphate and micro-elements [20,25]. CO2 is the main carbon source for microalgae and associated autotrophic bacteria, that can be recovered from biogas and combustion flue-gases via conventional physical-chemical technologies [26] or direct microalgae-based biogas upgrading [27]. Since the operational temperature of microalgae cultivation is a critical parameter, the availability of waste heat from biogas combustion is another major advantage of this integration scheme [28]. Moreover, the heterotrophic growth capability and high biomass concentrations reached for certain algae strains that are commonly exploited in microalgae-based wastewater treatment (e.g., Chlorella and Scenedesmus spp.) make them interesting for the treatment of wastewaters and industrial waste with high COD contents [[29], [30], [31], [32]].
The possibility of producing valuable bio-products from microalgae further contributes to the economic viability of microalgae-based bioremediation processes [16,19,33]. For example, the feasibility of biomass valorisation as bioplastics or biofuels has been technically demonstrated, although the high cost of finished products has pushed researchers to look for more suitable valorisation alternatives [34]. Despite biogas production from the algal biomass produced during LFD treatment would be a very interesting alternative in a circular economy viewpoint, the anaerobic digestion of algal biomass typically has very low biogas yields, mainly due to the presence of resistant algal cell walls, requiring energy-intensive pretreatments [35,36]. Biostimulants production is an interesting option because algae-based extracts have biostimulating effects promoting the growth and development of plants, also improving their tolerance to abiotic stress [[37], [38], [39]]. The regulation (EU) 2019/1009, laying down rules on fertilizing products in the EU market, include algae for biofertilizers/biostimulants production. The research on microalgal biostimulants has remarkably spread in the last years, as microalgae can be cultivated under controlled conditions, obtaining high-quality biomass and bioactive compounds [40]. Furthermore, microalgae are a renewable and sustainable biostimulants source, especially if obtained with waste or by-products in a biorefinery framework [[41], [42], [43]]. Within this context, some microalgae strains able to grow on LFD also have biostimulant properties on different crops, thanks to the production of different bioactive molecules (e.g., phytohormones, proteins, amino acids, polysaccharides, antioxidants, vitamins and enzymes, among others) [40]. For example, the application of aqueous Chlorella sp. And Scenedesmus sp. extracts showed significant improvements in germination [44,45], growth parameters and nutrient uptake [37,44]. Foliar applications of these microalgae extracts can improve the yield and stress resistance of different crops [[46], [47], [48], [49]].
In this work, the feasibility of integrating a microalgae-based biorefinery within a biomethane plant was evaluated from a technical and economic perspective. The model-based design of an innovative three-stage microalgae cultivation process is proposed, to generate algal biomass for the production of biostimulants. In the process, a continuous inoculation with selected algae strains is fed to a series of open raceway ponds treating the liquid fraction of digestate from anaerobic digestion and fixing CO2 from biogas upgrading. In a final heterotrophic cultivation step, the valorisation of low cost by-products from a local confectionery industry is realized to further increase the biomass productivity. Based on mathematical modelling of the proposed biorefinery scheme, a thoughtful process design and techno-economic assessment allowed to identify a cost range for biomass production and to discuss the opportunities and challenges of microalgal nutrient recovery to produce valuable products such as crop biostimulants.
2. Materials and methods
2.1. Proposed case study
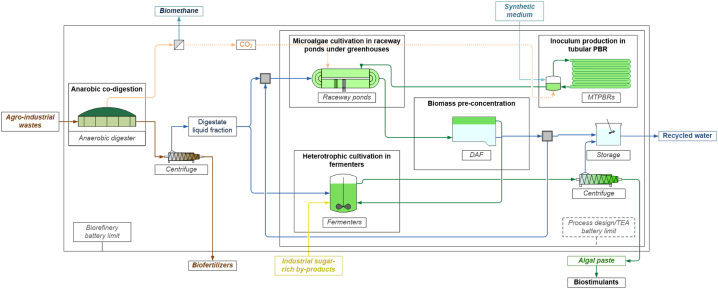
The proposed case is a microalgae-based biorefinery where the LFD and CO2 from AD of agricultural residues and selected confectionery industry by-products (IBP) are used as substrates for microalgae cultivation (Fig. 1). A three-stage cultivation process was designed for microalgae cultivation:
-
i)
A first autotrophic step, where a pure inoculum is grown on synthetic medium, to maintain the algal population stable and to avoid excessive contamination from other phototrophs,
-
ii)
A second autotrophic step, where massive cultivation is achieved using LFD and on-demand addition of CO2 in industrial-scale high-rate algal ponds (HRAP) covered by greenhouses, and
-
iii)
A third heterotrophic cultivation stage performed under controlled conditions in fermenters, where the pre-concentrated biomass obtained from the raceway ponds is fed with digestate and IBPs rich in simple sugars to further increase biomass productivity.
Fig. 1.
Schematic representation of the proposed microalgae-based biorefinery for the integration of microalgae cultivation in biomethane plants.
The biorefinery design, modelling, and techno-economic assessment thus include the three algal cultivation sections, as well as the required pre-concentration and harvesting sections, and auxiliary equipment to produce the algal biomass. Since no post-processing processes are explicitly considered for the current techno-economic assessment, the output of the study is the production cost of the algal paste, that can be applied to any other valorisation pathway. In the current case study, this is meant to be sold and externally post-processed for biostimulant production, but the proposed techno-economic model does not include such revenues.
Chlorella and Scenedesmus genera were considered as the most promising because of their documented ability to stand harsh environments and to grow photo-autotrophically or heterotrophically on digestate and waste streams [50], while being interesting for producing biostimulants [37].
2.2. Process design and modelling
2.2.1. Plant design and assumptions
The biorefinery is designed to be located next to an industrial AD plant producing biomethane located in Northern Italy. Next to the plant, approximately 3 ha of agricultural land are considered available for the implementation of the biorefinery. The plant, aimed at producing algal biomass to obtain biostimulants, has been designed according to the following assumptions:
-
-
An inoculum preparation under controlled conditions (closed photobioreactor, PBR, and synthetic medium) is used to produce approximately 10 % of the expected productivity of the following HRAP section (ensuring proper dominance of the desired algal strains on the culture), and designed according to conventional procedures [51].
-
-
The HRAP section is designed according to conventional criteria [52,53], while operational parameters and productivities are estimated via a model-based approach with a model validated on similar case-studies, i.e., growing green algae on LFD [21,54], as detailed in Section 2.2.2.
-
-
The heterotrophic section is designed in order to triple the algal biomass concentration and relies on mixed and aerated fed-batch fermenters. Heterotrophic growth parameters (e.g., rates and yields) were validated experimentally by using confectionary IBPs (Supplementary Data SI.1.2).
The main assumptions for each section are detailed below. Further details on design procedures are reported hereafter and in Supplementary Data (SI.1.1).
The biomethane production plant (AD). The biorefinery is designed to be located next to an industrial AD plant (1 MWel equivalent) producing biomethane in the Northern Italy. The main plant feedstocks include cow slurry (60 % vv), cow manure (10 % vv) and other agro-industrial by-products largely available from nearby factories. The plant produces approximately 150 t CO2·y−1 through biogas membrane filtration. Of the digestate produced by the digester, approximately 50 kt·y−1 of liquid digestate are considered to be available after S/L separation via screw press.
Liquid digestate preparation (DPT). This step is required to reduce the high solid concentrations in the liquid fraction from screw press, while improving its optical properties to favour light penetration in the HRAPs. To this purpose, polyelectrolyte-assisted centrifugation with cationic polyacrylamide (CPAM) is used, which is common in sludge dewatering. Preliminary experiments suggested a minimum dosage of 3 g CPAM·L−1 for effective clarification (data not shown). In this preliminary assessment, negative CPAM effects due to accumulation in the biomass have not been determined or included. Dilution was needed to adjust the hydraulic retention time (HRT) of the HRAPs, while adjusting the TAN concentration to tolerable levels for microalgae. The final flow of LFD (approximately 100 kt·y−1, with the characteristics reported in Table 1) is sent as nutrient source to both the RWP and HET sections.
Table 1.
Model parameters used in this work.
