Abstract
Objective
Lipoprotein assembly and secretion in the small intestine are critical for dietary fat absorption. Surfeit locus protein 4 (SURF4) serves as a cargo receptor, facilitating the cellular transport of multiple proteins and mediating hepatic lipid secretion in vivo. However, its involvement in intestinal lipid secretion is not fully understood. In this study, we investigated the role of SURF4 in intestinal lipid absorption.
Methods
We generated intestine-specific Surf4 knockout mice and characterized the phenotypes. Additionally, we investigated the underlying mechanisms of SURF4 in intestinal lipid secretion using proteomics and cellular models.
Results
We unveiled that SURF4 is indispensable for apolipoprotein transport and lipoprotein secretion. Intestine-specific Surf4 knockout mice exhibited ectopic lipid deposition in the small intestine and hypolipidemia. Deletion of SURF4 impeded the transport of apolipoprotein A1 (ApoA1), proline-rich acidic protein 1 (PRAP1), and apolipoprotein B48 (ApoB48) and hindered the assembly and secretion of chylomicrons and high-density lipoproteins.
Conclusions
SURF4 emerges as a pivotal regulator of intestinal lipid absorption via mediating the secretion of ApoA1, PRAP1 and ApoB48.
Keywords: SURF4, Lipoprotein, Apolipoprotein, Lipid secretion, Lipid absorption, Small intestine
Graphical abstract

Highlights
-
•
Intestinal Surf4 knockout mice recapitulate the phenotypes of chylomicron retention disease.
-
•
SURF4 regulates intestinal lipid secretion by mediating the transport of apolipoproteins PRAP1, ApoA1, and ApoB48.
-
•
Female Surf4 heterozygous mice are resistant to high-fat diet-induced hypercholesterolemia.
1. Introduction
The small intestine plays a crucial role in the absorption of dietary lipids. It is responsible for efficiently taking up both exogenous and endogenous triglycerides (TG), cholesterol (Chol), and phospholipids (PL). These lipids are loaded onto apolipoproteins to form chylomicrons or high-density lipoprotein (HDL), which are then secreted into the blood via the mesenteric lymphatic duct or portal vein to meet whole-body lipid demand and maintain metabolic homeostasis [1]. Dysregulation of dietary lipid absorption and chylomicron production is a significant contributor to dyslipidemia, which is associated with the prevalence of insulin resistance, type 2 diabetes, and obesity [2].
Lipoprotein assembly is a critical part of chylomicron formation and is tightly regulated. In the endoplasmic reticulum (ER), microsomal triglyceride transfer protein (MTTP) transfers lipids to apolipoprotein B48 (ApoB48), the primary apolipoprotein of chylomicrons, to form prechylomicrons [3]. The prechylomicron transport vesicle (PCTV), which encapsulates ApoB48, triglycerides, and a diverse array of proteins and lipids, undergoes budding from the ER to the Golgi. This process is finely orchestrated by a complex of multiple proteins, including the components of coat protein complex II (COPII), collectively responsible for ER-to-Golgi transport of PCTV [4]. In the Golgi, prechylomicrons acquire additional lipids as well as apolipoprotein A1 (ApoA1) to become mature chylomicrons [5]. ApoA1 is also engaged in HDL assembly by acquiring Chol [6]. Proline-rich acidic protein 1 (PRAP1), which is a TG and PL binding protein and highly expressed in the intestinal epithelium, is involved in chylomicron assembly and secretion by enhancing lipidation of ApoB48 mediated by MTTP and promoting chylomicron secretion [7]. In mice, PRAP1 deficiency impairs lipid absorption, leading to increased lipid accumulation in the small intestine and a decrease in TG content in chylomicrons [7]. Plasma PRAP1 levels are strongly associated with body mass index (BMI) in overweight/obese individuals [8].
Surfeit locus protein 4 (SURF4) is a housekeeping protein that acts as a cargo receptor in the ER. Global knockout of Surf4 or knockdown of Sft-4, the Caenorhabditis elegans homolog of mammalian Surf4, causes embryonic lethality, indicating the crucial role of SURF4 in embryonic development [9,10]. In vitro, SURF4 modulates the cellular transport of erythropoietin [11], α1-antitrypsin [12], proinsulin [13], progranulin [14], and sonic hedgehog [15]. In vivo, SURF4 regulates the trafficking of lipid-ferrying very-low-density lipoproteins (VLDLs) in the liver [16,17]. Hepatic inactivation of SURF4 in mice impairs lipoprotein secretion and reduces plasma TG levels due to the trapping of VLDLs in the ER [16]. Moreover, murine hepatic Surf4 exhibits a dose-dependent reduction in plasma lipids in response to its expression [16,18]. These results indicate the important role of SURF4 in hepatic lipid secretion. Recently, a study reported that SURF4 interacts with ApoB48 in differentiated Caco-2 cells [19]. Inducible knockout of intestinal Surf4 in adult mice inhibits lipid absorption and significantly reduces protein levels of ApoB48 in the serum [19]. However, it has not been explored whether SURF4 regulates the transport of ApoA1 and PRAP1 during lipoprotein assembly and secretion in the small intestine.
2. Materials and methods
2.1. Animal models
All animal experiments were approved by the Institutional Animal Care and Use Committee, Peking University, and followed the ethical guidelines for animal research. Mice were housed in standard cages under controlled environmental conditions, including 22 °C temperature, 40% humidity, and a 12-h light/dark cycle, with ad libitum access to food and water. Villin-cre mice (B6.Cg-Tg(Vil1-cre)997Gum/J, RRID: IMSR_JAX:004586) were obtained from the Jackson Laboratory, while villin-creERT2 mice (B6.Cg-Tg(Vil1-cre/ERT2)23Syr/J, RRID:IMSR_JAX:020282) were kindly provided by Dr. Ye-Guang Chen (School of Life Sciences, Tsinghua University). SURF4flox/flox mice were generated as previously described [16]. To specifically disrupt SURF4 in the intestine, villin-cre SURF4flox/flox (IKO) mice were generated by crossing villin-cre mice with SURF4flox/flox mice. To conditionally disrupt SURF4 in the intestine, villin-creERT2 SURF4flox/flox (CKO) mice were generated by crossing villin-creERT2 mice with SURF4flox/flox mice. To induce Cre recombinase expression, 5-week-old mice were intraperitoneally injected with 100 μg/g weight of tamoxifen (Sigma–Aldrich, T5648) dissolved in sunflower seed oil (Macklin, S875787) for 5 consecutive days. To induce metabolic disorders in mice, 4- to 5-week-old littermate IHet and WT mice were fed either a chow diet (CD) or a high-fat diet (HFD (Research Diets, D12492)) for 20 weeks.
2.2. Fat tolerance test, glucose tolerance test, and insulin tolerance test
To perform the fat tolerance test (FTT), 6-week-old mice were fasted for 4 h before being orally administered with olive oil (Macklin, O815210) at a dose of 10 μL/g body weight. Blood samples were collected from the tail vein at the indicated time points using heparinized capillary tubes (Fisher Scientific, 22-362566).
To perform the glucose tolerance test (GTT), 20-week-old mice were fasted overnight and intraperitoneally injected with glucose at 2 g/kg body weight. Blood collected from the tail vein was measured at the indicated time with a glucometer (Roche Diagnostics).
