Abstract
This study investigated sex differences in the relationship between post-vaccination adverse reactions, decision regret, and willingness to pay (WTP) for the booster dose of COVID-19 vaccines. This research carried out an online cross-sectional investigation among healthcare workers (HCWs) in Taizhou, China. In total, 1,054 respondents (165 males and 889 females) have received two-dose COVID-19 vaccination. We performed descriptive analysis, chi-square test, and mediation analysis on the exported data. In this study, 67 (40.6%) males and 429 (48.3%) females had WTP for the booster dose. Our study presented that decision regret mediated the effect of adverse reactions after vaccination on WTP for the booster dose in both male and female groups. In males, decision regret played a completely mediating role, while in females, it acted as a partial mediator. Sex differences in the relationship between post-vaccination side effects, decision regret, and WTP for the third dose were demonstrated in a sample of healthcare workers.
Keywords: Sex difference, COVID-19, Vaccination side effect, Decision regret, Willingness to pay
1. Introduction
It has been more than three years since the COVID-19 outbreak began in Wuhan, China, in late December 2019. The COVID-19 pandemic has created huge economic burdens globally (Harapan et al., 2020, Dong et al., 2020, Majumder and Minko, 2021). Available epidemiological data showed that the most common mechanism of transmission was from droplets generated during face-to-face conversation, coughing, or sneezing (Wiersinga et al., 2020). Among many, healthcare workers (HCWs) are at high risk of catching the disease due to their direct contact with a wide range of patients (Serrano-Ripoll et al., 2020). At this point, a safe and effective preventive vaccine would be considered a useful tool to reduce spread rates and subsequent infection (Polack et al., 2020, Russell and Greenwood, 2021, Palacios et al., 2020). Vaccines are therefore essential for HCWs to prevent COVID-19 infection and to keep health systems safe (Lazarus et al., 2021, Ripabelli et al., 2022, Noushad et al., 2021).
COVID-19 vaccines may be sold on the private market in the future, despite the fact that they are free in China. It is therefore important to evaluate the willingness to pay (WTP) for the COVID-19 vaccine. As we know, WTP is an indicator that people will consider paying for a service or health technology (He and Anderson, 2021). Knowing about people’s WTP for vaccines can help future pricing discussions and provide information to assist decision-making on COVID-19 vaccine pricing. Previous studies have focused on the acceptance and WTP for COVID-19 vaccines (Mengistu et al., 2022, Zhou et al., 2022). HCWs are vulnerable to the highly contagious virus since they are in direct contact with COVID-19 patients (Sabetian et al., 2021). Evidence regarding the WTP for COVID-19 vaccines among HCWs is critical to ensuring that it is accepted and valued, resulting in positive outcomes for the promotion of willingness to pay in the largest community.
So far, there have been a lot of studies concentrated on the WTP for vaccines. Reports showed that about 25 % of vaccinated people have experienced adverse reactions, such as pain in the muscles and fever (Kałucka et al., 2022, Maruyama et al., 2022). Therefore, investigating whether and how post-vaccination side effects influence individuals’ WTP for booster shots is necessary. Of interest, a previous study showed that men had more severe symptoms of COVID-19 than women (Rozenberg et al., 2020). Based on experience in monitoring post-vaccination adverse effects, many vaccines displayed gender differences (Zhu et al., 2021, Tadount et al., 2020). Our previous research has reported that post-vaccination side effects could influence WTP for the booster dose of COVID-19 vaccines, with decision regret mediating the above relations (Luo et al., 2022). Considering the potential sex difference in post-vaccination adverse reactions and WTP, sex disparity should also be taken into account in vaccine development and promotion. Hence, the objective of this study was to investigate sex differences in the relationship between post-vaccination adverse reactions, decision regret, and WTP for the booster dose of COVID-19 vaccines.
2. Methods
2.1. Study design
A cross-sectional online survey was carried out on the Wen-Juan Xing platform. The target participants were HCWs at a tertiary hospital in Taizhou, Zhejiang, China. Interviewees answered the questionnaires from August 31 to September 8, 2021. The information collected from the questionnaires was checked logically, including the exclusion of outliers; the exclusion of respondents under the age of 18; and the exclusion of respondents who took less than 120 s to fill out the questionnaire. This investigation was reviewed and approved by the Ethics Committee of Taizhou Hospital, Zhejiang Province, China (Approval number: K20210823). All related procedures were conducted according to the guidelines of the institutional Ethics Committee and the principles of the Declaration of Helsinki. We did not require separate written informed consent, as respondents were considered to have given informed consent by participating in the study. All participant information was kept anonymous.