| Biorefinery Section | Parameter | Unit | Value | Reference |
|---|---|---|---|---|
| HRAP cultivation system | Reactor type | – | Raceway pond | – |
| Reactor volume | m3 | 4,750 | – | |
| Liquid depth | cm | 20 | – | |
| Number of reactors | – | 10 | – | |
| Overall surface | ha | 3.25 | – | |
| Process design | Hydraulic retention time (HRT) | d | 20 | [54,55] |
| Biomass retention time (BRT) | d | 5 | [56] | |
| Greenhouse | No. of greenhouse covers | – | 1 | [28] |
| Climatic condition | Location of the system | – | Northern Italy | – |
| Climate classification | - | Csa (warm mediterranean) | [57] | |
| Average yearly temperature (min – max) | °C | 14.3; (−7.8 – 36.6) | – | |
| Average yearly PAR (min – max) | μE·m−1·s−1 | 759; (5 – 2033) | – | |
| Implemented models | Biological and physico-chemical HRAP model | – | ALBA | [58] |
| One-cover greenhouse-raceway pond model (GH – RWP) | – | Modified Li et al. | [28,59] | |
| Algal biomass | Cultivated strain | – | Chlorella – Scenedesmus consortium | [54] |
| Maximum specific growth rate | d−1 | 2.5 | [58] | |
| Biomass composition | – | C100H183O48 N11P | [58] | |
| PAR dependence | Model | – | Haldane | [60] |
| Initial P–I curve slope (α) | m2·s·μE−1 | 0.010 | [58] | |
| Optimal irradiance (IOPT) | μE·m−2·s−1 | 300 | [58] | |
| Temperature dependence | Model | – | CTMI | [61] |
| Minimum temperature (TMIN) | °C | −10.1 | [58] | |
| Optimal temperature (TOPT) | °C | 20.0 | [58] | |
| Maximum temperature (TMAX) | °C | 42.5 | [58] | |
| pH dependence | Model | – | CpHM | [62] |
| Minimum pH (pHMIN) | – | 2.0 | [58] | |
| Optimal pH (pHOPT) | – | 8.4 | [58] | |
| Maximum pH (pHMAX) | – | 12.0 | [58] | |
| DO dependence | Model | – | Hill | [63] |
| 50 % Inhibitory concentration (EC50,DO) | mg DO·L−1 | 20.0 | [58] | |
| Shape factor (n) | – | 15.0 | [58] | |
| Digestate source | Type | – | LFD from agro-waste co-digestion | – |
| Pre-treatments | – | Flocculation, centrifugation, dilution (1:2 vv) | – | |
| Digestate composition | TAN | mg N·L−1 | 800 | – |
| NO3- | mg N·L−1 | 0.0 | – | |
| PO43- | mg P·L−1 | 25 | – | |
| TIC | mg C·L−1 | 686 | – | |
| CODS | mg COD·L−1 | 3000 | – | |
| CODTOT | mg COD·L−1 | 3900 | – | |
| TSS | mg TSS·L−1 | 640 | – | |
| Other parameters | Biological data | – | Other biological and physical chemical parameters | [54,58] |
| Thermal data | – | Greenhouse and thermal model parameters | [28,59] | |
| Weather data | – | Hourly weather data | – |
Inoculum production in photobioreactors (PBR). The section of inoculum production was sized to obtain an algal production of 7.6 t DW·y−1. The inoculum is produced in a multilayer tubular photobioreactor (MTPBR) fed on synthetic medium (modified Bold's basal medium, MBBM, with ammoniacal nitrogen as nitrogen source instead of nitrate, to favour microalgae acclimation). The PBR was sized assuming a photosynthetic efficiency of 3.5 % [64] and a volumetric productivity of 0.4 kg DW·m−3·d−1 [51]. A design criterion was the dissolved O2 (DO) accumulation in the PBR, responsible for algal growth inhibition [65,66]. A maximum DO concentration corresponding to 200 % DO saturation was allowed at the end of the tube under the worst condition (35 °C, 6.7 mg DO·L−1). Stoichiometrically-computed oxygen production rates (OPRs) were incremented by daily and seasonal peak coefficients [67]. The resulting maximum tube length of 142 m was realized on different layers and the diameter chosen among commercial options was 6.5 cm, with a liquid velocity of 0.5 m·s−1. The total number of modules required to satisfy the production request was thus calculated. Degassing columns were designed (height: 3.5 m, diameter: 0.35 m), injecting air for stripping DO and other gases, and allowing for nutrients and thermal regulation (by submerged heat exchangers). CO2 from biogas upgrading is injected in the system according to algal stoichiometry, and to maintain the pH at the desired level. No additional light was considered, to limit capital/operational expenditures. The energy demand was separately calculated for degassing, pumping, and thermal control. As for degassing, a specific air flowrate of 0.07 vvm and a 60 % blower efficiency were assumed. The electric energy consumption by the recirculation pump was estimated according to conventional hydraulic criteria considering the tube length, diameter, and number of bends, allowing to estimate head losses (frictional coefficient λ = 0.02 and head loss coefficient for U-bends = 0.1). A specific chilling request of 2 MW·ha−1 was considered (personal communication from specialized providers).
Autotrophic microalgae cultivation in high-rate algal ponds (RWP). As mentioned, the RWP section represents the heart of the biorefinery, and the entire plant is sized based on this section. The HRAP design is based on the actual land availability for this section (3.1 ha, corresponding to a wet surface of 2.4 ha), including adequate spacing among ponds [68]. The resulting plant was constituted by 10 ponds with a length to width (L/W) ratio of 10. The ponds are realized with low-cost techniques, i.e., concrete walls keeping in place a waterproof lining, and paddlewheels for mixing and mass transfer. To improve the quality of the produced biomass and to limit potential contaminations (e.g., dust, birds, environmental microorganisms), a greenhouse was considered to cover the RWP. The impact of the greenhouse cover on the photosynthetically active radiation (PAR) availability and on the yearly temperature profile was assessed by mechanistic modelling of the greenhouse-pond system [28], as detailed in Section 2.2.2. The use of a single-cover greenhouse has been identified as the best trade-off to avoid low winter temperatures (in open-air outdoor cultivation) and hot summer temperatures (with double cover greenhouses). PVC was chosen as material for the cover, due to the higher PAR and near infrared (NIR) transmittance, allowing for higher heating dispersion during summer [28]. Mechanical ventilation was the most cost-effective solution to avoid summer overheating, while more advanced thermal regulation strategies would only reveal cost-effective if cheap/waste energy vectors are available. Model simulations with the state-of-the-art ALBA (algae-bacteria) model coupled with a GH thermal model [59] and optimization studies performed on digestate in a similar climate [54] were used to define operational parameters and expected productivities (details on this model are reported later in Section 2.2.2). According to simulations, this section is expected to produce 76 t DW·y−1 (9.8 g DW·m−2·d−1). The optimal liquid height of ponds was explored in the range 10 – 30 cm, and a value of 20 cm was eventually fixed, consistently with typical values [51]. No optimized liquid height management was considered, even if this could represent an interesting alternative to increase the productivity [69,70]. The retention times (HRT and BRT) were decoupled to simulate biomass or effluent recirculation [56]. In this case study, according to digestate characteristics providing fresh nutrients, the HRT was kept high (20 d) to limit water consumption and to adjust nutrient loads to the stoichiometric request. As confirmed by simulations, higher HRTs were excluded, possibly resulting in inhibition due to salt accumulation, as well as in nutrient limitation. As for the BRT, a value of 5 d has been considered as optimal value in a previous model-based optimization [54]. The electric energy consumption for running the pumps and paddlewheels was computed assuming 3.5 kW·ha−1 [68]. Ventilation was designed to achieve 30 greenhouse volume changes per hour, requiring 30 fans with 1.5 kW power each.
Biomass pre-concentration (BPC). The biomass produced in RWP must be concentrated before entering the subsequent heterotrophic stage to limit its footprint. Moreover, a higher initial biomass concentration is expected to favour the algal population with respect to undesired microorganisms (e.g., bacteria and fungi), potentially entering the fermenters with the LFD. The pre-concentration is realized by dissolved air floatation (DAF) assisted with flocculant (15 mg Al2(SO4)3·L−1, contact time: 15 min), as suggested in previous literature works [71], for increasing the biomass concentration up to 5 g DW·L−1.
Heterotrophic microalgae cultivation in fermenters (HET). This stage is performed in stainless steel fermenters operated in fed-batch with concentrated sugar-rich IBPs providing readily degradable carbon, and LFD providing nutrients. The fermenters have been sized to triple the initial algal concentration, thus producing further 168 t DW·y−1. The fermenter volume and reaction times were determined based on biomass yield coefficients, maximum specific growth rates, and lag phase durations, as proposed in literature [72,73]. The resulting HET volume is 707 m3, divided in 4 fermenters (height: 3.5 m, diameter: 8.0 m) operating in 3.5 days cycles. In the absence of an exact IBP composition, the request for sugar-rich by-products was based on stochiometric growth on glucose [74,75] and algal yields (Supplementary Data SI.1.2). The electricity consumption is based on design data and mass balances for: i) aeration, with a specific power consumption of 1 kWh·kg O2−1, a stoichiometric oxygen request of 1.1 kg O2·kg IBP−1, and a transfer efficiency of 20 %; ii) pumping, considering the fermenters height and flowrate according to the imposed HRT; and iii) mixing, with a specific power of 20 W·m−3 and 80 % motor efficiency [76]. Cleaning of the fermenters was considered using sodium hypochlorite (NaClO, 100 mg·L−1) after every fed-batch operation.
Biomass harvesting (BH). The final harvesting step is realized by centrifugation of the heterotrophic algal biomass, without further pre-concentration treatment, thanks to the high solid concentrations from the HET section. Centrifugation is required to obtain an algal paste at 200 g DW·kg−1 (20 % ww), that can be stored and shipped for post-processing. The electric consumption of the centrifuge is assumed as 1.5 kWh·m−3 of algal suspension harvested [68].
2.2.2. Mathematical modelling
To simulate the algal biomass production in the proposed biorefinery concept, the ALBA model was adopted [58]. The model was previously calibrated and validated on datasets from long-term experiments with different wastewaters and climatic conditions, obtaining robust predictions on nutrient removal and biomass production. The model is based on previous water treatment and quality models, integrating relevant biological processes (growth and respiration of microalgae, heterotrophic bacteria, and nitrifying bacteria; hydrolysis of slowly-biodegradable matter and organic nitrogen) and physical-chemical phenomena (pH equilibrium; atmospheric stripping and absorption). The model includes CO2 injection for pH control around optimal strain-specific values, as well as HRT/BRT decoupling. In this work, the ALBA model was coupled to mechanistic thermal models, allowing to simulate the effect of greenhouses or open-air configurations [28,59,69]. The combined thermal-biological model was used to simulate different scenarios of yearly temperature/irradiance profiles and operational conditions (HRT, BRT, liquid depth), in the presence/absence of the greenhouse. The model included the effect of the greenhouse cover transmittance, significantly decreasing the PAR at the pond surface [28,77]. The actual evaporation rate was dynamically estimated by the model and used to calculate make-up water requirements. Local hourly data (air temperature, global irradiance, relative air humidity, and wind velocity at 10 m above ground) were provided by the local regional environmental protection agency, as reported in Supplementary Data (SI.1.1).