To perform the insulin tolerance test (ITT), 21-week-old mice were fasted for 4 h and intraperitoneally injected with insulin (Novo Nordisk) at 1.5 U/kg body weight. Blood collected from the tail vein was measured at the indicated time with a glucometer (Roche Diagnostics).
2.3. Measurement of lipids
To measure serum or plasma triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and non-esterified fatty acids (NEFA), we used commercially available kits (FUJIFILM Wako Chemicals) and a chemical analyzer (Roche Diagnostics, cobas c 311 analyzer).
For fecal TG and TC analysis, each mouse was individually housed for 24 h. The collected feces were dried at 60 °C for 12 h, and 25 mg of the dried feces were homogenized in a 2 mL mixture of methanol:chloroform (1:2). Samples were then shaken overnight at 37 °C and allowed to stand for approximately 30 min. The supernatant was transferred into a fresh centrifuge tube and centrifuged at 6000 rpm for 2 min. The resulting supernatant was transferred into a fresh tube and evaporated at 60 °C for 6 h. The precipitates were dissolved in 10% TritonX-100 in isopropanol, which was used to measure TG and TC according to the manufacturer's instructions (Biosino).
To extract and measure enteric TG, we followed the manufacturer's instructions for an assay kit from Nanjing Jiancheng (A110-1-1). Briefly, duodenum samples were homogenized in absolute ethanol and then centrifuged at 2500 rpm for 10 min. The resulting supernatants were used for TG analysis. Enteric TC was extracted using methanol:chloroform (1:2) and measured according to the manufacturer's instructions (Biosino), in a similar manner to fecal TC, as described above.
Hepatic TG and TC, enteric TC were extracted with methanol:chloroform (1:2) and measured according to the manufacturer's instructions (Biosino) in a similar way to fecal TC as described above.
2.4. Blood glucose, plasma insulin, and liver function tests
Blood glucose levels were determined using test strips (Roche Diagnostics, ACCU-CHEK® Performa). Plasma insulin was measured according to the manufacturer's instructions (Millipore, EZRMI-13K). Plasma alanine transaminase (ALT) was measured by a chemical analyzer (Roche Diagnostics, cobas c 311 analyzer).
2.5. Endoplasmic reticulum isolation
Isolation of the endoplasmic reticulum was performed using the Minute™ ER Enrichment Kit (Invent Biotechnologies, ER-036). To meet the required 30–40 mg tissue quantity for each test, three separate samples from the proximal small intestine of neonatal mice, with each individual sample weighing around 10 mg, were pooled.
2.6. Histology
The proximal and distal small intestines and liver were excised and fixed in 4% paraformaldehyde for 48 h and then embedded in paraffin. The sections were sliced at a thickness of 3–5 μm and stained with hematoxylin and eosin (H&E) in accordance with the manufacturer's instructions (Baso).
For Oil Red O staining, the proximal small intestines samples were fixed in 4% paraformaldehyde for 48 h, then immersed in 15% and 30% sucrose in PBS for 12 h each, and subsequently embedded in OCT (SAKURA). The sections were cut at a thickness of 8–10 μm and stained according to the manufacturer's instructions (Baso, BA-4081).
Immunohistochemistry and immunofluorescence staining were performed as described previously by incubating the sections with primary antibodies at 4 °C overnight, followed by incubation with secondary antibodies at room temperature for 1 h [32,33]. The antibodies were listed in Table S4.
Periodic Acid Schiff (PAS) staining was performed in accordance with the manufacturer's instructions (Solarbio, G1281).
2.7. Electron microscopy
The proximal small intestine specimens were initially fixed in a solution of 2.5% glutaraldehyde (Sigma–Aldrich, G5882) in 0.1 M phosphate buffer (pH 7.4) for 1 h at room temperature, and then held at 4 °C overnight. Subsequently, the samples were rinsed 4 times in phosphate buffer for 10 min each, post-fixed with a solution of 2% (w/v) OsO4 (Ted Pella, 18456) and 1.5% (w/v) K4Fe(CN)6 (Sigma–Aldrich, 60279) for 2 h at room temperature while avoiding exposure to light. After that, specimens were en bloc stained with a 1% aqueous uranyl acetate at 4 °C overnight. The samples were then washed with ultra-pure water, dehydrated with a graded series of alcohol (30%, 50%, 70%, 85%, 95%, and 100%, for 10 min each) followed by pure acetone (2 × 100%, for 10 min each), infiltrated, embedded with an EMbed 812 embedding KIT (Electron Microscopy Sciences, 14120), and polymerized at 65 °C for 24 h. The resulting specimens were cut into 70 nm ultrathin sections using a Leica EM UC7 ultramicrotome equipped with a diamond knife (ultra 35°, Diatome, Switzerland) and mounted onto formvar-coated copper grids with a single slot. Before being observed under an electron microscope, the contrast of the grid-bound tissue was enhanced with uranyl acetate and lead citrate. Finally, the sections were examined using a Tecnai G2 Spirit BioTWIN electron microscope and captured using a digital camera (Orius 832, Gatan).
2.8. RNA extraction, cDNA synthesis, and qPCR
Total RNA was extracted from the small intestine and liver using FastPure Cell/Tissue Total RNA Isolation Kit in accordance with the manufacturer's instructions (Vazyme, RC112-01). The synthesis of cDNA was performed following the manufacturer's instructions (TransGen Biotech, AT311-04). Gene expression analysis by qPCR was carried out using the manufacturer's instructions (TIANGEN, FP205-02). The primer sequences were obtained from PrimerBank [34] and are listed in Table S1.
2.9. Protein extraction and immunoblotting
The proximal small intestine samples and HIEC-6 cells were lysed using RIPA buffer (Solarbio, R0010) and denatured by heating in the presence of protein loading buffer (Solarbio, P1016) according to the manufacturer's instructions. Serum and plasma samples were diluted with distilled water and subsequently denatured by heating in the presence of protein loading buffer (Solarbio, P1016). The culture media of HIEC-6 cells were collected and centrifuged at 12,000 rpm for 5 min at 4 °C. The supernatant was denatured by heating at 95 °C with protein loading buffer (Solarbio, P1016) according to the manufacturer's instructions. Antibodies for immunoblotting are listed in Table S2.
2.10. Proteomics and statistical analysis
The methodology for serum proteomics was conducted in accordance with the previous studies [35], while that for small intestine proteomics followed the protocol as described previously [36]. The proteomics data were then deposited on iProX-integrated proteome resources.
The differential protein expression analysis between the WT and IKO groups was conducted using the limma R package. A p-value cutoff of 0.05 and an absolute fold change value of 3/2 were used. The results are listed in Tables S5–S7. Gene set enrichment analysis (GSEA) was performed using the GSEA software with GO cellular component gene sets. Uniform manifold approximation and projection (UMAP) analysis was conducted using the umap R package. Differentially expressed protein enrichment analysis was carried out using the STRING database. The mice secretome was converted from the human secretome using the biomaRt R package, sourced from The Human Protein Atlas.
2.11. Cell culture, transfection, immunoblotting, immunofluorescence staining, and immunoprecipitation
HIEC-6 cells, obtained from ATCC (CRL-3266™), were cultured in RPMI 1640 medium (Solarbio, 31800) supplemented with 10% fetal bovine serum (FBS) (ExCell Bio, FSS500) at 37 °C in a 5% CO2 cell incubator. Transfection of plasmids was done with Lipofectamine™ 3000 (Thermo Fisher), according to the manufacturer's instructions. The plasmids are listed in Table S3.
