Abstract
Objective:
Inflammation plays a critical role in the progression of chronic liver diseases, and diet can modulate inflammation. Whether an inflammatory dietary pattern is associated with higher risk of hepatic steatosis or fibrosis remains unclear. We aimed to investigate the associations between inflammatory dietary pattern and the odds of hepatic steatosis and fibrosis.
Design:
In this nationwide cross-sectional study, diet was measured using two 24-h dietary recalls. Empirical dietary inflammatory pattern (EDIP) score was derived to assess the inflammatory potential of usual diet, which has been validated to highly predict inflammation markers in the study population. Controlled attenuation parameter (CAP) and liver stiffness measurement (LSM) were derived from FibroScan to define steatosis and fibrosis, respectively.
Setting:
US National Health and Nutrition Examination Survey.
Participants:
4171 participants aged ≥18 years.
Results:
A total of 1436 participants were diagnosed with S1 steatosis (CAP ≥ 274 dB/m), 255 with advanced fibrosis (LSM ≥ 9·7 kPa). Compared with those in the lowest tertile of EDIP-adherence scores, participants in the highest tertile had 74 % higher odds of steatosis (OR: 1·74, 95 % CI (1·26, 2·41)). Such positive association persisted among never drinkers, or participants who were free of hepatitis B and/or C. Similarly, EDIP was positively associated with CAP in multivariate linear model (P < 0·001). We found a non-significant association of EDIP score with advanced fibrosis or LSM (P = 0·837).
Conclusions:
Our findings suggest that a diet score that is associated with inflammatory markers is associated with hepatic steatosis. Reducing or avoiding pro-inflammatory diets intake might be an attractive strategy for fatty liver disease prevention.
Keywords: Cross-sectional study, Diet, Inflammation, Hepatic steatosis, Controlled attenuation parameter
Non-alcoholic fatty liver disease (NAFLD) imposes an enormous burden on health care systems and affects approximately 25 % of the population worldwide and 30 % of people in the USA(1). To date, due to the lack of approved drug therapy, lifestyle modification to achieve weight loss remains an optimal intervention for patients with NAFLD(1,2). Accumulating evidence indicates that chronic inflammation contributes substantially to NAFLD pathogenesis(3). Circulating concentrations of inflammation markers, such as IL-4, IL-6, C-reactive protein (CRP) and tumour necrosis factor-α receptor 2 (TNFα-R2), have been shown to be associated with NAFLD in prior studies(1,4,5). Moreover, in previous studies, lifestyles including diets can modulate inflammation(6–10). For instance, Mediterranean-type diets have anti-inflammatory properties and are effective in decreasing the risk of NAFLD and slowing its progression(11). Thus, we hypothesised that higher inflammatory potential of diet might be associated with increased risk of hepatic steatosis or fibrosis.
Recently, we re-derived and validated an empirical dietary inflammatory pattern (EDIP) in the US National Health and Nutrition Examination Survey (NHANES)(12), which is originally developed by Tabung et al. in three Harvard cohorts(13). EDIP is a hypothesis-driven a posteriori dietary pattern and was derived by entering thirty-nine predefined food groups into the reduced rank regression (RRR) followed by stepwise linear regression, which was highly predictive of concentration of two plasma inflammation markers including CRP and leucocytes count. EDIP has been suggested to be associated with higher risk of several chronic diseases including CVD(14), cancers(15–17) and type 2 diabetes(18). Moreover, we previously showed that EDIP is positively associated with risk of total and cancer-specific mortality(12), and hepatocellular carcinoma(16). However, to our knowledge, there have been no epidemiological studies regarding the association between EDIP and hepatic steatosis and fibrosis to date, although few studies(19–22) have investigated hepatic steatosis in relation to a literature-derived dietary inflammatory index (DII), which is an a priori dietary pattern (i.e. its development is based on the peer-reviewed articles on the association between dietary factors and inflammation). Given that DII is mainly nutrient-based (i.e. thirty-eight of its forty-five components are nutrients), findings from DII studies could be difficult to be translated readily into public health practice.
Herein, to add more evidence, we investigated the cross-sectional association between adherence to EDIP and odds of hepatic steatosis and fibrosis in a US nationwide sample.
Methods
Study population
This study used data from the 2017–2018 cycle of the US NHANES, in which hepatic transient elastography (TE) was performed for the first time in the survey. NHANES is a continuous cross-sectional survey conducted in the USA by the National Center for Health Statistics of the Centers for Disease Control and Prevention. The survey aimed to assess the health of a representative sample of about 5000 persons each year in the USA. Details of NHANES study design, study protocol and data collection approaches have previously been reported(23).
The flow chart of how we selected the study population was shown in Supplemental Fig. 1. Individuals aged 18 years or older were included. We excluded participants if they: (i) had missing data on diet (n 873); (ii) had implausible energy intake(16) (<600 or >3500 kcal/d for women; <800 or >4200 kcal/d for men, n 221); (iii) did not receive TE detection (n 264) or (iv) had invalid TE detection results (n 327). A total of 4171 participants were finally included in the analysis.
Assessments of diet and empirical dietary inflammatory pattern score
Dietary information was collected using two 24-h dietary recalls by skilled investigators. We used multiple-pass method to enhance complete and accurate data collection and decrease respondent burden(24). Dietary sampling weights were used to overcome the limitations including the dietary interview-specific nonresponse, day of the week for dietary recalls, unequal probability of selection and oversampling(24,25).
The development and validation of EDIP scores have been described previously(12). In short, thirty-nine pre-defined food groups(26) were entered into RRR model followed by stepwise linear regression analysis to identify a dietary pattern most predictive of two inflammation markers (i.e. CRP and leucocytes count). RRR can identify linear functions of predictors (i.e. food groups) that simultaneously explain as much response variation of inflammation markers as possible. The first factor (i.e. the RRR dietary pattern) identified by RRR then underwent further data reduction by stepwise linear regression to identify the most important component food groups of the RRR dietary pattern, with the RRR dietary pattern as the dependent variable, the thirty-nine food groups as independent variables, and a significance level of P = 0·01 for entry into, and retention in the model. A total of twenty-five food components were included in EDIP score (see online Supplemental Table 1). We used the regression coefficients in the final stepwise linear regression model as weights to calculate the EDIP scores. Higher EDIP scores (more positive) denote more pro-inflammatory potential of diets, while lower (more negative) scores indicate anti-inflammatory potential of diets.