In the simulated case study, an algae-bacteria consortium is expected to establish in the HRAPs due to the use of COD-rich and TAN-rich LFD, though the recalcitrant nature of digestate COD maintains the concentration of heterotrophic bacteria low [54]. Nominal model parameters have been used to describe the dependence of the algal growth rate on local temperature, PAR and other environmental conditions [63]. Relevant parameters used for simulations are reported in Table 1 and related references. At this stage, no dynamic modelling of the other two cultivation stages (PBR and HET sections) was implemented, so that literature assumptions and experimental parameters were applied in the design procedure (see Section 2.2.1 and 2.3.2).
2.3. Techno-economic assessment: methodology and assumptions
For the techno-economic assessment (TEA) of the algal biorefinery, the plant layout described in Section 2.2.1 is considered, excluding the anaerobic digestion and LFD preparation (i.e., assuming that LFD and CO2 are provided for free to the biorefinery). Cost evaluation was based on the expected yearly productivity, including dynamic outputs of the RWP and steady-state outputs for PBR and HET sub-models.
The framework for the economic assessment was based on robust TEA methodologies developed by the National Renewable Energy Laboratory (NREL) [67,68,78] and other research units [64,[79], [80], [81]], as well as on internal evaluations on similar case studies [21,82]. In the following sections, the main economic assumptions for each section are reported for the calculation of operational and capital expenditure (OPEX and CAPEX, respectively). More details are available in Supplementary Data (SI.1.2).
2.3.1. General plant assumptions
The calculated CAPEX is given by the sum of major equipment costs (MEC), land, civil works, instrumentation and control, as well as commissioning, transportation and engineering. The plant is constructed close to the AD plant and the land cost was calculated based on Eurostat data for the year 2021 [83]. The evaluation of MEC for specific sections is reported in Section 2.3.2. Other CAPEX were calculated based on preliminary internal surveys.
For items with no direct quotation, literature costs were used and an exponential scale law (cost exponent: 0.67) was considered to calculate the equipment price based on its size and available quotations for different sizes [80,84,85], providing a fair estimate of actual equipment costs. Available quotations in different currencies than € were converted and actualized to € (2023), based on the year of the reported quotation.
The calculation of OPEX included: labour, electricity for electro-mechanic equipment, nutrients and sugar-rich by-products, other reagents, and consumables. The labour cost was assumed as 90,000 €·y−1 (one skilled operator for plant management and production control, one supervisor, and one operator for biological supervision). The electricity cost was evaluated based on the operativity and energy demands from single units (Section 2.3.2). A combined heat and power (CHP) unit is used to cover internal electric and heat demand. A natural gas cost of 0.210 €·Sm−3 was considered, based on internal surveys on industrial gas prices within the next decades. The cost for CO2 delivery was included as 15 % increase of the specific energy consumption of the RWP section, with no additional costs for gas delivery infrastructures. The make-up water to compensate evaporation in HRAPs and to prepare nutrient solutions in PBR and HET was assumed to be groundwater from a local well, available at 0.50 €·m-3. Reagent requests were calculated for each section, based on related mass balances. For reagents and consumables, unitary costs were calculated based on chemical engineering plant cost index (CEPCI), in case of quotations available from different time periods [86]. Maintenance of the greenhouse and other equipment, as well as insurance, security and administration, and fees were quoted based on internal surveys (Supplementary Data, SI.1.2). The depreciation of investments was calculated with 10 % discount and 5 % interest rates over the biorefinery lifespan (20 years).
2.3.2. Assumptions for each section
Inoculum production in MTPBRs (PBR). Capital costs for this section include the MTPBR cost provided by specialized producers, with recirculation pumps, air pumps, degassing columns, and chillers. Influent and effluent pumps are not included and were quoted separately. The OPEX of the PBR section is the sum of the yearly cost for electricity and nutrients, based on N and P stoichiometric requirements (considering 90 % utilization efficiency).
Autotrophic microalgae cultivation in HRAPs (RWP). The low-cost design of the raceway ponds includes: concrete and impermeable liner to realize the RWPs, pipes and electric wires, pumps, and paddlewheels. The required amount of concrete (obtained at 120 €·m−3), impermeable lining (7 €·m−2), pipes and wires (100 €·m−1) were calculated based on pond geometries, allowing 3 m of distance among ponds. Electric wires have been assumed with a length of 50 % of pipes [68,82]. One paddlewheel for every pond was considered, with a specific installation cost of approximately 83 k€·unit−1 [68]. The overall cost for the pond systems, including concrete, lining, paddlewheels, pumps, pipes, electric wires resulted in approximately 170 k€·unit−1. The entire RWP section is located under a PVC greenhouse, with an installation cost provided by a commercial offer. The OPEX of the HRAP section include the electric energy consumption and the greenhouse maintenance (covers substitution every 4 years).
Biomass pre-concentration (BPC). The pre-concentration section is realized with DAF units, having a specific installation cost of 29 € per unit of flowrate treated (m3·d−1), as suggested in similar case studies [68,82]. The operational cost for biomass pre-concentration is given by circulation pumps and air insufflation, that is calculated based on the modelled DW concentration and the assumption of 130 kW h·t DW−1 [68].
Heterotrophic microalgae cultivation in fermenters (HET). The capital costs for the heterotrophic section include the cost for fermenters installation, pumps, mixing, and aerations systems [64]. Operational costs include the cost of electricity, sugar-rich IBPs, and water to prepare concentrated solutions. As for sugars, the specific cost for locally available IBPs was 60 €·t−1, and their demand was based on sugar conversion stoichiometry and experimental yields (see Supplementary Data SI.1.2).
Biomass harvesting (BH). The capital expenditures of this section are the installation costs for the pumps and for the microalgae centrifuge, as assumed from previous reports [64,68]. The OPEX of the section are represented by the electricity consumption for pumping and centrifuging.
2.3.3. Sensitivity and scenario analysis
Based on TEA results, relevant parameters having an impact on the final biomass production cost were identified, and their sensitivity was assessed based on ± 20 % cost variation. As detailed below, two further scenarios were coupled to the baseline one: a best-case and a worst-case scenario, in which all sensitive parameters were varied together, resulting in positive and negative additive impacts, respectively. This approach allows identifying reliable minimum-maximum range estimates, and has been used to assess uncertain biomass production costs in microalgae systems [21,80].
3. Results
3.1. Design results, modelling and integrated assessment
A complete scheme of the proposed biorefinery layout is presented in Fig. 2. The flowrates and the mass flows for the most relevant parameters (i.e., nitrogen, phosphorus, COD, and TSS) are summarized as Sankey diagrams in Fig. 3A–E.
Fig. 2.
Layout of the microalgae-based biorefinery plant designed in this study.
Fig. 3.
Sankey diagrams representing relevant mass balances for the proposed biorefinery concept. Flowrates (A) and mass flows of nitrogen (B), phosphorus (C), COD (D), and TSS (E). The nomenclature for nodes and links can be found in Fig. 2.
The inoculum production takes place in a large installation of MTPBRs (124 modules, length: 142 m each, total volume: 58 m3), that allows maintaining controlled conditions and producing a pure cultures with no external contamination. The PBR provides a constant inflow to the RWP of 1,910 m3·y−1, at the design concentration of 4 g DW·L−1 (resulting in a biomass production of 7.6 t DW·y−1). To do so, the MTPBR uses 0.7 t·y−1 of agricultural fertilizers and receives 40.8 t CO2·y−1 from biogas upgrading in AD. The water consumption of this section covers the entire flowrate entering the PBR. The PBR section accounts for the largest fraction of the overall energy consumption of the plant, with 506 MWh·y−1, mainly due to the temperature control and circulation pump systems. Large-scale autotrophic growth in open ponds (2.37 ha of cultivation area with 10 large-scale ponds, with a length of 154 m, a width of 15 m, and a total volume of 4,750 m3) allows achieving a massive production of microalgae. According to model-based simulations, the average expected RWP production is 76 t DW·y−1, in line with previous case studies [21,22,54,87]. Moreover, the development of undesired bacteria in the system is limited, thanks to the recalcitrant nature of organics in the digestate, that is composed of unbiodegradable COD for approximately 60 % [88,89]. In this section, nutrient removal efficiencies based on model simulations are: 80 % for TAN, 65 % for PO43−, and 15 % for soluble COD. The system is fed with 408 t CO2·y−1 from AD, which are transferred to the system and utilized with 35 % efficiency. The HRAP system is responsible for approximately 146 MWh·y−1, mostly represented by the paddlewheels and greenhouse ventilation energy. The HRAP effluent is concentrated via flocculant-assisted S/L separation realized by 5 DAF units (each with 16 m3 volume), concentrating the biomass up to 5 g DW·L−1. In the DAF process, 4.7 t·y−1 of Al2(SO4)3 are assumed to be dosed for efficient clarification. The energy request for the DAF is estimated as 52.6 MWh·y−1, mostly due to the aeration. The last heterotrophic biomass production stage (HET) is aimed at further increasing the biomass concentration and the yearly production of the biorefinery, thus reducing the overall biomass production cost (see also Section 3.3). The heterotrophic stage (carried out in 4 fermenters with a height of 3.5 m and a diameter of 8.0 m, for a total volume of 707 m3) was designed to produce 168 t·y−1 of biomass utilizing the nutrients (N and P) contained in the LFD and the organic matter contained in IBPs, thus promoting their recovery as valuable agricultural products.