For CRISPR/Cas9-mediated gene knockout, sgRNA was designed by CRISPick and cloned in pLenti-U6-gRNA-Cas9-P2A-EGFP-Neo. The sgRNA sequences are listed in Table S4. The transfected cells were subjected to trypsinization and sorted into a 96-well plate using a flow cytometer (BD FACSAria™ III Cell Sorter) based on the detection of positive green fluorescent protein (GFP) expression. The surviving clones were then sequenced and further propagated for subsequent experiments.
To detect ApoA1 through immunoblotting, non-targeting (NT) and SURF4 knockout (KO) cells were transfected with pCMV-APOA1-HA, or KO cells were transfected with pCMV-APOA1-HA and pCMV-N-GFP or pCMV-FLAG-SUFR4. After 2 days of culture, the lysate and media were harvested. To detect PRAP1 or ApoB through immunoblotting, NT and KO cells were transfected with pCMV3-SP-HA-PRAP1 or pCMV-MYC-ApoB48, or KO cells were transfected with pCMV3-SP-HA-PRAP1 or pCMV-MYC-ApoB48 and pCMV-N-GFP or pCMV-FLAG-SUFR4. After 1.5 days of culture, the cells were subjected to an overnight treatment with RPMI 1640 without FBS. Subsequently, the cells were incubated for 5 h in RPMI 1640 with 1% BSA and 100 μM OA. Then, the lysate and media were harvested.
To conduct immunofluorescence staining, the cells were fixed with ice-cold methanol for 5 min, followed by rinsing with PBS and subjected to serum-blocking buffer for 30 min. The blocked cells were thoroughly rinsed with PBS and incubated with primary antibody for 2 h at room temperature. Cells were then rinsed with PBS 3 times and incubated with secondary antibodies for 1 h at room temperature. Antibodies are listed in Table S2. Imaging of cells was carried out using a ZEISS LSM 880 confocal microscope.
For co-immunoprecipitation and colocalization analyses, plasmids pCMV-FLAG-SUFR4 and pCMV-APOA1-HA, pCMV-APOA1-HA, or pCMV-MYC-ApoB48 were transfected and cultured for 2 days. Co-IP was performed following the manufacturer's instructions (Beyotime, P2181, P2183, P2185). Antibodies for immunoblotting and colocalization immunofluorescence staining are listed in Table S2.
3. Results
3.1. Deletion of intestinal SURF4 impairs postnatal growth and induces hypolipidemia
SURF4 is critical for embryonic development and hepatic lipid metabolism [10,[16], [17], [18]]. It is highly expressed in the intestinal epithelium of both humans and mice. To investigate the role of SURF4 in the intestine, we generated intestine-specific Surf4 knockout (IKO) mice by crossing villin-Cre and Surf4flox/flox mice. The expression of Surf4 was significantly reduced in the small intestines of IKO mice (Figure S1A). The body size between IKO and their wild-type (WT) littermates was not different immediately at birth; however, IKO mice exhibited a smaller body size (Figure 1A) and lower body weight (Figure 1B) from 24 h after birth. Remarkably, almost all IKO mice died within 2 days after birth (Figure S1B). However, no significant difference was observed in the length of the intestines (Figure S1C). Hematoxylin and eosin (H&E) staining and Oil Red O staining revealed excessive lipid accumulation in the proximal small intestines of IKO mice (Figure 1C). The absence of SURF4 did not induce ER stress in the small intestines, as evidenced by the mRNA and protein expression levels of ER stress markers (Figure S1D-E). Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) staining showed no difference in cell death between IKO and WT intestines (Figure S1F). Furthermore, electron microscopy confirmed a large number of lipid droplets in the enterocytes of IKO mice (Figure 1C). Nutrient supply is critical for postnatal growth and survival. Therefore, we assessed circulating glucose and lipids and found that the levels of glucose, triglycerides (TG), total cholesterol (TC), and non-esterified fatty acids (NEFA) were significantly lower in IKO mice than in WT mice (Figure 1D–G). These results suggest that the deletion of SURF4 caused lipid accumulation in the intestine, leading to lower circulating lipid levels.
Figure 1.
IKO mice exhibit impaired postnatal growth and hypolipidemia. (A) Gross appearance at 1 day after birth. (B) Body weight (n = 47 for WT, n = 32 for IKO). (C) Lipid accumulation in the proximal small intestines of IKO mice. H&E and Oil Red O staining, scale bar = 100 μm. TEM (transmission electron microscope), scale bar = 1 μm. The asterisks indicate the lipid droplets. (D) Glucose (n = 26 for WT, n = 14 for IKO). (E–G) Serum TG (n = 29 for WT, n = 20 for IKO), NEFA (n = 24 for WT, n = 14 for IKO), TC (n = 30 for WT, n = 20 for IKO). ∗p < 0.05, ∗∗∗∗p < 0.0001, unpaired two-tailed Student's t-test. Data are presented as mean ± SEM.
The liver also plays a pivotal role in regulating glucose and lipid metabolism. To assess whether the anomalous circulating lipids were also impacted by the changes in the liver, we compared liver histology between IKO and WT mice and found no apparent differences (Figure S1G). Furthermore, we examined the expression of known rate-limiting enzymes of lipogenesis and gluconeogenesis in the livers by RT-qPCR and found that the expression of Acc1, Fasn, Hmgcr, Hmgcs1, G6pc, and Pck1 was not affected (Figure S1H). These findings further indicate that the low levels of circulating glucose and lipids in IKO mice were primarily caused by intestinal malabsorption.
The phenotypes were further confirmed by three survived male IKO mice. Similar to neonatal mice, 10-week-old adult IKO mice showed reduced body size (Figure S2A), lower body weight (Figure S2B), and markedly reduced plasma TG, TC, and NEFA levels (Figure S2C-2E). The small intestines of IKO mice were replete with lipids, as evidenced by the milk-white color of the intestines (Figure S2F). H&E, Oil Red O staining, and electron microscopy also showed large amounts of lipid accumulation in the small intestines (Figure S2G).
To ascertain if the lipid accumulation in the small intestines was caused by the abnormal development of the intestines, we examined the body weight, circulating glucose and lipids, and small intestinal morphology in embryos at embryonic day 18.5 (E18.5) and found that the body weight of IKO embryos was comparable to their WT littermates (Figure S3A), and blood glucose, serum TG, TC, and NEFA levels were also similar between IKO and WT embryos (Figure S3B-3E). Histological assessment by H&E, Ki67 immunohistochemistry (IHC), and Periodic Acid Schiff (PAS) staining confirmed that ablation of SURF4 did not affect the structural organization, cell proliferation, or goblet cells of the embryonic small intestines (Figure S3F). Moreover, the electron micrographs revealed that small intestinal microstructures were also similar between IKO and WT embryos (Figure S3F). Our findings suggest that the deletion of intestinal SURF4 did not affect the overall development of the small intestine.