In validation study of our prior publication(12), EDIP have shown a high ability to predict inflammatory markers (i.e. plasma high-sensitivity CRP and leucocytes count) in NHANES 2015–2018.
Assessments of covariates
Information on demographic and lifestyle factors, including age, sex, race/ethnicity, educational level, income, smoking and physical activity, were collected by standardised questionnaires during household interview. Information on alcohol intake, body weight and height was obtained from participants who received physical examinations in the NHANES Mobile Examination Center. Individuals who had smoked at least 100 cigarettes in life were defined as ever smokers, and never smokers were defined as those who did not have cigarettes consumption before the time of the interview. BMI was calculated as weight in kilograms divided by the square of the height in meters (kg/m2). The ratio of family income to poverty that accounts for family size and annual inflation and was calculated by dividing family income by the poverty thresholds. The poverty thresholds were defined as the dollar amounts set by the U.S. government to indicate the least amount of income a person or family needs to meet their basic needs, which were used to estimate the population’s income and poverty levels and related information. Physical activity was assessed by Global Physical Activity Questionnaire, which has been shown to have good reliability and validity in multiple populations(27). Individuals who performed <8·3, 8·3 to 16·7 and >16·7 metabolic equivalents of tasks hours of physical activity/week were classified as low, moderate and high levels according to the 2018 Physical Activity Guidelines for Americans(28). Hepatitis B virus (HBV) infection was defined as positive hepatitis B surface antigen (HBsAg), while both positive hepatitis C antibody and RNA indicated hepatitis C virus (HCV) infection. Diabetes was diagnosed if there were: (i) a self-reported history of diabetes; (ii) a fasting plasma glucose level of more than 126 mg/dl; (iii) a random glucose level of more than 200 mg/dl and (iv) a HbA1c level of more than 6·5 % for participants.
Ascertainments of hepatic steatosis and fibrosis
Vibration-controlled TE using the FibroScan® model 502 V2 Touch equipped with a medium (M) or extra-large (XL) wand (probe) was performed by technicians after a 2-d training program with an expert technician. Hepatic steatosis and fibrosis were measured using controlled attenuation parameter (CAP) and liver stiffness measurement (LSM), respectively. In accordance with previous studies(29–31), we used cut-off values of median CAP ≥ 274 for S1 steatosis, CAP ≥ 290 for S2 steatosis, LSM ≥ 8·2 kPa for significant fibrosis and LSM ≥ 9·7 kPa for advanced fibrosis. TE examinations were considered as reliable only when more than 10 LSMs were obtained after a fasting time no less than 3 h, with an interquartile range (IQR) to median ratio < 30 %.
By comparing CAP measurement for the detection of steatosis against biopsy, the area under the receiver operating characteristic curves was 0·87 (95 % CI (0·82, 0·92)) with a sensitivity and specificity of both 90 % for S ≥ S1 among patients with NAFLD(29). Similarly, when using LSM to define patients with fibrosis, the area under the receiver operating characteristic curves was 0·80 (95 % CI (0·75, 0·84)) for advanced fibrosis (F ≥ F3), with the corresponding sensitivity of 71 % and specificity of 75 %(29).
Statistical analysis
The prevalence of hepatic steatosis and fibrosis was standardised based on 2020 US population(31). Multiple linear regression was performed to evaluate the percentage change and 95 % CI for the associations of the EDIP scores with continuous CAP and LSM. Both CAP and LSM were natural logarithms transformed in the models given the deviation from normal distribution. We used multiple logistic regression to estimate the OR and 95 % CI for EDIP scores in relation to S1 and S2 steatosis. Covariates adjusted in the models were as follows: Model 1 was adjusted for age (18–39, 40–59 and ≥60). Model 2 was further adjusted for sex (male and female), smoking status (never smokers and ever smokers), race/ethnicity (non-Hispanic white, non-Hispanic black and other races), education (less than high school, high school diploma and more than high school), family income to poverty ratio (<1·30, 1·30–3·49 and ≥3·50), marital status (never married, married and widowed/divorced), physical activity (low level, moderate level and high level), total energy intake (tertiles), HBV (positive and negative) and HCV (positive and negative) infection. Of note, we did not adjust for alcohol consumption in our main analyses because wine and beer are components in EDIP. For covariates with missing values, a separate missing indicator variable was created and included in the models. We presented OR by tertile categories and per 1-sd increase of EDIP scores. Linear trends across increasing categories of EDIP scores were tested by entering EDIP scores as a continuous variable in the models, and P values for trend were calculated using a Wald test. We also used restricted cubic spline to identify the dose–response relationship between EDIP and hepatic steatosis.
Allowing for the potential intermediate role of BMI and diabetes in the association of EDIP and chronic liver disease(16), we did not adjust for BMI and diabetes in the main analyses but additionally adjusted for these two factors in the sensitivity analyses. To reduce measurement error and reflect dietary composition, we adjusted the EDIP scores for total energy intake using the nutrient residual method(32). Considering that HBV and HCV infections are important risk factors for liver diseases, we repeated analysis within individuals who are free of hepatitis B and/or C. Likewise, we investigated EDIP after removing their alcohol components (i.e. beer and wine) in relation to the prevalence of hepatic steatosis, with further adjustments for alcohol drinking status (never, low to moderate and heavy drinking), although beer and wine are included in the construct of EDIP. In addition, considering the possible etiological differences, we investigated the association of EDIP with non-alcoholic fatty liver and other steatosis separately. The non-alcoholic fatty liver diseases were defined if individuals: (i) were detected as steatosis through TE test; (ii) did not have significant alcohol consumption (>2 drinks/d for women and >3 drinks/d for men); (iii) were free of hepatitis B and/or C infection and (iv) did not take steatogenic medications (i.e. amiodarone, valproate, methotrexate, tamoxifen and corticosteroid) for at least 3 months or more before study enrollment(33,34).