This approach allows to divert 336 t·y−1 of industrial waste from disposal and to reduce the loads of nutrients associated to 20.7 kt·y−1 of digestate from field spreading or further treatment. On the other hand, CO2 production from the heterotrophic metabolism reaches up to 316 t CO2·y−1, thus reducing the net amount of CO2 captured from the plant. The energy consumption in HET, mainly due to aeration and mixing of the algal suspension, can reach up to 325 MWh·y−1, thus representing one of the most relevant OPEX for the plant. As demonstrated by model simulations and experimental results, feeding the heterotrophic reactors with pre-concentrated, autotrophically-grown biomass, limits the growth of heterotrophic bacteria, and thus their relative concentration in the harvested biomass. The algal biomass produced in the HET section is finally delivered to a centrifuge for biomass harvesting, producing an algal paste at 20 % ww of solids. This unit is energy-intensive, as demonstrated by the consumption of 65.1 MWh·y−1, and strongly contributes to the operational expenditure of the biorefinery (see also Section 3.3), as suggested in literature [90,91].
To produce biostimulants, the biomass stream must be further dried and post-processed via enzymatic hydrolysis and solvent extraction to obtain algal extracts. Although process design criteria and cost evaluations already exist for this post-processing route [92,93], the overall aim of this work was to evaluate the feasibility of producing the algal biomass and to evaluate the related production cost. Thus, the biorefinery battery limit was set to the algal paste production, without evaluating additional costs for downstream processing, nor additional revenues from selling the biomass.
3.2. Techno-economic assessment
The techno-economic assessment was first carried out on the baseline scenario, i.e., for the mass and energy flows depicted in the plant layout of Fig. 2. The results of the economic assessment are summarized in Table 2 and more details are available as Supplementary Data (SI.1.2).
Table 2.
Detail of costs evaluation for the baseline scenario.
| Cost type | Cost item | Best case | Baseline | Worst case | Unit |
|---|---|---|---|---|---|
| OPEX | Electricity | 47 | 59 | 71 | k€·y−1 |
| Water, reagents | 55 | 68 | 82 | k€·y−1 | |
| Labour | 72 | 90 | 108 | k€·y−1 | |
| Maintenance and cleaning | 38 | 38 | 38 | k€·y−1 | |
| Fees | 181 | 181 | 181 | k€·y−1 | |
| Depreciation | 210 | 247 | 283 | k€·y−1 | |
| Other costs | 15 | 15 | 15 | k€·y−1 | |
| Total yearly cost | 618 | 698 | 778 | k€·y−1 | |
| CAPEX | Photobioreactor (PBR) | 880 | 1,100 | 1,320 | k€ |
| Raceway ponds (RWP) | 1,360 | 1,700 | 2,040 | k€ | |
| Heterotrophic cultivation (HET) | 680 | 850 | 1,020 | k€ | |
| Pre-concentration and harvesting (BPC, BH) | 320 | 320 | 320 | k€ | |
| Land | 128 | 160 | 192 | k€ | |
| Other costs | 966 | 966 | 966 | k€ | |
| Total expenditure | 4,334 | 5,096 | 5,858 | k€ | |
| Specific costs | Total biomass production | 252 | 252 | 252 | t DW·y−1 |
| Specific biomass production cost | 2.5 | 2.8 | 3.1 | €· tDW−1 |
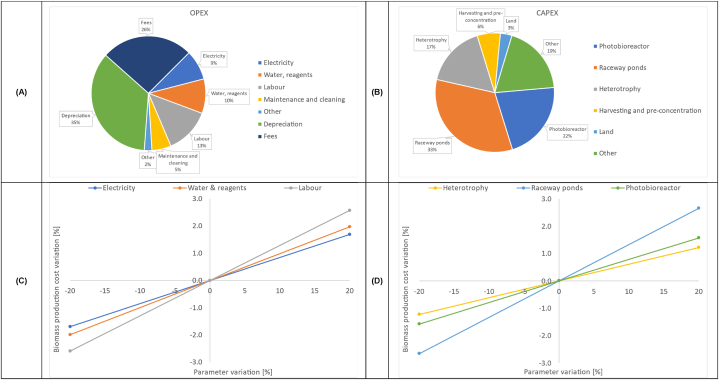
In the baseline scenario, a production cost of 2.8 €·kg DW−1 was calculated, in line with previous TEA of systems fed with wastewaters [82,86] and digestate [21]. Comparing these results with the available literature, a further cost reduction can be expected when designing larger systems, or when treating more suitable wastewaters, in terms of solids and TAN concentrations [18,19,82]. The total CAPEX for the biorefinery is 5.1 M€, with the largest contributors being the raceway ponds (33 %), followed by the photobioreactor units (22 %), heterotrophic fermenters (17 %), with a lower percentage for the biomass pre-concentration and harvesting sections (6 %). The total OPEX for the biorefinery is calculated as 698 k€·y−1, including: i) fixed costs for the investment depreciation (35 %), fees (26 %), and other costs for insurance, security, and administration (2 %); ii) operational costs for electricity (9 %), water and reagents (10 %), labour (13 %), and maintenance and cleaning (5 %). Fixed costs represent the majority of yearly costs, while the remaining OPEX are more evenly distributed. The complete distribution of OPEX and CAPEX for the baseline scenario is reported in Fig. 4A and B, respectively.
Fig. 4.
Cost breakdown and sensitivity analysis results for the baseline scenario. Distribution of OPEX (A) and CAPEX (B); sensitivity of the three most relevant cost voices in terms of OPEX (C) and CAPEX (D) contributions. The term “Other OPEX” include: insurance, security and administration; the term “Other CAPEX” include: instrumentation and control, commissioning and transportation, engineering, and civil works.
When performing the sensitivity analysis, parameters were chosen according to their ranking among OPEX or CAPEX, excluding parameters that cannot be varied (such as administrative costs, depreciation, land and other CAPEX constrained by engineering). The following parameters were thus chosen: i) the cost of electricity, water and reagents, and labour (OPEX); ii) the equipment cost of cultivation systems in the three stages: MTPBRs, HRAPs, and fermenters (CAPEX). As shown in Fig. 4C–D, the variation of these parameters showed a quite similar response on the final production cost, with only a weak improvement (or worsening) of biomass production costs, compared to the baseline scenario. Indeed, relative percent variations of the final production cost always remained within ± 2.5 - 3 % for all parameters, suggesting that other cost items prevail on the overall balance, buffering the effect of selected parameters. This narrows the possibility of effectively reducing the production cost, unless the production size is enlarged, or boundary conditions are optimized (e.g., nutrient assimilation, climatic conditions, etc.). Despite the relatively small contribution of the mentioned parameters, a best-case and a worst-case scenarios were identified by the concurrent variation (± 20 %) of all parameters. So, the biomass production cost ranges from 2.5 to 3.1 €·kg DW−1 (± 12 % variation around the baseline). This range can be considered an accurate and reasonable assessment of the real biomass production cost, by accounting for possible additive effects. It should be stressed that the obtained cost range seems compatible with downstream processing costs for biostimulants production, so that the economic feasibility of the biorefinery process is here demonstrated [92,93]. A detailed breakdown of costs for the three scenarios (best-case, baseline, and worst-case) is given in Table 2.
3.3. Discussion: opportunities, challenges, and further research needs
The proposed process allows for sustainable algal biomass generation, only using waste resources and a minimal input of reagents, water, and energy. The combination of the biomethane plant and the algae-based biorefinery allows to recover nutrients from agricultural feedstocks and industrial by-products, and to produce energy, biofertilizer/compost, algal biomass, and nutrient-depleted water for agricultural reuse. In particular, the range of biomass production costs obtained via techno-economic modelling (2.5 – 3.1 €·kg DW−1) was fairly low and suitable for further post-processing to obtain biostimulants or other bio-products from the algal biomass. Nonetheless, based on the proposed design and modelling results, as well as on experimental results, relevant technological challenges and barriers limiting the spread of algal biorefineries are to be highlighted and discussed. Indeed, in order to increase the reliability and efficiency of the algal biorefinery process, the following aspects are of primary importance.
At the digestate-level: An optimization of digestate composition may be required to maximize nutrient recovery [13,94]. Indeed, several digestate composition parameters may be enhanced, triggering a better algal growth performance. Among the most important factors affecting the growth of microalgae in digestate there are: i) unbalanced ratios among important macro-nutrients (i.e., the C/N and N/P ratios); ii) excessive concentrations of TAN, salts, and other toxic/inhibitory constituents (e.g., metal ions, and organic compounds); and iii) optical properties of the digestate. Several alternatives exist for alleviating these issues [13,20]. To mitigate nutrient unbalances, components present at too high concentrations can be removed (e.g., by stripping excess NH3 or biologically removing the excess COD) or missing nutrients can be recovered from other streams and supplemented (typically, phosphorus from precipitation processes) [95]. With respect to optical properties, dilution represents the easiest solution, allowing to regulate the concentrations of inhibitory compounds and to increase the light transmission within the suspension. However, dilution equally reduces the concentrations of all constituents and does not allow to regulate nutrient ratios. In addition, freshwater consumption poses economic and environmental concerns. Effluent recirculation increasing HRTs can be considered if accumulation of salts and non-reactive inhibitory compounds remains within acceptable ranges [56]. More efficient solutions for colour and solids removal include filtration, adsorption and advanced oxidation processes, still characterized by high OPEX [95]. Within the proposed biorefinery concept, digestate pre-treatment is obtained by flocculation at high flocculant dosages. Despite relatively high separation efficiencies obtained by this technology (see also Section 2.2.1), this option has high operational costs. In addition, flocculant toxicity should be preliminarily assessed, especially with metal-based or organic flocculants, that can result in inhibited growth [71].