3.2. Ablation of intestinal SURF4 disturbs lipoprotein secretion
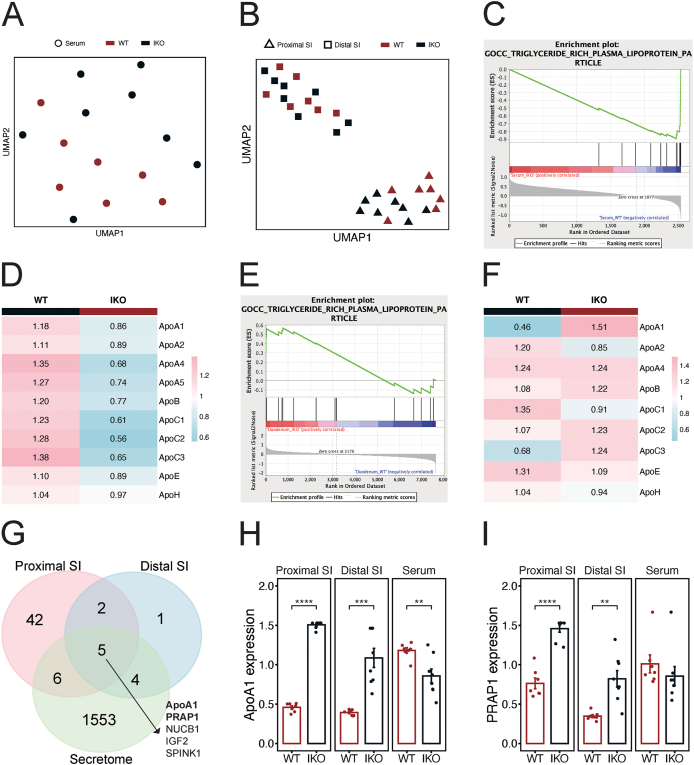
To investigate the underlying mechanism by which Surf4 knockout in the small intestine causes hypolipidemia, we used the total protein of the proximal and distal small intestines, and the serum to conduct tandem mass tag (TMT)-based proteomics. SURF4 deletion caused significant alterations in the proteomic profiles of the serum and the proximal small intestines, while the distal small intestines were only mildly affected (Figure 2A and B). Gene ontology (GO) analysis showed that the down-regulated proteins in the serum were mainly related to lipid transport and plasma lipoprotein particle remodeling, while the up-regulated proteins were mainly enriched in actin filament depolymerization (Figure S4A-C). Gene Set Enrichment Analysis (GSEA) revealed that the top suppressed GO Cellular Component pathways in the serum of IKO mice included triglyceride-rich plasma lipoprotein particles (Figure 2C), and most of the chylomicron apolipoproteins were reduced in the serum of IKO mice (Figure 2D). In addition, GO analysis showed that the up-regulated proteins in the proximal small intestines were mainly enriched in triglyceride homeostasis, whereas the down-regulated proteins were associated with SREBP-mediated de novo lipogenesis (Figure S4D-E). The differentially expressed proteins in the ileum were relatively few and did not show clear enrichment (Figure S4F). In contrast to the serum, GSEA analysis revealed that the top activated GO Cellular Component pathways in the proximal small intestines of IKO mice included triglyceride-rich plasma lipoprotein particles (Figure 2E), and more specifically, apolipoproteins such as ApoA1 and ApoC3 were increased in the proximal small intestines (Figure 2F).
Figure 2.
Deletion of intestinal SURF4 disturbs lipoproteins secretion. (A and B) Dimension reduction of the serum, the proximal and distal small intestines (SI) proteomics of WT and IKO mice using uniform manifold approximation and projection (UMAP). (C) GSEA enrichment plot of the serum proteomics using GO cellular component gene set. (D) Heatmap showing average expression of chylomicron apolipoproteins in the serum of WT and IKO mice. (E) GSEA enrichment plot of the proximal small intestines proteomics using GO cellular component gene set. (F) Heatmap showing average expression of chylomicron apolipoproteins in the proximal small intestines of WT and IKO mice. (G) Venn diagram showing the overlap between up-regulated proteins in the proximal and distal small intestines, and the mouse secretome. (H and I) Barplots of ApoA1 and PRAP1 in the proximal and distal small intestines, and the serum (n = 7 for WT, n = 8 for IKO). ∗∗p < 0.01, ∗∗∗p < 0.001, unpaired two-tailed Student's t-test. Data are presented as mean ± SEM.
Besides chylomicron apolipoproteins, lipid absorption and secretion in the small intestine involve numerous proteins that regulate the sequential processes, such as lipid uptake, intracellular transport, re-esterification, lipoprotein biogenesis, and lipid export [1,20]. Therefore, we examined the expression of cholesterol uptake protein NPC1L1; fatty acid uptake proteins CD36, FATP4, and GOT2; cholesterol export proteins ABCG5 and ABCG8; intracellular fatty acid transport proteins FABP1 and FABP2; cholesterol esterification protein ACAT2; fatty acid esterification proteins DGAT1, DGAT2, MGAT2, and MGAT3; phosphatidylcholine esterification protein LPCAT3; chylomicron assembly protein MTTP; and chylomicron trafficking proteins FABP1 and SAR1B, and found that the expression of all proteins was comparable in the proximal small intestines between WT and IKO mice (Figure S4G). These results suggest that the deletion of intestinal SURF4 did not affect the expression of proteins crucial for intestinal lipid absorption and secretion, except for apolipoproteins.
Given that SURF4 functions as a cargo receptor that transports specific cargo proteins from the ER to the Golgi, we analyzed that the proteins were increased in the intestines of IKO mice to identify the potential cargos for SURF4 by comparing them with the mouse secretome. We discovered that five secretory proteins accumulated in both the proximal and distal small intestines of IKO mice (Figure 2G–I). Among these five proteins, ApoA1 and PRAP1 play critical roles in lipoprotein assembly, and ApoA1 was significantly decreased in the serum of IKO mice (Figure 2H), while PRAP1 also showed a decreasing trend (Figure 2I), suggesting that SURF4 may serve as a regulator for ApoA1 and PRAP1 secretion in the small intestine. Overall, these results suggest that the disturbed lipid accumulation and secretion in IKO mice could be attributed to the disrupted transport of ApoA1 and PRAP1 caused by the absence of SURF4.
3.3. Intestinal SURF4 is critical for ApoA1 transport and secretion
To confirm the findings from the proteomics analysis, we conducted immunoblotting and found that ApoA1 was increased in the proximal small intestines of IKO mice, while it was decreased in the serum (Figure 3A). Importantly, we discovered that ApoA1 existed predominantly in the ER of IKO mice, as the intensity of the ApoA1 immunofluorescence staining was stronger in IKO mice and ApoA1 was mainly colocalized with the ER marker protein calnexin (Figure 3B). To investigate whether the absence of SURF4 affected ApoA1 transport, we designed SURF4-targeting and non-targeting gRNAs to generate a SURF4 knockout (KO) cell line and a negative control non-targeting (NT) cell line using HIEC-6 cell, a human small intestinal epithelial cell line. We transfected ApoA1 (HA-ApoA1) in both NT and KO cells and noticed reduced secretion and increased accumulation of ApoA1 in KO cells (Figure 3C), while transfection of SURF4 (FLAG-SURF4) in KO cells rescued the inhibition of ApoA1 secretion compared to the green fluorescent protein (GFP) transfected KO cells (Figure 3C). Furthermore, we transfected ApoA1 in KO and NT cells and discovered that ApoA1 was trapped mostly in the ER of KO cells compared to NT cells (Figure 3D). Additionally, we detected an interaction between SURF4 and ApoA1 using co-immunoprecipitation (co-IP; Figure 3E) and also co-localization of SURF4 and ApoA1 using immunofluorescence staining in SURF4 and ApoA1 co-transfected HIEC-6 cells (Figure 3F). These results indicate that SURF4 regulates ApoA1 transport and secretion in the small intestinal epithelial cells. Since ApoA1 participates in HDL assembly, deletion of SURF4 should result in increased ApoA1 in the small intestines and decreased ApoA1 and TC in the serum.