Previous studies suggested that several factors including age, sex, race, smoking status, drinking status, marital status, BMI and diabetes could modify the associations between inflammatory dietary pattern and chronic liver diseases(16,35–37). Therefore, we stratified analyses according to these factors and tested the potential interactions. Wald test was used to check whether the cross-product terms between these variables and exposures were statistically significant. We used the Bonferroni correction to define the statistical significance as P < 0·0031 (0·05/(2 outcomes x 8 groups)) for subgroup analysis to account for multiple comparisons. All statistical tests were two-tailed and performed using SAS version 9.4 (SAS Institute).
Results
Characteristics of participants
A total of 4171 participants aged 18 years or older (mean age, 49·4 years; sd, 18·3 years) were included in our study. The age-standardised prevalence was 42·5 % (1806 cases) for S1 steatosis, 33·8 % (1436 cases) for S2 steatosis and 5·6 % (255 cases) for advanced fibrosis. The median (IQR) of EDIP scores for the total population was −0·03 (IQR: −0·15 to 0·06), ranged from a median of −0·21 (IQR: −0·31 to −0·15) in the lowest tertile to 0·09 (IQR: 0·06 to 0·15) in the highest tertile. Participants with higher EDIP score were older, had higher BMI, were more likely to be ever smokers and non-Hispanic whites, were less educated, were more likely to be married, had lower ratio of family income to poverty, were less physically active and were more likely to have a history of diabetes and hepatitis C (Table 1).
Table 1.
Age-adjusted characteristics of participants according to tertiles of EDIP scores in NHANES 2017–2018*
| Characteristics | EDIP scores | |||||
|---|---|---|---|---|---|---|
| Tertile 1 (n 1390) | Tertile 2 (n 1391) | Tertile 3 (n 1390) | ||||
| Mean | IQR | Mean | IQR | Mean | IQR | |
| EDIP scores† | −0·21 | −0·31, −0·15 | −0·03 | −0·07, 0·00 | 0·09 | 0·06, 0·15 |
| Mean | sd | Mean | sd | Mean | sd | |
| Age (years)† | 48·1 | 17·7 | 48·3 | 18·6 | 51·8 | 18·2 |
| BMI (kg/m2) | 28·8 | 6·8 | 29·6 | 7·1 | 29·9 | 7·2 |
| METS-h/week|| | 78·1 | 124·9 | 63·7 | 103·7 | 78·8 | 124·7 |
| Energy (kcal/d) | 2295 | 708 | 1858 | 640 | 1804 | 686 |
| CAP (dB/m) | 259·2 | 61·8 | 263·7 | 61·7 | 268·2 | 64·3 |
| LSM (kPa) | 5·6 | 4·1 | 5·6 | 3·8 | 6·0 | 5·3 |
| n | % | n | % | n | % | |
| Male (%) | 749 | 53·9 | 618 | 44·4 | 666 | 47·9 |
| Drinking status (%) | ||||||
| Never | 143 | 10·3 | 160 | 11·5 | 154 | 11·1 |
| Low to moderate‡ | 1049 | 75·5 | 1085 | 78·0 | 1090 | 78·4 |
| Heavy | 198 | 14·1 | 146 | 10·5 | 146 | 10·5 |
| Ever smokers (%)§ | 489 | 35·2 | 522 | 37·5 | 674 | 48·5 |
| Race (%) | ||||||
| Non-Hispanic white | 407 | 29·3 | 408 | 29·3 | 645 | 46·4 |
| Non-Hispanic black | 363 | 26·1 | 374 | 26·9 | 214 | 15·4 |
| Other | 620 | 44·6 | 609 | 43·7 | 531 | 38·2 |
| Education (%) | ||||||
| Less than high school | 202 | 14·5 | 256 | 18·4 | 317 | 22·8 |
| High school diploma | 278 | 20·0 | 345 | 24·8 | 413 | 29·7 |
| More than high school | 910 | 65·5 | 790 | 56·8 | 660 | 47·5 |
| Family income to poverty ratio (%) | ||||||
| <1·30 | 318 | 22·9 | 417 | 30·0 | 453 | 32·6 |
| 1·30–3·49 | 539 | 38·8 | 551 | 39·6 | 580 | 41·7 |
| ≥3·50 | 533 | 38·3 | 423 | 30·4 | 357 | 25·7 |
| Marital status (%) | ||||||
| Never married | 374 | 26·9 | 391 | 28·1 | 370 | 26·6 |
| Married | 774 | 55·7 | 762 | 54·8 | 733 | 52·7 |
| Widowed or divorced | 242 | 17·4 | 238 | 17·1 | 287 | 20·7 |
| Physical activity (%) | ||||||
| Low level | 434 | 31·2 | 505 | 36·3 | 510 | 36·7 |
| Moderate level | 145 | 10·4 | 145 | 10·4 | 114 | 8·2 |
| High level | 811 | 58·4 | 741 | 53·3 | 766 | 55·1 |
| Diabetes (%) | 232 | 16·7 | 273 | 19·6 | 278 | 20·0 |
| HBV infection (%) | 10 | 0·75 | 2 | 0·15 | 6 | 0·41 |
| HCV infection (%) | 14 | 1·04 | 22 | 1·58 | 41 | 2·96 |
EDIP, empirical dietary inflammatory pattern; HBV, hepatitis B Virus; HCV, hepatitis C Virus; NHANES, US National Health and Nutrition Examination Survey.
Continuous variables were presented as means (sd) if they were normally distributed, otherwise median (IQR) estimate was used; All the variables were standardised to the age distribution of the study population except for EDIP scores and age; Notably, the summing proportions for some categories are not 100 % because of missing values or rounding.
Value was not age adjusted.
Individuals who never drank in the last year but reported a history of alcohol drinking previously were also assigned in this category.