2.2. Questionnaires
The main contents of the survey have been described previously (Luo et al., 2022). The collected information included the following four parts. (1) Basic characteristics, including sex, age, occupation, education, professional title, and underlying diseases; (2) Vaccination history, including the COVID-19 vaccination status and post-vaccination side effects; (3) Decision regret, including 5 items (Haun et al., 2019); and (4) The WTP for the booster dose.
2.3. Statistical analysis
The main objective was to investigate whether there were sex differences in the relationship between side effects after vaccination, decision regret, and WTP for the booster dose of COVID-19 vaccines. Here, we adopted mediation regression methods to study the above relations. The exposure () in this research was post-vaccination adverse reactions (yes or no); the potential mediator () was decision regret; and the outcome () was WTP for the booster dose (yes or no). We adopted the following equations for mediation analysis:
| (1) |
| (2) |
| (3) |
Category variables were described in terms of counts and percentages. Chi-square tests were used to compare differences between groups. Continuous variables were presented as mean(sd). We adopted the above equations to perform the mediation analysis. Variables with P-values < 0.05 were considered statistically significant.
3. Results
3.1. Basic characteristics
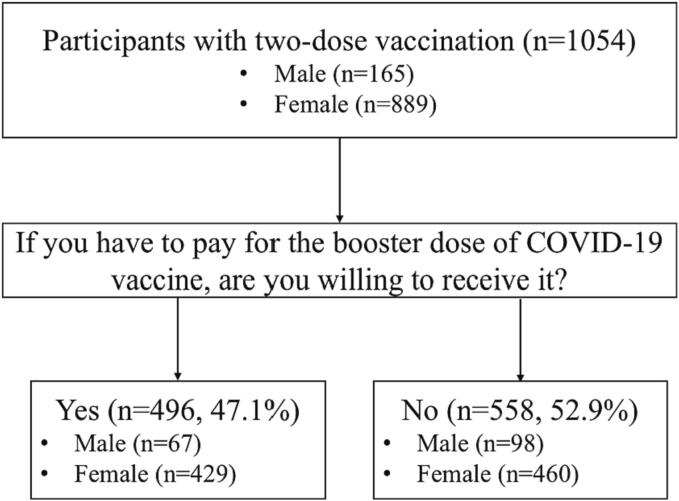
In this survey, a total of 1,054 participants have received two-dose COVID-19 vaccination. The collecting process of the WTP for the COVID-19 vaccine in males and females was shown in Fig. 1. There were 165 (15.7 %) males and 889 (84.3 %) females. Among the participants, only 47.1 % of them were willing to pay for the third dose, while another 52.9 % had no WTP for the booster vaccination. Among those who had no WTP for the booster dose, males and females accounted for 59.4 % (98/165) and 51.7 % (460/889), respectively.
Fig. 1.
Process of collecting the WTP in males and females.
The basic characteristics of the interviewees were presented in Table 1. The average(sd) age of respondents was 34.2(8.5) years old, and the median age was 34. The mean(sd) age for males and females was 36.0(9.6) and 33.8(8.1) years old, respectively. For males, the proportion of the WTP for the booster dose was 40.6 % (67/165). Among the male participants, most had undergraduate or above education levels. The majority occupation for male respondents was doctor. More than half of the respondents had medium or lower professional titles. Very few participants had underlying diseases, given that the participants were healthcare workers. Male participants who had post-vaccination adverse reactions accounted for 9.7 % (16/165). On the whole, male participants with older age, higher professional titles, no underlying diseases, and no post-vaccination adverse reactions had higher WTP for the booster dose. However, we did not see any statistically significant differences among the above subgroups. The average(sd) score of decision regret among males was 8.3(3.4). Of note, the mean(sd) score of males who had WTP for the booster dose was 7.5(3.4), while those without WTP had higher scores (8.9(3.3)). There were significant differences between the two groups.
Table 1.