Figure 3.
SURF4 regulates the secretion of ApoA1. (A) Immunoblotting analysis of ApoA1 in the proximal small intestines (n = 4 for WT, n = 4 for IKO) and serum (n = 6 for WT, n = 6 for IKO). (B) Immunofluorescence staining of intestinal sections of WT and IKO mice with anti-ApoA1 (red) and anti-calnexin (green) antibodies. Scale bar = 20 μm. (C) Immunoblotting analysis of ApoA1 in the cell lysate and culture medium of NT and KO cells (n = 3 for NT, n = 3 for KO) and KO cells transfected with GFP or FLAG-SURF4 (n = 3 for GFP, n = 3 for SURF4). (D) Immunofluorescence staining of ApoA1 and ER with anti-HA (red, ApoA1) and anti-calnexin (green) antibodies in NT and KO HIEC-6 cells. Scale bar = 10 μm. (E) Co-IP analysis showing the interaction of ApoA1 and SURF4 in HA-ApoA1 and FLAG-SURF4 co-transfected HIEC-6 cells. (F) Immunofluorescence staining in HA-ApoA1 and FLAG-SURF4 co-expressed HIEC-6 cells with anti-HA (green, ApoA1) and anti-FLAG (red, SURF4) antibodies. Scale bar = 10 μm ∗p < 0.05, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001, unpaired two-tailed Student's t-test. Data are presented as mean ± SEM.
3.4. Deletion of intestinal SURF4 hampers PRAP1 transport and secretion
PRAP1 is present in ER. It can bind both TG and PL and regulate their secretion by engaging in chylomicron assembly in the small intestine [7]. However, the regulation of PRAP1 transport had been unresolved. In this study, we found that the deletion of intestinal SURF4 caused dramatic accumulation of PRAP1 in the proximal small intestines (Figure 4A). After isolating the ER, we confirmed that the ER of IKO mice contained a higher amount of PRAP1 compared to the ER of WT mice (Figure 4B). We transfected PRAP1 (HA-PRAP1) in both NT and KO HIEC-6 cells and confirmed that the knockout of SURF4 resulted in a significant reduction in the secretion of PRAP1 (Figure 4C). Furthermore, overexpression of SURF4 in KO cells promoted PRAP1 secretion compared to GFP-transfected KO cells (Figure 4C). Additionally, we transfected PRAP1 in NT and KO cells and observed the retention of PRAP1 in the ER of KO cells through immunofluorescence staining (Figure 4D). Co-IP revealed the interaction between PRAP1 and SURF4 (Figure 4E), and immunofluorescence staining showed the colocalization of PRAP1 and SURF4 (Figure 4F) in PRAP1 and SURF4 co-transfected HIEC-6 cells. These results suggest that SURF4 mediates PRAP1 secretion.
Figure 4.
SURF4 mediates the transport of PRAP1. (A) Immunoblotting analysis of PRAP1 in the proximal small intestines (n = 4 for WT, n = 4 for IKO). (B) Immunoblotting analysis of PRAP1 in the intestinal ER of WT and IKO mice. The proximal small intestines from three mice were mixed to make one sample. (C) Immunoblotting analysis of PRAP1 in the cell lysate and culture media of NT and KO cells (n = 3 for NT, n = 3 for KO) and KO cells transfected with GFP or FLAG-SURF4 (n = 3 for GFP, n = 3 for SURF4). (D) Immunofluorescence staining of PRAP1 and ER with anti-HA (red, PRAP1) and anti-calnexin (green) antibodies in NT and KO HIEC-6 cells. Scale bar = 10 μm. (E) Co-IP analysis showing the interaction of PRAP1 and SURF4 in HA-PRAP1 and FLAG-SURF4 co-transfected HIEC-6 cells. (F) Immunofluorescence staining of PRAP1 and SURF4 in HA-PRAP1 and FLAG-SURF4 co-transfected HIEC-6 cells with anti-HA (green, PRAP1) and anti-FLAG (red, SURF4) antibodies. Scale bar = 10 μm ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001, unpaired two-tailed Student's t-test. Data are presented as mean ± SEM.
3.5. Ablation of intestinal SURF4 disrupts ApoB48 secretion
ApoB48 is essential for chylomicron assembly and plays a crucial role in intestinal lipid secretion. We observed a notable decrease in both ApoB100 and ApoB48 in the serum of IKO mice (Figure 5A), although we did not observe an increase in ApoB48 levels in the small intestines of IKO mice (Figure 5A). However, ApoB48 was accumulated when ApoB48 (MYC-ApoB48) was overexpressed in KO cells after incubation with oleic acid (Figure 5B), and the accumulation of ApoB48 was alleviated by the overexpression of SURF4 (Figure 5B). We further confirmed the interaction between SURF4 and ApoB48 through co-IP analysis in HIEC-6 cells with co-overexpression of SURF4 and ApoB48 (Figure 5C). These findings are consistent with the previous studies [16,17,19], implying that SURF4 contributes to ApoB48 secretion.
Figure 5.
SURF4 facilitates ApoB48 secretion. (A) Immunoblotting analysis of ApoB48/100 in the serum (n = 4 for WT, n = 4 for IKO) and the proximal small intestines (n = 4 for WT, n = 4 for IKO). (B) Immunoblotting analysis of ApoB48 in the cell lysate of NT and KO cells (n = 3 for WT, n = 3 for KO) and KO cells transfected with GFP or FLAG-SURF4 (n = 3 for GFP, n = 3 for SURF4). (C) Co-IP analysis showing the interaction of ApoB48 and SURF4 in MYC-ApoB48 and FLAG-SURF4 co-transfected HIEC-6 cells. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, unpaired two-tailed Student's t-test. Data are presented as mean ± SEM.
3.6. Deletion of adult intestinal SURF4 leads to ApoA1 and PRAP1 accumulation in the intestine
Due to the neonatal lethality observed in the IKO mice, we generated tamoxifen-induced conditional Surf4 knockout mice (CKO) by crossing villin-creERT2 and Surf4flox/flox mice to investigate the function of SURF4 during dietary lipid absorption, particularly its impact on apolipoprotein secretion in adult mice. We treated 5-week-old mice with tamoxifen for 5 consecutive days and performed experiments 2 days after the final injection. Consistent with the previous report [19], CKO mice showed significantly lower body weight (Figure S5A), whereas the length of their small intestines was significantly elongated compared to WT mice (Figure S5B). CKO mice also exhibited a whitish small intestine similar to that seen in survived adult IKO mice in gross observation (Figure S5C). Histological analyses, including H&E staining, Oil Red O staining, and electron microscopy, showed substantial lipid accumulation in the small intestines of CKO mice (Figure S5D). Accordantly, TG and TC levels were increased in both the small intestines (Figure 6A) and feces (Figure 6B) of CKO mice, while plasma TG, NEFA, TC, and high-density lipoprotein cholesterol (HDL-C) were all decreased in CKO mice (Figure 6C). Similar to the IKO mice, CKO mice exhibited decreased plasma ApoA1 and ApoB (Figure 6D), while ApoB remained unchanged and ApoA1 was increased in the small intestines of CKO mice (Figure 6D). Moreover, PRAP1 was increased in the small intestines of CKO mice (Figure 6D). Furthermore, olive oil gavage-induced increases in plasma TG were completely abolished in CKO mice (Figure 6E). These results further confirm the critical role of SURF4 in intestinal lipid absorption and secretion.