Individuals who had smoked at least 100 cigarettes in life.
Individuals who performed <8·3, 8·3 to 16·7, >16·7 METS-hours of physical activity/week were classified as low, medium, and high levels.
EDIP, hepatic steatosis and fibrosis
After adjusting for age, sex, and other covariates (Table 2), EDIP score was positively associated with CAP, with the percentage difference of 7·4 % (95 % CI (4·1, 10·9), P < 0·001) in participants with the highest tertile of EDIP scores, compared with those in the lowest tertile. This positive association was partly attenuated but remained statistically significant with further adjustments for BMI and diabetes (percentage difference: 3·5 %, 95 % CI (1·7, 5·4), P < 0·001). We found a non-significant association between EDIP score and LSM (percentage difference: 5·4 %, 95 % CI (−0·4 , 11·5), P = 0·837).
Table 2.
Percentage change (%) and 95 % CI for the associations of the empirical dietary inflammatory pattern with controlled attenuation parameter (CAP) and liver stiffness measurement (LSM) in NHANES (2017–2018)*
| Tertile 1 | Tertile 2 | Tertile 3 | Per 1-sd | P value † | ||||
|---|---|---|---|---|---|---|---|---|
| % | 95 % CI | % | 95 % CI | % | 95 % CI | |||
| CAP (dB/m) | ||||||||
| No. of participants | 1390 | 1391 | 1390 | |||||
| Model 1 | Reference | 2·6 | −0·2, 5·6 | 5·7 | 2·2, 9·2 | 2·5 | 1·2, 3·8 | <0·001 |
| Model 2 | Reference | 4·2 | 1·6, 6·9 | 7·4 | 4·1, 10·9 | 3·1 | 2·0, 4·3 | <0·001 |
| LSM (kPa) | ||||||||
| Model 1 | Reference | 1·7 | −4·9, 8·7 | 5·0 | −0·4, 10·7 | 0·6 | −2·1, 3·4 | 0·640 |
| Model 2 | Reference | 3·6 | −2·8, 10·5 | 5·4 | −0·4, 11·5 | 0·3 | −2·9, 3·6 | 0·837 |
EDIP, empirical dietary inflammatory pattern; HBV, hepatitis B Virus; HCV, hepatitis C Virus; NHANES, US National Health and Nutrition Examination Survey.
Model 1 was adjusted for age; Model 2 was further adjusted for sex, smoking status, race, education, family income to poverty ratio, marital status, physical activity, total energy, HBV, and HCV.
Linear trends across increasing categories of EDIP scores were tested by entering EDIP scores as a continuous variable into the models, and P values for trend were calculated using a Wald test.
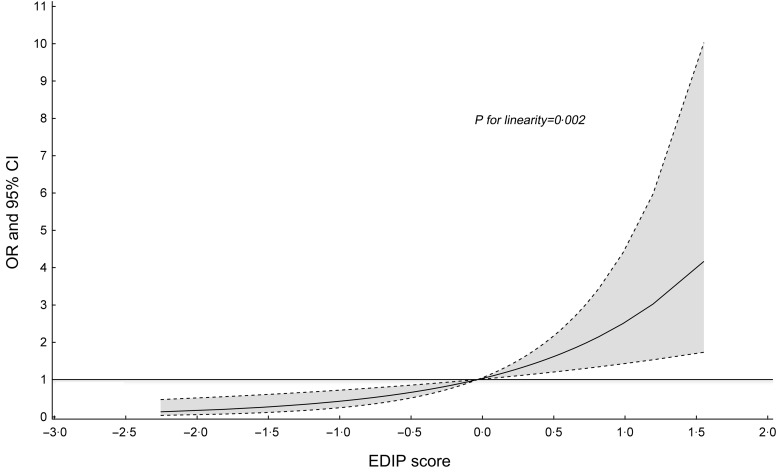
Similarly, participants with higher EDIP score had higher odds of hepatic steatosis with OR (comparing extreme tertile) of 1·74 (95 % CI (1·26, 2·41), P trend < 0·001, Table 3). When we additionally controlling BMI and diabetes, the magnitude of the positive association between EDIP and steatosis was partly attenuated (OR: 1·35, 95 % CI (1·04, 1·74), Ptrend = 0·001). Similar association was observed with the cut-off value of median CAP of no less than 290 dB/m (OR: 1·34, 95 % CI (1·06, 1·70), P trend = 0·004). Restricted cubic spline analysis did not support the non-linear association between EDIP and steatosis (P for linearity = 0·002, Fig. 1). We did not find any significant association between EDIP score and odds of significant fibrosis (LSM ≥ 8·2 kPa), advanced fibrosis (LSM ≥ 9·7 kPa) or cirrhosis (LSM ≥ 13·6 kPa, data not shown).
Table 3.
Odds ratios and 95 % confidence intervals for hepatic steatosis according to tertiles of EDIP scores in NHANES 2017–2018*
| Tertile 1 | Tertile 2 | Tertile 3 | Per 1-sd | P trend ‡ | ||||
|---|---|---|---|---|---|---|---|---|
| OR | 95 % CI | OR | 95 % CI | OR | 95 % CI | |||
| Steatosis† (≥S1) | ||||||||
| No. of cases | 565 | 596 | 645 | |||||
| Model 1 | Reference | 1·21 | 0·95, 1·55 | 1·47 | 1·09, 1·98 | 1·23 | 1·10, 1·38 | <0·001 |
| Model 2 | Reference | 1·36 | 1·09, 1·69 | 1·74 | 1·26, 2·41 | 1·33 | 1·16, 1·53 | <0·001 |
| Steatosis (≥S2) | ||||||||
| No. of cases | 438 | 468 | 530 | |||||
| Model 1 | Reference | 1·20 | 0·91, 1·57 | 1·52 | 1·16, 1·99 | 1·24 | 1·10, 1·39 | <0·001 |
| Model 2 | Reference | 1·33 | 1·04, 1·69 | 1·74 | 1·29, 2·34 | 1·32 | 1·14, 1·51 | <0·001 |
EDIP, empirical dietary inflammatory pattern; HBV, hepatitis B Virus; HCV, hepatitis C Virus; NHANES, US National Health and Nutrition Examination Survey.