Univariate analysis of factors associated with WTP for the booster dose of COVID-19 vaccine (n = 1054) in females and males, in Taizhou, Zhejiang, China: 2021.
| Variables | n | WTP for the booster dose in males |
n = 889 | WTP for the booster dose in females |
||||
|---|---|---|---|---|---|---|---|---|
| Yes [n (%)] |
No [n (%)] |
P-value | Yes [n (%)] |
No [n (%)] |
P-value | |||
| WTP for the booster dose | 165 | 67(40.6) | 98(59.4) | 429(48.3) | 460(51.7) | |||
| Age(years) | 0.456 | 0.676 | ||||||
| <34 | 71 | 26(36.6) | 45(63.4) | 465 | 228(49.0) | 237(51.0) | ||
| ≥34 | 94 | 41(43.6) | 53(56.4) | 424 | 201(47.4) | 223(52.6) | ||
| Education | 0.660 | 0.890 | ||||||
| Junior college or below | 18 | 8(44.4) | 10(55.6) | 215 | 105(48.8) | 110(51.2) | ||
| Undergraduate | 76 | 28(36.8) | 48(63.2) | 633 | 303(47.9) | 330(52.1) | ||
| Graduate | 71 | 31(43.7) | 40(56.3) | 41 | 21(51.2) | 20(48.8) | ||
| Occupation | 0.267 | 0.586 | ||||||
| Doctor | 105 | 46(43.8) | 59(56.2) | 69 | 31(44.9) | 38(55.1) | ||
| Nurse | 13 | 2(15.4) | 11(84.6) | 657 | 325(49.5) | 332(50.5) | ||
| Medical Technician | 38 | 15(39.5) | 23(60.5) | 89 | 42(47.2) | 47(52.8) | ||
| Others | 9 | 4(44.4) | 5(55.6) | 74 | 31(41.9) | 43(58.1) | ||
| Professional titles | 0.169 | 0.115 | ||||||
| Primary grade or below | 67 | 23(34.3) | 44(65.7) | 495 | 231(46.7) | 264(53.3) | ||
| Medium grade | 54 | 20(37.0) | 34(63.0) | 305 | 146(47.9) | 159(52.1) | ||
| Associate professor | 23 | 12(52.2) | 11(47.8) | 63 | 34(54.0) | 29(46.0) | ||
| Professor | 21 | 12(57.1) | 9(42.9) | 26 | 18(69.2) | 8(30.8) | ||
| Underlying disease | 0.130 | 0.450 | ||||||
| Yes | 30 | 8(26.7) | 22(73.3) | 91 | 40(44.0) | 51(56.0) | ||
| No | 135 | 59(43.7) | 76(56.3) | 798 | 389(48.7) | 409(51.3) | ||
| Post-vaccination adverse reaction | 0.108 | 0.002 | ||||||
| No | 149 | 64(43.0) | 85(57.0) | 782 | 393(50.3) | 389(49.7) | ||
| Yes | 16 | 3(18.8) | 13(81.2) | 107 | 36(33.6) | 71(66.4) | ||
| Decision regret | 8.3(3.4) | 7.5(3.4) | 8.9(3.3) | 0.008 | 8.6(3.5) | 7.8(3.2) | 9.3(3.6) | <0.001 |
Female participants who had the WTP for the third dose of COVID-19 vaccines accounted for 48.3 % (429/889). A similar distribution of data was found in the female group. Among the female participants, most had undergraduate education levels (71.2 %, 633/889). The majority occupation for females was the nurse (74.1 %, 659/889). Also, most of them had medium or lower professional titles and very few had underlying diseases. In total, females with older age, higher professional titles, and no underlying diseases had higher WTP for the booster dose, while the differences among the above subgroups were not found. In addition, female participants who had post-vaccination adverse reactions accounted for 12.0 % (107/889), which was higher than males. Among those who have experienced post-vaccination adverse reactions, only 33.6 % (36/107) of them had the WTP for the booster dose. Females without any side effects after vaccination had a higher WTP, with the percentage accounting for 50.3 % (393/782). The total average(sd) score of decision regret among females was 8.6(3.5). Females who had WTP obtained lower scores (7.8(3.2)) than those without WTP (9.3(3.6)). On the whole, female participants without post-vaccination adverse reactions or with lower scores of decision regret would be more willing to pay for the booster dose.
3.2. The mediation effect
Mediation analyses were performed based on Equation (1) - (3) on a subgroup of sex (male and female). Mediation regression models were adjusted for basic characteristics, including age, education, occupation, professional title, and underlying disease. The regression results were shown in Table 2, Table 3.