At the process-level: To increase the efficiency of microalgal biomass production in wastewater treating systems, several approaches have been attempted [94]. The optimization of environmental conditions (PAR intensity, temperature, pH, DO, and salinity) and of operational pond parameters (HRT, BRT, and liquid depth) according to species-specific optima can certainly play an important role [54,96]. Indeed, by assessing the impacts of these operational conditions at the bench-scale, it is possible to identify the values for the different parameters maximizing the biomass productivity and/or nutrient removal throughout the year [56,63]. Another aspect to be considered for advancing the knowledge on these systems is the use of mechanistic models, helping in the identification of relevant interactions among microalgae and associated wastewater bacteria, also being useful for the identification of “hidden” process rates reducing the overall process efficiency [58,97]. Mathematical modelling of the RWP system with the ALBA model provided extremely useful inputs for the biorefinery design in this work. The biomass production could be investigated under dynamic weather conditions, together with the removal efficiency of HRAPs. The simulation of a greenhouse-pond system with a coupled biological-thermal model allowed to define the most suitable greenhouse configuration (e.g., the number of covers to be installed, or the preferred temperature control strategies) [28]. The adoption of comprehensive mathematical models is thus strongly suggested to evaluate process outputs under a wide variety of operational conditions. The availability of advanced simulation tools may also help in the evaluation of further synergies that can be put in place within the biorefinery. For example, microalgae cultivation under mixotrophic conditions has been suggested as a way to increase the biomass concentration and productivity compared to other cultivation modes [98]. Using a validated growth model for comparing the expected productivity under different metabolic pathways would allow to assess different scenarios and to identify the optimal plant configuration reaching the technical and economic feasibility. Another interesting synergy for microalgae integration in biogas plants is the use of microalgal cultures to directly perform photo-biological biogas upgrading, that has been proposed as sustainable solution for integrated wastewater treatment, energy generation, and biomass production [27]. Last but not least, a thorough analysis of the expected environmental impacts is still lacking in the technical literature, with only a few works recently trying to draft the life-cycle assessment (LCA) of microalgae cultivation plants fed on wastewaters [12,99]. As emerged from the preliminary analysis of carbon fluxes in the proposed biorefinery, the net fixation of CO2 by the plant (occurring during the autotrophic stages) can be hindered by the release of CO2 during the heterotrophic metabolism. However, quantifying these contributions without proper LCA and plant-wide process models is not straightforward, this representing a relevant research need.
At the biomass-level: as suggested in previous studies, there is a large potential for increasing the biomass productivity in outdoor systems, by directly optimizing the algal metabolic activities [40]. Among advanced metabolomic tools, a large room for improvement is given by the selection of proper microalgae strains. For example, in this work it was demonstrated that green microalgal strains of the species Chlorella and Scenedesmus have the ability of growing on wastewater resources and releasing useful substances for biostimulants production. A large screening of microalgae strains should be thus conducted to search for other strains that can adapt to wastewaters, having more suitable characteristics for their valorisation compared to the strains tested in this study. For example, the use of extremophilic microalgae can help maintaining low contamination levels for pathogenic and other environmental bacteria. In this perspective, temperature and pH extremophiles have been proposed for the use in environmental applications [100,101], which however have to be carefully evaluated on a case-by-case basis, in light of their practical applicability (reagent requests, needs for further effluent post-treatment, environmental impacts arising from the operational conditions, e.g. NH3 stripping under alkaline pH or high temperatures).
At the bio-product level: Different factors should be considered and investigated, related to the target bio-products quality and requirements. First, a certain variability of the product characteristics must be foreseen and evaluated [45,102]. In particular, the optimization and standardization of both microalgae cultivation and downstream processing should be carried out, aimed at reducing the high variability in the product characteristics [102]. Within this topic, further actions may be needed to reduce the contamination risks, especially where the generated bio-product enters in contact with edible plant parts [84].
At the policy-level: When referring to algal cultivation on waste-recovered nutrients, several concerns have been raised, related to the safety of bio-products obtained from a wastewater-grown biomass. Indeed, a regulatory framework is still missing in this perspective, that would help defining the right nutrient valorisation pathways. Notably, the recent EU legislation (2019/1009) on biofertilizers and biostimulants gave precise indications about the level of pathogenic bacteria for agricultural products derived from microalgae grown on wastewater. However, other interesting valorisation routes are not specifically dealt with by regulatory authorities, making it difficult to assess whether a designed process may generate products that can effectively enter the market. Another strategy for policy makers to foster algae-based biorefineries comes from economic incentives, such as from carbon credits or from beneficial nutrient recovery [21,103].
4. Conclusions
A biorefinery process based on the integration of microalgae cultivation in biogas/biomethane plants was proposed. A full-scale plant was sized, based on innovative three-stage microalgae cultivation: a PBR inoculation phase (58 m3), a large-scale raceway pond system (2.4 ha, 4,750 m3), and a final heterotrophic stage in fermenters (707 m3). Within the process, 252 t·y−1 of algal biomass are produced, treating almost 100 kt·y−1 of digestate liquid fraction (i.e., approximately 50 kt·y−1 of raw liquid digestate), 400 t·y−1 of industrial by-products, and 474 t CO2·y−1 (of which 170 t·y−1 are effectively taken up in autotrophy). Process design was carried out with established technical and engineering criteria, using parameters validated at pilot-scale. The plant design was aided by mathematical modelling of raceway ponds, on which local weather conditions have the strongest impact. Dynamic modelling of microalgae growth in raceways was coupled to a thermal model predicting the evolution of temperature under a greenhouse to find optimal temperature control strategies. The assessment of uncertain parameters allowed to validate the use of waste resources (digestate and industrial by-products), and to reinforce preliminary design assumptions. By combining process design and modelling outputs, a comprehensive techno-economic assessment of the biorefinery was implemented, and a biomass production cost of 2.8 €·kg DW−1 was calculated. By performing a sensitivity analysis on the most relevant OPEX and CAPEX, a more accurate estimate for the biomass production cost lays in the range 2.5 – 3.1 €·kg DW−1, that is appealing for the valorisation of waste nutrients as biostimulants or other products.
CRediT authorship contribution statement
Simone Rossi: Writing - review & editing, Writing - original draft, Visualization, Validation, Supervision, Software, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Davide Carecci: Writing - review & editing, Validation, Software, Methodology, Investigation, Data curation, Conceptualization. Francesca Marazzi: Writing - review & editing, Writing - original draft, Validation, Supervision, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Francesca Di Benedetto: Writing - review & editing, Writing - original draft, Validation. Valeria Mezzanotte: Writing - review & editing, Validation, Supervision, Resources, Project administration, Methodology, Funding acquisition, Formal analysis, Conceptualization. Katia Parati: Writing - review & editing, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Conceptualization. Davide Alberti: Validation, Resources, Project administration, Funding acquisition. Ignazio Geraci: Validation, Supervision, Resources, Project administration, Methodology, Funding acquisition, Conceptualization. Elena Ficara: Writing - review & editing, Validation, Supervision, Software, Resources, Project administration, Methodology, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:SR, DC, FM, FdB, VM, KP, and EF report financial support was provided by A2A S. p.A.
Acknowledgements
This research was funded by A2A S.p.A and the European Union (National Recovery and Resilience Plan - PNRR, ECOSISTER project, Ecosistema Territoriale di Innovazione dell’Emilia-Romagna). Dr. Stefania Patronelli (A2A S.p.A.) is kindly acknowledged for the help in reviewing the manuscript. Dr. Luciano Foglio and Dr. Lorenzo Proietti (Istituto Sperimentale Italiano Lazzaro Spallanzani), and Dr. Daniel Andres Antolin (University of Valladolid) are kindly acknowledged for their valuable help in laboratory activities..