Model 1 was adjusted for age; Model 2 was further adjusted for sex, smoking status, race, education, family income to poverty ratio, marital status, physical activity, total energy, HBV, and HCV.
CAP values ≥ 274 dB/m and 290 dB/m were considered indicative of S1 and S2 steatosis, respectively.
Linear trends across increasing categories of EDIP scores were tested by entering EDIP scores as a continuous variable into the models, and P values for trend were calculated using a Wald test.
Fig. 1.
Association between empirical dietary inflammatory pattern scores and hepatic steatosis (≥S1) in NHANES (2017–2018)*. *Model was adjusted for age, sex, smoking status, race, education, family income to poverty ratio, marital status, physical activity, total energy, HBV, HCV, BMI and diabetes except for variables examined in the figure. Notably, the restricted multivariable cubic spline analysis showed significantly linear association between empirical dietary inflammatory pattern scores and hepatic steatosis (≥S1) (P for linearity = 0·002 and P for non-linearity = 0·157). Reference levels were set to the median EDIP value. Solid lines indicate OR, and dashed lines depict 95 % CI. EDIP, empirical dietary inflammatory pattern; HBV, hepatitis B Virus; HCV, hepatitis C Virus; NHANES, US National Health and Nutrition Examination Survey
Sensitivity and subgroup analyses
In sensitivity analysis, when repeated analysis using the energy-adjusted EDIP scores, the results were similar to those in the main analysis (see online Supplemental Table 2). Likewise, the results were not essentially changed among individuals who were free of hepatitis B and/or C (Fig. 2). After removing alcohol components in EDIP and further adjusted for alcohol intake in the models, the results were essentially unchanged (see online Supplemental Table 3). When examining non-alcoholic fatty liver and other steatosis separately, we did not find the significant heterogeneity on the associations of EDIP with odds of non-alcoholic fatty liver and other steatosis (P heterogeneity = 0·477) (see online Supplemental Table 4).
Fig. 2.
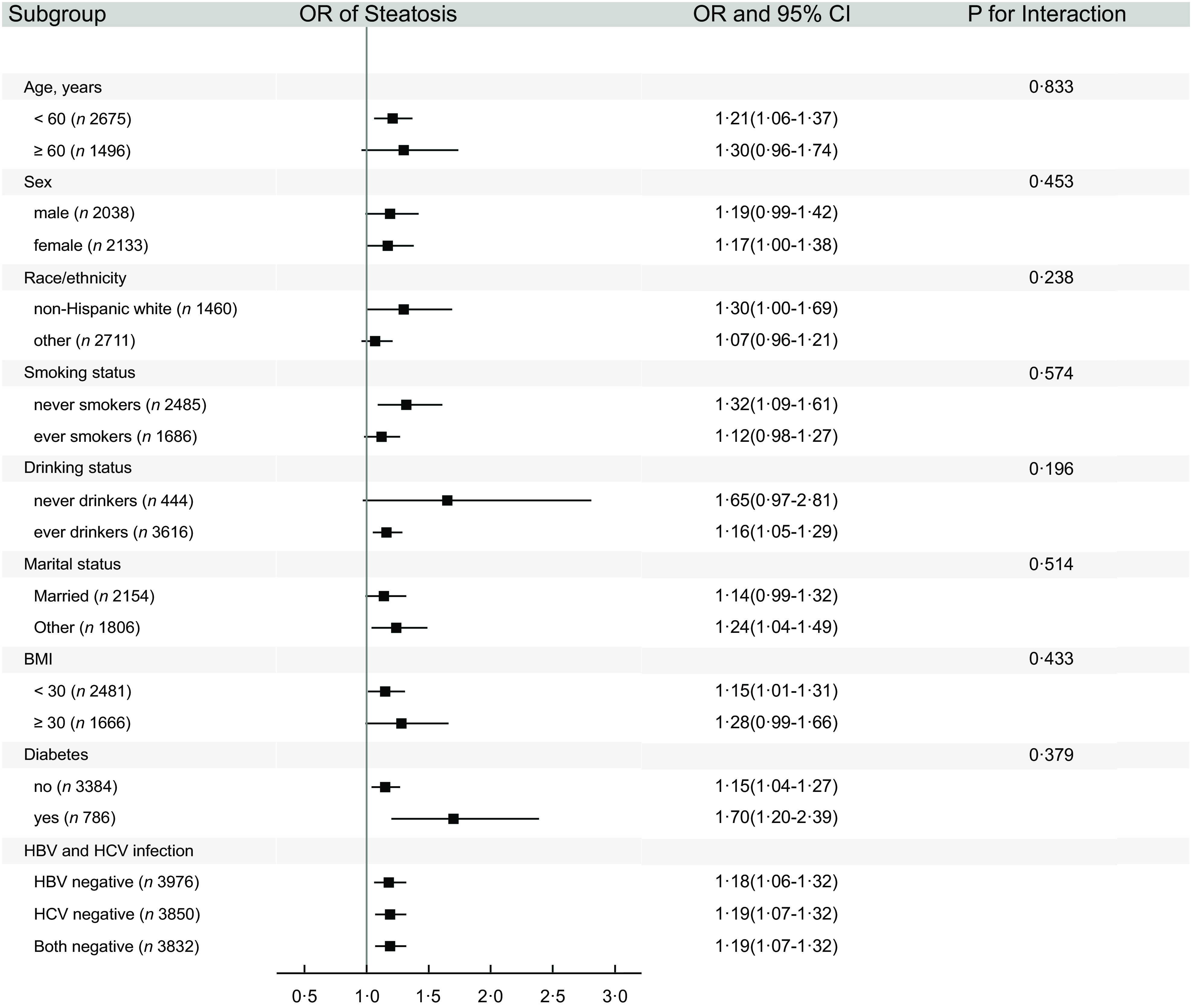
Subgroup analysis on the association of EDIP scores (per 1-sd increase) with hepatic steatosis (≥S1) in NHANES (2017–2018)*. *Model was adjusted for age, sex, smoking status, race, education, family income-poverty ratio, marital status, physical activity, total energy, HBV, HCV, BMI and diabetes except for variables examined in the figure. EDIP, empirical dietary inflammatory pattern; HBV, hepatitis B Virus; HCV, hepatitis C Virus; NHANES, US National Health and Nutrition Examination Survey
In subgroup analysis (Fig. 2), there was no differential association between EDIP and odds of hepatic steatosis when stratified by age, sex, race/ethnicity, smoking status, drinking status, marital status, BMI or diabetes (all the P values for interaction were greater than Bonferroni-corrected statistical significance of 0·0031).