Figure 6.
CKO mice exhibit impaired intestinal lipid absorption. (A) Proximal intestinal TG (n = 6 for WT, n = 7 for CKO) and TC (n = 3 for WT, n = 3 for CKO). (B) Fecal TG and TC (n = 3 for WT, n = 3 for CKO). (C) Plasma TG, NEFA, TC, and HDL-C (n = 9 for WT, n = 11 for CKO). (D) Immunoblotting analysis of ApoB48/100 and ApoA1 in plasma (n = 3 for WT, n = 3 for CKO) and ApoB48, SURF4, ApoA1, and PRAP1 in the proximal small intestines (n = 3 for WT, n = 3 for CKO). (E) Plasma TG and AUC of FTT (n = 10 for WT, n = 7 for CKO). AUC: area under the curve. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001, unpaired two-tailed Student's t-test. Data are presented as mean ± SEM.
3.7. Reducing Surf4 attenuates hypercholesterolemia induced by high-fat diet feeding in female mice
Our above findings indicated that the knockout of intestinal Surf4 caused severe hypolipidemia in both neonatal and adult mice. To explore the impact of reduced Surf4 on high-fat diet (HFD)-induced hyperlipidemia, we fed intestinal Surf4 heterogeneous (IHet) mice and their gender-matched WT littermates with either an HFD or chow diet (CD) as a control for 20 weeks, starting from 4 weeks of age. In male mice, reducing intestinal Surf4 did not result in notable differences in response to HFD in terms of body weight (Figure S6A), plasma lipids (Figure S6B-E), hepatosteatosis (Figure S6F), alanine transaminase (ALT) (Figure S6G), glucose tolerance test (Figure S6H), and insulin tolerance test (Figure S6I). H&E staining showed no discernible differences in small intestinal morphology and Ki-67 and lysozyme IHC staining displayed comparable proliferation of small intestine and localization and numbers of Paneth cells between HFD WT and HFD IHet mice (Figure S6J). However, female IHet mice exhibited a lower increase in the body weight upon HFD feeding compared to WT mice (Figure 7A). After 20 weeks of HFD feeding, IHet mice exhibited decreased plasma levels of TC and HDL-C (Figure 7B and C), along with increased TC in the proximal small intestines (Figure 7D). There were no differences in proximal intestinal TG (Figure S7A), fecal TG and TC (Figure S7B-C), or plasma TG and NEFA (Figure S7D-E). Additionally, IHet mice displayed resistance to HFD-induced hepatosteatosis (Figure 7E), accompanied by decreased TC and TG levels in the liver (Figure 7F–G) and enhanced liver function (Figure 7H). Moreover, there was a decrease in plasma ApoA1 (Figure 7I), while ApoB was not changed (Figure S7F). The results of the glucose tolerance test and insulin tolerance test revealed no differences between HFD WT and HFD IHet mice (Figure S7G-H). H&E, Ki-67 IHC, and lysozyme IHC staining results indicated that the morphology and proliferation of the small intestines and the Paneth cell localization, or numbers were comparable between HFD WT and HFD IHet mice (Figure S7I). The phenotypic differences between the females and males are likely attributed to varying levels of HFD-induced compensatory elevation of Surf4 in male IHet mice (Figure 7J).
Figure 7.
Reducing female Surf4 attenuated HFD-induced hypercholesterolemia. (A) Body weight (n = 20 for CD WT, n = 13 for CD IHet, n = 27 for HFD WT, n = 24 for HFD IHet). (B and C) Non-fasting plasma TC and HDL-C (n = 17 for CD WT, n = 11 for CD IHet, n = 21 for HFD WT, n = 19 for HFD IHet). (D) Proximal intestinal TC (n = 6 for CD WT, n = 5 for CD IHet, n = 16 for HFD WT, n = 16 for HFD IHet). (E) The hepatic H&E staining results of CD WT, CD IHet, HFD WT, and HFD IHet mice. Scale bar = 50 μm. (F and G) Hepatic TC and TG (n = 7 for CD WT, n = 4 for CD IHet, n = 6 for HFD WT, n = 7 for HFD IHet). (H) Plasma ALT (n = 17 for CD WT, n = 11 for CD IHet, n = 21 for HFD WT, n = 19 for HFD IHet. (I) Immunoblotting analysis of ApoA1 in the plasma (n = 3 for each group). (J) mRNA expression of Surf4 in the proximal intestines of male and female mice (n = 4 for each group). ∗p < 0.05, ∗∗p < 0.01, unpaired two-tailed Student's t-test. Data are presented as mean ± SEM.
Taken together, this study reveals that SURF4 is essential for intestinal lipid absorption, and targeting intestinal SURF4 may provide a new therapeutic strategy for hypercholesterolemia.
4. Discussion
Intestinal lipoprotein is important in facilitating the absorption of dietary lipids and their subsequent delivery into circulation. Lactation is known as the most effective approach to ensure the survival and growth of infants, where the lipids in breast milk are absorbed in the small intestine to supply the infant's energy requirements. Deficiency of proteins involved in key steps of lipid absorption can lead to infant growth retardation and death. Previous work has demonstrated that chylomicron secretion from enterocytes is critical for absorbing dietary lipids, and multiple proteins are involved in this process. For instance, lysophosphatidylcholine acyltransferase 3 (LPCAT3) plays a vital role in chylomicron secretion from enterocytes. Intestine-specific Lpcat3 knockout mice exhibit reduced plasma TG and growth retardation [21]. Knockout of intestinal Arfrp1 (ADP-ribosylation factor-related protein 1), a small trans-Golgi-associated GTPase responsible for chylomicron lipidation and maturation within the Golgi of intestinal epithelial cells, leads to hypolipidemia and growth retardation. More than half of these mice die within 1 week after birth [22]. Mutations in SAR1B result in defects in intracellular chylomicron trafficking and secretion and have been associated with chylomicron retention disease (CMRD) [[23], [24], [25]]. These studies revealed that the proper assembly and secretion of chylomicrons are essential for lipid absorption.
SURF4 is an ER transmembrane cargo receptor that regulates the ER export of diverse proteins. It modulates the cellular trafficking of various proteins, including erythropoietin [11], STING [26], α1-antitrypsin [12], progranulin [14], proinsulin [13], and sonic hedgehog [15]. Global Surf4 knockout mice displayed embryonic lethality [10]. Similarly, knockdown of C. elegans orthologous gene Sft-4 results in severe embryonic lethality [9], emphasizing its critical role in embryonic development. Recent studies have shown that SURF4 mediates the secretion of lipoproteins and regulates hepatic lipid metabolism. Hepatic knockout or knockdown of Surf4 hindered the secretion of VLDLs, resulting in a nearly complete depletion of circulating lipids in fasted conditions, and provided strong protection from atherosclerosis [16,17].