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e23240.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.EBA . European Biogas Association; 2022. Short-, Mid- and Long-Term Strategies to Speed up Biomethane Deployment in Europe. [Google Scholar]
- 2.EBA . European Biogas Association; 2022. EBA Statistical Report 2022 - Tracking Biogas and Biomethane Deployment across Europe.https://www.europeanbiogas.eu/wp-content/uploads/2022/12/EBA-Statistical-Report-2022_-Short-version.pdf [Google Scholar]
- 3.Drosg B., Fuchs W., Al Seadi T., Madsen M., Linke B. IEA Bioenergy; 2015. Nutrient Recovery by Biogas Digestate Processing | Bioenergy.https://www.ieabioenergy.com/blog/publications/nutrient-recovery-by-biogas-digestate-processing/ [Google Scholar]
- 4.Reuland G., Sigurnjak I., Dekker H., Michels E., Meers E. The potential of digestate and the liquid fraction of digestate as chemical fertiliser substitutes under the RENURE criteria. Agronomy. 2021;11(7):1374. doi: 10.3390/agronomy11071374. [DOI] [Google Scholar]
- 5.Guilayn F., Jimenez J., Monlau F., Vaneeckhaute C. In: Renewable Energy Technologies for Energy Efficient Sustainable Development. Sinharoy A., Lens P.N.L., editors. Springer International Publishing; Cham: 2022. Valorisation of anaerobic digestate: towards value-added products; pp. 227–262. (Applied Environmental Science and Engineering for a Sustainable Future). [DOI] [Google Scholar]
- 6.Kovačić Đ., Lončarić Z., Jović J., Samac D., Popović B., Tišma M. Digestate management and processing practices: a review. Appl. Sci. 2022;12(18):9216. doi: 10.3390/app12189216. [DOI] [Google Scholar]
- 7.ESPP . European Sustainable Phosphorus Platform; 2023. ESPP – DPP – NNP Nutrient Recovery Technology Catalogue; pp. 1–30.https://www.phosphorusplatform.eu/images/download/ESPP-NNP-DPP_nutrient-recovery_tech_catalogue.pdf [Google Scholar]
- 8.Verbeke M., van Dijk K., Brienza C. Deliverable WP3, D.3.2; Systemic project; 2021. (Scenario's and Schemes of Proven Nutrient Recovery and Reuse Techniques). [Google Scholar]
- 9.Lamolinara B., Pérez-Martínez A., Guardado-Yordi E., Guillén Fiallos C., Diéguez-Santana K., Ruiz-Mercado G.J. Anaerobic digestate management, environmental impacts, and techno-economic challenges. Waste Manag. 2022;140:14–30. doi: 10.1016/j.wasman.2021.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vondra M., Touš M., Teng S.Y. Digestate evaporation treatment in biogas plants: a techno-economic assessment by Monte Carlo, neural networks and decision trees. J. Clean. Prod. 2019;238 doi: 10.1016/j.jclepro.2019.117870. [DOI] [Google Scholar]
- 11.Duan N., Khoshnevisan B., Lin C., Liu Z., Liu H. Life cycle assessment of anaerobic digestion of pig manure coupled with different digestate treatment technologies. Environ. Int. 2020;137 doi: 10.1016/j.envint.2020.105522. [DOI] [PubMed] [Google Scholar]
- 12.Tua C., Ficara E., Mezzanotte V., Rigamonti L. Integration of a side-stream microalgae process into a municipal wastewater treatment plant: a life cycle analysis. J. Environ. Manag. 2021;279 doi: 10.1016/j.jenvman.2020.111605. [DOI] [PubMed] [Google Scholar]
- 13.Bauer L., Ranglová K., Masojídek J., Drosg B., Meixner K. Digestate as sustainable nutrient source for microalgae—challenges and prospects. Appl. Sci. 2021;11(3):1056. doi: 10.3390/app11031056. [DOI] [Google Scholar]
- 14.Behera B., Selvam S M., Paramasivan B. Research trends and market opportunities of microalgal biorefinery technologies from circular bioeconomy perspectives. Bioresour. Technol. 2022;351 doi: 10.1016/j.biortech.2022.127038. [DOI] [PubMed] [Google Scholar]
- 15.Catone C.M., Ripa M., Geremia E., Ulgiati S. Bio-products from algae-based biorefinery on wastewater: a review. J. Environ. Manag. 2021;293 doi: 10.1016/j.jenvman.2021.112792. [DOI] [PubMed] [Google Scholar]
- 16.Gouveia L., Graça S., Sousa C., Ambrosano L., Ribeiro B., Botrel E.P., Neto P.C., Ferreira A.F., Silva C.M. Microalgae biomass production using wastewater: treatment and costs: scale-up considerations. Algal Res. 2016;16:167–176. doi: 10.1016/j.algal.2016.03.010. [DOI] [Google Scholar]
- 17.Rezvani S., Moheimani N.R., Bahri P.A. Techno-economic assessment of CO2 bio-fixation using microalgae in connection with three different state-of-the-art power plants. Comput. Chem. Eng. 2016;84:290–301. doi: 10.1016/j.compchemeng.2015.09.001. [DOI] [Google Scholar]
- 18.Al Ketife A.M.D., Almomani F., El-Naas M., Judd S. A technoeconomic assessment of microalgal culture technology implementation for combined wastewater treatment and CO2 mitigation in the arabian gulf. Process Saf. Environ. Protect. 2019;127:90–102. doi: 10.1016/j.psep.2019.05.003. [DOI] [Google Scholar]
- 19.Chaudry S. Integrating microalgae cultivation with wastewater treatment: a peek into economics. Appl. Biochem. Biotechnol. 2021;193(10):3395–3406. doi: 10.1007/s12010-021-03612-x. [DOI] [PubMed] [Google Scholar]
- 20.Chong C.C., Cheng Y.W., Ishak S., Lam M.K., Lim J.W., Tan I.S., Show P.L., Lee K.T. Anaerobic digestate as a low-cost nutrient source for sustainable microalgae cultivation: a way forward through waste valorization approach. Sci. Total Environ. 2022;803 doi: 10.1016/j.scitotenv.2021.150070. [DOI] [PubMed] [Google Scholar]
- 21.Rossi S., Mantovani M., Marazzi F., Bellucci M., Casagli F., Mezzanotte V., Ficara E. Microalgal cultivation on digestate: process efficiency and economics. Chem. Eng. J. 2023;460 doi: 10.1016/j.cej.2023.141753. [DOI] [Google Scholar]
- 22.Mantovani M., Marazzi F., Fornaroli R., Bellucci M., Ficara E., Mezzanotte V. Outdoor pilot-scale raceway as a microalgae-bacteria sidestream treatment in a WWTP. Sci. Total Environ. 2020;710 doi: 10.1016/j.scitotenv.2019.135583. [DOI] [PubMed] [Google Scholar]
- 23.Ayre J.M., Moheimani N.R., Borowitzka M.A. Growth of microalgae on undiluted anaerobic digestate of piggery effluent with high ammonium concentrations. Algal Res. 2017;24:218–226. doi: 10.1016/j.algal.2017.03.023. [DOI] [Google Scholar]
- 24.Marazzi F., Bellucci M., Rossi S., Fornaroli R., Ficara E., Mezzanotte V. Outdoor pilot trial integrating a sidestream microalgae process for the treatment of centrate under non optimal climate conditions. Algal Res. 2019;39 doi: 10.1016/j.algal.2019.101430. [DOI] [Google Scholar]
- 25.Alburquerque J.A., de la Fuente C., Campoy M., Carrasco L., Nájera I., Baixauli C., Caravaca F., Roldán A., Cegarra J., Bernal M.P. Agricultural use of digestate for horticultural crop production and improvement of soil properties. Eur. J. Agron. 2012;43:119–128. doi: 10.1016/j.eja.2012.06.001. [DOI] [Google Scholar]
- 26.Andriani D., Wresta A., Atmaja T.D., Saepudin A. A review on optimization production and upgrading biogas through CO2 removal using various techniques. Appl. Biochem. Biotechnol. 2014;172(4):1909–1928. doi: 10.1007/s12010-013-0652-x. [DOI] [PubMed] [Google Scholar]
- 27.Muñoz, Meier L., Diaz I., Jeison D. A review on the state-of-the-art of physical/chemical and biological technologies for biogas upgrading. Rev. Environ. Sci. Biotechnol. 2015;14(4):727–759. doi: 10.1007/s11157-015-9379-1. [DOI] [Google Scholar]
- 28.Rossi S., Carecci D., Ficara E. Thermal response analysis and compilation of cardinal temperatures for 424 strains of microalgae, cyanobacteria, diatoms and other species. Sci. Total Environ. 2023;873 doi: 10.1016/j.scitotenv.2023.162275. [DOI] [PubMed] [Google Scholar]
- 29.Song M., Pei H. The growth and lipid accumulation of Scenedesmus quadricauda during batch mixotrophic/heterotrophic cultivation using xylose as a carbon source. Bioresour. Technol. 2018;263:525–531. doi: 10.1016/j.biortech.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 30.Agwa O., Ibi S.N., Abu G. Heterotrophic cultivation of Chlorella sp. Using different waste extracts. Int J Biochem Biotech. 2013;2:289–297. [Google Scholar]
- 31.de Mattos L.F.A., Bastos R.G. COD and nitrogen removal from sugarcane vinasse by heterotrophic green algae desmodesmus sp. Desalination Water Treat. 2016;57(20):9465–9473. doi: 10.1080/19443994.2015.1028454. [DOI] [Google Scholar]
- 32.Ende S.S.W., Noke A. Heterotrophic microalgae production on food waste and by-products. J. Appl. Phycol. 2019;31(3):1565–1571. doi: 10.1007/s10811-018-1697-6. [DOI] [Google Scholar]
- 33.Russo G.L., Langellotti A.L., Sacchi R., Masi P. Techno-economic assessment of DHA-rich aurantiochytrium sp. Production using food industry by-products and waste streams as alternative growth media. Bioresour. Technol. Rep. 2022 doi: 10.1016/j.biteb.2022.100997. [DOI] [Google Scholar]
- 34.Olguín E.J., Sánchez-Galván G., Arias-Olguín I.I., Melo F.J., González-Portela R.E., Cruz L., De Philippis R., Adessi A. Microalgae-based biorefineries: challenges and future trends to produce carbohydrate enriched biomass, high-added value products and bioactive compounds. Biology. 2022;11(8):1146. doi: 10.3390/biology11081146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ward A.J., Lewis D.M., Green F.B. Anaerobic digestion of algae biomass: a review. Algal Res. 2014;5:204–214. doi: 10.1016/j.algal.2014.02.001. [DOI] [Google Scholar]