Table 2.
Testing of the mediating role of decision regret in males (n = 165) in Taizhou, Zhejiang, China: 2021.
| Variable | Model 1 |
Model 2 |
Model 3 |
|||||
|---|---|---|---|---|---|---|---|---|
| OR | 95 %CI | Coef | 95 %CI | OR | 95 %CI | |||
| Independent variable | ||||||||
| Post-vaccination adverse reaction | ||||||||
| Yes vs. No | 0.24* | 0.05 ∼ 0.84 | 2.40** | 0.69 ∼ 4.11 | 0.35 | 0.07 ∼ 1.25 | ||
| Mediator | ||||||||
| Decision regret | — | — | — | — | 0.85** | 0.75 ∼ 0.95 | ||
Note: ***, P-value < 0.001; **, P-value < 0.01; *, P-value < 0.05. The outcome of Model 1 and 3 was WTP for the booster dose of COVID-19 vaccine (1 denotes “Yes”); the outcome of Model 2 was decision regret.
Abbreviation: OR, odds ratio; CI, confidence interval; Coef, standardized beta regression coefficient.
Table 3.
Testing of the mediating role of decision regret in females (n = 889) in Taizhou, Zhejiang, China: 2021.
| Variable | Model 1 |
Model 2 |
Model 3 |
|||||
|---|---|---|---|---|---|---|---|---|
| OR | 95 %CI | Coef | 95 %CI | OR | 95 %CI | |||
| Independent variable | ||||||||
| Post-vaccination adverse reaction | ||||||||
| Yes vs. No | 0.51** | 0.33 ∼ 0.78 | 1.36*** | 0.66 ∼ 2.06 | 0.59* | 0.37 ∼ 0.90 | ||
| Mediator | ||||||||
| Decision regret | — | — | — | — | 0.89*** | 0.85 ∼ 0.92 | ||
Note: ***, P-value < 0.001; **, P-value < 0.01; *, P-value < 0.05. The outcome of Model 1 and 3 was WTP for the booster dose of COVID-19 vaccine (1 denotes “Yes”); the outcome of Model 2 was decision regret.
Abbreviations: OR, odds ratio, CI, confidence interval, Coef, standardized beta regression coefficient.
In male group, respondents who experienced adverse reactions after vaccination had lower WTP for the booster dose than those without (OR = 0.24, 95 %CI: 0.05 ∼ 0.84). Besides, males who have experienced adverse reactions after vaccination would also have higher scores of decision regret (Coef = 2.40, 95 %CI: 0.69 ∼ 4.11). In addition, after controlling for post-vaccination adverse reactions, the effect of decision regret on WTP for the booster dose was also significant (OR = 0.85, 95 %CI: 0.75 ∼ 0.95). This means that the higher scores of decision regret, the less willingness to pay for vaccines would be achieved. However, the direct effect of post-vaccination adverse reactions on WTP had no statistical significance, which suggested that decision regret played a completely mediating role in the relationship between post-vaccination side effects and WTP.
We also carried out mediation analyses for female participants. Compared to females with no post-vaccination adverse reactions, those who experienced adverse reactions after vaccination had lower WTP (OR = 0.51, 95 %CI: 0.33 ∼ 0.78) and higher decision regret scores (Coef = 1.36, 95 %CI: 0.66 ∼ 2.06). Moreover, the direct effect of post-vaccination adverse reactions on WTP was significant (OR = 0.59, 95 %CI: 0.37 ∼ 0.90). After controlling for post-vaccination adverse reactions, the effect of decision regret on WTP was also significant (OR = 0.85, 95 %CI: 0.75 ∼ 0.95), which indicated that decision regret partially mediated the influence of post-vaccination adverse reactions on WTP for the booster dose.
4. Discussion
4.1. Clinical implications
To our best knowledge, although previous research has presented the mediating role of decision regret in the association between adverse post-vaccination reactions and WTP, few studies have investigated sex-specific differences in the above relations. The findings of this research showed that decision regret mediated the association between post-vaccination adverse reactions and WTP for the booster dose in both male and female groups. In males, decision regret played a completely mediating role, while in females, it acted as a partial mediator.