Discussion
In this nationwide cross-sectional study among US adults, we examined associations between EDIP and odds of hepatic steatosis and fibrosis. We found that persons with higher EDIP scores (i.e. consuming a pro-inflammatory diet) had a higher prevalence of hepatic steatosis. This positive association remained among individuals who were free of hepatitis B and/or C and persisted regardless of alcohol drinking status. EDIP seemed not to be associated with fibrosis, as indicated by LSM.
Previous studies have reported that several nutrients and foods, such as fructose(38,39), soft drinks(40) and red meat(40), have been associated with high risk for NAFLD. However, diets are complex combinations of nutrients and foods, which may interact mutually(41,42). Thus, dietary patterns considering multiple dietary factors may provide a more comprehensive assessment of diet and may thus be more predictive of diet–disease associations compared with the approach of using single nutrients or foods.
This is the first observational study to investigate the association of EDIP score with hepatic steatosis and fibrosis among the US adults, though few studies have assessed the association between DII and fatty liver diseases or their parameters, which all used cross-sectional design(19,21,22). The EDIP and DII both evaluate the inflammation potential of diet, while the two dietary patterns differ in several aspects. The EDIP is a hypothesis-driven a posteriori pattern (i.e. its development is based on RRR to identify food groups predictive of inflammation biomarkers) and is based exclusively on food groups. The DII is an a priori pattern (i.e. its development is based on the 1943 peer-reviewed articles on the association between dietary factors and inflammation) and is mainly nutrient-based. Different from our study, previous DII studies on fatty liver diseases used different approaches for outcome ascertainment, including Fatty Liver Index(19,21,22), the aspartate transaminase to alanine transaminase ratio(19) or fibrosis-4 score(19), with the exception of 2 studies(19,43). In the current study, we were able to derive CAP and LSM through TE (FibroScan®) to define hepatic steatosis and fibrosis with higher sensitivity and specificity(29,44). However, our study together with previous DII studies(19,21,22,43) consistently support that pro-inflammatory diets are associated with higher risk of fatty liver diseases.
In line with our study and previous DII studies(19,21,22,43), a randomised controlled trial(20) among younger adults with obesity showed the effectiveness of an energy-reduced anti-inflammatory diet with significant improvement of liver parameters, including Fatty Liver Index, liver fat score and fibrosis-4. Consistently, in two Harvard cohorts, the Nurses’ Health Study and the Health Professionals Follow-up Study of 119 316 participants with 142 incident hepatocellular carcinoma cases, we found a positive association between EDIP score and risk of hepatocellular carcinoma(16). These findings further support that a diet score that is associated with inflammatory markers is associated with hepatic steatosis.
The association between greater adherence to pro-inflammatory diet and higher odds of hepatic steatosis has its biological plausibility. It is accepted that insulin resistance is a crucial pathophysiological factor in the development of NAFLD(45,46), since the decreased insulin sensitivity of adipocyte causes an increased hepatic-free fatty acid flux creating favourable conditions for the progression of hepatic steatosis(46,47). Moreover, inflammation cytokines, such as IL and TNFα, may disrupt insulin action and mediate insulin resistance(24,48,49). Meantime, the elevated levels of inflammatory markers (i.e. CRP, IL-6 and TNFα) are observed among individuals with NAFLD(50,51). Thus, one possible mechanism is that diet can modulate inflammation and mediate insulin resistance, which in turn leads to hepatic steatosis. However, we did not find any significant association between EDIP-adherence score and the likelihood of fibrosis (data not shown). One possible reason is that coffee is included as a component in EDIP (see online Supplemental Table 1), whereas coffee could induce UDP glucuronosyltransferases, which may contribute to the protective, antioxidant effects in the progression of hepatic fibrosis(52–54). Alternatively, the lack of an association between EDIP and fibrosis may be due to the insufficient power caused by limited cases of fibrosis in the present study.
Strengths of our study include the use of validated food-based EDIP scores, a large nationally representative sample of US adults and valid TE detection to measure hepatic steatosis and fibrosis. However, our study has several limitations. First, dietary information was measured by 24-h recalls, which may limit the ability to capture habitual diets of individuals. To overcome this limitation, we used several methods, such as multiple-pass method and dietary sampling weights(24,25). We also used energy-adjusted EDIP in the models(32) and yielded similar results. Second, we are unable to completely rule out residual or unmeasured confounders (e.g. the use of anti-inflammation drugs). Third, the cross-sectional design in the current study does not allow the determination of causality.
In conclusion, findings from our study indicate that a diet score that is associated with inflammatory markers is associated with hepatic steatosis. Interventions to reduce the adverse effect of pro-inflammatory diet may reduce the likelihood of hepatic steatosis among US adults. However, our results should be interpreted with caution, given the measurement of diet using 24-h recalls and the cross-sectional design in the current study. More prospective cohort studies and clinical trials are needed to validate our findings.
Acknowledgements
We thank Meiling Li and Xueke Zeng for their help with the final English language version.
Financial support
This work was supported by the National Natural Science Foundation of China (WY, grant number 82073651); Anhui Provincial Natural Science Foundation (WY, grant number 2008085MH262), (ZZ, grant number 2108085QH357); Anhui Provincial Education Department (WY, grant number gxyqZD2021099) and Anhui Medical University (WY, grant numbers 2021xkjT007, XJ201935), (MT, grant numbers 2020lcxk033, 2019xkj161).