In this study, we discovered that SURF4 facilitates intestinal lipid absorption from the diet/milk by regulating the cellular transport and secretion of ApoB48, as well as two other apolipoproteins, ApoA1 and PRAP1. Intestinal Surf4 knockout mice display severe hypolipidemia and ectopic lipid deposition in the enterocytes, thereby disturbing lipid absorption. These findings were also partially confirmed by a recent study [19].
Through proteomic analysis, we identified five secretory proteins, ApoA1, PRAP1, NUCB1, IGF2, and SPINK1, which were increased in the intestines of IKO mice compared to WT mice. Within these five proteins, both NUCB1 and IGF2 can interact with SURF4, and NUCB1 has been identified as a cargo of SURF4; disruption of SURF4 affects NUCB1 secretion in vitro [15,27,28]. However, we only observed a decrease in ApoA1 and PRAP1, but not NUCB1, IGF2, and SPINK1 in the serum of IKO mice. Moreover, there are no current reports indicating that NUCB1 and SPINK1 are associated with lipid metabolism. SURF4 has been found as a cargo receptor for lipoprotein that bears ApoA1 in hepatocytes [16,29]. In IKO mice, circulating ApoA1 and TC were decreased, findings similar to those observed in intestine-specific Apoa1 knockout mice [30]. Through further investigation, we confirmed that SURF4 interacts with and regulates the trafficking of ApoA1 in intestinal epithelial cells.
PRAP1 has been shown to interact with MTTP in the presence of TG and promotes the lipidation of the chylomicron. PRAP1 deficiency or a mutation that impairs PRAP1 secretion impedes ApoB-containing lipoprotein assembly and secretion [7]. In this study, we reveal that SURF4 is the regulator for PRAP1 transport, as evidenced by the interaction of SURF4 and PRAP1. Deletion of intestinal SURF4 significantly increases PRAP1 accumulation in the small intestines and reduces plasma levels of PRAP1 and TG. This is also partially supported by a previous in vitro experiment, where PRAP1 is significantly increased in SURF4 KO Huh7 cells [28]. These findings affirm that PRAP1 is a newly identified interacting protein of SURF4, and SURF4 plays a critical role in regulating intestinal lipid homeostasis by regulating PRAP1 transport and secretion.
Previous studies using hepatic cells and murine liver lysates revealed that ApoB interacts with SURF4 [9,16]. Deletion of hepatic SURF4 resulted in decreased secretion of ApoB while increasing its accumulation in hepatocytes [16]. We also observed that the serum levels of ApoB are markedly reduced; however, the protein levels of ApoB48 are not changed in the small intestines of IKO mice. This could be attributed to insufficient lipidation and degradation of ApoB [31].
In addition, we observed attenuation in HFD-induced body weight gain and hyperlipidemia in female IHet mice. However, these metabolic changes were not evident in male IHet mice. Furthermore, we noticed that Surf4 expression in the small intestines of male IHet mice increased after HFD-feeding, while the expression was not affected by HFD-feeding in female IHet mice. The difference in Surf4 expression may explain the phenotypic differences between IHet males and females following HFD feeding. Further investigation into the underlying mechanism by which Surf4 expression is differently regulated by HFD in IHet males and females is needed. Nonetheless, our findings further confirm the crucial role of intestinal SURF4 in dietary cholesterol absorption and ApoA1 secretion. This highlights its potential as a promising target for the treatment of diet-related metabolic disorders.
Data and code availability
All data and additional information will be shared by the lead contact upon reasonable request.
This paper does not include the original code.
CRediT authorship contribution statement
Chun-Guang Guo: Conceptualization, Investigation, Visualization, Writing – original draft, Writing – review & editing. Rui Sun: Investigation, Writing – review & editing. Xiao Wang: Writing – review & editing, Resources. Ye Yuan: Investigation, Writing – review & editing. Yan Xu: Investigation. Shihan Li: Investigation. Xueting Sun: Investigation. Jue Wang: Writing – review & editing. Xinli Hu: Writing – review & editing. Tiannan Guo: Resources. Xiao-Wei Chen: Conceptualization, Resources, Writing – review & editing. Rui-Ping Xiao: Conceptualization, Writing – review & editing. Xiuqin Zhang: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank the Core Facilities at the School of Life Sciences, Peking University for their professional technical assistance in EM sample preparation and image analysis. We thank Dr. Ye-Guang Chen for the villin-creERT2 mice. This work was supported by the National Natural Science Foundation of China (81970690, 81630008, and 81770376) and the National Key Research and Development Program of China (2018YFA0801405).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2023.101847.
Contributor Information
Xiao-Wei Chen, Email: xiaowei_chen@pku.edu.cn.
Rui-Ping Xiao, Email: xiaor@pku.edu.cn.
Xiuqin Zhang, Email: zhangxq@pku.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Ko C.W., Qu J., Black D.D., Tso P. Regulation of intestinal lipid metabolism: current concepts and relevance to disease. Nat Rev Gastroenterol Hepatol. 2020;17:169–183. doi: 10.1038/s41575-019-0250-7. [DOI] [PubMed] [Google Scholar]
- 2.Wit M., Trujillo-Viera J., Strohmeyer A., Klingenspor M., Hankir M., Sumara G. When fat meets the gut-focus on intestinal lipid handling in metabolic health and disease. EMBO Mol Med. 2022;14 doi: 10.15252/emmm.202114742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dash S., Xiao C., Morgantini C., Lewis G.F. New insights into the regulation of chylomicron production. Annu Rev Nutr. 2015;35:265–294. doi: 10.1146/annurev-nutr-071714-034338. [DOI] [PubMed] [Google Scholar]
- 4.Siddiqi S., Saleem U., Abumrad N.A., Davidson N.O., Storch J., Siddiqi S.A., et al. A novel multiprotein complex is required to generate the prechylomicron transport vesicle from intestinal ER. J Lipid Res. 2010;51:1918–1928. doi: 10.1194/jlr.M005611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siddiqi S.A., Gorelick F.S., Mahan J.T., Mansbach C.M., 2nd COPII proteins are required for Golgi fusion but not for endoplasmic reticulum budding of the pre-chylomicron transport vesicle. J Cell Sci. 2003;116:415–427. doi: 10.1242/jcs.00215. [DOI] [PubMed] [Google Scholar]