- 36.Passos F., Uggetti E., Carrère H., Ferrer I. Pretreatment of microalgae to improve biogas production: a review. Bioresour. Technol. 2014;172:403–412. doi: 10.1016/j.biortech.2014.08.114. [DOI] [PubMed] [Google Scholar]
- 37.Barone V., Baglieri A., Stevanato P., Broccanello C., Bertoldo G., Bertaggia M., Cagnin M., Pizzeghello D., Moliterni V.M.C., Mandolino G., Fornasier F., Squartini A., Nardi S., Concheri G. Root morphological and molecular responses induced by microalgae extracts in sugar beet (beta vulgaris L.) J. Appl. Phycol. 2018;30(2):1061–1071. doi: 10.1007/s10811-017-1283-3. [DOI] [Google Scholar]
- 38.Craigie J.S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011;23(3):371–393. doi: 10.1007/s10811-010-9560-4. [DOI] [Google Scholar]
- 39.Michalak I., Chojnacka K., Dmytryk A., Wilk R., Gramza M., Rój E. Evaluation of supercritical extracts of algae as biostimulants of plant growth in field trials. Front. Plant Sci. 2016;7 doi: 10.3389/fpls.2016.01591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kapoore R.V., Wood E.E., Llewellyn C.A. Algae biostimulants: a critical look at microalgal biostimulants for sustainable agricultural practices. Biotechnol. Adv. 2021;49 doi: 10.1016/j.biotechadv.2021.107754. [DOI] [PubMed] [Google Scholar]
- 41.Morillas-España A., Lafarga T., Sánchez-Zurano A., Acién-Fernández F.G., González-López C. Microalgae based wastewater treatment coupled to the production of high value agricultural products: current needs and challenges. Chemosphere. 2022;291 doi: 10.1016/j.chemosphere.2021.132968. [DOI] [PubMed] [Google Scholar]
- 42.Rumin J., Nicolau E., Gonçalves de Oliveira Junior R., Fuentes-Grünewald C., Flynn K.J., Picot L. A bibliometric analysis of microalgae research in the world, Europe, and the European atlantic area. Mar. Drugs. 2020;18(2):79. doi: 10.3390/md18020079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stiles W.A.V., Styles D., Chapman S.P., Esteves S., Bywater A., Melville L., Silkina A., Lupatsch I., Fuentes Grünewald C., Lovitt R., Chaloner T., Bull A., Morris C., Llewellyn C.A. Using microalgae in the circular economy to valorise anaerobic digestate: challenges and opportunities. Bioresour. Technol. 2018;267:732–742. doi: 10.1016/j.biortech.2018.07.100. [DOI] [PubMed] [Google Scholar]
- 44.El-Naggar A.H., Osman M.E.H.E.-S., Gheda S.F. Influence of the aqueous extracts of ulva lactuca and Chlorella kessleri on growth and yield of vicia faba. Algol. Stud. Für Hydrobiol. Suppl. 2005:213–229. doi: 10.1127/1864-1318/2005/0116-0213. [DOI] [Google Scholar]
- 45.Ronga D., Biazzi E., Parati K., Carminati D., Carminati E., Tava A. Microalgal biostimulants and biofertilisers in crop productions. Agronomy. 2019;9(4):192. doi: 10.3390/agronomy9040192. [DOI] [Google Scholar]
- 46.Abd El-Baky H.H., El-Baz F.K., El Baroty G.S. Enhancing antioxidant availability in wheat grains from plants grown under seawater stress in response to microalgae extract treatments. J. Sci. Food Agric. 2010;90(2):299–303. doi: 10.1002/jsfa.3815. [DOI] [PubMed] [Google Scholar]
- 47.Garcia-Gonzalez J., Sommerfeld M. Biofertilizer and biostimulant properties of the microalga acutodesmus dimorphus. J. Appl. Phycol. 2016;28(2):1051–1061. doi: 10.1007/s10811-015-0625-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y., Xu S.-S., Gao J., Pan S., Wang G.-X. Chlorella triggers stomatal closure mediated by NADPH oxidase and improves instantaneous water use efficiency in vicia faba. Plant Signal. Behav. 2014;9 doi: 10.4161/psb.29078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shaaban M.M. Asian Network for Scientific Information; Pakistan: 2001. Nutritional Status and Growth of Maize Plants as Affected by Green Microalgae as Soil Additives.http://www.scialert.net/pdfs/jbs/2001/475-479.pdf [Google Scholar]
- 50.Mohsenpour S.F., Hennige S., Willoughby N., Adeloye A., Gutierrez T. Integrating micro-algae into wastewater treatment: a review. Sci. Total Environ. 2021;752 doi: 10.1016/j.scitotenv.2020.142168. [DOI] [PubMed] [Google Scholar]
- 51.Acién F.G., Molina E., Reis A., Torzillo G., Zittelli G.C., Sepúlveda C., Masojídek J. In: Microalgae-Based Biofuels and Bioproducts. Gonzalez-Fernandez C., Muñoz R., editors. Woodhead Publishing Series in Energy; Woodhead Publishing; 2017. 1 - photobioreactors for the production of microalgae; pp. 1–44. [DOI] [Google Scholar]
- 52.Chisti Y. Raceways-based production of algal crude oil. Greenpeace. 2013;3(3–4):195–216. doi: 10.1515/green-2013-0018. [DOI] [Google Scholar]
- 53.Hoffman J., Pate R.C., Drennen T., Quinn J.C. Techno-economic assessment of open microalgae production systems. Algal Res. 2017;23:51–57. doi: 10.1016/j.algal.2017.01.005. [DOI] [Google Scholar]
- 54.Casagli F., Rossi S., Steyer J.P., Bernard O., Ficara E. Balancing microalgae and nitrifiers for wastewater treatment: can inorganic carbon limitation cause an environmental threat? Environ. Sci. Technol. 2021;55(6):3940–3955. doi: 10.1021/acs.est.0c05264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Costache T.A., Acién Fernández F.G., Morales M.M., Fernández-Sevilla J.M., Stamatin I., Molina E. Comprehensive model of microalgae photosynthesis rate as a function of culture conditions in photobioreactors. Appl. Microbiol. Biotechnol. 2013;97(17):7627–7637. doi: 10.1007/s00253-013-5035-2. [DOI] [PubMed] [Google Scholar]
- 56.Casagli F., Beline F., Ficara E., Bernard O. Optimizing resource recovery from wastewater with algae-bacteria membrane reactors. Chem. Eng. J. 2023;451 doi: 10.1016/j.cej.2022.138488. [DOI] [Google Scholar]
- 57.Peel M.C., Finlayson B.L., McMahon T.A. Updated world map of the köppen-geiger climate classification. Hydrol. Earth Syst. Sci. 2007;11(5):1633–1644. doi: 10.5194/hess-11-1633-2007. [DOI] [Google Scholar]
- 58.Casagli F., Zuccaro G., Bernard O., Steyer J.-P., Ficara E.A.L.B.A. A comprehensive growth model to optimize algae-bacteria wastewater treatment in raceway ponds. Water Res. 2021;190 doi: 10.1016/j.watres.2020.116734. [DOI] [PubMed] [Google Scholar]
- 59.Li S., Willits D.H., Browdy C.L., Timmons M.B., Losordo T.M. Thermal modeling of greenhouse aquaculture raceway systems. Aquacult. Eng. 2009;41(1):1–13. doi: 10.1016/j.aquaeng.2009.04.002. [DOI] [Google Scholar]
- 60.Bernard O., Rémond B. Validation of a simple model accounting for light and temperature effect on microalgal growth. Bioresour. Technol. 2012;123:520–527. doi: 10.1016/j.biortech.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 61.Rosso L., Lobry J.R., Flandrois J.P. An unexpected correlation between cardinal temperatures of microbial growth highlighted by a new model. J. Theor. Biol. 1993;162(4):447–463. doi: 10.1006/jtbi.1993.1099. [DOI] [PubMed] [Google Scholar]
- 62.Rosso L., Lobry J.R., Bajard S., Flandrois J.P. Convenient model to describe the combined effects of temperature and pH on microbial growth. Appl. Environ. Microbiol. 1995;61(2):610–616. doi: 10.1128/aem.61.2.610-616.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rossi S., Casagli F., Mantovani M., Mezzanotte V., Ficara E. Selection of photosynthesis and respiration models to assess the effect of environmental conditions on mixed microalgae consortia grown on wastewater. Bioresour. Technol. 2020;305 doi: 10.1016/j.biortech.2020.122995. [DOI] [PubMed] [Google Scholar]
- 64.Norsker N.-H., Barbosa M.J., Vermuë M.H., Wijffels R.H. Microalgal production - a close look at the economics. Biotechnol. Adv. 2011;29(1):24–27. doi: 10.1016/j.biotechadv.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 65.Kazbar A., Cogne G., Urbain B., Marec H., Le-Gouic B., Tallec J., Takache H., Ismail A., Pruvost J. Effect of dissolved oxygen concentration on microalgal culture in photobioreactors. Algal Res. 2019;39 doi: 10.1016/j.algal.2019.101432. [DOI] [Google Scholar]
- 66.Sforza E., Pastore M., Franke S.M., Barbera E. Modeling the oxygen inhibition in microalgae: an experimental approach based on photorespirometry. N. Biotech. 2020;59:26–32. doi: 10.1016/j.nbt.2020.06.003. [DOI] [PubMed] [Google Scholar]
- 67.Clippinger J., Davis R. 2019. Techno-Economic Analysis for the Production of Algal Biomass via Closed Photobioreactors: Future Cost Potential Evaluated across a Range of Cultivation System Designs. NREL/TP--5100-72716. NREL/TP--5100-72716, 1566806. [DOI] [Google Scholar]
- 68.Davis R., Markham J., Kinchin C., Grundl N., Tan E.C.D., Humbird D. U.S. Department of Energy Office of Energy Efficiency & Renewable Energy; 2016. Process Design and Economics for the Production of Algal Biomass: Algal Biomass Production in Open Pond Systems and Processing through Dewatering for Downstream Conversion. NREL/TP--5100-64772. [DOI] [Google Scholar]