This study reported that the proportion of WTP for the booster vaccines was 47.1 % (40.6 % for males and 48.3 % for females), which was similar to previous research conducted among HCWs in eastern Ethiopia (Merga et al., 2022). The magnitude of WTP for COVID-19 vaccines in this research was higher than in studies focused on school teachers or other general populations (Shitu et al., 2021, Salman et al., 2022). Considering that HCWs were among the highest risk groups for contracting COVID-19, studies have found that vaccines are essential for them to protect themselves from infection (Voysey et al., 2021, Ramasamy et al., 2021, Yigit et al., 2022). However, the WTP rate is lower than other studies conducted in Indonesia, which showed that 66.2 % had WTP for a booster dose (Harapan et al., 2022). Therefore, investigation is needed to understand the influencing factors leading to the low WTP.
This study further demonstrated an interesting foundation that the occurrence of post-vaccination side effects has a significant impact on WTP for booster vaccines in both males and females. In this investigation, the incidence of post-vaccination adverse reactions among male participants (9.7 %) was lower than among females (12.0 %). This foundation was different from the previous study (Rozenberg et al., 2020). The possible reason might be the heterogeneity of the population. Also, the previous study indicated that the incidences of overall side effects among HCWs after the first and second doses were 15.6 % and 14.6 %, respectively (Zhang et al., 2021). Another study showed that the overall incidence of adverse effects among HCWs after the first and second vaccinations was 38.2 % and 31.0 %, respectively (Cheng et al., 2022). Also, a study conducted in Italy reported that 89.1 % of interviewed participants described at least one adverse event after the booster dose of either of the licensed mRNA Comirnaty (Pfzer/BioNTech) or Spikevax (Moderna) vaccines (Tamburro et al., 2022). It can be seen that the incidence of vaccine side effects fluctuates widely, so reducing the side effects of COVID-19 vaccines is an important prerequisite for improving the vaccination rate and WTP.
In addition to the risk factor of post-vaccination side effects on WTP, decision regret was found to play a mediating role in the association between post-vaccination side effects and WTP. Previous studies have shown that about a quarter of people vaccinated will experience an adverse reaction to the vaccine (Kałucka et al., 2022). Although all side effects disappear within a week, individuals may regret their previous vaccine choices. Poor physical health outcomes are one of the most commonly reported risk factors associated with decision regret (Becerra Pérez et al., 2016). Adverse reactions and decision regrets after vaccination were risk factors for WTP for booster vaccines in both males and females. Therefore, in future booster dose campaigns for COVID-19 vaccines, in addition to information on the efficacy and safety of the vaccine, adverse reactions after vaccination need to be included, which may increase the willingness to vaccinate.
4.2. Methodological considerations
The main strength of this study was that it used real-world designs to reflect real phenomena and investigated sex differences in the relationship among post-vaccination adverse reactions, decision regret, and WTP. However, this research was not without limitations. First of all, the study sample was focused on HCWs from one hospital, hence, sample representativeness is the main limitation. In addition, the sample might also not be representative enough, since there were many fewer men than women in this survey. Moreover, interviewees recruited for this survey are likely to be healthier than the general public, as they are healthy enough to work in medical institutions. Nevertheless, there may also be differences between HCWs and the general population. Further surveys with large sample sizes are essential not only to extrapolate findings from other parts of China, but also to better understand these relationships. Second, decision regret scores may vary over time, while our estimates are made at one-time points and do not reflect the long-term risk of various factors. Statistical methods of interest such as multiple repeated measurements and time-varying covariates can better address this type of problem. Third, in this investigation, only participants who have received two-dose COVID-19 vaccination were considered, while WTP for those without completing two-dose vaccination was ignored. At the same time, these are also important parts of exploring sex differences. Therefore, to further identify the role of decision regret in the relationship between adverse reactions after vaccination and WTP among males and females, the generalization and external validity should be further studied.
5. Conclusions
This study showed that the WTP for the booster dose in females was higher than in males. Both post-vaccination adverse reactions and decision regret were risk factors for WTP. In addition, the findings of this study found that decision regret mediated the relationship between adverse post-vaccination reactions and WTP for the booster dose in both male and female groups. Of note, decision regret played a completely mediating role in the male group, while in females, it acted as a partial mediator.
Ethical approval and consent to participate
This research was exempted from informed consent reviewed approved by the Ethics Committee of Taizhou Hospital of Zhejiang Province in China (Approval number: K20210823). All procedures were conducted in accordance with the guidelines of our institutional Ethics Committee and in compliance with the principles of the Declaration of Helsinki.