Conflicts of interest
There are no conflicts of interest.
Authorship
H.Y., T.Z. and W.S. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: W.Y. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: H.Y. and T.Z. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: H.Y. and Y.Z. Administrative, technical or material support: W.Y. Study supervision: W.Y.
Ethics of human subject participation
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving research study participants were approved by the (NCHS Research Ethics Review Board; Protocol #2011-17; Protocol #2018-01). Written informed consent was obtained from all subjects.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980023001970
References
- 1. Rinella ME (2015) Nonalcoholic fatty liver disease: a systematic review. JAMA 313, 2263–2273. [DOI] [PubMed] [Google Scholar]
- 2. Hallsworth K & Adams LA (2019) Lifestyle modification in NAFLD/NASH: facts and figures. JHEP Rep 1, 468–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Friedman SL, Neuschwander-Tetri BA, Rinella M et al. (2018) Mechanisms of NAFLD development and therapeutic strategies. Nat Med 24, 908–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cobbina E & Akhlaghi F (2017) Non-alcoholic fatty liver disease (NAFLD) – pathogenesis, classification, and effect on drug metabolizing enzymes and transporters. Drug Metab Rev 49, 197–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Duarte N, Coelho IC, Patarrao RS et al. (2015) How inflammation impinges on NAFLD: a role for Kupffer cells. Biomed Res Int 2015, 984578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dawson DR 3rd, Branch-Mays G, Gonzalez OA et al. (2014) Dietary modulation of the inflammatory cascade. Periodontol 2000 64, 161–197. [DOI] [PubMed] [Google Scholar]
- 7. Garcia-Arellano A, Ramallal R, Ruiz-Canela M et al. (2015) Dietary inflammatory index and incidence of cardiovascular disease in the PREDIMED study. Nutrients 7, 4124–4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Due A, Toubro S, Stender S et al. (2005) The effect of diets high in protein or carbohydrate on inflammatory markers in overweight subjects. Diabetes Obes Metab 7, 223–229. [DOI] [PubMed] [Google Scholar]
- 9. Asgharpour A, Cazanave SC, Pacana T et al. (2016) A diet-induced animal model of non-alcoholic fatty liver disease and hepatocellular cancer. J Hepatol 65, 579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kerr J, Anderson C & Lippman SM (2017) Physical activity, sedentary behaviour, diet, and cancer: an update and emerging new evidence. Lancet Oncol 18, e457–e471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ryan MC, Itsiopoulos C, Thodis T et al. (2013) The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol 59, 138–143. [DOI] [PubMed] [Google Scholar]
- 12. Li X, Chen B, Zhang J et al. (2022) Association of dietary inflammatory potential with risk of overall and cause-specific mortality. Br J Nutr 127, 1878–1887. [DOI] [PubMed] [Google Scholar]
- 13. Tabung FK, Smith-Warner SA, Chavarro JE et al. (2016) Development and validation of an empirical index of dietary inflammatory potential. J Nutr 146, 1560–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li J, Lee DH, Hu J et al. (2020) Dietary inflammatory potential and risk of cardiovascular disease among men and women in the U.S. J Am Coll Cardiol 76, 2181–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tabung FK, Liu L, Wang W et al. (2018) Association of dietary inflammatory potential with colorectal cancer risk in men and women. JAMA Oncol 4, 366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang W, Sui J, Zhao L et al. (2021) Association of inflammatory and insulinemic potential of diet and lifestyle with risk of hepatocellular carcinoma. Cancer Epidemiol Biomarkers Prev 30, 789–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tabung FK, Huang T, Giovannucci EL et al. (2017) The inflammatory potential of diet and ovarian cancer risk: results from two prospective cohort studies. Br J Cancer 117, 907–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee DH, Li J, Li Y et al. (2020) Dietary inflammatory and insulinemic potential and risk of type 2 diabetes: results from three prospective U.S. cohort studies. Diabetes Care 43, 2675–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ramirez-Velez R, Garcia-Hermoso A, Izquierdo M et al. (2022) The dietary inflammatory index and hepatic health in the US adult population. J Hum Nutr Diet 35, 968–979. [DOI] [PubMed] [Google Scholar]
- 20. Kendel Jovanovic G, Mrakovcic-Sutic I, Pavicic Zezelj S et al. (2021) Metabolic and hepatic effects of energy-reduced anti-inflammatory diet in younger adults with obesity. Can J Gastroenterol Hepatol 2021, 6649142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cantero I, Abete I, Babio N et al. (2018) Dietary inflammatory index and liver status in subjects with different adiposity levels within the PREDIMED trial. Clin Nutr 37, 1736–1743. [DOI] [PubMed] [Google Scholar]
- 22. Mazidi M, Shivappa N, Wirth MD et al. (2019) Diet with greater inflammatory potential is associated with higher prevalence of fatty liver among US adults. Eur J Clin Nutr 73, 1653–1656. [DOI] [PubMed] [Google Scholar]
- 23. Centers for Disease Control and Prevention (2020) National Health and Nutrition Examination Survey (NHANES) About the National Health and Nutrition Examination Survey. https://www.cdc.gov/nchs/nhanes/about_nhanes.htm (accessed September 2021).
- 24. Ahluwalia N, Dwyer J, Terry A et al. (2016) Update on NHANES dietary data: focus on collection, release, analytical considerations, and uses to inform public policy. Adv Nutr 7, 121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tooze JA, Midthune D, Dodd KW et al. (2006) A new statistical method for estimating the usual intake of episodically consumed foods with application to their distribution. J Am Diet Assoc 106, 1575–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hu FB, Rimm E, Smith-Warner SA et al. (1999) Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr 69, 243–249. [DOI] [PubMed] [Google Scholar]
- 27. Bull FC, Maslin TS & Armstrong T (2009) Global physical activity questionnaire (GPAQ): nine country reliability and validity study. J Phys Act Health 6, 790–804. [DOI] [PubMed] [Google Scholar]
- 28. US Department of Health and Human Services Physical Activity Guidelines for Americans (2018). https://health.gov/paguidelines/second-edition/pdf/Physical_Activity_Guidelines_2nd_edition.pdf (accessed September 2021).