- 69.Casagli F., Bernard O. Simulating biotechnological processes affected by meteorology: application to algae-bacteria systems. J. Clean. Prod. 2022 doi: 10.1016/j.jclepro.2022.134190. [DOI] [Google Scholar]
- 70.Rodríguez-Miranda E., Guzmán J.L., Acién F.G., Berenguel M., Visioli A. Indirect regulation of temperature in raceway reactors by optimal management of culture depth. Biotechnol. Bioeng. 2021;118(3):1186–1198. doi: 10.1002/bit.27642. [DOI] [PubMed] [Google Scholar]
- 71.Rossi S., Visigalli S., Castillo Cascino F., Mantovani M., Mezzanotte V., Parati K., Canziani R., Turolla A., Ficara E. Metal-based flocculation to harvest microalgae: a look beyond separation efficiency. Sci. Total Environ. 2021;799 doi: 10.1016/j.scitotenv.2021.149395. [DOI] [PubMed] [Google Scholar]
- 72.Perez-Garcia O., De-Bashan L.E., Hernandez J.-P., Bashan Y. Efficiency of growth and nutrient uptake from wastewater by heterotrophic, autotrophic, and mixotrophic cultivation of Chlorella vulgaris immobilized with azospirillum Brasilense1. J. Phycol. 2010;46(4):800–812. doi: 10.1111/j.1529-8817.2010.00862.x. [DOI] [Google Scholar]
- 73.Schediwy K., Trautmann A., Steinweg C., Posten C. Microalgal kinetics — a guideline for photobioreactor design and process development. Eng. Life Sci. 2019;19(12):830–843. doi: 10.1002/elsc.201900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chojnacka K. Kinetic and stoichiometric relationships of the energy and carbonmetabolism in the culture of microalgae. Biotechnology. 2004;3(1):21–34. [Google Scholar]
- 75.Yamane Y., Higashida K., Nakashimada Y., Kakizono T., Nishio N. Influence of oxygen and glucose on primary metabolism and astaxanthin production by phaffia rhodozyma in batch and fed-batch cultures: kinetic and stoichiometric analysis. Appl. Environ. Microbiol. 1997;63(11):4471–4478. doi: 10.1128/aem.63.11.4471-4478.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Metcalf Eddy. fifth ed. McGraw-Hill Education; New York, NY: 2014. Wastewater Engineering: Treatment and Resource Recovery. [Google Scholar]
- 77.Pessi B.A., Pruvost E., Talec A., Sciandra A., Bernard O. Does temperature shift justify microalgae production under greenhouse? Algal Res. 2022;61 doi: 10.1016/j.algal.2021.102579. [DOI] [Google Scholar]
- 78.Clippinger J., Davis R. 2021. Techno-Economic Assessment for Opportunities to Integrate Algae Farming with Wastewater Treatment; NREL/TP-5100-75237. MainId:7135. p NREL/TP-5100-75237, 1821631, MainId:7135. [DOI] [Google Scholar]
- 79.Leow S., Shoener B.D., Li Y., DeBellis J.L., Markham J., Davis R., Laurens L.M.L., Pienkos P.T., Cook S.M., Strathmann T.J., Guest J.S. A unified modeling framework to advance biofuel production from microalgae. Environ. Sci. Technol. 2018;52(22):13591–13599. doi: 10.1021/acs.est.8b03663. [DOI] [PubMed] [Google Scholar]
- 80.Ruiz J., Olivieri G., Vree J. de, Bosma R., Willems P., Reith J.H., Eppink M.H.M., Kleinegris D.M.M., Wijffels R.H., Barbosa M.J. Towards industrial products from microalgae. Energy Environ. Sci. 2016;9(10):3036–3043. doi: 10.1039/C6EE01493C. [DOI] [Google Scholar]
- 81.Ruiz J., Wijffels R.H., Dominguez M., Barbosa M.J. Heterotrophic vs autotrophic production of microalgae: bringing some light into the everlasting cost controversy. Algal Res. 2022;64 doi: 10.1016/j.algal.2022.102698. [DOI] [Google Scholar]
- 82.Rossi S., Pizzera A., Bellucci M., Marazzi F., Mezzanotte V., Parati K., Ficara E. Piggery wastewater treatment with algae-bacteria consortia: pilot-scale validation and techno-economic evaluation at farm level. Bioresour. Technol. 2022;351 doi: 10.1016/j.biortech.2022.127051. [DOI] [PubMed] [Google Scholar]
- 83.Eurostat Agricultural land prices by region. https://appsso.eurostat.ec.europa.eu/nui/submitViewTableAction.do
- 84.Acién F.G., Fernández J.M., Magán J.J., Molina E. Production cost of a real microalgae production plant and strategies to reduce it. Biotechnol. Adv. 2012;30(6):1344–1353. doi: 10.1016/j.biotechadv.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 85.Green D.W., Southard M.Z. ninth ed. McGraw-Hill Education; 2019. Perry's Chemical Engineers' Handbook. [Google Scholar]
- 86.Lee J.-C., Lee B., Kim H.-W., Jeon B.-H., Lim H. Techno-economic analysis of livestock urine and manure as a microalgal growth medium. Waste Manag. 2021;135:276–286. doi: 10.1016/j.wasman.2021.09.005. [DOI] [PubMed] [Google Scholar]
- 87.Pizzera A., Scaglione D., Bellucci M., Marazzi F., Mezzanotte V., Parati K., Ficara E. Digestate treatment with algae-bacteria consortia: a field pilot-scale experimentation in a sub-optimal climate area. Bioresour. Technol. 2019;274:232–243. doi: 10.1016/j.biortech.2018.11.067. [DOI] [PubMed] [Google Scholar]
- 88.Akhiar A., Battimelli A., Torrijos M., Carrere H. Comprehensive characterization of the liquid fraction of digestates from full-scale anaerobic Co-digestion. Waste Manag. 2017;59:118–128. doi: 10.1016/j.wasman.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 89.Henze M., Van Loosdrecht M.C.M., Ekama G.A., Brdjanovic D. Modeling and Design; 2020. Biological Wastewater Treatment: Principles. [DOI] [Google Scholar]
- 90.Barros A.I., Gonçalves A.L., Simões M., Pires J.C.M. Harvesting techniques applied to microalgae: a review. Renew. Sustain. Energy Rev. 2015;41:1489–1500. doi: 10.1016/j.rser.2014.09.037. [DOI] [Google Scholar]
- 91.Fasaei F., Bitter J.H., Slegers P.M., van Boxtel A.J.B. Techno-economic evaluation of microalgae harvesting and dewatering systems. Algal Res. 2018;31:347–362. doi: 10.1016/j.algal.2017.11.038. [DOI] [Google Scholar]
- 92.Rojo E.M., Molinos-Senante M., Filipigh A.A., Lafarga T., Fernández F.G.A., Bolado S. Agricultural products from algal biomass grown in piggery wastewater: a techno-economic analysis. Sci. Total Environ. 2023;887 doi: 10.1016/j.scitotenv.2023.164159. [DOI] [PubMed] [Google Scholar]
- 93.Romero-García J.M., González-López C.V., Brindley C., Fernández-Sevilla J.M., Acién-Fernández F.G. Simulation and techno-economical evaluation of a microalgal biofertilizer production process. Biology. 2022;11(9):1359. doi: 10.3390/biology11091359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Christenson L., Sims R. Production and harvesting of microalgae for wastewater treatment, biofuels, and bioproducts. Biotechnol. Adv. 2011;29(6):686–702. doi: 10.1016/j.biotechadv.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 95.Al-Mallahi J., Ishii K. Attempts to alleviate inhibitory factors of anaerobic digestate for enhanced microalgae cultivation and nutrients removal: a review. J. Environ. Manag. 2022;304 doi: 10.1016/j.jenvman.2021.114266. [DOI] [PubMed] [Google Scholar]
- 96.Sánchez Zurano A., Gómez Serrano C., Acién-Fernández F.G., Fernández-Sevilla J.M., Molina-Grima E. Modeling of photosynthesis and respiration rate for microalgae-bacteria consortia. Biotechnol. Bioeng. 2021;118(2):952–962. doi: 10.1002/bit.27625. [DOI] [PubMed] [Google Scholar]
- 97.Sánchez-Zurano A., Rodríguez-Miranda E., Guzmán J.L., Acién-Fernández F.G., Fernández-Sevilla J.M., Molina Grima E. ABACO: a new model of microalgae-bacteria consortia for biological treatment of wastewaters. Appl. Sci. 2021;11(3):998. doi: 10.3390/app11030998. [DOI] [Google Scholar]
- 98.Lowrey J., Brooks M.S., McGinn P.J. Heterotrophic and mixotrophic cultivation of microalgae for biodiesel production in agricultural wastewaters and associated challenges—a critical review. J. Appl. Phycol. 2015;27(4):1485–1498. doi: 10.1007/s10811-014-0459-3. [DOI] [Google Scholar]
- 99.Arashiro L.T., Josa I., Ferrer I., Van Hulle S.W.H., Rousseau D.P.L., Garfí M. Life cycle assessment of microalgae systems for wastewater treatment and bioproducts recovery: natural pigments, biofertilizer and biogas. Sci. Total Environ. 2022;847 doi: 10.1016/j.scitotenv.2022.157615. [DOI] [PubMed] [Google Scholar]
- 100.Malavasi V., Soru S., Cao G. Extremophile microalgae: the potential for biotechnological application. J. Phycol. 2020;56(3):559–573. doi: 10.1111/jpy.12965. [DOI] [PubMed] [Google Scholar]
- 101.Varshney P., Mikulic P., Vonshak A., Beardall J., Wangikar P.P. Extremophilic micro-algae and their potential contribution in biotechnology. Bioresour. Technol. 2015;184:363–372. doi: 10.1016/j.biortech.2014.11.040. [DOI] [PubMed] [Google Scholar]
- 102.Colla G., Rouphael Y. Microalgae: new source of plant biostimulants. Agronomy. 2020;10(9):1240. doi: 10.3390/agronomy10091240. [DOI] [Google Scholar]
- 103.Silva M.I., Gonçalves A.L., Vilar V.J.P., Pires J.C.M. Experimental and techno-economic study on the use of microalgae for paper industry effluents remediation. Sustainability. 2021;13(3):1314. doi: 10.3390/su13031314. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.