CRediT authorship contribution statement
Chengwen Luo: . Hai-Xiao Chen: Writing – review & editing, Visualization, Investigation. Tao-Hsin Tung: Writing – review & editing, Writing – original draft, Validation, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to thank participants for their cooperation and support.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on request.
References
- Becerra Pérez M., Menear M., Brehaut J., Légaré F. Extent and predictors of decision regret about health care decisions: a systematic review. Med. Decis. Making. 2016;36(6):777–790. doi: 10.1177/0272989X16636113. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Li T., Zheng Y., Xu B., Bi Y., Hu Y., et al. Self-Reported adverse events among Chinese healthcare workers immunized with COVID-19 vaccines composed of inactivated SARS-CoV-2. Hum. Vaccin. Immunother. 2022;18(5):2064134. doi: 10.1080/21645515.2022.2064134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020;20(5):533–554. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harapan H., Itoh N., Yufika A., Winardi W., Keamg S., Te H., et al. Coronavirus disease 2019 (COVID-19): a literature review. J. Infect. Public Health. 2020;13(5):667–673. doi: 10.1016/j.jiph.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harapan H., Sallam M., Fathima R., Kusuma H.I., Anwar S., Nalapraya W.Y., et al. Willingness to pay (WTP) for COVID-19 vaccine booster dose and its determinants in Indonesia. Infectious Disease Reports. 2022;14(6):1017–1032. doi: 10.3390/idr14060101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haun M., Schakowski A., Preibsch A., Friederich H., Hartmann M. Assessing decision regret in caregivers of deceased German people with cancer-a psychometric validation of the decision regret scale for caregivers. Health Expect. 2019;22(5):1089–1099. doi: 10.1111/hex.12941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S., Anderson E. Conceptualizing and measuring pathways for how object attachment affects willingness to pay (WTP) Curr. Opin. Psychol. 2021;39:121–214. doi: 10.1016/j.copsyc.2020.09.008. [DOI] [PubMed] [Google Scholar]
- Kałucka S., Kusideł E., Głowacka A., Oczoś P., Grzegorczyk-Karolak I. Pre-vaccination stress, post-vaccination adverse reactions, and attitudes towards vaccination after receiving the COVID-19 vaccine among health care workers. Vaccines (basel). 2022;10(3):401. doi: 10.3390/vaccines10030401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus J., Ratzan S., Palayew A., Gostin L., Larson H., Rabin K., et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat. Med. 2021;27(2):225–228. doi: 10.1038/s41591-020-1124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C., Jiang W., Chen H., Tung T. Post-vaccination adverse reactions, decision regret, and willingness to pay for the booster dose of COVID-19 vaccine among healthcare workers: A mediation analysis. Hum. Vaccin. Immunother. 2022;18(6):2146964. doi: 10.1080/21645515.2022.2146964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder J., Minko T. Recent developments on therapeutic and diagnostic approaches for COVID-19. AAPS J. 2021;23(1):14. doi: 10.1208/s12248-020-00532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama A., Sawa T., Teramukai S., Katoh N. Adverse reactions to the first and second doses of Pfizer-BioNTech COVID-19 vaccine among healthcare workers. J. Infect. Chemother. 2022:00094. doi: 10.1016/j.jiac.2022.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengistu D., Demmu Y., Asefa Y. Global COVID-19 vaccine acceptance rate: Systematic review and meta-analysis. Front. Public Health. 2022;10:1044193. doi: 10.3389/fpubh.2022.1044193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merga B., Ayana G., Negash B., Alemu A., Abdurke M., Abdu A., et al. Health-care workers' willingness to pay for COVID-19 vaccines in Eastern Ethiopia: using contingent valuation method. Clinicoecon Outcomes Res. 2022;14:395–404. doi: 10.2147/CEOR.S361199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noushad M., Rastam S., Nassani M.Z., Al-Saqqaf I.S., Hussain M., Yaroko A.A., et al. A global survey of COVID-19 vaccine acceptance among healthcare workers. Front. Public Health. 2021;9 doi: 10.3389/fpubh.2021.794673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios R., Patiño E., de Oliveira P., Conde M., Batista A., Zeng G., et al. Double-blind, randomized, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of treating healthcare professionals with the adsorbed COVID-19 (inactivated) vaccine manufactured by Sinovac - PROFISCOV: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21(1):853. doi: 10.1186/s13063-020-04775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack F., Thomas S., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N. Engl. J. Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy M., Minassian A., Ewer K., Flaxman A., Folegatti P., Owens D., et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396(10267):1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripabelli G., Tamburro M., Buccieri N., Adesso C., Caggiano V., Cannizzaro F., et al. Active surveillance of adverse events in healthcare workers recipients after vaccination with COVID-19 BNT162b2 vaccine (Pfizer-BioNTech, Comirnaty): a cross-sectional study. J. Community Health. 2022;47(2):211–225. doi: 10.1007/s10900-021-01039-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenberg S., Vandromme J., Martin C. Are we equal in adversity? Does Covid-19 affect women and men differently? Maturitas. 2020;138:62–68. doi: 10.1016/j.maturitas.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell F., Greenwood B. Who should be prioritised for COVID-19 vaccination? Hum. Vaccin. Immunother. 2021;17(5):1317–1321. doi: 10.1080/21645515.2020.1827882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabetian G., Moghadami M., Hashemizadeh Fard Haghighi L., Shahriarirad R., Fallahi M., Asmarian N., et al. COVID-19 infection among healthcare workers: a cross-sectional study in southwest Iran. Virol. J. 2021 doi: 10.1186/s12985-021-01532-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salman M., Mallhi T., Tanveer N., Shehzadi N., Khan H., Ul Mustafa Z., et al. Evaluation of conspiracy beliefs, vaccine hesitancy, and willingness to pay towards COVID-19 vaccines in six countries from Asian and African regions: a large multinational analysis. Vaccines (basel). 2022;10(11):1866. doi: 10.3390/vaccines10111866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Ripoll M., Meneses-Echavez J., Ricci-Cabello I., Fraile-Navarro D., Fiol-deRoque M., Pastor-Moreno G., et al. Impact of viral epidemic outbreaks on mental health of healthcare workers: a rapid systematic review and meta-analysis. J. Affect. Disord. 2020;277:347–357. doi: 10.1016/j.jad.2020.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shitu K., Wolde M., Handebo S., Kassie A. Acceptance and willingness to pay for COVID-19 vaccine among school teachers in Gondar City, Northwest Ethiopia. Trop. Med. Health. 2021;49(1):63. doi: 10.1186/s41182-021-00337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadount F., Doyon-Plourde P., Rafferty E., MacDonald S., Sadarangani M., Quach C. Is there a difference in the immune response, efficacy, effectiveness and safety of seasonal influenza vaccine in males and females? - a systematic review. Vaccine. 2020;38(3):444–459. doi: 10.1016/j.vaccine.2019.10.091. [DOI] [PubMed] [Google Scholar]
- Tamburro M., Ripabelli G., D'Amico A., De Dona R., Iafigliola M., Parente A., et al. A cross-sectional study of untoward reactions following homologous and heterologous COVID-19 booster immunizations in recipients seventeen years of age and older. J. Community Health. 2022;47(5):814–821. doi: 10.1007/s10900-022-01112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voysey M., Costa Clemens S., Madhi S., Weckx L., Folegatti P., Aley P., et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397(10277):881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersinga W., Rhodes A., Cheng A., Peacock S., Prescott H. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. J. Am. Med. Assoc. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- Yigit M., Ozkaya-Parlakay A., Cosgun Y., Ince Y., Bulut Y., Senel E. Should a third booster dose be scheduled after two doses of CoronaVac? A single-center experience. J Med Virol. 2022;94(1):287–290. doi: 10.1002/jmv.27318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M.X., Zhang T.T., Shi G.F., Cheng F.M., Zheng Y.M., Tung T.H., et al. Safety of an inactivated SARS-CoV-2 vaccine among healthcare workers in China. Expert Rev. Vaccines. 2021;20(7):891–898. doi: 10.1080/14760584.2021.1925112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Pan L., Shi H., Luo J., Wang P., Porter H., et al. Willingness to pay for and willingness to vaccinate with the COVID-19 vaccine booster dose in China. Front. Pharmacol. 2022;13:1013485. doi: 10.3389/fphar.2022.1013485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Zhang M., Chien C., Yang W., Shi G., Qiu S., et al. Sex differences in adverse reactions to an inactivated SARS-CoV-2 vaccine among medical staff in China. Front. Med. (lausanne). 2021;8 doi: 10.3389/fmed.2021.731593. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author on request.