- 29. Eddowes PJ, Sasso M, Allison M et al. (2019) Accuracy of fibroscan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology 156, 1717–1730. [DOI] [PubMed] [Google Scholar]
- 30. Ciardullo S, Monti T & Perseghin G (2021) High prevalence of advanced liver fibrosis assessed by transient elastography among U.S. adults with type 2 diabetes. Diabetes Care 44, 519–525. [DOI] [PubMed] [Google Scholar]
- 31. Zhu Y, Peng Z, Lu Y et al. (2022) Higher dietary insulinaemic potential is associated with increased risk of liver steatosis and fibrosis. Liver Int 42, 69–79. [DOI] [PubMed] [Google Scholar]
- 32. Willett WC, Howe GR & Kushi LH (1997) Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 65, 1220S–1228S. [DOI] [PubMed] [Google Scholar]
- 33. Vilar-Gomez E, Nephew LD, Vuppalanchi R et al. (2022) High-Quality diet, physical activity, and college education are associated with low risk of NAFLD among the US population. Hepatology 75, 1491–1506. [DOI] [PubMed] [Google Scholar]
- 34. Kim D, Konyn P, Cholankeril G et al. (2022) Physical activity is associated with nonalcoholic fatty liver disease and significant fibrosis measured by fibroscan. Clin Gastroenterol Hepatol 20, e1438–e1455. [DOI] [PubMed] [Google Scholar]
- 35. Long L, Liu X, Petrick J et al. (2023) Dietary inflammatory and insulinemic potential, risk of hepatocellular carcinoma, and chronic liver disease mortality. JNCI Cancer Spectr 7, pkad023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shivappa N, Hebert JR, Polesel J et al. (2016) Inflammatory potential of diet and risk for hepatocellular cancer in a case-control study from Italy. Br J Nutr 115, 324–331. [DOI] [PubMed] [Google Scholar]
- 37. Zhang S, Meng G, Zhang Q et al. (2022) Inflammatory potential of diet and risk of nonalcoholic fatty liver disease: a prospective cohort study. Eur J Clin Nutr 76, 1125–1132. [DOI] [PubMed] [Google Scholar]
- 38. Noakes TD & Windt J (2017) Evidence that supports the prescription of low-carbohydrate high-fat diets: a narrative review. Br J Sports Med 51, 133–139. [DOI] [PubMed] [Google Scholar]
- 39. Vos MB & Lavine JE (2013) Dietary fructose in nonalcoholic fatty liver disease. Hepatology 57, 2525–2531. [DOI] [PubMed] [Google Scholar]
- 40. Zelber-Sagi S, Nitzan-Kaluski D, Goldsmith R et al. (2007) Long term nutritional intake and the risk for non-alcoholic fatty liver disease (NAFLD): a population based study. J Hepatol 47, 711–717. [DOI] [PubMed] [Google Scholar]
- 41. Hu FB (2002) Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol 13, 3–9. [DOI] [PubMed] [Google Scholar]
- 42. Tapsell LC, Neale EP, Satija A et al. (2016) Foods, nutrients, and dietary patterns: interconnections and implications for dietary guidelines. Adv Nutr 7, 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vahid F, Shivappa N, Hekmatdoost A et al. (2018) Association of pro-inflammatory dietary intake and non-alcoholic fatty liver disease: findings from Iranian case-control study. Int J Vitam Nutr Res 88, 144–150. [DOI] [PubMed] [Google Scholar]
- 44. Chang TY, Chang SH, Lin YH et al. (2021) Utility of quantitative ultrasound in community screening for hepatic steatosis. Ultrasonics 111, 106329. [DOI] [PubMed] [Google Scholar]
- 45. Marchesini G & Forlani G (2002) NASH: from liver diseases to metabolic disorders and back to clinical hepatology. Hepatology 35, 497–499. [DOI] [PubMed] [Google Scholar]
- 46. Petta S, Gastaldelli A, Rebelos E et al. (2016) Pathophysiology of non alcoholic fatty liver disease. Int J Mol Sci 17, 2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tilg H & Moschen AR (2008) Insulin resistance, inflammation, and non-alcoholic fatty liver disease. Trends Endocrinol Metab 19, 371–379. [DOI] [PubMed] [Google Scholar]
- 48. Taniguchi CM, Emanuelli B & Kahn CR (2006) Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol 7, 85–96. [DOI] [PubMed] [Google Scholar]
- 49. White MF (2002) IRS proteins and the common path to diabetes. Am J Physiol Endocrinol Metab 283, E413–422. [DOI] [PubMed] [Google Scholar]
- 50. Lizardi-Cervera J, Chavez-Tapia NC, Perez-Bautista O et al. (2007) Association among C-reactive protein, Fatty liver disease, and cardiovascular risk. Dig Dis Sci 52, 2375–2379. [DOI] [PubMed] [Google Scholar]
- 51. Utzschneider KM & Kahn SE (2006) Review: the role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab 91, 4753–4761. [DOI] [PubMed] [Google Scholar]
- 52. Molloy JW, Calcagno CJ, Williams CD et al. (2012) Association of coffee and caffeine consumption with fatty liver disease, nonalcoholic steatohepatitis, and degree of hepatic fibrosis. Hepatology 55, 429–436. [DOI] [PubMed] [Google Scholar]
- 53. Kalthoff S, Ehmer U, Freiberg N et al. (2010) Coffee induces expression of glucuronosyltransferases by the aryl hydrocarbon receptor and Nrf2 in liver and stomach. Gastroenterology 139, 1699–1710. [DOI] [PubMed] [Google Scholar]
- 54. Attar BM & Van Thiel DH (2013) Current concepts and management approaches in nonalcoholic fatty liver disease. ScientificWorldJournal 2013, 481893. [DOI] [PMC free article] [PubMed] [Google Scholar]