- 6.Giammanco A., Cefalù A.B., Noto D., Averna M.R. The pathophysiology of intestinal lipoprotein production. Front Physiol. 2015;6:61. doi: 10.3389/fphys.2015.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng H., Chiu T.Y., Liang Y.J., Lee C.J., Liu C.S., Suen C.S., et al. PRAP1 is a novel lipid-binding protein that promotes lipid absorption by facilitating MTTP-mediated lipid transport. J Biol Chem. 2021;296 doi: 10.1074/jbc.RA120.015002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cominetti O., Núñez G.A., Corthésy J., Valsesia A., Irincheeva I., Kussmann M., et al. Obesity shows preserved plasma proteome in large independent clinical cohorts. Sci Rep. 2018;8 doi: 10.1038/s41598-018-35321-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saegusa K., Sato M., Morooka N., Hara T., Sato K. SFT-4/Surf4 control ER export of soluble cargo proteins and participate in ER exit site organization. J Cell Biol. 2018;217:2073–2085. doi: 10.1083/jcb.201708115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emmer B.T., Lascuna P.J., Tang V.T., Kotnik E.N., Saunders T.L., Khoriaty R., et al. Murine Surf4 is essential for early embryonic development. PLoS One. 2020;15 doi: 10.1371/journal.pone.0227450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin Z., King R., Tang V., Myers G., Balbin-Cuesta G., Friedman A., et al. The endoplasmic reticulum cargo receptor SURF4 facilitates efficient erythropoietin secretion. Mol Cell Biol. 2020;40 doi: 10.1128/MCB.00180-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ordóñez A., Harding H.P., Marciniak S.J., Ron D. Cargo receptor-assisted endoplasmic reticulum export of pathogenic α1-antitrypsin polymers. Cell Rep. 2021;35 doi: 10.1016/j.celrep.2021.109144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saegusa K., Matsunaga K., Maeda M., Saito K., Izumi T., Sato K. Cargo receptor Surf4 regulates endoplasmic reticulum export of proinsulin in pancreatic β-cells. Commun Biol. 2022;5:458. doi: 10.1038/s42003-022-03417-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devireddy S., Ferguson S.M. Efficient progranulin exit from the ER requires its interaction with prosaposin, a Surf4 cargo. J Cell Biol. 2022;221 doi: 10.1083/jcb.202104044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang X., Chen R., Mesias V.S.D., Wang T., Wang Y., Poljak K., et al. A SURF4-to-proteoglycan relay mechanism that mediates the sorting and secretion of a tagged variant of sonic hedgehog. Proc Natl Acad Sci U S A. 2022;119 doi: 10.1073/pnas.2113991119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X., Wang H., Xu B., Huang D., Nie C., Pu L., et al. Receptor-mediated ER export of lipoproteins controls lipid homeostasis in mice and humans. Cell Metabol. 2021;33:350–366.e7. doi: 10.1016/j.cmet.2020.10.020. [DOI] [PubMed] [Google Scholar]
- 17.Wang B., Shen Y., Zhai L., Xia X., Gu H.M., Wang M., et al. Atherosclerosis-associated hepatic secretion of VLDL but not PCSK9 is dependent on cargo receptor protein Surf4. J Lipid Res. 2021;62 doi: 10.1016/j.jlr.2021.100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang V.T., McCormick J., Xu B., Wang Y., Fang H., Wang X., et al. Hepatic inactivation of murine Surf4 results in marked reduction in plasma cholesterol. Elife. 2022;11 doi: 10.7554/eLife.82269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tao G., Wang H., Shen Y., Zhai L., Liu B., Wang B., et al. Surf4 (surfeit locus protein 4) deficiency reduces intestinal lipid absorption and secretion and decreases metabolism in mice. Arterioscler Thromb Vasc Biol. 2023;43:562–580. doi: 10.1161/ATVBAHA.123.318980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abumrad N.A., Davidson N.O. Role of the gut in lipid homeostasis. Physiol Rev. 2012;92:1061–1085. doi: 10.1152/Fphysrev.00019.2011. [online] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rong X., Wang B., Dunham M.M., Hedde P.N., Wong J.S., Gratton E., et al. Lpcat3-dependent production of arachidonoyl phospholipids is a key determinant of triglyceride secretion. Elife. 2015;4 doi: 10.7554/eLife.06557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaschke A., Chung B., Hesse D., Kluge R., Zahn C., Moser M., et al. The GTPase ARFRP1 controls the lipidation of chylomicrons in the Golgi of the intestinal epithelium. Hum Mol Genet. 2012;21:3128–3142. doi: 10.1093/hmg/dds140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sassolas A., Di M., Aggerbeck L.P., Peretti N., Samson-Boum M.E. Mutations in human genetic disease. InTech; 2012. [online] [DOI] [Google Scholar]
- 24.Jones B., Jones E.L., Bonney S.A., Patel H.N., Mensenkamp A.R., Eichenbaum-Voline S., et al. Mutations in a Sar1 GTPase of COPII vesicles are associated with lipid absorption disorders. Nat Genet. 2003;34:29–31. doi: 10.1038/ng1145. [DOI] [PubMed] [Google Scholar]
- 25.Levy E., Poinsot P., Spahis S. Chylomicron retention disease: genetics, biochemistry, and clinical spectrum. Curr Opin Lipidol. 2019;30:134–139. doi: 10.1097/MOL.0000000000000578. [DOI] [PubMed] [Google Scholar]
- 26.Mukai K., Ogawa E., Uematsu R., Kuchitsu Y., Kiku F., Uemura T., et al. Homeostatic regulation of STING by retrograde membrane traffic to the ER. Nat Commun. 2021;12:61. doi: 10.1038/s41467-020-20234-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang Y., Yin H., Li B., Wu Q., Liu Y., Poljak K., et al. An in vitro vesicle formation assay reveals cargo clients and factors that mediate vesicular trafficking. Proc Natl Acad Sci U S A. 2021;118 doi: 10.1073/pnas.2101287118. [online] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomez-Navarro N., Maldutyte J., Poljak K., Peak-Chew S.Y., Orme J., Bisnett B.J., et al. Selective inhibition of protein secretion by abrogating receptor-coat interactions during ER export. Proc Natl Acad Sci U S A. 2022;119 doi: 10.1073/pnas.2202080119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang V.T., Abbineni P.S., da Veiga Leprevost F., Basrur V., Emmer B.T., Nesvizhskii A.I., et al. 2023. Identification of LMAN1 and SURF4 dependent secretory cargoes. [online] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsujita M., Vaisman B., Chengyu L., Vickers K.C., Okuhira K.I., Braesch-Andersen S., et al. Apolipoprotein A-I in mouse cerebrospinal fluid derives from the liver and intestine via plasma high-density lipoproteins assembled by ABCA1 and LCAT. FEBS Lett. 2021;595:773–788. doi: 10.1002/1873-3468.13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ginsberg H.N., Fisher E.A. The ever-expanding role of degradation in the regulation of apolipoprotein B metabolism. J Lipid Res. 2009;50(Suppl):S162–S166. doi: 10.1194/jlr.R800090-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mao J., Hu X., Xiao Y., Yang C., Ding Y., Hou N., et al. Overnutrition stimulates intestinal epithelium proliferation through β-catenin signaling in obese mice. Diabetes. 2013;62:3736–3746. doi: 10.2337/db13-0035. [online] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao Y., Wang C., Chen J.Y., Lu F., Wang J., Hou N., et al. Deficiency of PRKD2 triggers hyperinsulinemia and metabolic disorders. Nat Commun. 2018;9:2015. doi: 10.1038/s41467-018-04352-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang X., Spandidos A., Wang H., Seed B. PrimerBank: a PCR primer database for quantitative gene expression analysis, 2012 update. Nucleic Acids Res. 2012;40:D1144–D1149. doi: 10.1093/nar/gkr1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen B., Yi X., Sun Y., Bi X., Du J., Zhang C., et al. Proteomic and metabolomic characterization of COVID-19 patient sera. Cell. 2020;182:59–72.e15. doi: 10.1016/j.cell.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nie X., Qian L., Sun R., Huang B., Dong X., Xiao Q., et al. Multi-organ proteomic landscape of COVID-19 autopsies. Cell. 2021;184:775–791.e14. doi: 10.1016/j.cell.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and additional information will be shared by the lead contact upon reasonable request.
This paper does not include the original code.
Data will be made available on request.